Abstract
Restricted and repetitive behaviors (RRBs) are hallmark symptoms of autism spectrum disorders (ASDs); however, it has proven difficult to understand the mechanisms underlying these behaviors. One hypothesis suggests that RRBs are the result of a core deficit in attention. Alternatively, abnormalities of the motor system may constitute the central mechanism underlying RRBs, given motor deficits observed in ASDs. In this experiment, we investigated the etiology of RRBs and the relationship between attention and motor deficits. Movement impairments (a) may be indirectly related to attention deficits, (b) may result from a shared compromised process, or (c) may be independent. Twenty-two adolescents with ASD and 20 typically developing participants performed a spatial attention task. Movement impairments were assessed with a rhythmic tapping task. Attentional orienting and motor control were found to be related and supported the hypothesis that these impairments in ASD arise from a shared process. In contrast, measures of attention switching and motor control were found to be independent. Stereotyped behaviors, as assessed by parental ratings, were related more to the degree of motor impairment than to deficits of attention. These results suggest that both attentional orienting deficits and stereotyped RRBs are related to a compromised motor system.
According to current DSM (DSM-IV-TR; American Psychiatric Association, 2000) criteria, in addition to social and communication deficits, individuals with autism spectrum disorder (ASD) must exhibit at least one symptom from a heterogeneous set of restricted, repetitive, and stereotyped patterns of behavior or interests, which are restricted and repetitive behaviors (RRBs). These behaviors range from higher order cognitive symptoms, such as an encompassing preoccupation with certain interests and nonfunctional routines, to lower order motor symptoms, including stereotyped and repetitive motor mannerisms (Turner, 1999). It has proven difficult to specify the mechanism or mechanisms underlying this multifaceted symptom domain (Carcani-Rathwell, Rabe-Hasketh, & Santosh, 2006; Mosconi et al., 2009). In this paper, we investigate the degree to which RRBs are related to deficits in attention and motor control.
Some researchers have hypothesized that RRBs are a consequence of disordered selective attention (Courchesne & Allen, 1997; Ozonoff et al., 2004). Selective attention refers to the ability to attend to a directed location or dimension in feature space, while ignoring task-irrelevant information. Attention switching refers to the ability to change attentional set. Thus, restricted behaviors that indicate an undue fascination with objects, interests, or rituals may be a function of the diminished ability of those with ASD to shift attentional focus. Several studies have found that children and adolescents with ASD are slower to covertly direct spatial attention and switch attention to newly relevant locations (Harris, Courchesne, Townsend, Carper, & Lord, 1999; Townsend et al., 1999; Townsend, Courchesne, & Egaas, 1996; Wainwright-Sharp & Bryson, 1993). Moreover, these deficits are not restricted to visuospatial attention. Children with ASD have difficulty on selective attention tasks when the targets are defined by shape or color (Allen & Courchesne, 2003) or on tasks requiring that they continually switch attention between two streams of information (e.g., visual and auditory; Courchesne et al., 1994). Thus, selective attention deficits have been well documented in ASD, but the idea that an impairment of attention underlies RRBs has not been studied.
Although not included in the diagnostic criteria, motor impairments are frequently observed in individuals with ASD (Gidley Larson & Mostofsky, 2008). For example, instruments designed to measure motor function reveal general impairments in ASD (Gidley Larson et al., 2008; Haas et al., 1996; Hallett et al., 1993). Children with ASD score higher on the Physical and Neurologic Examination of Subtle Signs (Denckla, 1985) compared to those with attention-deficit/hyperactivity disorder and control participants (Mostofsky, Burgess, & Gidley Larson, 2007, Mostofsky et al., 2009). Although early motor development (Ozonoff et al., 2008) and acquisition of motor milestones (Freitag, Kleser, Schnieder, & von Gontard, 2007) are not necessarily delayed, the development of more complex skills like riding a bike is compromised (Gidley Larson & Mostofsky, 2008). Individuals with ASDs show greater inconsistency in producing rhythmic movements (Sheridan & McAuley, 1997) than do typically developing controls, and they exhibit impaired performance of motor skills and gestures consistent with a developmental dyspraxia (Dowell, Mahone, & Mostofsky, 2009; Minshew, Goldstein, & Siegel, 1997; Rogers, Bennetto, McEvoy, & Pennington, 1996). Furthermore, there appears to be a genetic association between motor clumsiness and autistic-like traits (Moruzzi, Ogliari, Ronald, Happe, & Battaglia, 2011). In sum, research of a pervasive motor control deficit in ASD continues to accumulate, but it is unclear whether these deficits are related to lower-level RRBs such as body rocking or circle turning and higher-level RRBs of restricted attentional focus.
In addition to the uncertainty surrounding the etiology of RRBs, little is known about the relationship between attention and motor deficits in ASD. Here we propose three potential models that could explain their co-occurrence and how these might be associated with the expression of RRBs: (a) a core impairment of coordination or selective attention may tax mental resources, resulting in impaired performance in other, unaffected domains (resource allocation account); (b) a core impairment of a shared process common to both movement coordination and selective attention may lead to deficits in both domains (shared process account); or (c) there is no core impairment that can explain observed deficits in both movement coordination and attention (independent account).
First, according to the resource allocation account, a core impairment of one system could indirectly affect performance on the other. For example, although a skilled bike rider may have little difficulty listening to an e-book while cruising on the bike path, the situation can change dramatically when navigating down a tricky mountain trail. Devoting mental resources to movement may appear to produce an attentional failure (if asked about the book). Alternatively, allocating resources to a demanding attentional task (e.g., cross-modal task switching) might reduce resources available to coordinate movement. In support of this account, Ravizza and Ivry (2001) assessed cross-modal attention switching in a group of individuals with movement coordination problems due to either cerebellar pathology or Parkinson disease. When motor demands were high, those with cerebellar ataxia performed worse than they did in a condition in which the attentional task remained unchanged, but the movement requirements were reduced. The motor-related improvement for the ataxia group is consistent with a resource allocation hypothesis; presumably, at least some component of their attentional deficit was related to the fact that they had to devote additional resources to producing movement. In contrast, patients with Parkinson disease exhibited a switching deficit in both conditions, suggesting that the disease affected task switching in a more direct manner.
Second, another possibility is that deficits in attention and motor control arise from a common, compromised process. Anatomical abnormalities in the parietal lobe and the cerebellum, two neural regions associated with attention and movement coordination, are implicated in the diverse neuropathology associated with ASD (Amaral, Schumann, & Nordahl, 2008). An impairment in one or both of these regions, or the communication between these regions, could disrupt a process necessary for both movement and attention. For example, spatial processing in the parietal cortex could be important for reaching as well as the ability to direct spatial attention. According to the shared process account, performance would suffer on movement and attention tasks that require the compromised process.
Third, it is possible that motor and attention deficits are independent. The widespread neural pathology in ASD could, and likely does, affect multiple functional networks, giving rise to a diverse constellation of compromised function. The independence account predicts that motor and attentional deficits are dissociable and may result from heterogeneous and varied neuropathology.
In the current experiment, we tested motor and attentional function in a group of adolescents with ASD, asking how their performance on these tasks was associated with RRBs. Our focus on attentional and motor performance in adolescents was motivated by two reasons. Although adolescents with ASD are included in many studies of selective attention, the age range in these studies is quite large, and often adults in their late 30s and early 40s are included in the sample (Allen & Courchesne, 2003; Harris et al., 1999; Townsend et al., 1996, 1999, 2001; Wainwright-Sharp & Bryson, 1993). Without a pure measure of attentional function in adolescence, interpreting these findings from a developmental perspective is complicated. The present experiment restricted the age range of participants so that attentional performance at the adolescent stage could be determined. Our focus on adolescence was also motivated by reports of motor improvement with age in ASD (Landa & Garrett-Mayer, 2006; Ming, Brimacombe, & Wagner, 2007). Although motor control may by disturbed in adults with ASD (Hallett et al., 1993), a more sensitive measure of the relationship among motor skill, attention, and RRBs will be obtained by testing an age group in which motor abnormalities are still reliably present. To get a fuller understanding of how these disorders develop in ASD during adolescence, age was correlated with performance in all attention and motor tasks. Although we are assessing attention and motor function at only one stage of development, this analysis will provide preliminary information about the development of these processes during this 5-year interval.
To assess motor function, we used a simple repetitive finger-tapping task that emphasizes movement timing. For attention function, we used the Posner spatial cueing task (Posner, 1980), using performance gains on validly cues trials as a measure of attentional orienting and the cost on invalidly cued trials as a measure of attentional switching. We probed the relationship between movement and attention in two ways: first, the interval between successive targets was manipulated to see if those with ASD would improve on the attention task, under the assumption that a slower rate would reduce the demands on motor control; second, we correlated performance on the rhythmic tapping task with measures of attentional orienting and switching.
Our goal was to adjudicate between the three hypotheses, which make different predictions about how performance on the attention task should be related to performance on the motor task (see Table 1). The resource-allocation account predicts that measures of attention should improve when motor demands are reduced because resources can be reallocated. Moreover, rhythmic tapping performance should be related to performance on the selective attention task, assuming that those exhibiting the greatest motor problems require the greatest shift of resources away from the attention task. The shared processing account also predicts a positive correlation between the measures of motor and attentional function, although it does not predict that attention performance will improve when motor demands are reduced. Finally, the independent account predicts that measures of motor and attentional function will not be related, nor should there be an improvement in attentional function when motor demands are reduced. Our previous work supported the resource allocation account for the relationship of movement and attention deficits in ataxia (Ravizza & Ivry, 2001). Although we use a different attention task in the current study, we predict a similar finding here, given that cerebellar abnormalities are widely documented in ASD (for a review, see Amaral et al., 2008).
Table 1.
Predictions based on the resource allocation, shared processing, and independent accounts of the relationship between movement coordination and attention
| Resource Allocation | Shared Processing | Independent | |
|---|---|---|---|
| Do low motor demands improve performance? | Yes | No | No |
| Are attention and tapping correlated? | Yes | Yes | No |
| Is tapping correlated with ___? | Both RRBs | Both RRBs | Stereotyped |
| Is attention correlated with ___? | Both RRBs | Both RRBs | Restricted |
Note: RRBs, restricted and repetitive behaviors.
To assess how impairments of movement and attention impact RRBs, we correlated parental observations of repetitive behavior as measured with the Repetitive Behavior Scale—Revised (RBS-R) with our measures of motor control and attention. We focused on two categories of RRBs that have emerged from factor analyses of this scale: stereotypic behaviors and restricted interests. These categories seem to best represent lower order motor and higher order cognitive symptoms (Lam & Aman, 2007). Stereotypic behaviors are purposeless movements such as hand flapping or body rocking that are produced repeatedly in a similar manner. Restricted behaviors are defined as behaviors that are limited in their range of focus, interest, or activity, and include strong attachment to objects (e.g., playing the same music or film continuously).
The three explanations of the relationship of movement and attention also predict how stereotyped and restricted behaviors will be related to performance. Both the resource allocation and the shared process accounts posit a relationship between movement and attention; therefore, they predict that RRBs will be related to performance in both domains. The independent account predicts that stereotyped behaviors will be related to tapping performance, whereas restricted behaviors should be more highly related to attention effects.
Methods
Participants
Participants were between the ages of 12 and 17 years, 11 months with a verbal IQ > 85 as assessed by the Wechsler Abbreviated Scale of Intelligence (Table 1). None of the participants had a co-occurring neurological disorder. Twenty-two participants (2 female) were diagnosed with ASD and met the following criteria: high-functioning autism (n = 13) or Asperger syndrome (n = 9) according to DSM-IV-TR; ASD according to the Autism Diagnostic Observation Schedule—Generic (Lord et al., 2000); and autism in all domains on the Autism Diagnostic Interview—Revised (Lord, Rutter, & LeCouteur, 1994). The decision to include individuals with both high-functioning autism and Asperger syndrome derives from studies showing that it is difficult to reliably distinguish between these two disorders (Howlin, 2003; Ozonoff & Griffith, 2000; South, Ozonoff, & McMahon, 2005). Asperger syndrome has been eliminated in DSM-5 (American Psychiatric Association, 2013) and subsumed under ASDs (Swedo, 2010). Six participants with ASD took atypical antipsychotic medication. We report secondary analyses in which we compare subgroups of the ASD sample to examine the effect of diagnostic type(Asperger or high functioning) and medication.
Typically developing participants (n = 20, 2 female) had no history of ASD or other neurodevelopmental or neurological disorders. The two experimental groups were matched on age, t (40) = 0.49, p = .63; gender; verbal IQ, t (40) = −0.02, p = .982; and full-scale IQ, t (40) = 0.64, p = .53 (see Table 2).
Table 2.
Demographic information for each participant in the ASD group and the group average
| Subject | Age | Grade | Sex | ADOS | SCQ | FSIQ | VIQ | Diagnosis | Medications |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 16 | 10 | M | 7 | 29 | 97 | 99 | HFA | None |
| 2 | 13 | 8 | M | 10 | 21 | 106 | 100 | AS | Ceraqual, Stratera, Advera |
| 3 | 14 | 9 | M | 13 | 29 | 109 | 112 | HFA | None |
| 4 | 14 | 8 | M | 8 | 21 | 120 | 119 | AS | None |
| 5 | 16 | 10 | M | 17 | 30 | 79 | 88 | HFA | Zoloft, Risperdal |
| 6 | 15 | 10 | M | 8 | 15 | 116 | 106 | AS | None |
| 7 | 16 | 10 | M | 10 | 24 | 106 | 105 | AS | None |
| 8 | 12 | 6 | M | 9 | 22 | 87 | 106 | HFA | Concerta |
| 9 | 16 | 11 | M | 11 | 16 | 122 | 122 | AS | Zoloft |
| 10 | 16 | 10 | M | 13 | 9 | 104 | 103 | AS | None |
| 11 | 14 | 9 | M | 7 | 26 | 89 | 106 | HFA | None |
| 12 | 13 | 7 | M | 17 | 32 | 89 | 98 | HFA | None |
| 13 | 12 | 7 | M | 7 | 21 | 121 | 123 | AS | Zoloft |
| 14 | 15 | 9 | F | 8 | 22 | 114 | 109 | AS | None |
| 15 | 13 | 8 | M | 9 | 24 | 89 | 87 | HFA | None |
| 16 | 16 | 10 | M | 12 | 23 | 108 | 110 | HFA | Risperdal, Aderal, Prozac, Abilify |
| 17 | 12 | 8 | M | 9 | 26 | 126 | 120 | AS | Risperdal, Concerta, Klonpin |
| 18 | 12 | 7 | M | 10 | 22 | 113 | NA | HFA | Risperdal |
| 19 | 17 | 12 | F | 13 | 28 | 96 | 96 | HFA | None |
| 20 | 15 | 10 | M | 7 | 19 | 109 | 119 | HFA | None |
| 21 | 15 | 10 | M | 9 | 27 | 101 | 104 | HFA | Buspar, Abilify, Wellbutrin |
| 22 | 13 | 8 | M | 7 | 15 | 132 | 132 | HFA | None |
| Average | 14.38 | 9 | 20 M, 2 F | 104.81 | 106.6 | ||||
| Controls | 14.55 | 9.1 | 18 M, 2 F | 108.6 | 107.7 |
Note: The average data for the typically developing control group is presented in the last row of the table. ASD, autism spectrum disorder; ADOS, Autism Diagnostic Observation Schedule; SCQ, Social Communication Questionnaire; FSIQ, full-scale IQ; VIQ, verbal IQ; HFA, high-functioning autism; AS, Asperger syndrome.
Procedure
Spatial attention
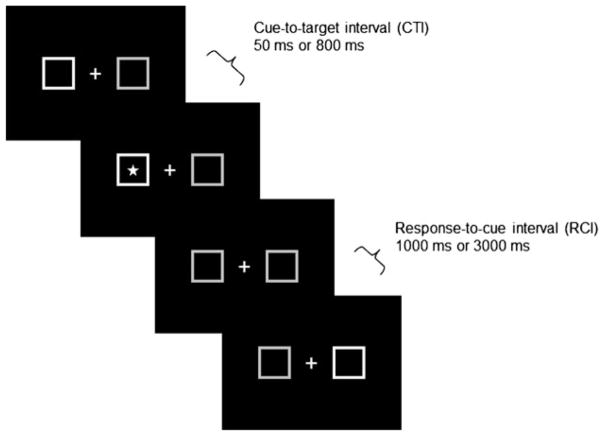
In the Posner Curing Paradigm (Posner, 1980), participants were instructed to fixate on a cross presented at the center of the computer screen and to press the space bar when a star appeared (Figure 1). The star appeared with equal probability inside a square on either the left or the right side of the cross. The outlines of one of the squares brightened in advance of the target on 84% of the trials, providing a spatial cue. The target appeared at this cued location on 80% of the cued trials (valid trials) and at the uncued location on 20% of the cued trials (invalid trials). Participants were told that the cue was usually predictive of the target location and that this information could help them respond faster. The other 16% of the trials were divided equally into trials in which both squares brightened or neither brightened.
Figure 1.
An example of the display and timing of the Posner spatial cuing task.
The time between the cue (i.e., squares brightening) and target presentation, which is the cue-to-target interval (CTI), was either short (50 ms) or long (800 ms). The target remained visible until the participant responded or until 2 s elapsed. The time between the response and the next cue, which is the response-to-cue interval (RCI), could also be short (1000 ms) or long (3000 ms). Participants completed six blocks of 64 trials each.
Orienting and validity effects were used to assess attention. The orienting effect indexes the advantage of a long interval in which to direct attention to a validly cued location. It was calculated by subtracting response times (RTs) from the long CTI condition from the short CTI condition in the valid conditions. It is assumed that a long CTI will be more beneficial (i.e., a larger orienting effect) for those who are slower to orient attention to the cued location (Townsend et al., 1999). Attention switching was measured by subtracting RT for targets appearing in validly cued locations from RT for invalidly cued locations (validity effect). Larger validity effects indicate that more time was needed to switch attention to an unexpected location. Data from the no-cue and both conditions were not analyzed.
In addition to these attentional measures, the effect of motor demand on performance was measured by calculating the difference in RT between the long and short RCI. We assume that the short RCI condition is more taxing on the motor system, given the relatively fast trial rate. As such, participants who have restricted resources should show a larger benefit on trials with a long RCI.
Tapping task
Each trial of the rhythmic tapping task was composed of a synchronization and a continuation phase (Ivry & Keele, 1989; Wing & Kristofferson, 1973). To start the trial, a series of 50 ms tones was presented, separated by 550 ms intervals. Participants were asked to tap in synchrony with the tones. After the participant produced his/her first tap, 12 more tones were presented (and thus the participant would usually produce 12 paced intervals). The metronome was then terminated, and the participant was required to continue tapping at the instructed pace until he/she had produced 30 unpaced intervals. Participants completed three blocks of six trials each.
Motor ability was assessed by determining how well participants matched and maintained the target pace during the continuation phase of the task. Intertap intervals were averaged across trials for each individual, and duration error was calculated by taking the difference between this interval and the target interval (550 ms). Given that some people erred by tapping too slow and others too fast, we took the absolute value of duration error to estimate how well the participant matched the target sequence. We also measured the variability of the intertap intervals during the continuation phase. For this measure, the raw tapping data (30 taps) were first fit to a regression line, and the standard deviation from this trend line was calculated on each trial and averaged across trials for each participant (Wing & Kristofferson, 1973). This detrending procedure eliminates global effects on variability that would arise if a participant sped up or slowed down over the course of a trial, allowing us to estimate local variability attributed to internal timing and response implementation. Trials in which there were three missing taps (e.g., gaps of greater than 1000 ms) or outlying values (<275 or >825 ms) were eliminated from the analysis. In the case of more than 3 taps or outliers, the average of the adjacent intervals was substituted for the missing response (Lundy-Ekman, Ivry, Keele, & Woollacott, 1991). On average, less than 1% of trials were excluded because of missing or outlying values. The number of discarded trials did not differ by group, t (37) = 0.82, p = .42.
RBS-R
The RBS-R (Bodfish, Symons, & Lewis, 1999) is a 43-item questionnaire that assesses six dimensions of repetitive behavior, including stereotyped motor behavior, self-injurious behavior, compulsive acts, ritualistic behavior, sameness behavior, and restricted behavior. Ratings were completed by parents who used a 4-point Likert scale that ranged from 0 (behavior does not occur) to 3 (behavior occurs and is a severe problem). Parents of both the ASD children and the typically developing children completed the questionnaire. Given that the RBS-R assesses a wide range of behaviors, we restricted our analyses to the two factors that seemed to best isolate attention and motor function. Motor abilities were assessed by the stereotyped behaviors sub-scale, using 9 items (max score = 27) from the RBS-R that load on this factor (Lam & Aman, 2007). Attention was assessed using the restricted behaviors scale, which was composed of 3 items (max score = 9; Lam & Aman, 2007).
Results
Spatial attention switching
Data from one control participant had to be excluded because of a technical difficulty with the computer program. Errors (no response) were rare, occurring on less than 1% of the trials. RTs below 150 ms or three standard deviations above the subject’s overall mean were discarded (4% total, range = 1%–22%). More than 10% of the trials were excluded for three control participants, with most of these being RT that were very fast (<150 ms). We assume that these individuals were responding to the cue rather than the target on many of these trials. For all participants, a minimum of 9 trials in the invalid conditions and 36 trials in the valid conditions were obtained in each condition.
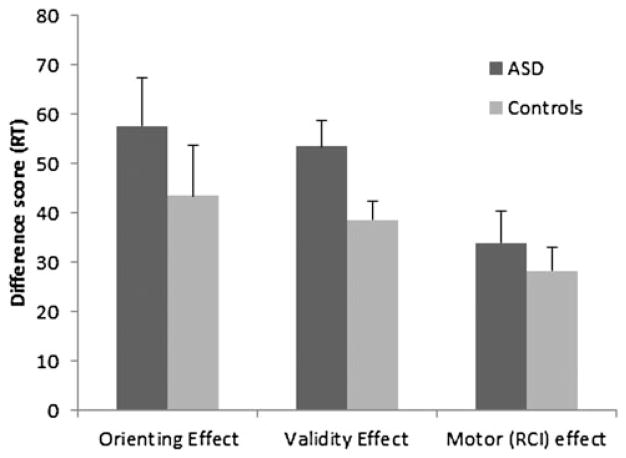
Group differences in the orienting effect were assessed using a mixed-factor analysis of variance with CTI and RCI as the within-subject factors and ASD diagnosis as the between-subject factor. This analysis was restricted to valid trials, given that this is the only condition in which attention can be directed to a predictable location. There was no main effect of group, F (1, 39) = 0.18, p = .67, suggesting that the ASD group was not generally slower than controls. RTs were faster when the CTI was longer, F (1, 39) = 50.92, p < .05; however, the Group×CTI interaction was not significant, F (1, 39) = 1.05, p = .31. In contrast to other reports showing a larger orienting effect in ASD (Townsend et al., 1996, 1999; Wainwright-Sharp & Bryson, 1993), our results suggest that the speed of orienting attention was equivalent between groups (Figure 2). We also determined whether the intact orienting effect was due to our inclusion of participants with Asperger syndrome. When we repeated the analysis without these participants (n = 9), the Group ×CTI interaction was not significant, F (1, 30) = 0.46, p = .5. Similarly, the results remained unchanged if we excluded individuals taking atypical medications (n = 6). Although the mean orienting effect was larger in the ASD group compared to the control group, variability was high. Note that the ASD and the control groups had equivalent IQs, whereas groups were not matched on IQ in most of the previous studies showing deficits of attentional orienting (Townsend et al., 1996, 1999, 2001; Wainwright-Sharp & Bryson, 1993).
Figure 2.
The orienting (valid short cue-to-target interval [CTI]–valid long CTI), validity (invalid–valid), and motor (short response-to-cue interval [RCI]–long RCI) effects in the attention task for the autism spectrum disorder (ASD) and typically developing (control) groups. RT, response time.
The validity and RCI effects were assessed using a mixed-factor analysis of variance with CTI (short, long), RCI (short, long), and cue validity (valid, invalid) as within-subject factors and ASD diagnosis as the between-subject factor. A main effect of cue validity indicated that participants were faster on trials with valid cues compared to invalid cues, F (1, 39) = 190.72, p < .05. A significant interaction of cue validity and group, F (1, 39) = 5.11, p < .05, indicated that those with ASD took longer to switch attention to targets appearing at unpredictable locations (Figure 2). Both groups were significantly slower on invalidly cued trials than on valid trials, ASD: t (21) = 10.25, p < .05; control: t (18) = 10.66, p < .05, and the magnitude of the difference was larger for those with ASD than for control participants (54 vs. 39 ms), t (39) = 2.27, p < .05 (see Figure 3). This is consistent with previous work showing larger validity effects in ASD (Townsend et al., 1996, 1999).
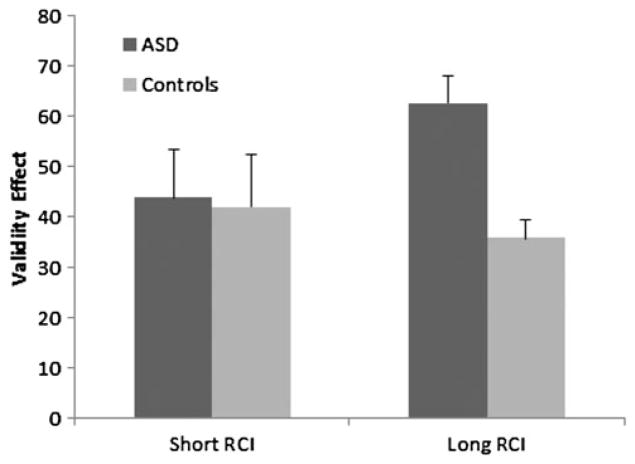
Figure 3.
The validity effect as a function of resource demands (length of response-to-cue interval [RCI]). ASD, autism spectrum disorder.
The resource allocation account predicted that a lengthier RCI would improve performance for those with ASD because of the longer interval between the response on trial n and the onset of the orienting cue on trial n + 1. This interval should minimize the overlapping demands on shared resources between the motor and the attentional domains. Participants were faster with the longer RCI, F (1, 39) = 46.51, p < .05. However, this improvement did not selectively improve performance for those with ASD, given that the RCI×Group interaction was not significant, F (1, 39) =0.02, p =.882. Although the RCI×Group×Validity interaction was marginally significant, F (1, 39) = 3.84, p = .057, the validity effect was larger for the ASD group with the long RCI, the condition in which motor demands are reduced (Figure 3). When we excluded participants (n = 6) taking atypical antipsychotic medication, the three-way interaction was significant, F (1, 33) = 4.46, p < .05. Rather than showing a benefit of RCI, attention switching became slower for the ASD group when motor demands were reduced. These results argue against the resource allocation account.
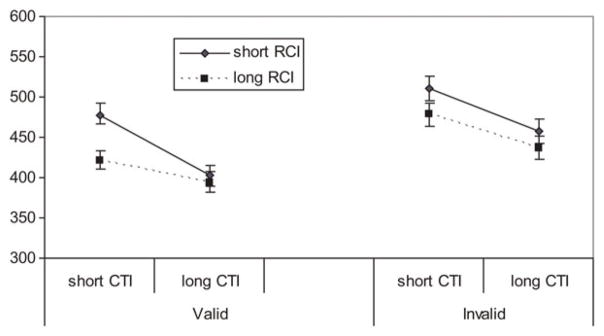
The three-way interaction of CTI×RCI×Validity was significant, F (1, 39) = 11.26, p < .05. As can be seen in Figure 4, the advantage of a long CTI for validly cued trials was greater at the short RCI than at the long RCI, t (40) = 9.01, p < .05, but not at invalid trials, t (40) = 1.14, p = .26. Thus, lowering motor demands improved the orienting of attention for all participants, but it had no effect on switching attention.
Figure 4.
The response time as a function of cue-to-target interval (CTI) length, response-to-cue interval (RCI) length, and cue validity for all participants.
Rhythmic tapping
Data from three control participants were discarded due to technical difficulties. During the continuation phase, participants with ASD tapped slightly faster than did control participants (mean interval duration: 532 ms vs. 542 ms), but this difference was not reliable, t (37) =1.5, p =.14. The absolute value of the duration error also did not differ between groups (23 vs. 14 ms), t (37) = 1.6, p = .11. In terms of temporal variability, the ASD children and typically developing children performed similarly, t (37) = 0.69, p = .495, with mean Weber fractions (SD/M) of 0.082 and 0.074, respectively. Excluding those with Asperger syndrome or taking atypical medications did not change any of the results.
Correlations between attention and movement
We next examined the relationship between the dependent variables in the movement and attention tasks for the ASD group. The shared process account of concomitant attention and movement disorders predicts that performance on these tasks should be related, whereas the independent account predicts that performance on these measures need not be related.
Given that we are using the orienting and validity effects as separate assays of attentional function, we first assessed the degree to which these measures were related to each other. The orienting and validity effects were significantly related, r (21) = .48, p < .05. Thus, in the following analyses, we used partial correlations in order to attain a purer measure of the relationship of attentional orienting or switching to motor control.
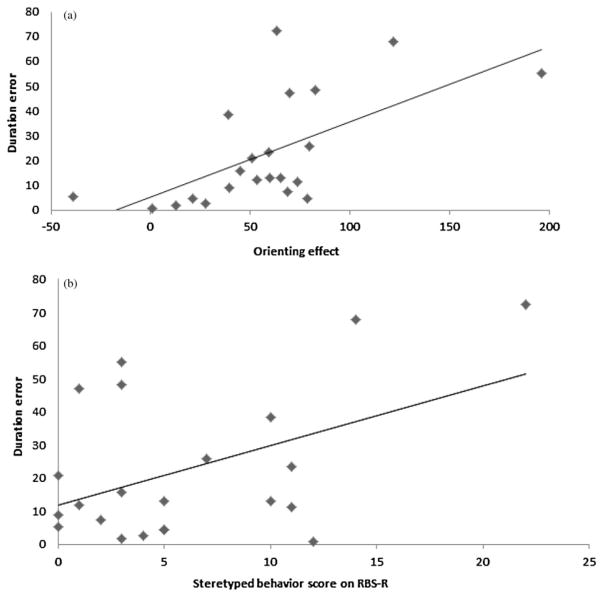
A relationship between motor and attention performance would support both the resource allocation and the shared process accounts. In support of these accounts, a positive correlation was observed between the size of the orienting effect and duration error, r (19) = .55, p < .05, even after adjusting for the size of the validity effect (Figure 5a). Variability of the intertap interval was also positively correlated with the orienting effect, r (19) = .45, p < .05. In contrast, the size of the validity effect was not significantly correlated with the duration error, r (21) = .10, p = .65, or the tapping variability, r (19) = .06, p = .81, after adjusting for any contribution from the orienting effect. In sum, orienting attention was related to motor performance; however, switching attention was independent of motor performance.
Figure 5.
The relationship in the autism spectrum disorder group between the error duration in the rhythmic tapping task and (a) the size of the orienting effect in the attention task and (b) parental ratings of stereotyped behaviors. RBS-R, Repetitive Behavior Scale—Revised.
The resource allocation account predicts that participants with greater motor impairments will benefit more from a long RCI in the attention task. Contrary to this prediction, the size of the RCI effect was not related to tapping performance, in terms of either duration error or tapping variability (all ps >.1). Thus, the degree to which participants improved on the attention task when the motor demands were reduced was unrelated to the participants’ performance on the motor task.
Relationship of repetitive behaviors to motor and attention performance
The preceding results suggest that motor coordination and one aspect of attentional function, orienting, are impaired in a correlated manner in ASD. This raises the question of whether these deficits are related to RRBs. Given the behavioral and neural evidence associating RRBs with failures of motor inhibition (Agam, Joseph, Barton, & Manoach, 2010; Lopez, Lincoln, Ozonoff, & Lai, 2005; Mosconi et al., 2009; Shafritz, Dichter, Baranek, & Belger, 2008; Thakkar et al., 2008; Yerys et al., 2009), we predicted that RRBs would be associated with performance on the motor tapping task. We found that rating severity for stereotyped movements was significantly correlated with duration error on the tapping task, r (21) = .46, p < .05 (Figure 5b). When we used a partial correlation measure to ask if this relationship held, even after controlling for the effects of attentional factors (i.e., orienting and validity effects), the correlation between stereotypy ratings and tapping performance remained significant, r (19) =.47, p <.05. Thus, the severity of stereotyped behaviors (e.g., body rocking and hand flapping) was associated with more difficulty in producing the target tapping rate in the motor task. Duration error was positively, but not reliably, correlated with the restricted interest component of the RRB assessments, r (21) = .34, p = .12. Tapping variability was positively correlated with stereotyped behaviors (r = .24) and restricted interests (r = .15); however, neither of these correlations were reliable (all ps > .1).
There was little evidence for a relationship between RRBs and performance on the attention task. Neither the validity, stereotyped: r (21) =.26, p =.24; restricted interest: r (21) =.09, p = .7, nor the orienting effects, stereotyped: r (21) = .13, p = .56; restricted interest: r (21) = .38, p = .09, were reliably related to RRB ratings. Note that the correlation between the orienting effect and ratings on the restricted interest subscale was relatively high and approached significance. To examine this relationship when motor performance was factored out, we performed partial correlations between restricted movements and the orienting effect, although controlling for duration error. The correlation between the orienting effect and restricted interests was actually reduced when controlling for motor performance, r (19) = .23, p = .33.
Relationship of age and performance
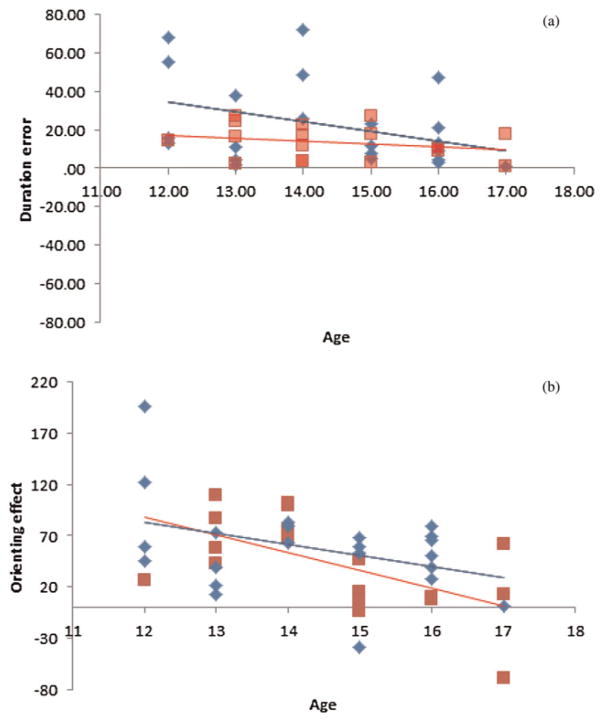
Motor skill is reported to improve with age (Landa & Garrett-Mayer, 2006; Ming et al., 2007). Thus, childhood or adolescence may be a critical time for assessing the relationship of motor deficits to cognitive dysfunction. To see how attentional control, motor skill, and RRBs improved with age, correlations were performed between these variables (Figure 6). Given our small sample size and restricted age range, these correlations did not reach significance. All correlations between age and performance were negative, orienting effect: r (21) = −.38, p = .079; validity effect: r = −.41, p = .058; tapping error: r (21) = −.365, p = .095; tapping variability: r (21) = −.349, p = .112; stereotypy ratings: r (21) = −.27, p = .226; restrict interest ratings: r (21) = −.18, p = .433; that is, attention, motor control, and RRBs tended to improve with age in the ASD group.
Figure 6.
The relationship of age to (a) the size of the orienting effect and (b) error duration in the rhythmic tapping task. Diamonds (blue online only) indicate individuals in the autism spectrum disorder group, and squares (red online only) denote individuals in the typically developing group. [A color version of this figure can be viewed online at http://journals.cambridge.org/dpp]
Discussion
The goal of this study was to assess the relationship of motor control and attention in ASD and to determine whether performance in these domains was associated with RRBs. Motor performance and attention were related in ASD; the speed at which attention was oriented by a predictive spatial cue was related to error and variability in reproducing a target pace in a rhythmic tapping task. This result is in line with both the resource allocation and the shared process hypotheses for concomitant motor and attention deficits in ASD. The resource allocation account predicted that attentional orienting would improve when motor demands were reduced; however, the ASD group did not show a greater benefit of lower motor demands than did controls, an observation at odds with this hypothesis. Thus, attentional orienting and rhythmic tapping performance most likely require a shared process. In contrast, attention switching was not reliably associated with tapping performance and seems to be independent from motor control. Tapping performance was not strongly related to the restricted interests score, but it was related to ratings of stereotyped behaviors, thought to reflect lower order motor control issues. There was no evidence that deficits of attention underlie RRBs.
The shared process account predicted that deficits of attention and motor performance should be related, and we found support for this hypothesis when considering attentional orienting. Note that we are claiming that deficits in attention and motor control can be explained by a shared process despite the fact that a “deficit” was not observed in the ASD group on either the measure of attentional orienting or the rhythmic tapping performance. Although the ASD group tended to show poorer performance on these measures, the group differences were not reliable. Note that ASD is a complex disorder, and individuals with ASD exhibit greater or lesser degrees of behavioral, social, and cognitive impairment. The ability to detect group differences in attention or motor skill may be a function of the heterogeneity of the ASD group. This heterogeneity in performance may reflect different rates of neural development between individuals with ASD and typically developing participants. For example, our data show improvements in performance with age even within our restricted 5-year age range. If adolescents with ASD in the upper age range (15–17) have reached a level of performance that is equivalent to controls, then group differences are less likely to be observed when combining the data of older and younger adolescents. Moreover, typically developing participants can also vary in neural development, even though they do not have a clinical disorder. For example, children vary in their level of clumsiness, which is thought to be due to differences in cerebellar development (Williams, Woollacott, & Ivry, 1992). To the extent that development is slow in similar neural regions, less of a difference between groups should be expected. Focusing on the question of how attentional control varies as a function of motor development is a promising way to understand the consequences of slow or abnormal development in both the typically developing and the ASD groups.
The present study was designed to establish functional relationships between attentional and motor task domains. Future work is required to explore specific mechanistic hypotheses that might underlie the shared process. One possibility to consider is whether a core deficit in temporal processing affects performance in both domains. It has been argued that individuals with ASD have difficulty understanding that successive events are part of a unitary process (Allman, 2011). In our attention task, participants must use the cue to predict the location of a subsequent target in order to deploy their attention appropriately. Inefficient temporal binding of the cue and target events may slow attentional orienting. Similarly, reproducing a target pace requires maintaining and adapting the interval durations across successive movements.
An alternative, yet related idea is that the co-occurrence of action and attention impairments in ASD may be due to problems with predicting the internal conditions necessary to deal with upcoming events (Akshoomoff, 2000. According to this hypothesis, the cerebellum, a region found to be structurally and functionally atypical in ASD (Amaral et al., 2008; Courchesne & Allen, 1997), is thought to prepare task-relevant neural systems. Such preparatory function would facilitate the rapid deployment of attention and coordination of rapid movements. A related idea posits that the cerebellum may code the precise timing necessary for responding to anticipated sensory stimuli and for producing coordinated movements (Ghajar & Ivry, 2009). Although this hypothesis has emerged from consideration of cerebellar links to attention and motor control, it may also be relevant with respect to other neural regions. Striatum and parietal and prefrontal cortices have also been linked to attention and motor tasks, and shown to be abnormal in ASD (for a review, see Amaral et al., 2008). Abnormal functioning of corticostriatal loops has been argued to underlie the presence of RRBs in clinical disorders rather than the cerebellum (Langen, Durston, Kas, van Engleland, & Staal, 2011).
Consistent with other studies, the ASD group was significantly slower at switching attention to invalidly cued locations compared to validly cued locations. However, motor abilities were not highly related to the validity effect. This pattern suggests that movement and switching attention are independent processes. Moreover, switching did not improve when motor demands were reduced, suggesting that a resource allocation problem is not responsible for the attentional deficit. We recognize that our assessment of motor function is quite limited here. It would be important to assess other aspects of motor performance in future studies. The tapping task used here might be most effective in assessing motor timing, and this aspect of motor control might not be critical for attention switching.
The resource allocation account predicted that attentional performance should improve when the time between successive movements is lengthened. This longer interval would allow resources to be diverted back to target detection rather than motor control. Unlike our study of those with cerebellar ataxia (Ravizza & Ivry, 2001), attentional performance did not improve in a selective manner for the ASD group when motor demands were reduced (Figure 3). The ASD group became slower to shift attention when motor demands were reduced. Although we have interpreted this as evidence against the resource allocation account, it may be that reducing motor demands frees attentional resources that can then be used for engaging attention, and this indirectly effects attention switching. In this variant of the resource allocation idea, the ASD group may be disproportionately slower when asked to disengage attention from validly cued locations, especially when greater attentional resources are available to engage attention to those locations. If so, it might be expected that the validity effect with a short CTI would be smaller than for a long CTI. In other words, it might be more difficult to switch attention when CTI is long because there is more time to orient attention to the target location and, consequently, more difficult to switch attention from that location. However, validity effects did not differ by CTI for the ASD group when motor demands were low; they were actually in the opposite direction than predicted from this hypothesis (validity effect at short CTI = 69 ms, validity effect at long CTI = 56 ms). Thus, attentional deficits in ASD do not appear to be the result of resources being commandeered to movement selection or execution.
The hypothesis that RRBs are related to a core deficit of attention was not supported by the results of this study. We instead found that motor deficits were related to stereotyped behaviors. These lower order repetitive mannerisms may be a product of disordered motor control at the level of either response selection or generating and executing alternative motor plans. Thus, motor control rather than attention performance was related to stereotyped behaviors, arguing against a core deficit in attention as a factor in behavioral rigidity.
We did not find a relationship between motor control and restricted interests. It may be that restricted interests tap the ability to switch attention, which was not highly related to motor control in the present study. Alternatively, the restricted interest subscale of the RBS may not be sufficiently sensitive to measure this construct because of the lesser number of items comprising this scale (Lam & Aman, 2007). The lack of a significant relationship between restricted behaviors and performance should therefore be interpreted with caution.
Research has focused on the relationship of RRBs to executive functioning, often relating the prevalence of RRBs to disorders of response selection or inhibition (Agam et al., 2010; Lopez et al., 2005; Mosconi et al., 2009; Shafritz et al., 2008; Thakkar et al., 2008). For example, errors due to a failure in inhibiting eye movements toward a target on an antisaccade task were positively correlated with RRBs (Mosconi et al., 2009). Moreover, RRBs are associated with hypo- or hyperactivity of the anterior cingulate in the antisaccade task (Agam et al., 2010; Shafritz et al., 2008; Thakkar et al., 2008), a region reliably engaged by response conflict (Carter et al., 1998; Kerns et al., 2004). Other studies have examined RRBs and the ability to shift attentional set in discrimination learning tasks like the Wisconsin Card Sorting test (South et al., 2007; Yerys et al., 2009). However, these tasks also place high demands on response inhibition; participants must overcome previously learned stimulus–response associations in order to respond correctly to a shift in attentional set. There are multiple ways of interpreting performance on these tasks (Miller & Cohen, 2001), and it is unclear whether the relationship between RRBs and set shifting in these studies is due to a disorder of attention switching apart from a problem with response selection and inhibition.
Studies of the neural underpinning of repetitive behaviors have suggested a few possible candidates. Using an antisaccade task, correlations were observed between activity of the rostral anterior cingulate and ratings of repetitive behaviors (Agam et al., 2010; Shafritz et al., 2008; Thakkar et al., 2008). Abnormalities of the cerebellum have also been linked to repetitive behaviors (Martin, Goldowitz, & Mittelman, 2010; Pierce & Courchesne, 2001). The observation of both anterior cingulate and cerebellar contributions to RRBs suggests that these regions may work together to support flexible responding. For example, RRBs may generate conflict in the motor system between previously activated responses and contextual goals that call for response flexibility. The anterior cingulate may be important for detecting such conflict and triggering cerebellar recruitment in preparing alternative responses (Helmuth, Ivry, & Shimizu, 1997). Given reports of intact implicit motor learning in ASD (Barnes et al., 2008; Travers, Klinger, Mussey, & Klinger, 2010), an anterior cingulate–cerebellum circuit may facilitate behavioral flexibility by determining when flexibility is necessary and generating response alternatives in nonautomatic learning conditions.
This study has several limitations. First, our sample was small, and this limited the types of statistical analyses that were possible. Second, we did not correct for multiple comparisons in our correlational analyses but interpreted our correlation coefficients as effect sizes (Cohen, 1992). We felt that guarding stringently against Type I error would reduce power and thereby increase false negatives. Given that the relationship between attention and motor control has received little attention in autism research, we opted to take a less conservative approach here. Third, our sample was restricted to those with above average or better IQs, limiting the generalizability of our findings. This is important in the study of RRBs because profiles of such behaviors are known to differ based on cognitive level (Bishop, Richler, & Lord, 2006).
Conclusion
Psychiatric disorders are often associated with widespread neural pathology. As a result, disrupted performance across disparate task domains may reflect multiple levels of impairment that may not be related to one another. This study provides some insight into the relationship between attention and movement impairments in ASD. Instead of movement impairments indirectly producing an apparent deficit in attention, our results support the shared process account of the relationship between attentional orienting and movement. Motor performance was related to ratings of stereotyped behaviors, arguing against ideas that behavioral rigidity is a product of a core attentional deficit. Instead, our results suggest that motor abilities affect both movement coordination and the production of stereotyped behaviors.
Acknowledgments
This study was funded by the UC Davis MIND Institute (S-CCMIN07 to C.C.) and the National Institutes of Child Health and Human Development (R01HD060306 to R.I). We thank Petrina Kaluzhny, Neil Cummings, and Stanford Ly for help with data collection.
References
- Agam Y, Joseph RM, Barton JJ, Manoach DS. Reduced cognitive control of response inhibition by the anterior cingulate cortex in autism spectrum disorders. NeuroImage. 2010;52:336–347. doi: 10.1016/j.neuroimage.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akshoomoff N. Neurological underpinnings of autism. In: Prizant B, Wetherby A, Dynlap G, Mirenda P, Rydell P, Williamson G, editors. Autism spectrum disorders: A transactional developmental perspective. Baltimore, MD: Paul H. Brookes; 2000. pp. 167–190. [Google Scholar]
- Allen G, Courchesne E. Differential effects of developmental cerebellar abnormality on cognitive and motor functions in the cerebellum: An fMRI study of autism. American Journal of Psychiatry. 2003;160:262–273. doi: 10.1176/appi.ajp.160.2.262. [DOI] [PubMed] [Google Scholar]
- Allman MJ. Deficits in temporal processing associated with autistic disorder. Frontiers in Integrative Neuroscience. 2011;5:2. doi: 10.3389/fnint.2011.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral DG, Schumann CM, Nordahl CW. Neuroanatomy of autism. Trends in Neuroscience. 2008;31:137–145. doi: 10.1016/j.tins.2007.12.005. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: Author; 2000. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5. Washington DC: Author; 2013. [Google Scholar]
- Barnes KA, Howard JH, Jr, Howard DV, Gilotty L, Kenworthy L, Gaillard WD, et al. Intact implicit learning of spatial context and temporal sequences in childhood autism spectrum disorder. Neuropsychology. 2008;22:563–570. doi: 10.1037/0894-4105.22.5.563. [DOI] [PubMed] [Google Scholar]
- Behrmann M, Thomas C, Humphreys K. Seeing it differently: Visual processing in autism. Trends in Cognitive Science. 2006;10:258–264. doi: 10.1016/j.tics.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Bishop SL, Richler J, Lord C. Association between restricted and repetitive behaviors and nonverbal IQ in children with autism spectrum disorders. Child Neuropsychology. 2006;12:247–267. doi: 10.1080/09297040600630288. [DOI] [PubMed] [Google Scholar]
- Bodfish JW, Symons FJ, Lewis MH. Western Carolina Center Research Reports. Cullowhee, NC: Western Carolina University; 1999. The Repetitive Behavior Scale. [Google Scholar]
- Carcani-Rathwell I, Rabe-Hasketh S, Santosh PJ. Repetitive and stereotyped behaviours in pervasive developmental disorders. Journal of Child Psychology and Psychiatry. 2006;47:573–581. doi: 10.1111/j.1469-7610.2005.01565.x. [DOI] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis. Current Directions in Psychological Science. 1992;1:98–101. [Google Scholar]
- Courchesne E, Allen G. Prediction and preparation, fundamental functions of the cerebellum. Learning & Memory. 1997;4:1–35. doi: 10.1101/lm.4.1.1. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Townsend J, Akshoomoff NA, Saitoh O, Yeung-Courchesne R, Lincoln AJ, et al. Impairment in shifting attention in autistic and cerebellar patients. Behavior Neuroscience. 1994;108:848–865. doi: 10.1037//0735-7044.108.5.848. [DOI] [PubMed] [Google Scholar]
- Denckla MB. Revised neurological examination for subtle signs. Psychopharmacology Bulletin. 1985;21:773–800. [PubMed] [Google Scholar]
- Dowell LR, Mahone EM, Mostofsky SH. Associations of postural knowledge and basic motor skill with dyspraxia in autism: Implication for abnormalities in distributed connectivity and motor learning. Neuropsychology. 2009;23:563–570. doi: 10.1037/a0015640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitag CM, Kleser C, Schneider M, von Gontard A. Quantitative assessment of neuromotor function in adolescents with high functioning autism and Asperger syndrome. Journal of Autism and Developmental Disorders. 2007;37:948–959. doi: 10.1007/s10803-006-0235-6. [DOI] [PubMed] [Google Scholar]
- Ghajar J, Ivry RB. The predictive brain state: Asynchrony in disorders of attention? Neuroscientist. 2009;15:232–242. doi: 10.1177/1073858408326429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidley Larson JC, Mostofsky SH. Evidence that the pattern of visuomotor sequence learning is altered in children with autism. Autism Research. 2008;1:341–353. doi: 10.1002/aur.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas RH, Townsend J, Courchesne E, Lincoln AJ, Schreibman L, Yeung-Courchesne R. Neurologic abnormalities in infantile autism. Journal of Child Neurology. 1996;11:84–92. doi: 10.1177/088307389601100204. [DOI] [PubMed] [Google Scholar]
- Hallett M, Lebiedowska MK, Thomas SL, Stanhope SJ, Denckla MB, Rumsey J. Locomotion of autistic adults. Archives of Neurology. 1993;50:1304–1308. doi: 10.1001/archneur.1993.00540120019007. [DOI] [PubMed] [Google Scholar]
- Harris NS, Courchesne E, Townsend J, Carper RA, Lord C. Neuroanatomic contributions to slowed orienting of attention in children with autism. Cognitive Brain Research. 1999;8:61–71. doi: 10.1016/s0926-6410(99)00006-3. [DOI] [PubMed] [Google Scholar]
- Helmuth LL, Ivry RB, Shimuzu N. Preserved performance by cerebellar patients on tests of word generation, discrimination learning, and attention. Learning & Memory. 1997;3:456–474. doi: 10.1101/lm.3.6.456. [DOI] [PubMed] [Google Scholar]
- Howlin P. Outcome in high-functioning adults with autism with and without early language delays: Implications for the differentiation between autism and Asperger syndrome. Journal of Autism and Developmental Disorders. 2003;33:3–13. doi: 10.1023/a:1022270118899. [DOI] [PubMed] [Google Scholar]
- Ivry RB, Keele SW. Timing functions of the cerebellum. Journal of Cognitive Neuroscience. 1989;1:136–152. doi: 10.1162/jocn.1989.1.2.136. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, III, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Lam KS, Aman MG. The Repetitive Behavior Scale—Revised: Independent validation in individuals with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2007;37:855–866. doi: 10.1007/s10803-006-0213-z. [DOI] [PubMed] [Google Scholar]
- Landa R, Garrett-Mayer E. Development in infants with autism spectrum disorders: A prospective study. Journal of Child Psychology and Psychiatry. 2006;47:629–638. doi: 10.1111/j.1469-7610.2006.01531.x. [DOI] [PubMed] [Google Scholar]
- Langen M, Durston S, Kas MJH, van Engeland H, Staal WG. The neurobiology of repetitive behavior: . . . and men. Neuroscience & Biobehavioral Reviews. 2011;35:356–365. doi: 10.1016/j.neubiorev.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Lopez BR, Lincoln AJ, Ozonoff S, Lai Z. Examining the relationship between executive functions and restricted, repetitive symptoms of autistic disorder. Journal of Autism and Developmental Disorders. 2005;35:445–460. doi: 10.1007/s10803-005-5035-x. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, et al. The Autism Diagnostic Observation Schedule—Generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30:205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview—Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Lundy-Ekman L, Ivry R, Keele S, Woollacott M. Timing and force control in clumsy children. Journal of Cognitive Neuroscience. 1991;3:367–376. doi: 10.1162/jocn.1991.3.4.367. [DOI] [PubMed] [Google Scholar]
- Martin LA, Goldowitz D, Mittleman G. Repetitive behavior and increased activity in mice with Purkinje cell loss: A model for understanding the role of cerebellar pathology in autism. European Journal of Neuroscience. 2010;31:544–555. doi: 10.1111/j.1460-9568.2009.07073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Ming X, Brimacombe M, Wagner GC. Prevalence of motor impairment in autism spectrum disorders. Brain and Development. 2007;29:565–570. doi: 10.1016/j.braindev.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Minshew NJ, Goldstein G, Siegel DJ. Neuropsychologic functioning in autism: Profile of a complex information processing disorder. Journal of the International Neuropsychology Society. 1997;3:303–316. [PubMed] [Google Scholar]
- Moruzzi S, Ogliari A, Ronald A, Happe F, Battaglia M. The nature of covariation between autistic traits and clumsiness: A twin study in a general population sample. Journal of Autism and Developmental Disorders. 2011;41:1665–1674. doi: 10.1007/s10803-011-1199-8. [DOI] [PubMed] [Google Scholar]
- Mosconi MW, Kay M, D’Cruz AM, Seidenfeld A, Guter S, Stanford LD, et al. Impaired inhibitory control is associated with higher-order repetitive behaviors in autism spectrum disorders. Psychological Medicine. 2009;39:1559–1566. doi: 10.1017/S0033291708004984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostofsky SH, Burgess MP, Gidley Larson JC. Increased motor cortex white matter volume predicts motor impairment in autism. Brain. 2007;130:2117–2122. doi: 10.1093/brain/awm129. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Powell SK, Simmonds DJ, Goldberg MC, Caffo B, Pekar JJ. Decreased connectivity and cerebellar activity in autism during motor task performance. Brain. 2009;132:2413–2425. doi: 10.1093/brain/awp088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Cook I, Coon H, Dawson G, Joseph RM, Klin A, et al. Performance on Cambridge Neuropsychological Test Automated Battery subtests sensitive to frontal lobe function in people with autistic disorder: Evidence from the Collaborative Programs of Excellence in Autism Network. Journal of Autism and Developmental Disorders. 2004;34:139–150. doi: 10.1023/b:jadd.0000022605.81989.cc. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Griffith EM. Neuropsychological function and the external validity of Asperger syndrome. In: Klin A, Volkmar FR, Sparrow SS, editors. Asperger syndrome. New York: Guilford Press; 2000. pp. 72–96. [Google Scholar]
- Ozonoff S, Young GS, Goldring S, Greiss-Hess L, Herrera AM, Steele J, et al. Gross motor development, movement abnormalities, and early identification of autism. Journal of Autism and Developmental Disorders. 2008;38:644–656. doi: 10.1007/s10803-007-0430-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce K, Courchesne E. Evidence for a cerebellar role in reduced exploration and stereotyped behavior in autism. Biological Psychiatry. 2001;49:655–664. doi: 10.1016/s0006-3223(00)01008-8. [DOI] [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. Quarterly Journal of Experimental Psychology. 1980;32:3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Ravizza SM, Ivry RB. Comparison of the basal ganglia and cerebellum in shifting attention. Journal of Cognitive Neuroscience. 2001;13:285–297. doi: 10.1162/08989290151137340. [DOI] [PubMed] [Google Scholar]
- Rogers SJ, Bennetto L, McEvoy R, Pennington BF. Imitation and pantomime in high-functioning adolescents with autism spectrum disorders. Child Development. 1996;67:2060–2073. [PubMed] [Google Scholar]
- Shafritz KM, Dichter GS, Baranek GT, Belger A. The neural circuitry mediating shifts in behavioral response and cognitive set in autism. Biological Psychiatry. 2008;63:974–980. doi: 10.1016/j.biopsych.2007.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan J, McAuley JD. Rhythm as a cognitive skill: Temporal processing deficits in autism. Paper presented at the 4th Australasian Cognitive Science Conference.1997. [Google Scholar]
- South M, Ozonoff S, McMahon WM. Repetitive behavior profiles in Asperger syndrome and high-functioning autism. Journal of Autism and Developmental Disorders. 2005;35:145–158. doi: 10.1007/s10803-004-1992-8. [DOI] [PubMed] [Google Scholar]
- Thakkar KN, Polli FE, Joseph RM, Tuch DS, Hadjikhani N, Barton JJ, et al. Response monitoring, repetitive behaviour and anterior cingulate abnormalities in autism spectrum disorders (ASD) Brain. 2008;131:2464–2478. doi: 10.1093/brain/awn099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend J, Courchesne E, Covington J, Westerfield M, Harris NS, Lyden P, et al. Spatial attention deficits in patients with acquired or developmental cerebellar abnormality. Journal of Neuroscience. 1999;19:5632–5643. doi: 10.1523/JNEUROSCI.19-13-05632.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend J, Courchesne E, Egaas B. Slowed orienting of covert visual–spatial attention in autism: Specific deficits associated with cerebellar and parietal abnormality. Development and Psychopathology. 1996;8:563–584. [Google Scholar]
- Townsend J, Westerfield M, Leaver E, Makeig S, Jung T, Pierce K, et al. Event-related brain response abnormalities in autism: Evidence for impaired cerebello-frontal spatial attention networks. Cognitive Brain Research. 2001;11:127–145. doi: 10.1016/s0926-6410(00)00072-0. [DOI] [PubMed] [Google Scholar]
- Travers BG, Klinger MR, Mussey JL, Klinger LG. Motor-linked implicit learning in persons with autism spectrum disorders. Autism Research. 2010;3:68–77. doi: 10.1002/aur.123. [DOI] [PubMed] [Google Scholar]
- Turner MA. Generating novel ideas: Fluency performance in high-functioning and learning disabled individuals with autism. Journal of Child Psychology and Psychiatry. 1999;40:189–201. [PubMed] [Google Scholar]
- Wainwright-Sharp JA, Bryson SE. Visual orienting deficits in high-functioning people with autism. Journal of Autism and Developmental Disorders. 1993;23:1–13. doi: 10.1007/BF01066415. [DOI] [PubMed] [Google Scholar]
- Williams HG, Woollacott MH, Ivry R. Timing and motor control in clumsy children. Journal of Motor Behavior. 1992;24:165–172. doi: 10.1080/00222895.1992.9941612. [DOI] [PubMed] [Google Scholar]
- Wing AM, Kristofferson AB. Response delays and the timing of discrete motor responses. Perception & Psychophysics. 1973;14:5–12. [Google Scholar]
- Yerys BE, Wallace GL, Harrison B, Celano MJ, Giedd JN, Kenworthy LE. Set-shifting in children with autism spectrum disorders: Reversal shifting deficits on the Intradimensional/Extradimensional Shift Test correlate with repetitive behaviors. Autism. 2009;13:523–538. doi: 10.1177/1362361309335716. [DOI] [PMC free article] [PubMed] [Google Scholar]