Abstract
Exposure to cadmium (Cd) has been associated with development of chronic obstructive lung disease (COPD). The mechanisms and signaling pathways whereby Cd causes pathological peribronchiolar fibrosis, airway remodeling, and subsequent airflow obstruction remain unclear. We aimed to evaluate whether low-dose Cd exposure induces vimentin phosphorylation and Yes-associated protein 1 (YAP1) activation leading to peribronchiolar fibrosis and subsequent airway remodeling. Our data demonstrate that Cd induces myofibroblast differentiation and extracellular matrix (ECM) deposition around small (<2 mm in diameter) airways. Upon Cd exposure, α-smooth muscle actin (α-SMA) expression and the production of ECM proteins, including fibronectin and collagen-1, are markedly induced in primary human lung fibroblasts. Cd induces Smad2/3 activation and the translocation of both Smad2/3 and Yes-associated protein 1 (YAP1) into the nucleus. In parallel, Cd induces AKT and cdc2 phosphorylation and downstream vimentin phosphorylation at Ser39 and Ser55, respectively. AKT and cdc2 inhibitors block Cd-induced vimentin fragmentation and secretion in association with inhibition of α-SMA expression, ECM deposition, and collagen secretion. Furthermore, vimentin silencing abrogates Cd-induced α-SMA expression and decreases ECM production. Vimentin-deficient mice are protected from Cd-induced peribronchiolar fibrosis and remodeling. These findings identify two specific sites on vimentin that are phosphorylated by Cd and highlight the functional significance of vimentin phosphorylation in YAP1/Smad3 signaling that mediates Cd-induced peribronchiolar fibrosis and airway remodeling.
Keywords: cadmium, fibrosis, phosphorylation, vimentin, chronic obstructive lung disease
the lung is a portal of entry for many inhaled environmental toxins including heavy metals such as cadmium (Cd). Cd is a toxic heavy metal that is ranked seventh on the Agency for Toxic Substances and Disease Registry (ATSDR) Substance Priority List (29). Cd exposure can occur through smoking, food, and water contamination or outdoor air pollution that can reach the small airways (2, 5, 21). Each cigarette contains 2 μg of Cd (27). Cd has a very long biological half-life (>25 yr) in the body (25) and is found in many target organs, including the lung (3). Cd levels are higher in the lungs of patients with Global Initiative for Chronic Obstructive Lung Disease (GOLD) Grade 4 COPD (late) than in GOLD stage 1 (early) patients (20, 38), suggesting a role of Cd in COPD pathogenesis.
The important role of peribronchiolar fibrosis in the loss of the lumen of small airways, and subsequent airway remodeling in COPD has been well described (6, 26, 47). Extracellular matrix (ECM) deposition around bronchioles by activated fibroblasts is one of the causes of the irreversible narrowing of the airway lumen that defines COPD (6). Airway wall fibrosis is also considered a feature of the bronchiolar obstruction in smokers who develop COPD (4, 10, 40). Interestingly, peribronchiolar fibrosis can occur before emphysema (21) and has been documented to be present even in the presence of alveolar wall loss (30, 45). The remodeling process first narrows and then removes a large number of bronchioles leaving relatively thickened airways as demonstrated on microcomputed tomography and histology (21, 22). Although evidence has indicated that reactive oxygen species (27, 49) may be involved in Cd-induced tissue injury, whether Cd causes peribronchiolar fibrosis with airway remodeling and the molecular signaling mechanisms underlying Cd-induced peribronchiolar fibrosis remain poorly understood.
Yes-associated protein 1 (YAP1), the transcriptional coactivator of Hippo signaling pathway (39), has been found to mediate TGF-β-induced signaling in several organ systems including the lung (36, 50). Phosphorylated YAP1 can be sequestered with 14-3-3 in the cytoplasm and YAP1 subsequently translocated to the nucleus where it regulates transcription upon dephosphorylation (1). However, whether YAP1 is involved in Cd-induced peribronchiolar fibrosis remains to be elucidated.
Vimentin is a major intracellular intermediate filament protein. It is abundantly expressed by mesenchymal cells such as fibroblasts (18). It is also expressed on the cell surface of macrophage (34) and endothelial cells (ECs) (48). Upon activation, vimentin is secreted from these cells. Although the respective biological functions of cell surface and secreted vimentin continue to be elucidated, current evidence suggests the extracellular vimentin may regulate many important activities such as bacterial killing (34), endothelial signaling (48), and NLRP3 inflammasome activation (13). Critical functions such as wound healing and cell migration are defective in vimentin-deficient fibroblasts (15). Vimentin can be phosphorylated and has been identified as a substrate for a wide range of kinases, including cell division cycle protein 2 (cdc2) kinase (44), protein kinase C (PKC) (35), cAMP-dependent protein kinase A (PKA) (16), and protein kinase B (PKB; also known as AKT) (51). It has been suggested that both extracellular signal-regulated kinase (ERK) and AKT pathways can be activated and involved in Cd-induced tumor growth and angiogenesis in human lung cells (27).
Accumulating evidence suggests that the effects of Cd differ dependent on the dose, species used, and exposure pathways (27, 41). In this study, we hypothesized that Cd is a potent inducer of peribronchiolar fibrosis and airway remodeling in which phosphorylated vimentin and YAP1 play important roles. To address these speculations, mice were administrated low doses of cadmium chloride (CdCl2) for 8 wk via intratracheal instillation. Both small interfering RNA (siRNA) vimentin transfection strategies and vimentin knockout mice were used to demonstrate functional contributions of vimentin. Pharmacologic inhibitor analysis of AKT and cdc2 events in Cd-stimulated human lung fibroblasts revealed that vimentin is a downstream target of AKT and cdc2 signaling. Furthermore, inhibition of AKT and cdc2 resulted in reduced Cd-driven vimentin fragmentation and secretion. We report a novel vimentin-driven mechanism of Cd-induced COPD through peribronchiolar fibrosis and airway remodeling. Site-specific vimentin phosphorylation and activation of YAP1 mediated by both AKT and cdc2-dependent pathways are critical elements of Cd-induced peribronchiolar fibrosis.
MATERIALS AND METHODS
Mouse model.
C57BL/6 mice, Vimentin knockout mice (Vimentin−/−, 129S-Vimtm1Cba/MesDmarkJ) and genetic wild type (WT), 10–12 wk of age, weighing 25–30 g, were purchased from the Jackson Laboratory. All animal procedures were reviewed and approved by the Institutional Committee of Animal Care of the University of Alabama at Birmingham. Mice were exposed to CdCl2 (0.009 or 0.018 mg/kg body wt) via nonsurgical intratracheal instillation in saline every other day for 8 wk. These doses used in mice were chosen to produce similar tissue levels of Cd as seen in human lungs of patients with COPD (8). Nonsurgical intratracheal instillation is noninvasive intratracheal inoculation via the oro-tracheal route, after the tongue was gently pulled out of the mouse (23, 31). Mice were weighed to calculate the CdCl2 dose required for each mouse and anesthetized with isoflurane and oxygen. Upon adequate anesthesia was observed, CdCl2 (volume not to exceed 50 μl) was instilled into the trachea via the larynx. Control mice received sterile saline. On weeks 1, 2, 4, and 8, mice were killed and lungs were removed for histology. Plasma was prepared and bronchoalveolar lavage (BAL) was carried out as described previously by our laboratory (42).
Immunohistochemistry and subepithelial collagen thickness measurement.
Lung specimens were fixed with formalin and embedded in paraffin, and immunohistochemistry was performed using Dako Envision visualization kit following the standard protocol. Slides were stained with hematoxylin-eosin or incubated with primary antibodies against α-smooth muscle actin (α-SMA; 1:400; Santa Cruz Biotechnology, Santa Cruz, CA), vimentin (1:500; Santa Cruz Bioetchnology), or collagen-1 (1:200; Rockland, Limerick, PA). Picro-sirius red was also used to determine collagen expression using a Picro-Sirius Red Stain Kit (Abcam, Cambridge, MA). The images were viewed at ×200, and Bioquant Imaging Analysis software (Bioquant, Nashville, TN) was used to quantitate the staining intensity. The mean subepithelial collagen thickness was traced and determined as previous described (37).
Airways resistance measurements.
Details of airways resistance alterations in Cd-exposed mice were evaluated. Following ketamine/xylazine anesthesia and tracheal cannulization, the FlexiVent system (SCIREQ; 160 breaths/min, tidal volume of 6 ml/kg, and positive end-expiratory pressure of 2.5 cm H2O) was used and methacholine was administered by inhalation in 5-min intervals (8, 42).
Cd level measurement and primary human lung fibroblast isolation.
Human lung tissues were obtained from the University of Alabama at Birmingham Tissue Procurement Core following an approved Institutional Review Board protocol. Cd levels in lung tissue from patients undergoing lung transplantation for COPD were measured using inductively coupled plasma mass spectrometry. Primary human lung fibroblasts were isolated as described previously (11). Briefly, small pieces of lung tissue were suspended in 1 mg/ml of collagenase HBSS solution for 30 min at 37°C. The pieces of lung were removed following filtration with 100-μm cell filter, and the solution was centrifuged at 500 g for 5 min. To remove lymphocytes/macrophages, cells were cultured in CD16/32-and CD45-coated Petri dishes in Eagle’s MEM with 10% FBS before passage. Primary fibroblasts were used after passage two to increase the purity and allowed to grow until 80–90% confluency and then trypsinized and plated as needed. IMR-90, human fetal lung fibroblast cell line, was purchased from the American Type Culture Collection (ATCC, Manassas, VA). The cells were cultured in MEM with 2 mM glutamine and 10% fetal bovine serum. Cell viability was evaluated by using thiazolyl blue tetrazolium bromide (MTT; Sigma-Aldrich, St. Louis, MO) assay. Briefly, cells were incubated with or without CdCl2 at different concentration for 3 h followed by the addition of fresh media and cultured for different time points. MTT (5 mg/ml) was added for additional 4 h before addition of 100 μl of DMSO. Optical density values at 570 nm were read.
Enzyme-linked immunosorbent assay analysis.
Vimentin concentrations in mice plasma and BAL were quantified using a commercially available mouse vimentin enzyme-linked immunosorbent assays (ELISA) kit (MyBioSource, San Diego, CA) according to the manufacturer’s recommendation.
Measurement of collagen.
For collagen quantification, the right lung from mice was removed, weighted, and homogenized. Collagen content was assessed by the Sircol collagen assay (Biocolor, Northern Ireland, UK) following the manufacturer’s instructions. The soluble collagen levels in supernatants from Cd treatment cells were also evaluated.
Western blotting and immunoprecipitation.
Primary lung fibroblasts or IMR-90 cells were incubated with CdCl2 for 3 h and then allowed to recover for the different periods of time. For some experiments, cells were pretreated with or without the MEK 1 inhibitor PD 985059 (Cayman Chemical, Ann Arbor, MI), the PKC inhibitor G06983 (Tocris; Avonmouth, Bristol, UK), the AKT inhibitor LY294002 (Sigma-Aldrich), the cdc2 inhibitor Roscovitine (Santa Cruz Biotechnology), or calf intestinal phosphatase (CIP; New England BioLabs, Ipswich, MA). To examine collagen and vimentin secretion, supernatants from cells treated with different CdCl2 concentrations at different time points were collected and concentrated by ultrafiltration using a Millipore centrifugal concentrator (Labsupplyoutlaws, Cleveland, OH). Nuclear and cytoplasmic fractionations were conducted using the NE-PER nuclear and cytoplasmic extraction reagents kit (Pierce Biotechnology, Rockford, IL). For immunoprecipitation, nuclear or cytoplasmic fractionations were precleared with 30 μl protein A/G agarose (Santa Cruz Biotechnology) for 2 h at 4°C. Supernatants were then incubated with 2 μg of YAP1 or vimentin overnight at 4°C in RIPA buffer containing 1.0 mM NaVO3, 0.1% 2-mercaptoethanol, protease, and phosphatase inhibitor (ThermoFisher Scientific, Rockford, IL). Cell lysates and concentrated supernatants were prepared and processed according to standard immunoblotting procedures using the following primary antibodies: anti-vimentin (5G3F10), anti-fibronectin, anti-collagen 1, anti-α-SMA, anti-PKC βII (F-7), anti-phospho-vimentin at Ser38, anti-pan 14-3-3, and anti-lamin (Santa Cruz Biotechnology); anti-phospho-P44/42 MAPK (Thr202/Tyr204), anti-P44/42 MAPK, anti-phospho-AKT (Ser473), anti-AKT, anti-phospho-cdc2 (Thr161), anti-cdc2 (POH1), anti-phospho-PKC (pan, βII Ser660), anti-phospho-PKA (Thr197) (D45D3), anti-PKA, anti-phospho-vimentin at Ser39, anti-phospho-Smad2 (Ser465/467), anti-Smad2/3, and cleaved caspase-3 (Asp 175) (Cell Signaling Technology, Beverly, MA); anti-phospho-vimentin at Ser33, Ser50, and Ser55 (MBL international, Woburn, MA); and anti-α-tubulin, anti-β-actin, anti-YAP1 (Sigma-Aldrich).
Flow cytometric analysis.
To detect intracellular reactive oxygen species, primary lung fibroblasts were stained using a cell permeant reagent, 2′,7′-dichlorodihydrofluorescein diacetate (DCFDA; Abcam, Cambridge, MA). Briefly, cells were pretreated with 20 μM DCFDA for 30 min at 37°C and then cultured for 3 h with or without CdCl2 (10 or 20 μM) before analysis by flow cytometry.
siRNA transfection studies.
IMR-90 cells were plated at 70% confluence and incubated with the vimentin siRNA or nontargeting control siRNA (NT-siRNA) to the dilute transfection reagent (Santa Cruz Biotechnology). Transfection was performed according to the manufacturer’s instructions. After 24 h of transfection, the cells were treated with CdCl2 and harvested for Western blotting.
Statistical analysis.
Data are expressed as means ± SD. One-way ANOVA was used to compare the differences between groups. After the ANOVA analysis, the post hoc multiple comparisons were performed by using Tukey’s honestly significant difference test to determine the statistical difference from each other among subgroups. P < 0.05 was considered significant.
RESULTS
Cd induces peribronchiolar fibrosis.
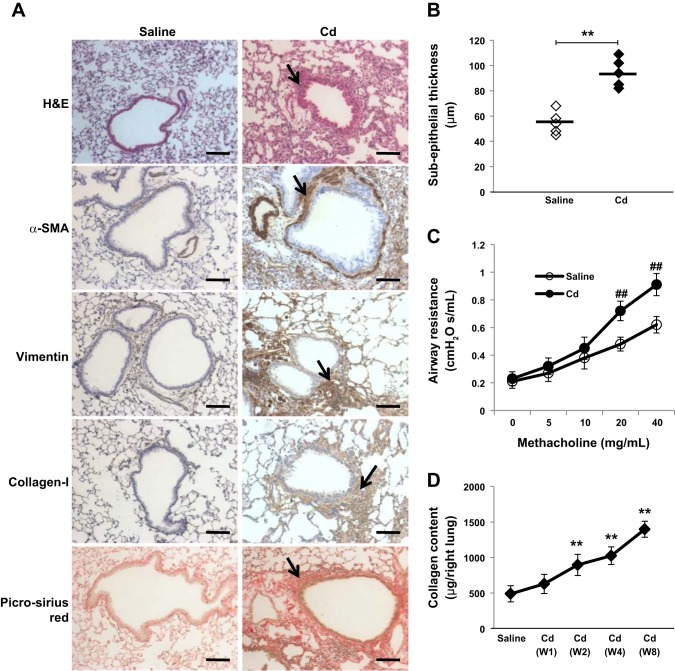
To understand how inhalation of Cd leads to airway remodeling, we exposed mice to low doses of CdCl2 via nonsurgical intratracheal instillation every other day for 8 wk. We chose the dose to achieve Cd lung tissue levels seen in explant of patients with COPD and peribronchiolar fibrosis (n = 3, Cd level: 0.85 ± 0.3 ng/mg lung tissue protein). In preliminary experiments, we found that a low dose of Cd (0.009 mg/kg) by intratracheal instillation injection every other day allowed us to achieve similar lung tissue levels as those seen in humans with COPD. At 8 wk, Cd levels in control mice were 0.18 ± 0.05, while in intratracheal instillation CdCl2 mice were 0.77 ± 0.1 ng/mg lung tissue protein. Histological analysis demonstrated small airway wall thickening in mice exposed to CdCl2 (Fig. 1A). The subepithelium was significantly thicker in Cd exposure mice compared with saline control mice (Fig. 1B). Furthermore, increased airway resistance was observed in Cd-exposed mice (Fig. 1C). Of interest, these experiments showed that Cd induces peribronchiolar fibrosis as assessed by vimentin, collagen-1 immunostaining, and picro-sirius red staining. We also found increased staining for α-SMA, a marker for myofibroblasts differentiation (37). As well, collagen secretion increased in a time-dependent manner (Fig. 1D). These data suggest that Cd induces peribronchiolar fibrosis with increases in collagen deposition and α-SMA expression.
Fig. 1.
Cadmium (Cd) induces peribronchiolar fibrosis. A: mice (n = 5) were treated intratracheally with saline or CdCl2 (0.009 mg/kg body wt) every other day for 8 wk. Cryosections from weeks 1, 2, 4, and 8 mice lungs were stained with hematoxylin-eosin, α-smooth muscle actin (α-SMA), vimentin, collagen-1, or picro-sirius red. Representative photomicrographs from 5 animals on week 4 are shown, and peribronchiolar fibrosis is indicated by arrows. Bar = 100 μm. B: the peribronchiolar subepithelial layer in mice (n = 5) was measured and comparable to the saline control. Each symbol represents an individual subjects, and horizontal bars indicate the mean values. C: respiratory mechanics demonstrating increased airway resistance following methacholine challenge. ##P < 0.01 vs. saline mice with methacholine at 20 or 40 mg/ml. D: mice were treated as shown in A, and collagen content in the right lung at different time points was assessed using Sircol collagen assay. **P < 0.01 vs. saline control.
Cd induces myofibroblast differentiation and ECM protein deposition.
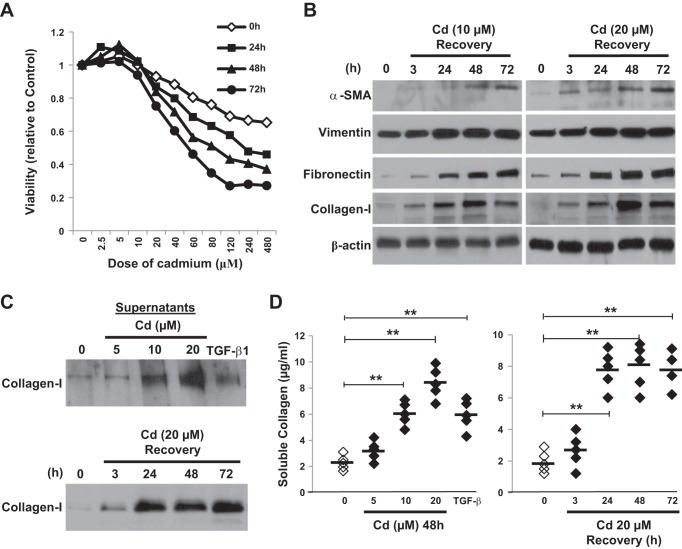
To explore the mechanism of Cd-induced peribronchiolar fibrosis, we investigated the effects of CdCl2 on the expression of α-SMA and ECM-related proteins. We first checked the sensitivity of human fibroblasts to Cd. Primary human lung fibroblasts were exposed to various concentrations of CdCl2 for 3 h and then cultured in CdCl2 free medium for different period of time. Dose-response graphs were established and cell viability was determined by MTT cytotoxicity assay. The treatments with doses <20 μM CdCl2 for 24 h or longer showed no significant evidence of cytotoxicity (Fig. 2A), and therefore, 10 and 20 μM CdCl2 were chosen for the following experiments. Western blotting (Fig. 2B) revealed dose- and time-dependent increases in α-SMA protein expression. The levels of ECM proteins, such as vimentin, fibronectin, and collagen-1, also increased significantly in a dose- and time-dependent manner during recovery from Cd treatment. These increases persist up to 72 h after Cd exposure. Similar to TGF-β treatment, exposure to Cd also induced collagen secretion measured by Western blotting (Fig. 2C) and Sircol collagen assay (Fig. 2D).
Fig. 2.
Cd induces α-SMA expression and increases extracellular matrix (ECM) protein accumulation as well as collagen secretion. A: primary human fibroblasts were incubated with or without CdCl2 at different concentrations for 3 h and then allowed to recover for 24, 48, or 72 h. Cell viability was measured by thiazolyl blue tetrazolium bromide (MTT) assay, and percentage of viability relative to control was shown. Results are expressed as mean of triplicate samples and are representative of those from 3 independent experiments. B: cells were incubated with or without CdCl2 (10 or 20 μM) for 3 h and then allowed to recover for the indicated periods of time. Immunoblotting were performed using antibodies shown above. C: cells were treated with CdCl2 at different concentrations (top) or 20 μM at different time points (bottom), and supernatants were collected, concentrated by ultrafiltration and analyzed by Western blotting. D: cells were treated as above and the soluble collagen levels (n = 5) were evaluated by Sircol collagen assay. Horizontal bars indicate the mean values. Cells were also treated with TGF-β (2 ng/ml) for 48 h as a positive control. **P < 0.01 vs. control.
Cd induces phosphorylation of AKT, cdc2, and vimentin phosphorylation specifically at Ser39 and Ser55.
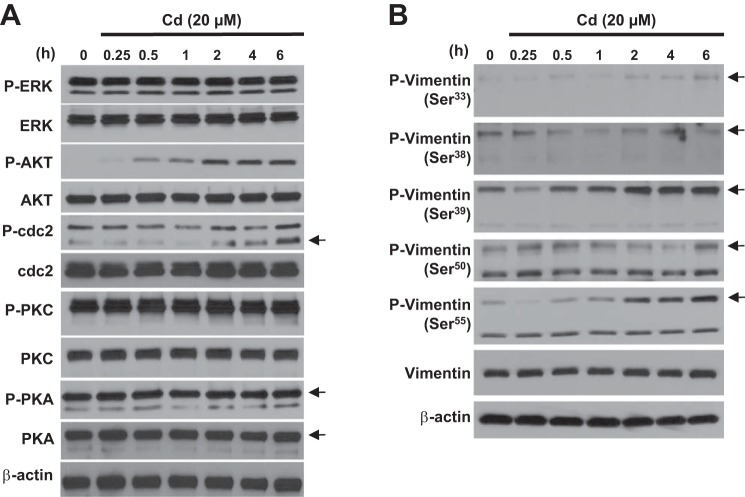
The signaling events modulating α-SMA expression and ECM protein deposition following Cd exposure are still unclear. We show that exposure to CdCl2 in primary lung fibroblast-induced phosphorylation of several kinases, such as AKT and cdc2 (Fig. 3A). Immunoblotting confirmed that AKT was rapidly phosphorylated, which was clearly detected 30 min after Cd stimulation and sustained until 6 h. However, Cd only-induced cdc2 phosphorylation at a later time period, which was detectable 2 h after stimulation and peaked at 6 h. In contrast, stimulation with Cd led to less phosphorylation of PKC and PKA. We next determined whether Cd stimulation induced vimentin phosphorylation. Immunoblotting analysis clearly showed that Cd stimulation led to vimentin phosphorylation specifically at Ser39 and Ser55 but not at Ser33, Ser38, or Ser50 (Fig. 3B). We further confirmed these findings using a human lung fibroblast cell line, IMR-90 (data not shown).
Fig. 3.
Cd induces phosphorylation of AKT and cdc2 as well as vimentin phosphorylation at Ser39 and Ser55. A and B: after 24 h of serum starvation, primary lung fibroblasts were treated with CdCl2 (20 μM) for the indicated times. Immunoblotting were performed using antibodies shown above. The results shown in A and B are representative of those from 3 independent experiments. Arrows represent specific band.
Both AKT and cdc2 inhibitors inhibit Cd-induced vimentin Ser39 and Ser55 phosphorylation, α-SMA expression, ECM protein accumulation, and collagen secretion.
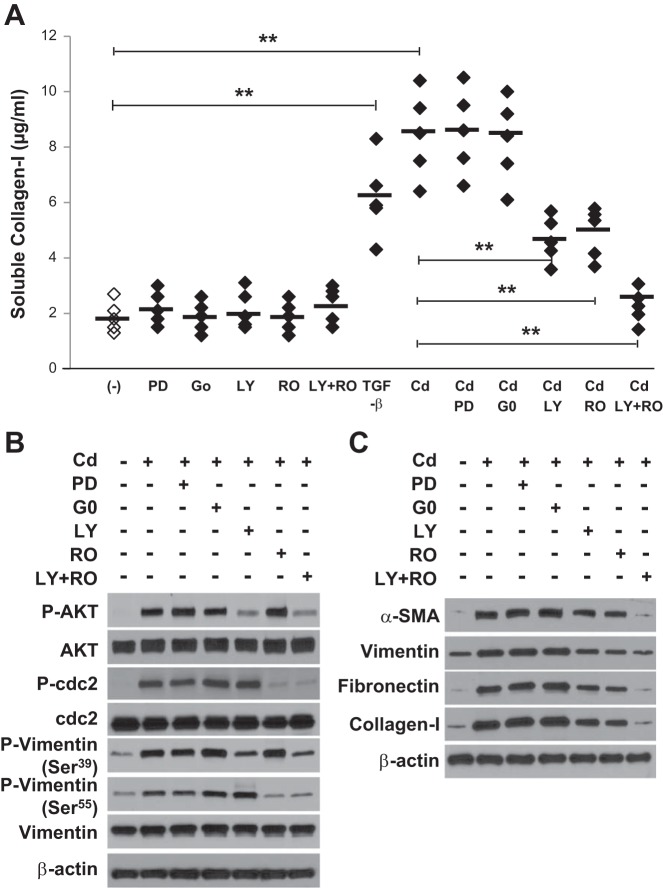
Primary lung fibroblasts were pretreated with the MEK 1 inhibitor, PKC inhibitor, AKT inhibitor, cdc2 inhibitor, or combination of AKT and cdc2 inhibitors. As shown in Fig. 4A, pretreatment with these inhibitors only in the absence of Cd had no effect on the collagen secretion, while Cd-induced collagen secretion in the supernatants of cultured fibroblasts was dramatically blocked by pretreatment with AKT and cdc2 inhibitors but not with MEK1 and PKC inhibitors. We further assessed the specific signaling pathways involved in Cd-induced vimentin serine phosphorylation. Although both MEK1 and PKC inhibitors did not affect the response to Cd, the AKT inhibitor and cdc2 inhibitor significantly reduced vimentin phosphorylation at Ser39 and Ser55, respectively (Fig. 4B), suggesting that vimentin serine phosphorylation was dependent on AKT and cdc2 kinases activity.
Fig. 4.
Both AKT and cdc2 inhibitors inhibit Cd-induced vimentin phosphorylation at Ser39 and Ser55, α-SMA activation, ECM accumulation, and collagen secretion. A; after 24 h of serum starvation, primary lung fibroblasts (n = 5) were pretreated with the MEK 1 inhibitor PD 985059 (PD; 20 μM), the PKC inhibitor G06983 (G0; 250 nM), the AKT inhibitor LY294002 (LY; 20 μM), the cdc2 inhibitor Roscovitine (RO; 10 μM) only, or combination of AKT and cdc2 inhibitors (LY + RO) 1 h before 20 μM CdCl2 treatment for 3 h and then allowed to recover for 48 h. The soluble collagen levels were evaluated by Sircol collagen assay. Horizontal bars indicate the mean values. **P < 0.01 vs. control. Cells were also treated with TGF-β (2 ng/ml) for 48 h as a positive control. B and C: cells were pretreated with inhibitor shown as above before 20 μM CdCl2 treatment for 2 h (B) or 3 h and then allowed to recover for 48 h (C), followed by immunoblot analysis for indicated antibodies. The results shown in A–C are representative of those from 3 independent experiments.
We further evaluated the potential effects of both AKT and cdc2 inhibitors on Cd-induced α-SMA activation and ECM protein deposition. The expression of Cd-induced α-SMA, vimentin, fibronectin, and collagen-1 was significantly inhibited by both inhibitors compared with Cd alone (Fig. 4C). These findings suggest that Cd-induced myofibroblast differentiation, ECM protein deposition and collagen secretion can be attenuated by both AKT and cdc2 inhibitors.
Cd induces AKT and cdc2-dependent vimentin fragmentation and secretion.
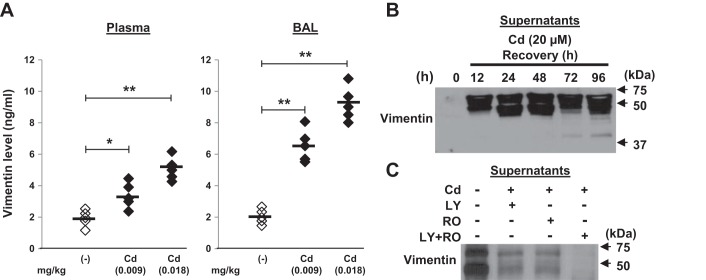
We next determined whether vimentin was secreted in response to Cd stimulation. Analyses of plasma and BAL specimens by ELISA showed there was dose-dependent increase in vimentin levels in Cd-treated mice compared with the saline controls (Fig. 5A). Secretion of vimentin was also confirmed by immunoblotting of concentrated supernatants (Fig. 5B). Vimentin, a caspase substrate, can be cleaved by multiple caspases (7). Western blot analysis showed a full-length vimentin (57 kDa) and new fragments of vimentin (49 kDa or less) in supernatants in response to Cd stimulation, suggesting that vimentin can be fragmented by Cd and importantly secreted by human fibroblasts. Moreover, pretreating with the AKT inhibitor, the cdc2 inhibitor, and the combination of AKT and cdc2 could effectively reverse Cd-induced vimentin fragmentation and secretion (Fig. 5C), suggesting that Cd-induced vimentin fragmentation and secretion are both AKT and cdc2 kinase dependent.
Fig. 5.
Cd-induced vimentin fragmentation and secretion can be inhibited by both AKT and cdc2 inhibitors. A: mice (n = 5) were treated intratracheally with saline or CdCl2 (0.009 or 0.018 mg/kg) as shown in Fig. 1. Plasma and bronchoalveolar lavage (BAL) were collected at week 4 and the vimentin levels were examined by ELISA. Horizontal bars indicate the mean values. B: primary lung fibroblasts were incubated with or without CdCl2 (20 μM) for 3 h and then allowed to recover for the indicated periods of time, and the supernatants were collected, concentrated by ultrafiltration and equal aliquots were analyzed by Western blotting using an anti-vimentin antibody. C: cells were pretreated with the AKT inhibitor (LY; 20 μM), the cdc2 inhibitor (RO; 10 μM), or a combination of AKT and cdc2 inhibitors (LY + RO) for 1 h, followed by 20 μM of CdCl2 treatment for 3 h and then allowed to recover for 48 h. Supernatants was analyzed as above. *P < 0.05, **P < 0.01. Arrows represent positions for the indicated molecular weight. The data are representative of 3 independent experiments.
Silencing of vimentin inhibits Cd-induced α-SMA expression, ECM protein deposition, and peribronchiolar fibrosis.
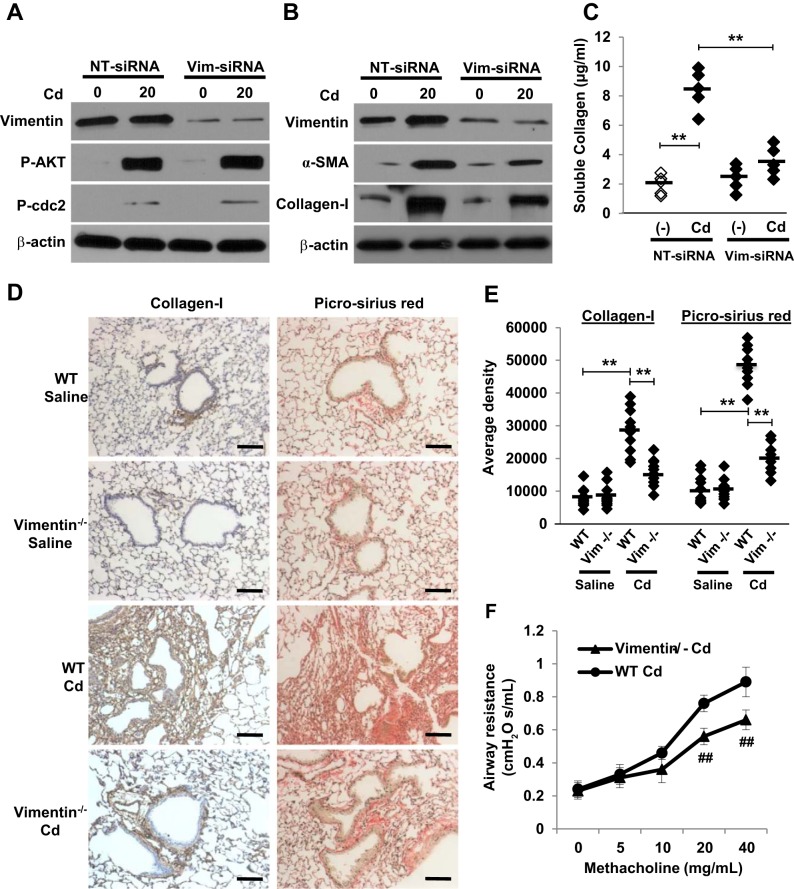
Having established that activation of both AKT and cdc2 kinases was crucial for the fragmentation and secretion of vimentin, we next tested whether vimentin function was important for Cd-induced peribronchiolar fibrosis. Depletion of vimentin with vimentin siRNA markedly decreased vimentin expression but had no effect on AKT and cdc2 phosphorylation (Fig. 6A). As expected, vimentin-depleted cells had lower expression of α-SMA and collagen-1 (Fig. 6B). Vimentin silencing was also found to efficiently reverse Cd-induced collagen secretion (Fig. 6C). More importantly, depletion of vimentin significantly reduced the ability of mice lung fibroblasts to form fibrous collagen networks and peribronchiolar fibrosis (Fig. 6, D and E). Depletion of vimentin also blocked Cd-induced airway resistance (Fig. 6F). These observations support the idea that vimentin is critical for Cd-induced myofibroblast differentiation, matrix deposition, peribronchiolar fibrosis, and subsequent airway remodeling.
Fig. 6.
Vimentin is required for Cd-induced peribronchiolar fibrosis. A and B: IMR-90 cells were transfected with vimentin siRNA or NT-siRNA and followed by 20 μM of CdCl2 treatment for 2 h (A) or 3 h and then allowed to recover for 48 h (B). Western blots were performed using antibodies shown above. C: the soluble collagen levels were evaluated by Sircol collagen assay (n = 5). **P < 0.01. D: wild-type (WT) and Vimentin−/− mice (n = 5 of each group) were treated intratracheally with saline or CdCl2 (0.009 mg/kg body wt) every other day for 8 wk. Cryosections from weeks 1, 2, 4, and 8 mice lungs were stained with collagen-1 or picro-sirius red. Bar = 100 μm. Representative photomicrographs from 5 animals on week 4 are shown. E: 3,3′-diaminobenzidine staining density was quantified and the average positive area density was calculated from 10 random areas. Densities are denoted in arbitrary units. **P < 0.01. F: WT and Vimentin−/− mice (n = 5 of each group) were treated as above and airway resistance following methacholine challenge was measured. ##P < 0.01 vs. WT Cd mice with methacholine at 20 or 40 mg/ml. Horizontal bars indicate the mean values.
Vimentin phosphorylation, localization, and association of YAP1 and Smad2/3 in the nucleus are required for Cd-induced peribronchiolar fibrosis.
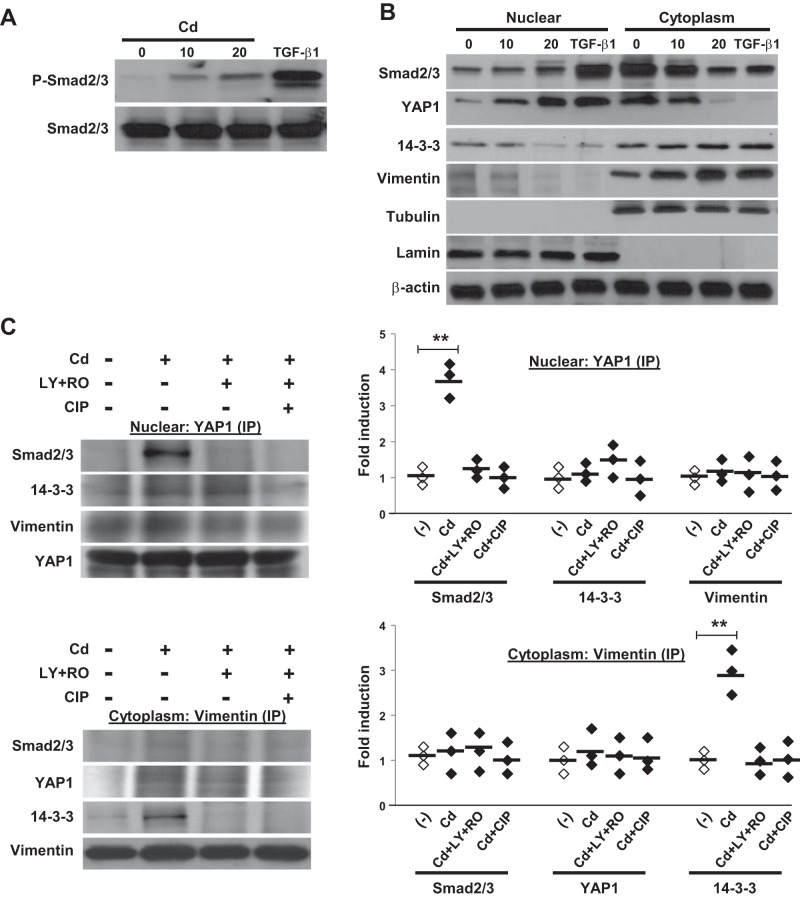
To understand how vimentin phosphorylation drives Cd-induced peribronchiolar fibrosis, we investigated the interaction of YAP1 with its downstream regulators in fibroblasts. We determined the subcellular localization of YAP1 in response to Cd stimulation. Cell lysates were fractionated into cytoplasmic and nuclear fractions. These experiments showed that Cd treatment not only induced Smad2/3 phosphorylation (Fig. 7A) but also induced the nuclear translocation of both Smad2/3 and YAP1 (Fig. 7B). 14-3-3, however, mainly remained in the cytoplasm. Interestingly, Cd also induced vimentin accumulation in the cytoplasm and showed a slight nuclear decrease. Furthermore, Cd treatment resulted in the association of YAP1 and Smad2/3 in the nuclear fraction and association of vimentin and 14-3-3 in cytoplasmic fraction (Fig. 7C). No complexes of YAP1 with Smad2/3 in cytoplasm and vimentin with 14-3-3 in nuclear were observed (data not shown). These associations were dependent on vimentin phosphorylation, as it was abolished after pretreatment with AKT and cdc2 inhibitors or CIP phosphatase.
Fig. 7.
Cd induces nuclear localization and association of Yes-associated protein 1 (YAP1) and Smad2/3. A: primary lung fibroblasts were incubated with or without CdCl2 (10 or 20 μM) or TGF-β (2 ng/ml) for 2 h and followed by immunoblot analysis with anti-P-Smad2/3 or anti-Smad2/3. B: cells were subjected to subcellular fractionation to obtain nuclear and cytoplasm fractions. Equivalent protein amounts of each fraction were separated by SDS-PAGE, and immunoblotted with anti-Smad2/3, YAP1, 14-3-3, or anti-vimentin. Tubulin, lamin, and β-actin were used as loading controls. C: cells were pretreated with AKT inhibitor (LY; 20 μM) and cdc2 inhibitor (RO; 10 μM) or phosphatase inhibitor [calf intestinal phosphatase (CIP); 10 units/ml] for 1 h and then stimulated with CdCl2 for 2 h, lysed, and subjected to YAP1 or vimentin immunoprecipitation followed by Smad2/3, 14-3-3, vimentin, or YAP1 Western analysis. The densities of protein bands were determined by densitometry and the data represent a 1-fold increase from the control density. **P < 0.01. Horizontal bars indicate the mean values. The results shown in A–C are representative of those from 3 independent experiments.
DISCUSSION
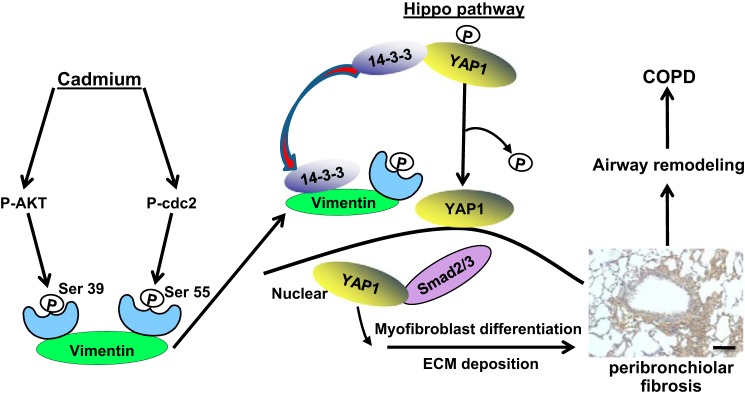
Chronic low-dose exposure to Cd through smoking or through environmental exposure has been shown to cause obstructive lung disease ranging from airway hyperreactivity to GOLD Grade 4 COPD (8, 20). COPD is diagnosed in these subjects by the presence of irreversible expiratory airflow obstruction on spirometry (19, 32, 46). Importantly, patients with a diagnosis of COPD may not necessarily have a history of smoking (33), and environmental exposures, including to Cd, are well recognized to cause airflow obstruction (12, 20). In the present study, we demonstrated that in vivo exposure to low-dose Cd causes the development of peribronchiolar fibrosis that is mediated by myofibroblast differentiation of fibroblasts and deposition of ECM proteins. We also provided evidence to support the hypothesis that specific AKT and cdc2 activation along with the downstream vimentin phosphorylation at Ser39 and Ser55 leading to YAP1 activation is required for Cd-induced peribronchiolar fibrosis and airway remodeling (Fig. 8).
Fig. 8.
Proposed model for the role of vimentin phosphorylation and YAP1 in Cd-induced peribronchiolar fibrosis, airway remodeling, and subsequent COPD. Prolonged exposure to Cd results in fibrosis around the airways. Cd-induced phosphorylation of AKT and cdc2 kinase is required for the vimentin phosphorylation at Ser39 and Ser55, respectively. Such phosphorylated vimentin will bind 14-3-3 and facilitate the nuclear translocation of YAP1 and Smad2/3 and complex formation of YAP1 and Smad2/3 in the nucleus, which activates myofibroblast and induces ECM protein deposition.
Exposure to environment toxins causes the development of pathological small airway remodeling and subsequent COPD (2, 5, 21). In this process, fibroblasts are the main target for airway remodeling (22). In the present experiments, in vivo studies demonstrated that low-dose Cd induced significant fibrotic changes and collagen accumulation after 2 wk and remained at high levels at 8 wk. Primarily peribronchiolar distribution in fibrosis was observed, the significant increase noted in small airways, <2 mm in diameter, suggesting that low-dose of Cd causes peribronchiolar thickening and subsequent narrowing of the lumen of small airways. It has been reported that ECM homeostasis is crucial for fibrotic diseases and that deposition of collagens and other ECM proteins contribute to the luminal ablation of small bronchioles (28, 43). Our data in vitro also confirm the increase in expression of α-SMA and accumulation of ECM proteins (28, 43). Furthermore, our results show that these processes can be effectively inhibited by both AKT and cdc2 kinase inhibitors suggesting that the use of AKT and Cd inhibitors may be beneficial to the Cd-induced peribronchiolar fibrosis.
Previous studies have implicated that, in addition to P38 pathway in human promonocytic cells (17), other signaling pathways, such as NF-κB (9), ERK, AKT, and p70S6K1 (27), are also involved in Cd-induced injury to bronchiolar epithelial cells. Here we provide new evidence to demonstrate that in Cd-exposed human lung fibroblasts AKT and cdc2 but not ERK, PKC, and PKA kinases are activated. We confirmed vimentin as a potential AKT and cdc2 downstream target. Furthermore, AKT-induced vimentin phosphorylation at Ser39 and cdc2-induced vimentin phosphorylation at Ser55 were identified. Our findings indicate that the inhibition of AKT and cdc2 activity and vimentin knockout impair Cd-induced vimentin serine phosphorylation as well as its subsequent α-SMA activation and ECM collagen deposition. These data suggest a key regulatory role of vimentin phosphorylation in Cd-induced peribronchiolar fibrosis and highlight that AKT and cdc2 activation are essential for vimentin phosphorylation.
Our data indicate that upon Cd stimulation, Cd-induced vimentin phosphorylation forms a complex with 14-3-3 proteins. In the meantime, protein phosphatase 2 (PP2) binds YAP1 and dephosphorylates it (39), allowing YAP1 to translocate from the cytoplasm to nucleus where it binds to Smad2/3 and activates fibrosis-mediated transcription factors (Fig. 7). YAP1 activation is required for matrix stiffening and stiff matrices further promote YAP1 nuclear localization (14). It is possible that Cd induced collagen generation, and further increases in matrix stiffness may be responsible for the YAP1 activation. Our work is the first report to highlight the emerging role of YAP1 in Cd-induced peribronchiolar fibrosis and airway remodeling in Cd-induced COPD.
Furthermore, we have shown that vimentin is secreted by fibroblasts upon Cd stimulation. AKT and cdc2 kinase inhibitors suppress Cd-induced vimentin fragmentation and secretion. These data indicate that Cd-induced vimentin fragmentation and secretion are upregulated by both AKT and cdc2. It has been proposed that secreted vimentin is exported via endoplasmic reticulum and Golgi complex (34). We show that Cd induces vimentin cytoplasm accumulation suggesting a nuclear-cytoplasmic shuttling, as described with other proteins (24). Importantly, our results suggest the importance of regulation of phosphorylation in Cd-induced vimentin secretion. This is supported by our results that kinase inhibitors and CIP phosphatase can effectively prevent this secretion.
Taken together, the data provided herein report for the first time that exposure to low doses of Cd is fibrogenic and causes lung peribronchiolar fibrosis in which myofibroblast differentiation and ECM deposition play a critical role. It is important to note that Cd-induced phosphorylation of AKT and cdc2 kinase are required for the vimentin phosphorylation at Ser39 and Ser55, respectively, highlighting AKT and cdc2 as attractive potential therapeutic targets for Cd-induced peribronchiolar fibrosis. Furthermore, we demonstrate the important role of vimentin phosphorylation in Cd-induced ECM deposition. Such phosphorylated vimentin binds 14-3-3 and facilitates the complex formation of YAP1 and Smad2/3 in the nucleus and regulates ECM downstream targets. Our study delineates on the role of Cd activated lung fibroblasts in the development of airway bronchiolar remodeling and luminal loss through peribronchiolar fibrosis and subsequent COPD. This study recognizes the important role of Cd-induced lung disease and the increasingly recognized role that environmental pollution plays in the development of COPD.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant P01-HL-114470 (to V. J. Thannickal and V. B. Antony).
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the authors.
AUTHOR CONTRIBUTIONS
F.J.L. and V.B.A. conceived and designed research; F.J.L., R.S., H.L., and Z.W. performed experiments; F.J.L., G.L., R.M.L., and V.B.A. analyzed data; F.J.L. interpreted results of experiments; F.J.L. prepared figures; F.J.L. drafted manuscript; F.J.L., G.L., R.M.L., S.B.M., M.A., V.J.T., and V.B.A. edited and revised manuscript; V.J.T. and V.B.A. approved final version of manuscript.
REFFRENCES
- 1.Basu S, Totty NF, Irwin MS, Sudol M, Downward J. Akt phosphorylates the Yes-associated protein, YAP, to induce interaction with 14-3-3 and attenuation of p73-mediated apoptosis. Mol Cell 11: 11–23, 2003. doi: 10.1016/S1097-2765(02)00776-1. [DOI] [PubMed] [Google Scholar]
- 2.Bentayeb M, Simoni M, Baiz N, Norback D, Baldacci S, Maio S, Viegi G, Annesi-Maesano I; Geriatric Study in Europe on Health Effects of Air Quality in Nursing Homes Group . Adverse respiratory effects of outdoor air pollution in the elderly. Int J Tuberc Lung Dis 16: 1149–1161, 2012. doi: 10.5588/ijtld.11.0666. [DOI] [PubMed] [Google Scholar]
- 3.Beveridge R, Pintos J, Parent ME, Asselin J, Siemiatycki J. Lung cancer risk associated with occupational exposure to nickel, chromium VI, and cadmium in two population-based case-control studies in Montreal. Am J Ind Med 53: 476–485, 2010. doi: 10.1002/ajim.20801. [DOI] [PubMed] [Google Scholar]
- 4.Bosken CH, Wiggs BR, Paré PD, Hogg JC. Small airway dimensions in smokers with obstruction to airflow. Am Rev Respir Dis 142: 563–570, 1990. doi: 10.1164/ajrccm/142.3.563. [DOI] [PubMed] [Google Scholar]
- 5.Brauer M, Freedman G, Frostad J, van Donkelaar A, Martin RV, Dentener F, van Dingenen R, Estep K, Amini H, Apte JS, Balakrishnan K, Barregard L, Broday D, Feigin V, Ghosh S, Hopke PK, Knibbs LD, Kokubo Y, Liu Y, Ma S, Morawska L, Sangrador JL, Shaddick G, Anderson HR, Vos T, Forouzanfar MH, Burnett RT, Cohen A. Ambient air pollution exposure estimation for the global burden of disease 2013. Environ Sci Technol 50: 79–88, 2016. doi: 10.1021/acs.est.5b03709. [DOI] [PubMed] [Google Scholar]
- 6.Brewster CE, Howarth PH, Djukanovic R, Wilson J, Holgate ST, Roche WR. Myofibroblasts and subepithelial fibrosis in bronchial asthma. Am J Respir Cell Mol Biol 3: 507–511, 1990. doi: 10.1165/ajrcmb/3.5.507. [DOI] [PubMed] [Google Scholar]
- 7.Byun Y, Chen F, Chang R, Trivedi M, Green KJ, Cryns VL. Caspase cleavage of vimentin disrupts intermediate filaments and promotes apoptosis. Cell Death Differ 8: 443–450, 2001. doi: 10.1038/sj.cdd.4400840. [DOI] [PubMed] [Google Scholar]
- 8.Chandler JD, Wongtrakool C, Banton SA, Li S, Orr ML, Barr DB, Neujahr DC, Sutliff RL, Go YM, Jones DP. Low-dose oral cadmium increases airway reactivity and lung neuronal gene expression in mice. Physiol Rep 4: e12821, 2016. doi: 10.14814/phy2.12821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen DJ, Xu YM, Du JY, Huang DY, Lau AT. Cadmium induces cytotoxicity in human bronchial epithelial cells through upregulation of eIF5A1 and NF-kappaB. Biochem Biophys Res Commun 445: 95–99, 2014. doi: 10.1016/j.bbrc.2014.01.146. [DOI] [PubMed] [Google Scholar]
- 10.Cosio M, Ghezzo H, Hogg JC, Corbin R, Loveland M, Dosman J, Macklem PT. The relations between structural changes in small airways and pulmonary-function tests. N Engl J Med 298: 1277–1281, 1978. doi: 10.1056/NEJM197806082982303. [DOI] [PubMed] [Google Scholar]
- 11.Cui H, Ge J, Xie N, Banerjee S, Zhou Y, Antony VB, Thannickal VJ, Liu G. miR-34a inhibits lung fibrosis by inducing lung fibroblast senescence. Am J Respir Cell Mol Biol 56: 168–178, 2017. doi: 10.1165/rcmb.2016-0163OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Das D, Bindhani B, Mukherjee B, Saha H, Biswas P, Dutta K, Prasad P, Sinha D, Ray MR. Chronic low-level arsenic exposure reduces lung function in male population without skin lesions. Int J Public Health 59: 655–663, 2014. doi: 10.1007/s00038-014-0567-5. [DOI] [PubMed] [Google Scholar]
- 13.dos Santos G, Rogel MR, Baker MA, Troken JR, Urich D, Morales-Nebreda L, Sennello JA, Kutuzov MA, Sitikov A, Davis JM, Lam AP, Cheresh P, Kamp D, Shumaker DK, Budinger GR, Ridge KM. Vimentin regulates activation of the NLRP3 inflammasome. Nat Commun 6: 6574, 2015. doi: 10.1038/ncomms7574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, Elvassore N, Piccolo S. Role of YAP/TAZ in mechanotransduction. Nature 474: 179–183, 2011. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 15.Eckes B, Dogic D, Colucci-Guyon E, Wang N, Maniotis A, Ingber D, Merckling A, Langa F, Aumailley M, Delouvée A, Koteliansky V, Babinet C, Krieg T. Impaired mechanical stability, migration and contractile capacity in vimentin-deficient fibroblasts. J Cell Sci 111: 1897–1907, 1998. [DOI] [PubMed] [Google Scholar]
- 16.Ermakova S, Choi BY, Choi HS, Kang BS, Bode AM, Dong Z. The intermediate filament protein vimentin is a new target for epigallocatechin gallate. J Biol Chem 280: 16882–16890, 2005. doi: 10.1074/jbc.M414185200. [DOI] [PubMed] [Google Scholar]
- 17.Galán A, García-Bermejo ML, Troyano A, Vilaboa NE, de Blas E, Kazanietz MG, Aller P. Stimulation of p38 mitogen-activated protein kinase is an early regulatory event for the cadmium-induced apoptosis in human promonocytic cells. J Biol Chem 275: 11418–11424, 2000. doi: 10.1074/jbc.275.15.11418. [DOI] [PubMed] [Google Scholar]
- 18.Goodpaster T, Legesse-Miller A, Hameed MR, Aisner SC, Randolph-Habecker J, Coller HA. An immunohistochemical method for identifying fibroblasts in formalin-fixed, paraffin-embedded tissue. J Histochem Cytochem 56: 347–358, 2008. doi: 10.1369/jhc.7A7287.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han MK, Agusti A, Calverley PM, Celli BR, Criner G, Curtis JL, Fabbri LM, Goldin JG, Jones PW, Macnee W, Make BJ, Rabe KF, Rennard SI, Sciurba FC, Silverman EK, Vestbo J, Washko GR, Wouters EF, Martinez FJ. Chronic obstructive pulmonary disease phenotypes: the future of COPD. Am J Respir Crit Care Med 182: 598–604, 2010. doi: 10.1164/rccm.200912-1843CC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hassan F, Xu X, Nuovo G, Killilea DW, Tyrrell J, Da Tan C, Tarran R, Diaz P, Jee J, Knoell D, Boyaka PN, Cormet-Boyaka E. Accumulation of metals in GOLD4 COPD lungs is associated with decreased CFTR levels. Respir Res 15: 69, 2014. doi: 10.1186/1465-9921-15-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, Paré PD. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med 350: 2645–2653, 2004. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- 22.Hogg JC, Timens W. The pathology of chronic obstructive pulmonary disease. Annu Rev Pathol 4: 435–459, 2009. doi: 10.1146/annurev.pathol.4.110807.092145. [DOI] [PubMed] [Google Scholar]
- 23.Huang WT, Vayalil PK, Miyata T, Hagood J, Liu RM. Therapeutic value of small molecule inhibitor to plasminogen activator inhibitor-1 for lung fibrosis. Am J Respir Cell Mol Biol 46: 87–95, 2012. doi: 10.1165/rcmb.2011-0139OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Janiszewska M, De Vito C, Le Bitoux MA, Fusco C, Stamenkovic I. Transportin regulates nuclear import of CD44. J Biol Chem 285: 30548–30557, 2010. doi: 10.1074/jbc.M109.075838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Järup L, Berglund M, Elinder CG, Nordberg G, Vahter M. Health effects of cadmium exposure–a review of the literature and a risk estimate. Scand J Work Environ Health 24, Suppl 1: 1–51, 1998. [PubMed] [Google Scholar]
- 26.Jeffery PK. Remodeling and inflammation of bronchi in asthma and chronic obstructive pulmonary disease. Proc Am Thorac Soc 1: 176–183, 2004. doi: 10.1513/pats.200402-009MS. [DOI] [PubMed] [Google Scholar]
- 27.Jing Y, Liu LZ, Jiang Y, Zhu Y, Guo NL, Barnett J, Rojanasakul Y, Agani F, Jiang BH. Cadmium increases HIF-1 and VEGF expression through ROS, ERK, and AKT signaling pathways and induces malignant transformation of human bronchial epithelial cells. Toxicol Sci 125: 10–19, 2012. doi: 10.1093/toxsci/kfr256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kolahian S, Fernandez IE, Eickelberg O, Hartl D. Immune mechanisms in pulmonary fibrosis. Am J Respir Cell Mol Biol 55: 309–322, 2016. doi: 10.1165/rcmb.2016-0121TR. [DOI] [PubMed] [Google Scholar]
- 29.Lampe BJ, Park SK, Robins T, Mukherjee B, Litonjua AA, Amarasiriwardena C, Weisskopf M, Sparrow D, Hu H. Association between 24-hour urinary cadmium and pulmonary function among community-exposed men: the VA Normative Aging Study. Environ Health Perspect 116: 1226–1230, 2008. doi: 10.1289/ehp.11265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lang MR, Fiaux GW, Gillooly M, Stewart JA, Hulmes DJ, Lamb D. Collagen content of alveolar wall tissue in emphysematous and non-emphysematous lungs. Thorax 49: 319–326, 1994. doi: 10.1136/thx.49.4.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu RM, Vayalil PK, Ballinger C, Dickinson DA, Huang WT, Wang S, Kavanagh TJ, Matthews QL, Postlethwait EM. Transforming growth factor β suppresses glutamate-cysteine ligase gene expression and induces oxidative stress in a lung fibrosis model. Free Radic Biol Med 53: 554–563, 2012. doi: 10.1016/j.freeradbiomed.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mannino DM. COPD: epidemiology, prevalence, morbidity and mortality, and disease heterogeneity. Chest 121, Suppl: 121S–126S, 2002. doi: 10.1378/chest.121.5_suppl.121S. [DOI] [PubMed] [Google Scholar]
- 33.Meyer PA, Mannino DM, Redd SC, Olson DR. Characteristics of adults dying with COPD. Chest 122: 2003–2008, 2002. doi: 10.1378/chest.122.6.2003. [DOI] [PubMed] [Google Scholar]
- 34.Mor-Vaknin N, Punturieri A, Sitwala K, Markovitz DM. Vimentin is secreted by activated macrophages. Nat Cell Biol 5: 59–63, 2003. doi: 10.1038/ncb898. [DOI] [PubMed] [Google Scholar]
- 35.Ogawara M, Inagaki N, Tsujimura K, Takai Y, Sekimata M, Ha MH, Imajoh-Ohmi S, Hirai S, Ohno S, Sugiura H, Yamauchi T, Inagaki M. Differential targeting of protein kinase C and CaM kinase II signalings to vimentin. J Cell Biol 131: 1055–1066, 1995. doi: 10.1083/jcb.131.4.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pefani DE, Pankova D, Abraham AG, Grawenda AM, Vlahov N, Scrace S, O’ Neill E. TGF-β targets the Hippo pathway scaffold RASSF1A to facilitate YAP/SMAD2 nuclear translocation. Mol Cell 63: 156–166, 2016. doi: 10.1016/j.molcel.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 37.Pini L, Pinelli V, Modina D, Bezzi M, Tiberio L, Tantucci C. Central airways remodeling in COPD patients. Int J Chron Obstruct Pulmon Dis 9: 927–932, 2014. doi: 10.2147/COPD.S52478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rokadia H, Agarwal S. Serum heavy metals and obstructive lung disease: results from the National Health and Nutrition Examination Survey. Chest 143: 388–397, 2013. doi: 10.1378/chest.12-0595. [DOI] [PubMed] [Google Scholar]
- 39.Schlegelmilch K, Mohseni M, Kirak O, Pruszak J, Rodriguez JR, Zhou D, Kreger BT, Vasioukhin V, Avruch J, Brummelkamp TR, Camargo FD. Yap1 acts downstream of α-catenin to control epidermal proliferation. Cell 144: 782–795, 2011. doi: 10.1016/j.cell.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Snider GL. Chronic obstructive pulmonary disease–a continuing challenge. Am Rev Respir Dis 133: 942–944, 1986. [PubMed] [Google Scholar]
- 41.Snider GL, Lucey EC, Faris B, Jung-Legg Y, Stone PJ, Franzblau C. Cadmium-chloride-induced air-space enlargement with interstitial pulmonary fibrosis is not associated with destruction of lung elastin. Implications for the pathogenesis of human emphysema. Am Rev Respir Dis 137: 918–923, 1988. doi: 10.1164/ajrccm/137.4.918. [DOI] [PubMed] [Google Scholar]
- 42.Surolia R, Karki S, Kim H, Yu Z, Kulkarni T, Mirov SB, Carter AB, Rowe SM, Matalon S, Thannickal VJ, Agarwal A, Antony VB. Heme oxygenase-1-mediated autophagy protects against pulmonary endothelial cell death and development of emphysema in cadmium-treated mice. Am J Physiol Lung Cell Mol Physiol 309: L280–L292, 2015. doi: 10.1152/ajplung.00097.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Togo S, Holz O, Liu X, Sugiura H, Kamio K, Wang X, Kawasaki S, Ahn Y, Fredriksson K, Skold CM, Mueller KC, Branscheid D, Welker L, Watz H, Magnussen H, Rennard SI. Lung fibroblast repair functions in patients with chronic obstructive pulmonary disease are altered by multiple mechanisms. Am J Respir Crit Care Med 178: 248–260, 2008. doi: 10.1164/rccm.200706-929OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsujimura K, Ogawara M, Takeuchi Y, Imajoh-Ohmi S, Ha MH, Inagaki M. Visualization and function of vimentin phosphorylation by cdc2 kinase during mitosis. J Biol Chem 269: 31097–31106, 1994. [PubMed] [Google Scholar]
- 45.Vlahovic G, Russell ML, Mercer RR, Crapo JD. Cellular and connective tissue changes in alveolar septal walls in emphysema. Am J Respir Crit Care Med 160: 2086–2092, 1999. doi: 10.1164/ajrccm.160.6.9706031. [DOI] [PubMed] [Google Scholar]
- 46.Washko GR, Criner GJ, Mohsenifar Z, Sciurba FC, Sharafkhaneh A, Make BJ, Hoffman EA, Reilly JJ; The NETT Research Group . Computed tomographic-based quantification of emphysema and correlation to pulmonary function and mechanics. COPD 5: 177–186, 2008. doi: 10.1080/15412550802093025. [DOI] [PubMed] [Google Scholar]
- 47.Wright JL. Emphysema: concepts under change–a pathologist’s perspective. Mod Pathol 8: 873–880, 1995. [PubMed] [Google Scholar]
- 48.Xu B, deWaal RM, Mor-Vaknin N, Hibbard C, Markovitz DM, Kahn ML. The endothelial cell-specific antibody PAL-E identifies a secreted form of vimentin in the blood vasculature. Mol Cell Biol 24: 9198–9206, 2004. doi: 10.1128/MCB.24.20.9198-9206.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang CF, Shen HM, Shen Y, Zhuang ZX, Ong CN. Cadmium-induced oxidative cellular damage in human fetal lung fibroblasts (MRC-5 cells). Environ Health Perspect 105: 712–716, 1997. doi: 10.1289/ehp.97105712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang H, von Gise A, Liu Q, Hu T, Tian X, He L, Pu W, Huang X, He L, Cai CL, Camargo FD, Pu WT, Zhou B. Yap1 is required for endothelial to mesenchymal transition of the atrioventricular cushion. J Biol Chem 289: 18681–18692, 2014. doi: 10.1074/jbc.M114.554584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhu QS, Rosenblatt K, Huang KL, Lahat G, Brobey R, Bolshakov S, Nguyen T, Ding Z, Belousov R, Bill K, Luo X, Lazar A, Dicker A, Mills GB, Hung MC, Lev D. Vimentin is a novel AKT1 target mediating motility and invasion. Oncogene 30: 457–470, 2011. doi: 10.1038/onc.2010.421. [DOI] [PMC free article] [PubMed] [Google Scholar]