Abstract
Although different preclinical models have demonstrated a favorable role for bone marrow-derived mesenchymal stem cells (B-MSC) in preventing fibrosis, this protective effect is not observed with late administration of these cells, when fibrotic changes are consolidated. We sought to investigate whether the late administration of B-MSCs overexpressing microRNAs (miRNAs) let-7d (antifibrotic) or miR-154 (profibrotic) could alter lung fibrosis in a murine bleomycin model. Using lentiviral vectors, we transduced miRNAs (let-7d or miR-154) or a control sequence into human B-MSCs. Overexpression of let-7d or miR-154 was associated with changes in the mesenchymal properties of B-MSCs and in their cytokine expression. Modified B-MSCs were intravenously administered to mice at day 7 after bleomycin instillation, and the mice were euthanized at day 14. Bleomycin-injured animals that were treated with let-7d cells were found to recover quicker from the initial weight loss compared with the other treatment groups. Interestingly, animals treated with miR-154 cells had the lowest survival rate. Although a slight reduction in collagen mRNA levels was observed in lung tissue from let-7d mice, no significant differences were observed in Ashcroft score and OH-proline. However, the distinctive expression in cytokines and CD45-positive cells in the lung suggests that the differential effects observed in both miRNA mice groups were related to an effect on the immunomodulation function. Our results establish the use of miRNA-modified mesenchymal stem cells as a potential future research in lung fibrosis.
Keywords: bone marrow-derived mesenchymal stem cells, microRNA, lung fibrosis, immunomodulation
Bone marrow-derived-mesenchymal stem cells (B-MSCs), a group of multipotent cells with the ability to differentiate in vitro into multiple cell types and modulate immune response in vivo, have been suggested as an attractive therapeutic candidate for a variety of lung diseases, including idiopathic pulmonary fibrosis (IPF) (1, 23). Our group and other investigators have shown that infusion of B-MSCs can protect the lung from severe inflammation and fibrotic scarring induced by bleomycin in mice (24, 25, 30), the most commonly used animal model for pulmonary fibrosis (3). However, late administration of B-MSCs in the lung fibrosis bleomycin model—when fibrotic changes already exist and there is a decrease in inflammatory response—does not have any protective effect (5, 24). These findings are important as they suggest a scenario by which fibrosis is already consolidated, and more adequately resembles IPF (3).
The manipulation of B-MSCs by overexpressing certain microRNAs (miRNAs) is a novel approach to modify or enhance the capacities of these cells. miRNAs are short, noncoding RNA strands that have an epigenetic regulation of gene expression through posttranscriptional gene silencing. They are known to have multiple target genes and are involved in multiple cellular pathways (16). In stem cells, miRNAs play a role in maintaining stemness, self-renewal, and differentiation (11). Several studies have demonstrated that manipulation of specific miRNA expression can alter the properties of B-MSCs (9, 13, 38). However, whether modification of B-MSCs using specific miRNAs could alter their antifibrotic properties has not been previously studied. Some preliminary studies (14, 20) have assessed the effects of miRNA modification in fibroblasts, the pivotal cells in IPF. Previously, we described that overexpression of miRNA let-7d in fibroblasts causes changes in their mesenchymal and phenotypic properties, reducing their fibrotic capacities (14). Conversely, the overexpression of miRNA-154 in the same cells has the opposite effect. Rather than reducing fibrotic capacities, fibroblast proliferation and migration were enhanced (20). As these miRNAs seem to be implicated in the regulation of pulmonary fibrosis (26), the study of B-MSC modification using these miRNAs and their effect on fibrotic properties is relevant.
Accordingly, we sought to investigate whether the overexpression of miRNA let-7d or miR-154 in B-MSCs could modify their antifibrotic properties. In addition, we assessed the effect of these modified B-MSCs in a murine model 7 days after bleomycin instillation, when fibrosis already exists and more adequately resembles IPF, a period in which naive B-MSCs have not shown beneficial effects in reversing bleomycin injury (5, 24).
MATERIALS AND METHODS
Human B-MSC cultures and transfection.
Human B-MSCs (Tulane University, New Orleans, LA) were cultured with αMEM, 16% FBS, 2 mM l-glutamine, and 100 µg/ml penicillin G-streptomycin sulfate (Invitrogen, Waltham, MA) in a humidified chamber at 37°C with 5% CO2.
Approximately 20 µl/ml (2 million particles total) of concentrated let-7d, miR-154, or a control sequence lentiviral vector (Open Biosystems, Huntsville, TX) were transduced into human B-MSCs that were ~85% confluent in a T-25 flask. Cells were incubated overnight with concentrated virus and 6 mg/ml of polybrene. Cells were then selected using 2 µg/ml of puromycin solution (Sigma-Aldrich, St. Louis, MO) in growth media. Cells were grown in puromycin selection media until most of them were dead, and all living cells appeared green under the fluorescent scope [vector carries a green gluorescent protein (GFP) for selection].
As previously reported (7), endotoxin (LPS) activates B-MSCs. To assess cytokine expression of B-MSCs, we stimulated modified B-MSCs with 200 ng/ml LPS (Sigma-Aldrich) for 24 h (7). Cells were serum-starved in αMEM with 1% antibiotic-antimycotic for 12–16 h before stimulation.
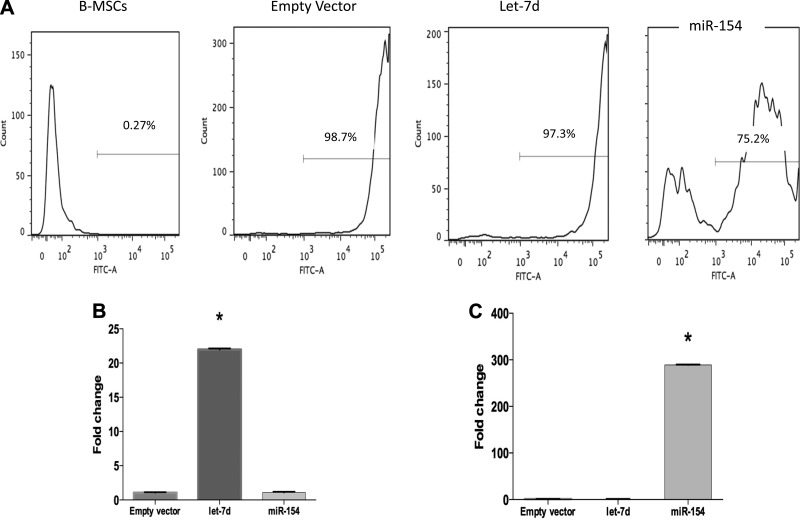
Using FACS, we confirmed transfection efficiency (GFP-positive cells compared with the total cell population) post-lentivirus transduction (Fig. 1A). Using qPCR, we confirmed a higher expression of let-7d and miR-154 post-transduction and puromycin selection compared with empty vector cells (Fig. 1, B and C).
Fig. 1.
Stable transfection of let-7d (antifibrotic) and miR-154 in B-MSCs is successful. Human bone-mesenchymal stem cells (B-MSCs) were stably transfected with let-7d, miR-154, or an empty vector control using lentiviral particles. To confirm successful transduction, we evaluated the presence of green fluorescent protein (GFP) using a fluorescent microscope, due to the expression of GFP in the expression lentivirus construct. Using FACS, we quantified the efficiency of the transduction (A). We also evaluated let-7d and miR-154 expression levels using qPCR (n = 3) (B and C). *P < 0.05.
Animal model.
Eight- to ten-week-old female C57BL/6 mice (Jackson Laboratory, Bar Harbor, ME), weighing 18–22 g, were anesthetized by ketamine via intraperitoneal injection and treated with 50 µl of bleomycin hydrochloride solution (63323-136-10; APP Pharmaceuticals; Schaumburg, IL) containing 2.15 U/kg of bleomycin dissolved in sterile saline or sterile saline alone, delivered by direct injection into the trachea using a 0.9-mm needle. Mice were treated intravenously with 500,000 modified and nonmodified B-MSCs on day 7 after bleomycin injection. Control group mice received the same volume of sterile media solution. The body weight of the mice was measured daily for 14 days after the day of intratracheal administration of bleomycin. Animals were euthanized at day 14. The left lung was used for OH-proline assay. The right lung was cut into pieces: half inflated with formaline for histological analysis and half for RNA analysis.
All animal protocols were reviewed and approved by the Institutional Animal Care and Use Committee.
RNA isolation and real-time quantitative RT-PCR analysis in B-MSCs.
Total RNAs and miRNAs were isolated using the miRNeasy mini kit (Qiagen, Valencia, CA), according to the manufacturer’s instructions. RNA quantity was determined by a Nanodrop ND-1000 spectrophotometer (Thermo Fisher Scientific, Wilmington, DE), and its quality was determined using Agilent Bioanalyzer 2100 (Agilent Technologies, Wilmington, DE).
TaqMan miRNA assays [Applied Biosystems (ABI), Foster City, CA] were used to determine the relative expression levels of let-7d and miR-154 in cells. The results were analyzed by the ΔΔCt method using RNU43 control RNA to normalize the results. Fold change was calculated taking empty lentiviral vector transduction as the baseline.
TaqMan gene expression assays (ABI) were used to determine the relative expression levels of α-smooth muscle actin (α-SMA), N-cadherin (N-cad), fibroblast surface protein-1 (FSP-1) (mesenchymal markers), tight junction protein-1, keratin 19 (KRT-19) (epithelial markers), and SLUG (transcriptional factor implicated in epithelial-mesenchymal transition) in cells. These markers were chosen on the basis of our previous publications (14). Additionally, we tested the expression of two representative cytokines in B-MSCs: IL-6 (proinflammatory) and IL-1RN (anti-inflammatory) (4). TaqMan gene expression assays (ABI) were also used to determine the relative expression levels of α-SMA, N-cad, FSP-1, high-mobility group-A2 protein (HMGA-2), and collagen 1 (Col-1) in mice lung samples. Results were analyzed by the ΔΔCt method, and GUSB was used for normalization. Fold change was normalized to empty lentiviral vector transduction control.
Microarrays.
Total RNAs and miRNAs were isolated as described above. RNA quantity and integrity were determined by NanoDrop and Bioanalyzer analysis, respectively. Labeling was performed using the Agilent Low RNA input linear amplification kit PLUS, one color (5184-3523, Agilent Technologies), according to the manufacturer’s protocol. To perform mRNA microarray profiling, 50 ng of total RNA from B-MSCs transduced with let-7d, miR-154, or empty virus was extracted and used as a template for double-stranded cDNA synthesis. The cDNA was used as a template to generate Cy3-labeled cRNA (using the low-input kit) to be used after for hybridization. Subsequently, after purification and fragmentation of the samples was performed, they were hybridized to Agilent. Each array, after hybridization, was sequentially washed and scanned by Agilent Microarray Scanner. Images were processed using Agilent’s feature extraction software version 10.7.3.1. After the labeled cDNA was purified and fragmented, aliquots of each sample were hybridized to Agilent SurePrint G3 Human Gene Expression 8 × 60 K v. 2 Microarray at 65°C for 17 h (Agilent Technologies). All arrays were scanned by an Agilent Microarray Scanner and were visually inspected individually for hybridization defects and quality control failures. For the array readout, we used Agilent feature extraction 10.7.3.1. software. Signals were processed and normalized using GeneSpring software (Agilent Technologies), as well as the identification of differentially expressed genes. A q value of 5 that corresponds to a false discovery rate of 5% was set as the threshold for significance. Differentially regulated genes were analyzed by ingenuity pathway analysis (IPA) to determine gene expression signature signaling and pathways.
Gene network analysis.
Agilent SurePrint G3 Human GE v. 2 probes were collapsed to genes (19), then 2,138 genes included in the gene ontology (GO) term “immune system process” (GO:0002376) (http://www.ebi.ac.uk/QuickGO) were selected and used to build the coexpression networks for cells transduced with empty vector, let-7d, or miR-154. In each case, Pearson correlation was calculated from triplicates using the R platform, and the significance thresholds were |R| > 0.8, P < 0.01 or P < 0.001, as indicated in the text. For further analysis, correlations involving a differentially expressed gene (in mir-154 vs. empty vector or let-7d vs. empty vector) were considered. Results were graphically represented with Cytoscape (31). The list of miR-154 and let-7d targets was obtained from http://www.microrna.org (18).
OH-proline assay.
Collagen content of the left lung tissue was measured spectrophotometrically by absorbance at 550 nm to quantify the OH-proline content of the lung 14 days after bleomycin treatment. In short, the minced left lung lobes were homogenized in 6 mol/l HCl and hydrolyzed for 5 h at 130°C. The pH was adjusted to 6.5–7.0 with NaOH, and the sample volume was adjusted to 30 ml with distilled water. The sample solution (1.0 ml) was mixed with 1.0 ml of chloramine T solution (0.05 mol/l), and then the mixture was incubated for 20 min at room temperature. The absorbance at 550 nm was analyzed. The results were expressed as micrograms OH-proline per milligrams of wet lung weight using OH-proline standards (Sigma).
Histology and inmunohistochemistry.
For microscopic observation, the lung samples were fixed in 4% paraformaldehyde for 24 h, and then lungs were sectioned, placed on microscope slides, and stained with hematoxylin and eosin (H&E) for routine histological examination. Masson’s trichrome staining (Sigma-Aldrich) was used to delineate collagen content.
Additional Col-1A staining (Thermo Fisher Scientific) was performed on slides from sectioned lungs. To assess collagen deposition quantitatively, images were taken using an Axio Zeiss Observer A1 microscope (Zeiss, Oberkochen, Germany). Collagen staining was quantified and determined by percentage of stained areas from four different images per animal. Images were taken at ×20 magnification. Images were analyzed with ImageJ software (ImageJ 1.51f version; National Institutes of Health (NIH), Bethesda, MD).
Lung sections from mice were also incubated in conjugated anti-CD-45 antibodies (Thermo Fisher Scientific) and DAPI (Life Technologies, Eugene, OR). Slides were assessed with an Axio Observer immunofluorescence microscope with an Axio Cam 503 camera. The percentage of CD-45-positive cells was determined by comparison to total cells per field of view from five different images per mouse at ×20 magnification. Images were analyzed using ImageJ software (ImageJ 1.51f version; NIH).
Fibrosis scoring.
Ashcroft scoring was performed, as described previously by Ashcroft (2). All lobes were sectioned on a single slide, and 20 nonoverlapping ×10 fields were scored per sample, and the average score of all fields indicates the Ashcroft Score of a single sample. The scoring scale was as follows: 0 = no abnormalities, 1 = slight thickening of alveolar membranes, 2 = small areas of fibrosis (<10%), 3 = 10–20% fibrotic area, 4 = 20–40% fibrotic area, 5 = 40–60% fibrosis, 6 = 60–80% fibrosis, 7 = >80% fibrosis, and 8 = complete fibrosis.
Statistical analysis.
Statistical analyses were done using GraphPad Prism version 5 (GraphPad Software, San Diego, CA). Group comparisons were made using an unpaired, two-tailed Student’s t-test or one-way ANOVA with Bonferroni correction for multiple comparisons. Weight loss was analyzed with two-way ANOVA and Bonferroni-corrected multiple-comparison tests among different groups. Survival analysis of mice was performed using Kaplan-Meier curves and the log-rank (Mantel-Cox) test. Significant enrichment of overexpression of miRNAs in specific chromosomal locations was determined using Fisher’s exact test. A P value of less than 0.05 was considered to be significant.
RESULTS
Overexpression of let-7d in B-MSCs causes changes in global gene expression.
To investigate the impact of let-7d in B-MSC biology, first, we assessed by qPCR the modification in several mesenchymal and epithelial markers, measured via qPCR. Treatment with let-7d resulted in a significant decrease in α-SMA expression, while a significant increase in KRT19 was noted. Furthermore, SLUG, a transcription factor that is involved in the epithelial-mesenchymal transition process, was found to be statistically downregulated after let-7d transduction (Fig. 2A). Next, we assessed changes in whole human gene expression in these cells using arrays. Let-7d transduction differentially expressed (DE) 3981 genes compared with the empty vector transduction (Fig. 2B). Using IPA, we have identified the top pathways enriched from the DE gene list, as shown in Fig. 2C. Interestingly, TGF-β signaling pathway, commonly associated with pulmonary fibrosis (33), was part of the pathways list generated by the DE let-7d-modified B-MSCs group. (R = 25). Specifically, we observed downregulation of TGF-β2 and TGF-β receptor 2 gene. Conversely, TGF-β3, which opposes the effects of TGF-β1 and TGF-β2, was upregulated.
Fig. 2.
Gene expression of epithelial/mesenchymal markers after let-7d transduction. B-MSCs were transfected with let-7d and an empty vector (n = 3). Gene expression was evaluated 24 h posttransfection using quantitative PCR analysis. The results are shown as means ± range derived from the change in cycle threshold (ΔCT) SD (A). Microarray analysis was used to evaluate global gene expression changes; 3,981 differentially expressed genes were demonstrated using a heat map, which represents statistically significant (P ≤ 0.05), differentially expressed mRNAs. Upregulated RNAs are shown in yellow, and downregulated RNAs are shown in purple (B). Ingenuity pathway analysis (IPA) was used to evaluate pathways and networks that were significantly changed post transduction. P is calculated by Fisher’s exact test (right-tailed); R is the ratio of the number of genes in the indicated pathway divided by the total number of genes that make up that pathway (C). ***P < 0.001.
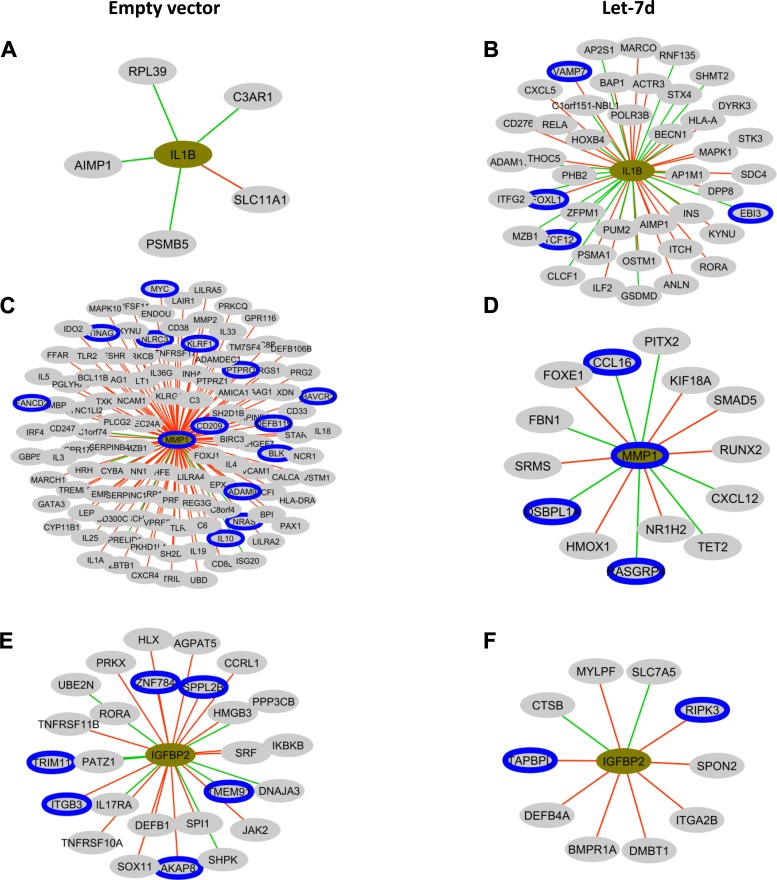
To assess the existence of immunity-related alterations in let-7d B-MSCs transcriptomics, we obtained the list of the 2,138 genes included in the GO term “immune system process” (GO:0002376) and with them built the let-7d B-MSCs or empty vector B-MSCs coexpression networks with DE genes defined in the previous analysis detailed in methods. The most relevant network correlations of DE genes in let-7d compared with empty vector were IL-1β, MMP1, and IGFBP2. A higher number of correlated genes were observed in IL-1β in let-7d cells (Fig 3, A and B). In contrast, a lower number of correlations were observed in MMP1 (Fig. 3, C and D) and IGFBP2 (Fig 3, E and F).
Fig. 3.
Pearson coexpresssion networks in B-MSCs transfected with empty vector or let-7d. Cytoscape representation of the Pearson correlations with |r|>0.8 and P < 0.01 observed for IL-1B, matrix metalloproteinase 1, and insulin-like growth factor binding protein 2 in mesenchymal stem cells transfected with the empty vector (A, C, E) or with let-7d (B, D, F). In the network, genes are colored in relation to its fold change. Gray denotes no statistically significant differences, green edges denote negative correlations, red edges denote positive correlations and a blue border denotes that the gene is a putative let-7d target, as detailed in methods.
Overexpression of miR-154 in B-MSCs causes changes in global gene expression.
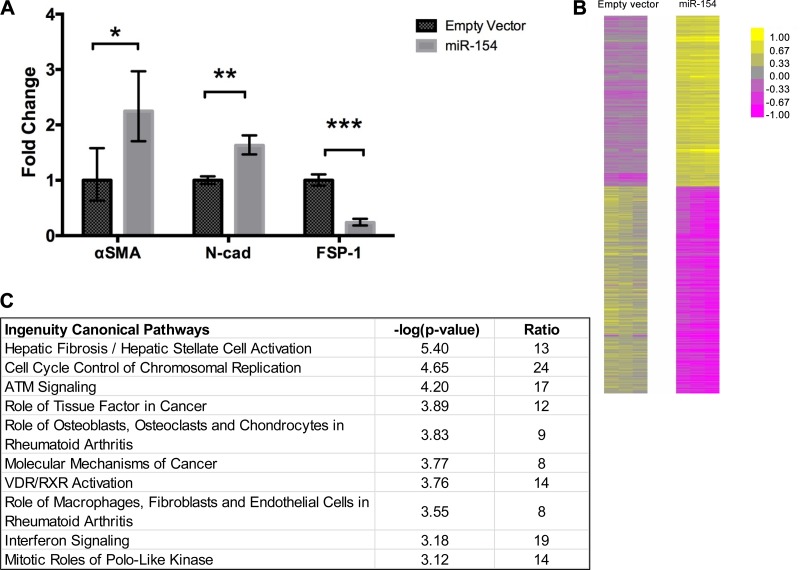
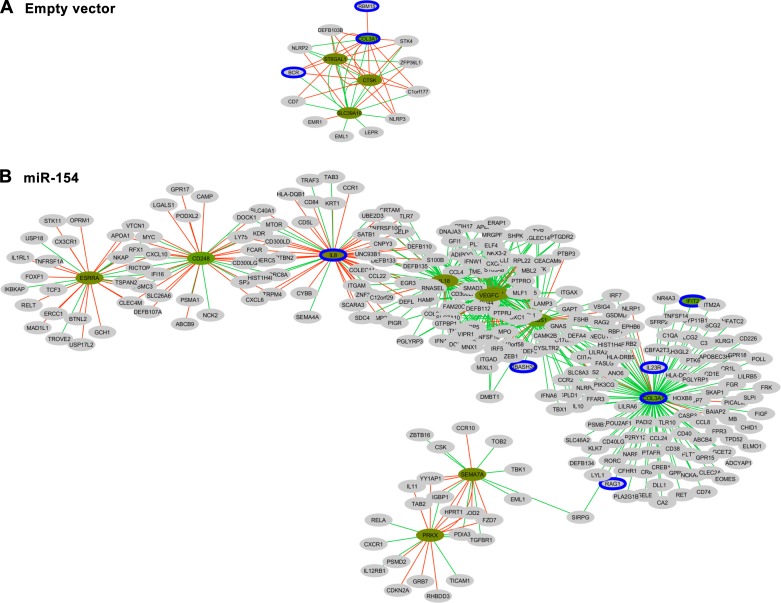
Several mesenchymal and epithelial markers were also measured via qPCR in miR-154 B-MSCs. Contrary to the effects of let-7d, overexpression of miR-154 resulted in a significant increase in α-SMA and N-cad expression compared with the empty vector group. However, FSP-1 expression decreased compared with the empty vector. (Fig. 4A). Whole human gene expression arrays showed that miR-154 transduction results in 1,396 DE genes compared with the empty vector transfection (Fig. 4B). In Fig. 4C, IPA analysis revealed the top pathways associated with the DE genes. Although no pathways directly related to IPF were included in the top pathways, other common pathways associated with IPF were found, specifically hepatic fibrosis/hepatic stellate cell activation (R = 13) and the role of macrophages, fibroblasts, and endothelial cells in rheumatoid arthritis (R = 8). However, the number of global DE genes and the different ratio of the pathways were lower than the let-7d B-MSCs group. Similarly to let-7d B-MSCs analysis, we selected 2,138 (GO:002376 immune system response) genes to build the gene coexpression correlation networks between empty vector and miR-154. The most relevant network involving DE genes in miR-154 compared with empty vector included genes as COL-3A, VEGFC, IL-8, and IL-18. These DE genes have a higher connectivity in miR-154 than in empty vector cells (Fig. 5).
Fig. 4.
Gene expression of epithelial/mesenchymal markers after miR-154 transduction. B-MSCs were transfected with mir-154 and an empty vector (n = 3). Gene expression was evaluated 24-h post transfection using quantitative PCR analysis. The results are shown as means ± range derived from ΔCT SD (A). Microarray analysis was used to evaluate global gene expression changes; 1,396 differentially expressed genes were demonstrated using a heat map, which represents statistically significant (P ≤ 0.05), differentially expressed mRNAs. Upregulated RNAs are shown in yellow, and downregulated RNAs are shown in purple (B). Ingenuity pathway analysis (IPA) was used to evaluate pathways and networks that were significantly changed post transduction. P is calculated by Fisher’s exact test (right-tailed); R is the ratio of the number of genes in the indicated pathway divided by the total number of genes that make up that pathway. (C). *P < 0.05, **P < 0.01, ***P < 0.001.
Fig. 5.
Pearson coexpression networks in B-MSCs transfected with empty vector or miR-154. Cytoscape representation of the Pearson correlations with |r|>0.8 and P < 0.01 observed for COL3A in mesenchymal stem cells transfected with the empty vector (A) or with miR-154 (B). In the network, genes are colored in relation to its fold change. Gray denotes no statistically significant differences, green edges denote negative correlations, red edges denote positive correlations and a blue border denotes that the gene is a putative miR-154 target, as detailed in methods.
Modified B-MSCs overexpressing let-7d and miR-154 could modulate cytokine expression.
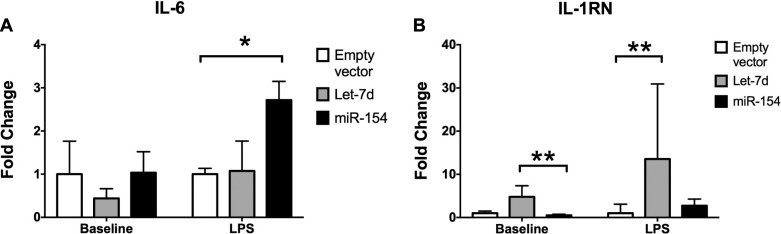
To evaluate the cytokine expression in modified B-MSCs, we assessed the expression of IL-6 and IL-1RN. In addition, we assessed the response of both cytokines after LPS stimulation in modified B-MSCs (Fig. 6). Let-7d B-MSCs had a lower expression of IL-6. IL-6 levels were not elevated after LPS stimulation. However, miR-154 significantly elevated IL-6 levels after LPS stimulation compared with the empty vector control (Fig. 6A). Contrastingly, IL-1RN expression levels were increased in Let-7d B-MSCs, but not in miR-154. After LPS stimulation, an even bigger fold change difference was observed in comparison to the empty vector control group. No significant change was found in miR-154 B-MSCs group (Fig. 6B).
Fig. 6.
IL-6 and IL-1RN expression in miRNA-modified B-MSCs. B-MSCs were transfected with mir-154, let-7d, and an empty vector. Each cell line was divided in a group stimulated during 24 h with LPS (200 ng/ml) (n = 3) and a group with no stimulation (n = 3). Extracted RNA from the cells was used to examine the expression levels of IL-6 (A) and IL-1RN (B). The results are shown as means ± range derived from ΔCT SD, *P < 0.05, **P < 0.01.
Weight loss is reversed after treatment with B-MSCs modified with let-7d during bleomycin injury.
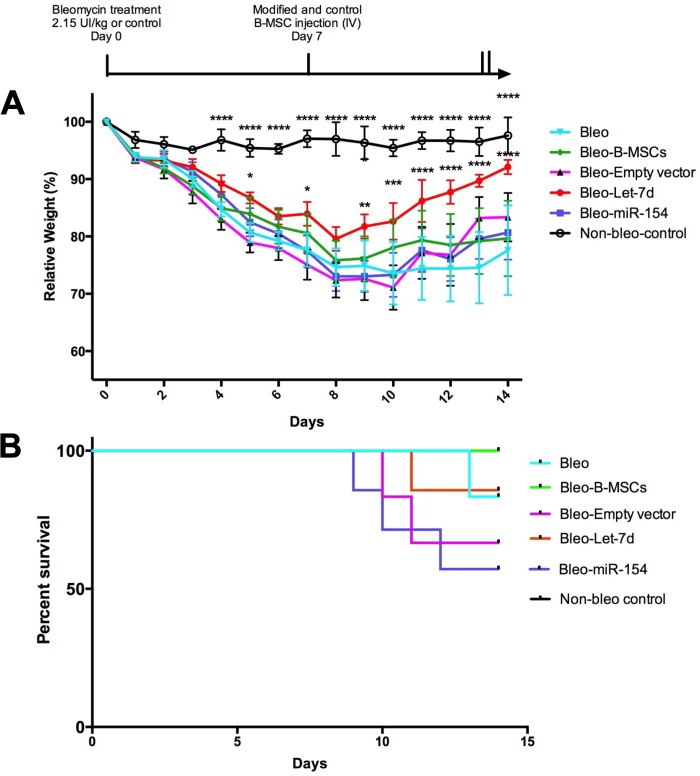
Mice were injected (intratracheally) at day 0 with bleomycin (2.15 U/kg) or sterile saline (control group, n = 3). At day 7, mice were infused (500,000 B-MSCs in each group, intravenously) with control B-MSCs (n = 6) and modified B-MSCs [B-MSCs transduced with an empty vector (n = 6), let-7d (n = 7), and miR-154 (n = 7)] or did not receive any B-MSC infusion (bleomycin group, n = 6). Mice were weighed daily, and all started to lose weight after bleomycin treatment. The control group showed significantly higher weight compared with the bleomycin group from day 4 to 14 (P < 0.0001). After the infusion of B-MSCs, the only group that showed recovery was the group treated with B-MSCs transduced with let-7d. This group showed significantly higher weight values from day 9 to 14 compared with the “untreated” bleomycin group. No significant weight changes were observed when the groups treated with nonmodified B-MSCs were compared with the bleomycin group (Fig. 7A).
Fig. 7.
Mice weight and survival is affected by injected miRNA-modified B-MSCs. Experimental design: Mice were injected intratracheally at day 0 with bleomycin (2.15 units/kg) or sterile saline (control group, n = 3). At day 7, mice were injected intravenously with control B-MSCs (n = 6) and modified B-MSCs [B-MSCs transduced with an empty vector (n = 6), let-7d (n = 7), and miR-154 (n = 7)] or not received any injection (bleomycin group, n = 6). A: body weight curves of mice are shown as the percentage of day 0 weight set as 100%. Two-way ANOVA Bonferroni corrected for multiple comparisons was performed, using bleomycin as the reference group, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. The results are shown as means ± SE B: Kaplan Meier survival curves. Log-rank test (P value = not significant).
Mice treated with modified B-MSCs with miR-154 had the worst survival among the different groups.
Fig. 7B shows the Kaplan-Meier survival curves of the different experimental groups. No statistically significant difference was reached when the log-rank test was calculated, although survival rates were quite different at day 14. The control group (no bleomycin) had 100% survival probability along with the B-MSC group (without lentivirus). Both bleomycin and let-7d groups had 85% survival probability. On the other hand, the group of mice injected with empty vector-transduced B-MSCs showed 67% survival probability. The group of mice injected with miR-154-transduced B-MSCs showed the lowest survival probability (57.5%). When the survival of the miR-154 group was individually compared with the group of mice with 100% survival (B-MSCs and control group together), the log-rank test obtained a P value of 0.03.
Modified B-MSCs effect on lung injury and CD45-positive cells.
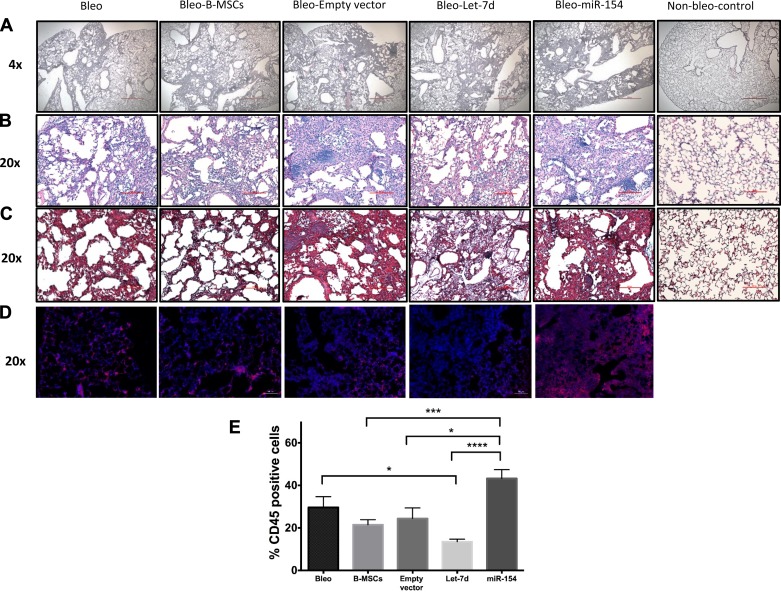
Histological sections were made from the right lung of each mouse. To assess overall lung condition and collagen expression, H&E (Fig. 8, A and B) and trichrome staining (Fig. 8C) were performed on the lung sections. As expected, mice from the bleomycin group showed an increase in lung fibrosis. When assessing the histological sections, no fibrotic differences were observed among groups, except in the control group, where no fibrosis was observed. As CD45 is a surface marker for inflammatory cells (15), we assessed the presence of CD45-positive cells in lungs of each group as a measure of cell inflammation associated with bleomycin injury and the influence of modified B-MSCs in inflammatory cells (Fig. 8D). Interestingly, in let-7d B-MSC-treated mice, the rate of CD45-positive cells was lower compared with bleomycin nontreated group. By contrast, the number of CD45-positive cells was higher in the mice treated with miR-154 B-MSCs (Fig. 8E).
Fig. 8.
miRNA modified B-MSCs and fibrosis in the lung. Mice were injected intratracheally at day 0 with bleomycin or sterile saline (control group, n = 3). At day 7, mice were injected intravenously with control B-MSCs (n = 6) and modified B-MSCs [B-MSCs transduced with an empty vector (n = 6), let-7d (n = 7), and miR-154 (n = 7)] or not received any injection (bleomycin group, n = 6). Mice were euthanized at day 14. Lungs from all the groups of mice were used for histological evaluation using hematoxylin and eosin (A and B) and Masson’s trichrome staining (C). CD45-positive cells (red) over total cells (blue) were assessed in lung slides obtained from the different mice model groups (D and E). Scale bars: 500 µm in A, 100 µm in B and C, 50 µm in D. *P < 0.05. ***P < 0.001. ****P < 0.0001.
Modified B-MSCs overexpressing let-7d do not reverse established fibrosis after bleomycin injury, but reduce collagen transcript levels in mouse lungs.
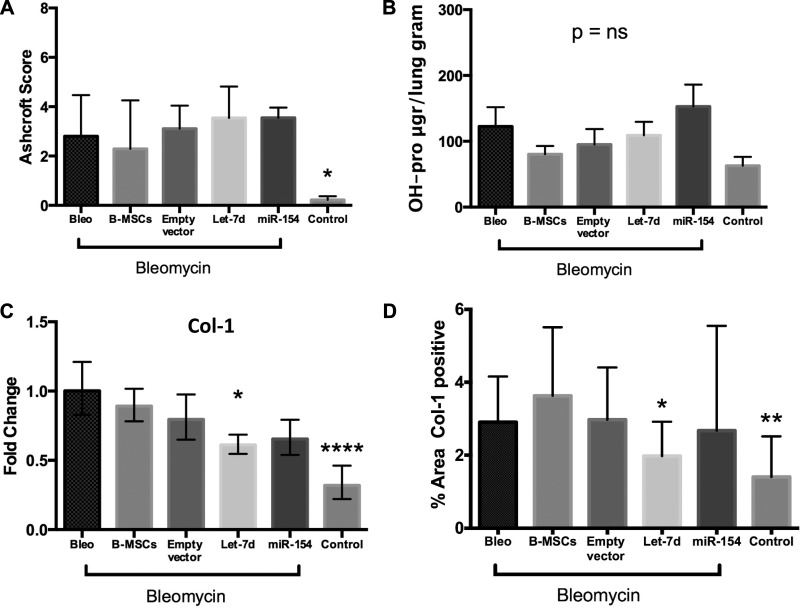
To quantify these findings, we scored fibrosis of the different slides using the Ashcroft score method. No differences were observed when the bleomycin group was compared with the other B-MSC groups. Concordantly, the Ashcroft Scores in “nonbleomycin” controls were lower compared with the rest of the groups (Fig. 9A). Furthermore, no significant changes among groups were observed in OH-proline assay, although the control group presented the lowest OH-proline levels (Fig. 9B).
Fig. 9.
miRNA modified B-MSCs and quantification of fibrosis in the lung. Mice were injected intratracheally at day 0 with bleomycin or sterile saline (control group, n = 3). At day 7, mice were injected intravenously with control B-MSCs (n = 6) and modified B-MSCs [B-MSCs transduced with an empty vector (n = 6), let-7d (n = 7), and miR-154 (n = 7)] or not received any injection (bleomycin group, n = 6). Mice were euthanized at day 14. A: Ashcroft fibrosis score was measured at day 14. The results are shown as means ± SD. B: quantitative OH-proline (OH-pro) measurements were obtained from the left lung in all groups. The results are shown as means ± SE. C: extracted RNA from those lungs was used to examine, by qPCR analysis, the expression levels of collagen-1. The results are shown as means ± range derived from ΔCT SD. D: lung slides from the different groups were also stained for collagen-1 and the percentage of lung areas positive for collagen-1 was calculated from four images by each mouse at ×20 magnification per slide with ImageJ software. *P < 0.05, **P < 0.01, ****P < 0.0001.
When mesenchymal markers were measured in lung samples, we observed changes in Col-1 expression. Col-1 was significantly downregulated in the let-7d B-MSCs group, as well as the control group. (Fig. 9C). Interestingly, Col-1 staining quantification revealed similar trends as the qPCR Col-1 data (Fig. 9D). No differences were observed in α-SMA, HMGA-2, N-cad, and FSP-1 expression (data not shown).
DISCUSSION
The potential therapeutic use of B-MSCs in lung fibrosis is currently evolving, and the initial clinical trials are ongoing (34). Our study represents a new approach to a novel concept, that is, the modification of B-MSCs using miRNAs to alter the properties of these cells and enhance the ability to induce their effects. This ability could be important in those models where the usefulness of B-MSCs has been shown to be limited, such as in radiation-induced lung fibrosis models, where fibrosis is established before the intervention of therapy, and B-MSCs acquire a myofibroblast phenotype and contribute to fibrosis development (8, 37). In our current study, we have been able to modify the phenotype of B-MSCs using miRNA transduction into the cells and partially reducing the effects of bleomycin lung injury after 7 days of intravenous infusion of let-7d modified-B-MSCs.
The bleomycin model in mice is a sequence of two events after the injury. The initial elevation of proinflammatory cytokines is followed by increased expression of profibrotic markers (transforming growth factor-1, fibronectin, and procollagen-1) with a peak around day 14. The “shift” between inflammation and fibrosis appears to occur around day 9 after bleomycin (21). In our model, we have treated with B-MSCs around the reported “shift” time point. Interestingly, no beneficial effect was reported in previous studies when B-MSCs were used at this time point (5, 24). At this stage, we have been able to see differential effects using modified B-MSCs that we did not observe in nonmodified B-MSCs. The similar Ashcroft score and OH-proline levels among groups suggest that our modified B-MSCs could not reverse fibrosis areas after bleomycin lung injury once consolidated. However, we observed an effect on survival (miR-154) and weight gain (let-7d) (Fig. 7). Additionally, we observe a slight effect on Col-1 in let-7d B-MSC-treated group (Fig. 9, C and D). The results observed in the network analysis, the expression of cytokines, and CD45-positive cells, suggest that after modification of B-MSCs, the immunomodulation in the mice model was different among groups. These results are concordant with the known immunomodulatory effect of B-MSCs (17, 29, 36). In addition, we have previously demonstrated the protective capacity of IL-1RN secreted by B-MSCs in a ARDS model (4). Accordingly, the role of these modified B-MSCs in the bleomycin lung injury seems to be related to a paracrine effect rather than a consequence of a potential cell differentiation, as many authors have demonstrated in B-MSCs (27). In addition, network analysis (Figs. 3 and 5) revealed differential immune correlations between let-7d and miR-154 groups, showing the differential immunity-related capacity. Although many therapies are tested in the early stage of the bleomycin model, this is not what is recommended, especially in the case of therapies that are being considered for future use in IPF (3). The use of B-MSCs that we are proposing is concordant with the current vision in therapies for IPF, as the potential therapeutic use of B-MSCs has been considered more as stabilization than as regeneration of damaged tissue (35). In fact, therapies accepted in IPF, such as nintedanib and pirfenidone, do not reverse fibrosis, but only slow down the progression of the disease (28).
In our experiment, we only modified one miRNA in each group of B-MSCs. Expression of different miRNAs in the lung has been demonstrated to be vital for cell properties and phenotypes in IPF (22, 26). Furthermore, a single miRNA administration is probably not sufficient to cause a full therapeutic effect. Given that IPF is influenced by different miRNAs (26), the use of several miRNAs simultaneously may be required to achieve a more significant change in lung fibrosis. Our ability to modify the natural role of B-MSCs during lung injury to a more therapeutic degree by using miRNA is what should be emphasized. In previous studies, we were able to change fibroblast phenotype and properties that caused fibroblasts to be less mesenchymal and more similar to epithelial cells through using miRNA let-7d (14). After transduction, we observed a clear decrease in the mesenchymal markers α-SMA and SLUG, while the epithelial marker KRT-19 was upregulated to a similar level, as described in the previous study with let-7d in fibroblasts (14). α-SMA is a typical marker of myofibroblasts (14), so the downregulation of α-SMA suggests that the overexpression of let-7d would prevent the differentiation of B-MSCs into cells with myofibroblast properties that could enhance the fibrotic process (37). SLUG is a member of the Snail family that is activated through the TGF-β-SMAD-signaling pathway (32), which is directly associated with lung fibrosis (10). On the other hand, KRT-19 is a specific protein present in epithelial cells (14). This is concordant with what we observed in microarray analysis after ingenuity pathway analysis. Several genes from modified B-MSCs that changed after let-7d transduction are implicated in the TGF-β signaling pathway (Fig. 2). TGF-β receptor 2 and TGF-β2, which are directly associated with the SMAD signaling pathway (33), were also downregulated in let-7d-modified B-MSCs. Conversely, TGF-β3, which opposes the effects of TGF-β1 and TGF-β2 (6), was upregulated in let-7d B-MSCs. By contrast, as hypothesized, the overexpression of miR-154 in B-MSCs had opposite effects compared with let-7d B-MSCs. α-SMA and N-Cad, typical mesenchymal markers, were increased in miR-154 B-MSCs. In addition, we observed a decrease in FSP-1 expression, suggesting that miR-154 induced a subpopulation of α-SMA-positive and FSP-1-negative B-MSCs.
To date, this is the first study to assess how the modification of B-MSCs by different miRNAs can alter their properties and affect lung fibrosis in a bleomycin murine model, a strategy that may be relevant in the therapeutic use of B-MSCs. Nevertheless, some limitations need to be considered. First, although the most relevant results were observed in the mice group treated with let-7d B-MSCs, and weight gain is usually a precursor of increased survival (12), we did not observe increased final survival in this group. One explanation for the lack of change in survival could be that animals were euthanized after 7 days of B-MSC treatment, before we could observe any effect on survival rate. Second, we did not explore the mechanisms in vivo by which modified B-MSCs alter lung injury in a bleomycin model, as it was not the main outcome in this initial feasibility study. Recently, Ortiz and colleagues (27) have shown how MSCs could secrete specific miRNA via exosomes and reduce inflammation by affecting other cell types, so this could be one potential mechanism through which B-MSCs could have this effect. In fact, miRNA let-7d is specifically expressed in microvesicles of mesenchymal stem cells (11). However, the exact mechanisms by which B-MSCs exert a paracrine signal that could modify lung fibroblasts require further research.
In summary, the study shows that the transfection of miRNAs into B-MSCs causes changes in their profile and gene expression. Moreover, let-7d miRNA was able to modify the role of B-MSCs postbleomycin injury in mice. In particular, it reveals shifts in animal weight loss, collagen activity after treatment, and decrease in CD45-positive cells. Thus, these results may suggest the use of miRNA-modified B-MSCs as a potential therapeutic strategy.
GRANTS
This work was supported by grants R01 HL-083019, 1RO1 HL-123766-O1A1, K01 HL-084683-05 and a grant from the American Federation of Aging. R. Faner is the recipient of the Miguel Servet Research Program (Institut de Salud Carlos III FEDER, CP16/00039).
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the authors.
AUTHOR CONTRIBUTIONS
L.H., N.C., D.A., K.S., and G.Y. performed experiments; L.H., J.S., D.A., R.F., M.G.K., and M.R. analyzed data; L.H., J.S., N.C., D.A., R.F., K.S., G.Y., M.G.K., N.K., and M.R. interpreted results of experiments; L.H., J.S., R.F., K.S., and G.Y. prepared figures; L.H., J.S., and R.F. drafted manuscript; L.H., J.S., N.C., D.A., R.F., N.K., and M.R. edited and revised manuscript; L.H., J.S., N.C., D.A., R.F., K.S., G.Y., M.G.K., N.K., and M.R. approved final version of manuscript; M.R. conceived and designed research.
ACKNOWLEDGMENTS
We thank Chandler Caufield (Department of Medicine, Division of Pulmonary, Allergy, and Critical Care Medicine; University of Pittsburgh Medical Center; Pittsburgh, PA) and Roger Marshall for assistance in the editing the manuscript.
REFERENCES
- 1.Álvarez D, Levine M, Rojas M. Regenerative medicine in the treatment of idiopathic pulmonary fibrosis: current position. Stem Cells Cloning 8: 61–65, 2015. doi: 10.2147/SCCAA.S49801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashcroft T, Simpson JM, Timbrell V. Simple method of estimating severity of pulmonary fibrosis on a numerical scale. J Clin Pathol 41: 467–470, 1988. doi: 10.1136/jcp.41.4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blackwell TS, Tager AM, Borok Z, Moore BB, Schwartz DA, Anstrom KJ, Bar-Joseph Z, Bitterman P, Blackburn MR, Bradford W, Brown KK, Chapman HA, Collard HR, Cosgrove GP, Deterding R, Doyle R, Flaherty KR, Garcia CK, Hagood JS, Henke CA, Herzog E, Hogaboam CM, Horowitz JC, King TE Jr, Loyd JE, Lawson WE, Marsh CB, Noble PW, Noth I, Sheppard D, Olsson J, Ortiz LA, O’Riordan TG, Oury TD, Raghu G, Roman J, Sime PJ, Sisson TH, Tschumperlin D, Violette SM, Weaver TE, Wells RG, White ES, Kaminski N, Martinez FJ, Wynn TA, Thannickal VJ, Eu JP. Future directions in idiopathic pulmonary fibrosis research. An NHLBI workshop report. Am J Respir Crit Care Med 189: 214–222, 2014. doi: 10.1164/rccm.201306-1141WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bustos ML, Huleihel L, Meyer EM, Donnenberg AD, Donnenberg VS, Sciurba JD, Mroz L, McVerry BJ, Ellis BM, Kaminski N, Rojas M. Activation of human mesenchymal stem cells impacts their therapeutic abilities in lung injury by increasing interleukin (IL)-10 and IL-1RN levels. Stem Cells Transl Med 2: 884–895, 2013. doi: 10.5966/sctm.2013-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cabral RM, Branco É, Rizzo MS, Ferreira GJ, Gregores GB, Samoto VY, Stopiglia AJ, Maiorka PC, Fioretto ET, Capelozzi VL, Borges JB, Gomes S, Beraldo MA, Carvalho CR, Miglino MA. Cell therapy for fibrotic interstitial pulmonary disease: experimental study. Microsc Res Tech 74: 957–962, 2011. doi: 10.1002/jemt.20981. [DOI] [PubMed] [Google Scholar]
- 6.Clark DA, Coker R. Transforming growth factor-beta (TGF-β). Int J Biochem Cell Biol 30: 293–298, 1998. doi: 10.1016/S1357-2725(97)00128-3. [DOI] [PubMed] [Google Scholar]
- 7.Crisostomo PR, Wang Y, Markel TA, Wang M, Lahm T, Meldrum DR. Human mesenchymal stem cells stimulated by TNF-α, LPS, or hypoxia produce growth factors by an NF-κB- but not JNK-dependent mechanism. Am J Physiol Cell Physiol 294: C675–C682, 2008. doi: 10.1152/ajpcell.00437.2007. [DOI] [PubMed] [Google Scholar]
- 8.Epperly MW, Guo H, Gretton JE, Greenberger JS. Bone marrow origin of myofibroblasts in irradiation pulmonary fibrosis. Am J Respir Cell Mol Biol 29: 213–224, 2003. doi: 10.1165/rcmb.2002-0069OC. [DOI] [PubMed] [Google Scholar]
- 9.Eskildsen T, Taipaleenmäki H, Stenvang J, Abdallah BM, Ditzel N, Nossent AY, Bak M, Kauppinen S, Kassem M. MicroRNA-138 regulates osteogenic differentiation of human stromal (mesenchymal) stem cells in vivo. Proc Natl Acad Sci USA 108: 6139–6144, 2011. doi: 10.1073/pnas.1016758108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernandez IE, Eickelberg O. The impact of TGF-β on lung fibrosis: from targeting to biomarkers. Proc Am Thorac Soc 9: 111–116, 2012. doi: 10.1513/pats.201203-023AW. [DOI] [PubMed] [Google Scholar]
- 11.Guo L, Zhao RC, Wu Y. The role of microRNAs in self-renewal and differentiation of mesenchymal stem cells. Exp Hematol 39: 608–616, 2011. doi: 10.1016/j.exphem.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 12.Guzy RD, Stoilov I, Elton TJ, Mecham RP, Ornitz DM. Fibroblast growth factor 2 is required for epithelial recovery, but not for pulmonary fibrosis, in response to bleomycin. Am J Respir Cell Mol Biol 52: 116–128, 2015. doi: 10.1165/rcmb.2014-0184OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang F, Fang ZF, Hu XQ, Tang L, Zhou SH, Huang JP. Overexpression of miR-126 promotes the differentiation of mesenchymal stem cells toward endothelial cells via activation of PI3K/Akt and MAPK/ERK pathways and release of paracrine factors. Biol Chem 394: 1223–1233, 2013. doi: 10.1515/hsz-2013-0107. [DOI] [PubMed] [Google Scholar]
- 14.Huleihel L, Ben-Yehudah A, Milosevic J, Yu G, Pandit K, Sakamoto K, Yousef H, LeJeune M, Coon TA, Redinger CJ, Chensny L, Manor E, Schatten G, Kaminski N. Let-7d microRNA affects mesenchymal phenotypic properties of lung fibroblasts. Am J Physiol Lung Cell Mol Physiol 306: L534–L542, 2014. doi: 10.1152/ajplung.00149.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huntington ND, Tarlinton DM. CD45: direct and indirect government of immune regulation. Immunol Lett 94: 167–174, 2004. doi: 10.1016/j.imlet.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 16.Hwang HW, Mendell JT. MicroRNAs in cell proliferation, cell death, and tumorigenesis. Br J Cancer 94: 776–780, 2006. doi: 10.1038/sj.bjc.6603023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kyurkchiev D, Bochev I, Ivanova-Todorova E, Mourdjeva M, Oreshkova T, Belemezova K, Kyurkchiev S. Secretion of immunoregulatory cytokines by mesenchymal stem cells. World J Stem Cells 6: 552–570, 2014. doi: 10.4252/wjsc.v6.i5.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Landgraf P, Rusu M, Sheridan R, Sewer A, Iovino N, Aravin A, Pfeffer S, Rice A, Kamphorst AO, Landthaler M, Lin C, Socci ND, Hermida L, Fulci V, Chiaretti S, Foà R, Schliwka J, Fuchs U, Novosel A, Müller RU, Schermer B, Bissels U, Inman J, Phan Q, Chien M, Weir DB, Choksi R, De Vita G, Frezzetti D, Trompeter HI, Hornung V, Teng G, Hartmann G, Palkovits M, Di Lauro R, Wernet P, Macino G, Rogler CE, Nagle JW, Ju J, Papavasiliou FN, Benzing T, Lichter P, Tam W, Brownstein MJ, Bosio A, Borkhardt A, Russo JJ, Sander C, Zavolan M, Tuschl T. A mammalian microRNA expression atlas based on small RNA library sequencing. Cell 129: 1401–1414, 2007. doi: 10.1016/j.cell.2007.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Langfelder P, Horvath S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics 9: 559, 2008. doi: 10.1186/1471-2105-9-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Milosevic J, Pandit K, Magister M, Rabinovich E, Ellwanger DC, Yu G, Vuga LJ, Weksler B, Benos PV, Gibson KF, McMillan M, Kahn M, Kaminski N. Profibrotic role of miR-154 in pulmonary fibrosis. Am J Respir Cell Mol Biol 47: 879–887, 2012. doi: 10.1165/rcmb.2011-0377OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moeller A, Ask K, Warburton D, Gauldie J, Kolb M. The bleomycin animal model: a useful tool to investigate treatment options for idiopathic pulmonary fibrosis? Int J Biochem Cell Biol 40: 362–382, 2008. doi: 10.1016/j.biocel.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Montgomery RL, Yu G, Latimer PA, Stack C, Robinson K, Dalby CM, Kaminski N, van Rooij E. MicroRNA mimicry blocks pulmonary fibrosis. EMBO Mol Med 6: 1347–1356, 2014. doi: 10.15252/emmm.201303604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mora AL, Rojas M. Adult stem cells for chronic lung diseases. Respirology 18: 1041–1046, 2013. doi: 10.1111/resp.12112. [DOI] [PubMed] [Google Scholar]
- 24.Ortiz LA, Gambelli F, McBride C, Gaupp D, Baddoo M, Kaminski N, Phinney DG. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci USA 100: 8407–8411, 2003. doi: 10.1073/pnas.1432929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ortiz LA, Dutreil M, Fattman C, Pandey AC, Torres G, Go K, Phinney DG. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc Natl Acad Sci USA 104: 11,002–11,007, 2007. doi: 10.1073/pnas.0704421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pandit KV, Milosevic J, Kaminski N. MicroRNAs in idiopathic pulmonary fibrosis. Transl Res 157: 191–199, 2011. doi: 10.1016/j.trsl.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 27.Phinney DG, Di Giuseppe M, Njah J, Sala E, Shiva S, St Croix CM, Stolz DB, Watkins SC, Di YP, Leikauf GD, Kolls J, Riches DW, Deiuliis G, Kaminski N, Boregowda SV, McKenna DH, Ortiz LA. Mesenchymal stem cells use extracellular vesicles to outsource mitophagy and shuttle microRNAs. Nat Commun 6: 8472, 2015. doi: 10.1038/ncomms9472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raghu G, Rochwerg B, Zhang Y, Garcia CAC, Azuma A, Behr J, Brozek JL, Collard HR, Cunningham W, Homma S, Johkoh T, Martinez FJ, Myers J, Protzko SL, Richeldi L, Rind D, Selman M, Theodore A, Wells AU, Hoogsteden H, Schünemann HJ; American Thoracic Society; European Respiratory society; Japanese Respiratory Society; Latin American Thoracic Association . An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline: Treatment of Idiopathic Pulmonary Fibrosis. An Update of the 2011 Clinical Practice Guideline. Am J Respir Crit Care Med 192: e3–e19, 2015. doi: 10.1164/rccm.201506-1063ST. [DOI] [PubMed] [Google Scholar]
- 29.Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, Zhao RC, Shi Y. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell 2: 141–150, 2008. doi: 10.1016/j.stem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 30.Rojas M, Xu J, Woods CR, Mora AL, Spears W, Roman J, Brigham KL. Bone marrow-derived mesenchymal stem cells in repair of the injured lung. Am J Respir Cell Mol Biol 33: 145–152, 2005. doi: 10.1165/rcmb.2004-0330OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 13: 2498–2504, 2003. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shih J-Y, Yang P-C. The EMT regulator slug and lung carcinogenesis. Carcinogenesis 32: 1299–1304, 2011. doi: 10.1093/carcin/bgr110. [DOI] [PubMed] [Google Scholar]
- 33.Tatler AL, Jenkins G. TGF-β activation and lung fibrosis. Proc Am Thorac Soc 9: 130–136, 2012. doi: 10.1513/pats.201201-003AW. [DOI] [PubMed] [Google Scholar]
- 34.Wecht S, Rojas M. Mesenchymal stem cells in the treatment of chronic lung disease. Respirology 21: 1366–1375, 2016. doi: 10.1111/resp.12911. [DOI] [PubMed] [Google Scholar]
- 35.Weiss DJ, Ortiz LA. Cell therapy trials for lung diseases: progress and cautions. Am J Respir Crit Care Med 188: 123–125, 2013. doi: 10.1164/rccm.201302-0351ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yagi H, Soto-Gutierrez A, Parekkadan B, Kitagawa Y, Tompkins RG, Kobayashi N, Yarmush ML. Mesenchymal stem cells: Mechanisms of immunomodulation and homing. Cell Transplant 19: 667–679, 2010. doi: 10.3727/096368910X508762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yan X, Liu Y, Han Q, Jia M, Liao L, Qi M, Zhao RC. Injured microenvironment directly guides the differentiation of engrafted Flk-1+ mesenchymal stem cell in lung. Exp Hematol 35: 1466–1475, 2007. doi: 10.1016/j.exphem.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 38.Zhang JF, Fu WM, He ML, Xie WD, Lv Q, Wan G, Li G, Wang H, Lu G, Hu X, Jiang S, Li JN, Lin MC, Zhang YO, Kung HF. MiRNA-20a promotes osteogenic differentiation of human mesenchymal stem cells by co-regulating BMP signaling. RNA Biol 8: 829–838, 2011. doi: 10.4161/rna.8.5.16043. [DOI] [PubMed] [Google Scholar]