Abstract
OBJECTIVES
To assess electrocochleography (ECochG) to tones as an instrument to account for CI speech perception outcomes in children with auditory neuropathy spectrum disorder (ANSD).
MATERIALS & METHODS
Children (<18 years) receiving CIs for ANSD (n=30) and non-ANSD (n=74) etiologies of hearing loss were evaluated with ECochG using tone bursts (0.25–4 kHz). The total response (TR) is the sum of spectral peaks of responses across frequencies. The compound action potential (CAP) and the auditory nerve neurophonic (ANN) in ECochG waveforms were used to estimate nerve activity and calculate nerve score. Performance on open-set monosyllabic word tests was the outcome measure. Standard statistical methods were applied.
RESULTS
On average, TR was larger in ANSD than in non-ANSD subjects. Most ANSD (73.3%) and non-ANSD (87.8%) subjects achieved open-set speech perception; TR accounted for 33% and 20% of variability in the outcomes, respectively. In the ANSD group, the PTA accounted for 69.3% of the variability, but there was no relationship with outcomes in the non-ANSD group.
In both populations, nerve score was sensitive in identifying subjects at risk for not acquiring open-set speech perception, while the CAP and the ANN were more specific.
CONCLUSION
In both subject groups, the TRs correlated with outcomes but these measures were notably larger in the ANSD group. There was also strong correlation between PTA and speech perception outcome in ANSD group. In both subject populations, weaker evidence of neural activity was related to failure to achieve open-set speech perception.
Keywords: Auditory neuropathy spectrum disorder, cochlear implants, electrocochleography, compound action potential, auditory nerve neurophonic
1. INTRODUCTION
Auditory neuropathy spectrum disorder (ANSD) is hearing dysfunction characterized by compromised integrity of transmission to or within the auditory nerve with relative sparing of cochlear function [1, 2]. The deficit is thought to stem from a dysynchronous neural response to the acoustic signal, resulting in increased gap detection thresholds, poor sound localization and speech discrimination. In particular, postlingually deafened patients with ANSD demonstrate impaired speech perception out of proportion with the increase in their detection thresholds [3–5]. Commonly, ANSD is identified in infants who fail newborn hearing screening (NBHS) and are evaluated with auditory brainstem response (ABR) testing that reveals a cochlear microphonic (CM) but abnormal wave V [5, 6]. Otoacoustic emissions may also be present indicating healthy outer hair cells [5, 7, 8]. ANSD is reported to affect 6.5% to 15% of newborns with hearing loss [9, 10]. It is associated with a wide range of etiologies and risk factors including premature birth, hyperbilirubinemia, genetic mutations and cochlear nerve deficiency (CND) [11–14].
Patients with ANSD are often treated with cochlear implants (CI), however, their outcomes are highly variable; some subjects perform as well as the best CI users with hearing loss due to non-ANSD etiologies, while others never achieve open-set speech perception [8, 10–12, 15, 16]. When comorbidities are absent or controlled for, the outcomes in ANSD and non-ANSD CI recipients are not significantly different [17–19]. Still, no specific factors highly predictive of speech perception outcomes in ANSD patients have been identified [12, 16, 20]. The site-of-lesion appears to have prognostic significance: abnormalities restricted to the inner hair cells, the synapse, and the terminal dendrites of the auditory nerve allow for better CI outcomes than pathologies of spiral ganglion cells, myelination, or the central nervous system (CNS) [2, 7, 11, 19, 21–24]. However, most ANSD subjects do not have clinical indicators of the site-of-lesion which limits the utility of this distinction in current clinical practice.
Electrocochleography (ECochG) provides a possible objective measure to evaluate the peripheral auditory system of CI candidates including those diagnosed with ANSD [1, 16, 22, 25, 26]. By examining the origins and magnitude of the potentials recorded with ECochG we may improve our understanding of the variation in speech perception outcomes. The stimuli previously used for evaluation have typically been clicks or high frequency tone pips; the ECochG components produced by these are the CM, summating potential (SP) and compound action potential (CAP) [16, 27–30]. Our approach is to utilize a wide range of tone frequencies, because most responses in CI subjects are to low frequencies [25, 26, 31, 32]. In addition to the CM, SP and CAP, the response to low frequency tones also includes the auditory nerve neurophonic (ANN). From the responses to this series of tones, we’ve developed a ‘total response’ (TR) measure calculated by summing the significant spectral peaks of responses across a wide frequency range (250 Hz to 4000 Hz) as a measure of overall cochlear health [25, 26, 32]. The objective of this study is to evaluate features of the inner ear electrophysiology including TR, CAP, and ANN as well as their influence on speech perception outcomes of pediatric CI recipients with ANSD.
2. MATERIALS AND METHODS
All aspects of this prospective observational cohort study were approved by the Institutional Review Board. Parental consent was obtained for all subjects and patient assent was obtained from children at least seven years old. All pediatric patients (eight months to eighteen years) who satisfied the criteria for CI at our institution were eligible for the study. Children with non-English speaking guardians, severe inner ear malformations or undergoing revision surgery were excluded from the study. Each subject underwent comprehensive evaluation at the Children’s CI Center at University of North Carolina (CCIC at UNC) which included review of birth, medical, family and social history, molecular and genetic testing, ABR, MRI and/or CT imaging where indicated. ANSD was diagnosed based on ABR testing demonstrating a reduced/absent wave V in the presence of CM [7]. In ANSD subjects, the presence of risk factors such as hyperbilirubinemia, prematurity, neonatal intensive care unit stay with requirement of mechanical ventilation (NICU), and temporal bone abnormalities was noted. Additional comorbidities such as CNS abnormalities, systemic illnesses, prelingual deafness and developmental delay were also considered. Bilateral implantations were considered as individual cases. Audiometric thresholds were obtained using developmentally appropriate techniques for each subject. When the highest level of stimulation elicited no response, 120 dB HL was recorded as the threshold for that particular frequency. Pure tone average (PTA) was calculated as the average of threshold at 500Hz, 1000Hz and 2000Hz.
2.1 Operative set up and ECochG recording
All ECochG recording procedures were performed during each subjects’ CI procedure under general anesthesia. Acoustic stimulation and recording were performed using a Biologic Navigator PRO (Natus Medical Inc., San Carlos, CA). The stimuli were delivered through a foam insert attached to a speaker (Etymotic 3b). A ground electrode was placed on the glabella and the reference electrode on the mastoid contralateral to surgical site. The round window was exposed to insert the CI by using the standard transmastoid facial recess approach. Just prior to insertion of the CI electrode array, a recording probe (Neurosign; Magstim Co., Wales, UK) was placed at the round window. Electrode impedances were less than 16 kOhm.
Acoustic stimuli included tone bursts (250 to 4000Hz) presented with alternating phases at 90 dB nHL (95–107 dB SPL). In most cases, 2 to 3 frequencies at 90 dB SPL were tested with the sound tube crimped to test for the absence of electrical artifact.
2.2 Signal Analysis
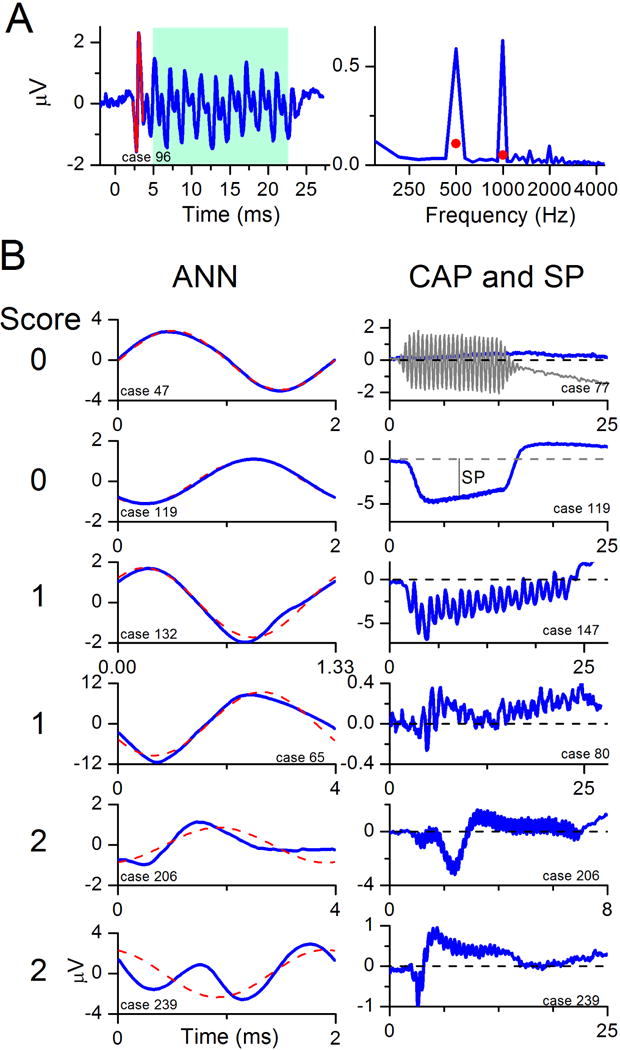
Recorded responses to the acoustic stimuli were processed using custom MATLAB programs (Mathworks, Natick, MA, USA). An example of recorded waveform and its spectrum is shown in Figure 1A. The energy spectrum is derived from the ongoing, or steady-state portion of the response (shaded box), that occurs after the CAP and lasts until the stimulus is turned off. The TR was derived by adding the amplitudes of significant responses at the first three harmonics, summed across all stimulus frequencies (only the response to 500 Hz, with two significant peaks, is shown). To be considered ‘significant’, the spectral peaks had to exceed the mean noise level by at least 3 standard deviations. The noise and its variance were determined from six bins, three on each side of the target frequency, starting two bins away from the peak. The lowest detectable signal levels were typically on the order of 0.02 μV.
Figure 1.
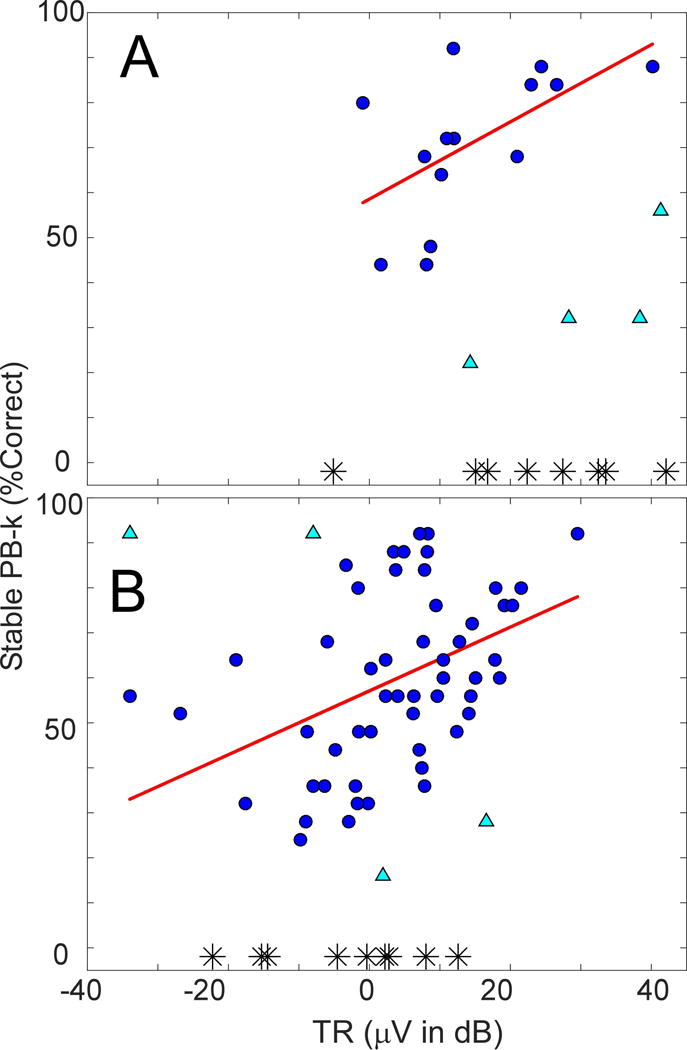
ECochG waveform and energy spectra characteristics of CI subjects. All stimuli were presented at 90 dB nHL.
A. ECochG response and to 500 Hz and the corresponding FFT spectrum. Red portion of the waveform at the onset of the response indicates a CAP. Blue portion within the blue background indicates the ongoing, or steady-state portion of the ECochG response containing the CM and ANN. The red circles in the spectrum indicate the level required for the peaks to be significant (see Methods).
B. ECochG recordings with increasing evidence of neural activity in the form of CAP and ANN. From top to bottom panel the visually apparent magnitudes of CAP and ANN increase resulting in a higher scores noted on the left of the panels.
(Left) The ANN was determined using the ‘average cycle’ to low frequency tones. The average cycle is the average of all cycles in the ongoing portion of the response, as in the blue box in A. The responses shown are to different cases, and were to 500 (top two and bottom tracing), 750 (third from top), and 250 Hz (fourth and fifth from top). The degree that the average cycle deviated from the best-fit sinusoid (red dashed line) was used to determine the score for the ANN.
(Right) The CAP was most apparent in the sum of the responses to alternating phase stimuli (blue lines). The score for the CAP is given at the left. The top and the bottom two panels are responses to 2000 Hz tone bursts, and the third and fourth panels are the responses to 500 Hz tone bursts.
Neural activity was assessed based on the presence of CAP and ANN in the ECochG waveforms. Only subjects with a TR greater than −6 dB (0.5 μV) were included in this analysis because in smaller responses the signal to noise ratio was too low for these neural features to be detected. In CI subjects a CAP is often visible as a pronounced negative deflection of the potential at the onset of the response (Figure 1A, portion in red), but across subjects the morphology is highly variable and difficult to quantify [33]. Similarly, the presence of an ANN is detectable by distortions in the ongoing portion of the response but its magnitude is difficult to quantify because it is mixed with the CM (Figure 1 A, in the blue box). Consequently, a subjective scale ranging from 0 to 2 was used to independently grade the evidence of CAP and ANN in each waveform. On this scale, 0 indicates complete absence, 1 indicates presence of even the smallest evidence of the potential, and 2 indicates that the potential were clearly discernable. The scores for CAP and ANN were added to produce a composite ‘nerve’ score. Examples of the different morphologies and scores are provided in Figure 1B.
2.3 Speech Perception Testing
A battery of age and developmentally appropriate speech perception tests was employed at follow-up audiology appointments [34]. Results from open-set monosyllabic word tests, usually the phonetically balanced kindergarten word test (PB-k), were selected as the outcome measure. The PB-k lists are composed of 25 words found in average vocabulary of a kindergarten-aged child and is scored as ‘percent correct’ [35–37]. Pediatric CI recipients may continue improving in their speech perception performance for longer than 5 years after implantation [38]. However, for the purposes of this study, we used the PB-k scores that were part of a stable of performance as indicated by PB-k scores within 10 points of each other during two consecutive testing sessions, collected after at least 1 year of CI use. The majority of the subjects were implanted at less than 4 years old and required 1–3 years of exposure to spoken language and time to develop auditory skills before being able to complete open-set speech perception testing. Scores obtained in recorded condition were preferred, however, in some cases, children could only be tested in monitored live voice (MLV) condition. In several cases, speech perception was evaluated with consonant-nucleus-consonant (CNC) word test rather than PB-k, these were used to indicate a child’s achievement of open-set speech perception but were not included in regression analysis between TR and PB-k scores.
2.4 Statistical Analyses
Univariate analyses were performed on continuous (TR, unaided preoperative PTA) and categorical (e.g., CAP, ANN, nerve score, and SP) variables to identify factors that were associated with speech perception performance. Cook’s distance analysis was used to identify the outlier data points. ANOVA and Pearson’s Chi-square analysis were employed for continuous and categorical variables and to calculate φ coefficient of association between binary variables. Paired and independent samples t-tests were utilized where appropriate. Equal variances were not assumed. Tests of sensitivity and specificity of identifying subjects at risk for not achieving open-set speech perception were applied to the CAP, ANN and nerve score.
3. RESULTS
The study includes 30 ANSD and 74 non-ANSD subjects. The clinical and electrophysiological characteristics of the ANSD subjects are listed in Table 1. Subjects with ANSD were typically diagnosed with hearing loss during NBHS (24/30), and most had at least one clinical risk factor known to be associated with ANSD (21/30). Additionally, six individuals had major comorbidities of the CNS and three subjects were diagnosed with developmental delay not attributable to hearing loss.
Table 1.
Clinical and electrophysiological characteristics of pediatric CI recipients diagnosed with ANSD.
| Subject No. | Age at CI (years) | NBHS result | Preop. PTA (dB HL) | Temporal bone and CNS abnormalities (CT and MRI) | ANSD risk factors and comorbidities | TR | Nerve Score | Speech perception outcome |
|---|---|---|---|---|---|---|---|---|
| 46 | 2 | referred | 105 | CND, EVA | 32.5 | 2 | – | |
| 53 | 2 | passed | 113 | Basal ganglia changes | HB, G6PD deficiency, CP, DD | 42.1 | 1 | – |
| 77 | 2 | referred | 113 | CND, corpus callosum agenesis, holoprosencephaly, microcephaly, and Chiari I malformation | ADOA, HB, DD | 33.5 | 1 | – |
| 85 | 4 | passed | 93 | White matter change | −5.2 | 2 | – | |
| 119 | 2 | referred | 100 | CND, dysplastic cochlea & SCCs | CHARGE syndrome | 15.1 | 0 | – |
| 137 | 5 | referred | 102 | CND | 27.4 | 3 | – | |
| 202 | 2 | referred | 65 | Grade II ICH | prematurity, NICU | 22.4 | 2 | – |
| 239 | 9 | passed | 113 | bilateral EVA | HB, NICU | 16.8 | 4 | – |
| 206 Left | 4 | referred | 70 | prematurity | 14.3 | 3 | PB-k (MLV): 22% | |
| 50 | 3 | referred | 85 | HB, prematurity, NICU, CP, DD | 28.3 | 1 | PB-k (Recorded): 32% | |
| 206 Right | 3 | referred | 77 | prematurity | 38.4 | 4 | PB-k (MLV): 32% | |
| 4 Right | 1 | referred | 107 | HB | 8.1 | 1 | PB-k (MLV): 44% | |
| 4 Left | 2 | referred | 120 | HB | 1.7 | 1 | PB-k (MLV): 44% | |
| 131 | 3 | referred | 102 | HB | 8.7 | 3 | PB-k (MLV): 48% | |
| 226 | 8 | passed | 62 | HB, CP, DD | 41.3 | 1 | PB-k (MLV): 56% | |
| 130 | 2 | referred | 92 | prematurity | 6.3 | 2 | CNC: 58% | |
| 96 | 1.5 | referred | 97 | 10.2 | 3 | PB-k (MLV): 64% | ||
| 334 | 11 | referred | 75 | HB, prematurity, NICU | 31.6 | 1 | CNC: 64% | |
| 173 | 3 | referred | 120 | prematurity | 4.8 | 3 | CNC: 72% | |
| 47 | 2 | referred | 83 | 21 | 3 | PB-k (MLV): 68% | ||
| 81 Right | 5 | referred | 85 | 7.8 | 3 | PB-k (Recorded): 68% | ||
| 55 | 6 | passed | 93 | 11.6 | 3 | PB-k (Recorded): 72% | ||
| 81 Left | 3 | referred | 85 | 11.5 | 2 | PB-k (MLV): 72% | ||
| 80 | 3 | referred | 107 | HB, prematurity, NICU | −0.9 | 2 | PB-k (MLV): 80% | |
| 65 Left | 4 | referred | 77 | Chiari I malformation, cerebellar encephalomalatia | prematurity, NICU | 26.6 | 2 | PB-k (Recorded): 84% |
| 65 Right | 4 | referred | 67 | Chiari I malformation, cerebellar encephalomalatia | prematurity, NICU | 23 | 1 | PB-k (Recorded): 84% |
| 132 | 7 | referred | 73 | HB, prematurity, NICU | 24.4 | 2 | PB-k (Recorded): 88% | |
| 79 Left | 11 | referred | 73 | Dysplastic cochlea | prematurity | 40.2 | 1 | PB-k (Recorded): 88% |
| 39 | 7 | passed | 70 | 11.5 | 4 | PB-k (Recorded): 92% | ||
| 79 Right | 10 | referred | 68 | Dysplastic cochlea | prematurity | 11.9 | 1 | PB-k (Recorded): 92% |
NBHS: newborn hearing screening, Preop PTA: preoperative pure tone average, CNS: central nervous system, CT: Computed tomography, MRI: magnetic resonance imaging, PB-k: phonetically balanced kindergarten word test, MLV: monitored live voice, CNC: consonant-nucleus-consonant word test, HB: hyperbilirubinemia, NICU: neonatal intensive care unit with mechanical ventilation, CND: cochlear nerve deficiency, CHARGE: syndrome consisting of colomboma of the eye, heart defects, atresia of choanae, slowed growth and development, Ear abnormalities and deafness, SCC: semicircular canals, EVA: enlarged vestibular aqueduct, G6PD: glucose-6-phosphage dehydrogenase, ADOA: autosomal dominant optic atrophy, ICH: intercranial hemorrhage, DD: developmental delay, CP: cerebral palsy, TR: total response from ECochG, SP: summating potential
3.1 Speech perceptions outcomes as a function of TR and preoperative PTA
The mean TR of the ANSD cohort was higher than the non-ANSD group (18.9 ± 13.1 dB μV compared to 2.7 ± 12.9 dB μV) and the difference was significant (t-test, 2-tailed, df=52, t=5.6, p<0.001). Figure 2 depicts the regression analysis of TR and PB-k scores in the two groups. Fourteen ANSD subjects, (circles in Figure 2A) were included in the analysis. Three subjects were tested with CNC only, and one had incomplete set of ECochG recordings to calculate the TR (not shown). There were four statistically determined outliers (triangles), and eight without open-set speech perception (asterisks). For the group included in the regression, the correlation (r) between TR and PB-k score was 0.58 (df =13, F=6.0, p=0.03), accounting for 33% of the variability (r2).
Figure 2.
Regression analysis of TR and speech perception outcomes in ANSD (A) and non-ANSD (B) pediatric CI recipients.
Circle markers-subjects included in regression analysis.
Triangle markers-subjects excluded from regression analysis based on Cook’s distance.
Asterisk markers-subjects unable to complete speech perception testing.
Fifty-three non-ANSD subjects (Figure 2B) were included in the regression; excluded from the analysis were four statistically determined outliers, eight subjects tested with CNC (not shown), and nine that did not achieve open-set speech perception (asterisks). The regression analysis yielded a correlation (r) of 0.44, accounting for 19.5% (r2) of the variability (df =52, F=12.4, p=0.001).
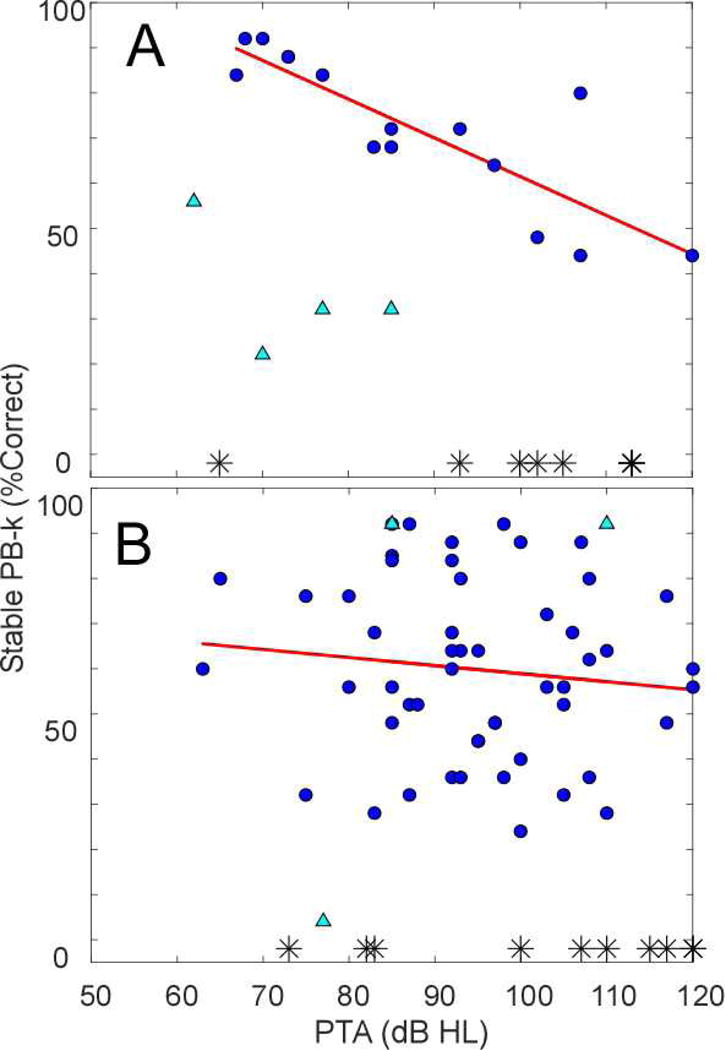
Figure 3 demonstrates the results of linear regression analysis of PTA and PB-k scores in both groups. In the ANSD group, the correlation was 0.83, accounting for 69.3% of variability (df=13, F=27.1, p<0.001). In the non-ANSD group, the analysis did not reach level of statistical significance. The PTA and TR were highly correlated in ANSD (r=0.75, df=13, F=15.1, p=0.002) but the correlation was not significant in the non-ANSD group.
Figure 3.
Regression analysis of PTA and speech perception outcomes in ANSD (A) and non-ANSD (B) pediatric CI recipients
Circle markers-subjects included in regression analysis.
Triangle markers-data points excluded from regression analysis based on Cook’s distance. There were no outliers in non-ANSD group based on Cook’s distance analysis of PTA and PB-k, the triangles denote the outliers based on the TR and PB-k analysis included here to provide context.
Asterisk markers-subjects unable to complete speech perception testing.
3.2 Analysis of the Outliers
In the ANSD population, the same four outliers were identified by Cook’s distance analysis of TR and PB-k as the analysis of TR and PTA. These outlier data points may have been affected by external factors. Two of these data points belong to one subject (subject 206, Left and Right in Table 1) with bilateral CIs who has prominent attention and behavioral challenges. His symmetrically poor performance is most likely the result of these global barriers to progress. The other two outlier data points (subjects 50 and 226 in Table 1) came from subjects whose ECochG waveforms did not have a CAP but showed evidence of ANN. Both of these subjects experienced partial loss of device functionality. Replacement of the processor improved open-set speech perception performance (CNC) for one of them. There were two non-ANSD outlier points with PB-k scores better than their TR would predict, both of these data points belonged to a subject with hearing loss due to meningitis. One of the remaining two outliers in the non-ANSD group had evidence of a CAP but ANN was not evident in that nor the other subject’s waveforms. Cook’s distance analysis of PTA and PB-k in non-ANSD group did not identify any outliers.
3.3 Analysis of neural activity
All 30 subjects with ANSD and 59 of the non-ANSD subjects had signals strong enough to assess CAP and ANN scores. These measures and the nerve score resulting from their summation varied widely in both subject groups. The distribution of CAP and ANN scores for the two groups were not significantly different (Mann-Whitney, p>0.05 in both cases).
The two major outcome categories were subjects with open-set speech perception and subjects not able to achieve this goal despite adequate duration of CI experience. Most of the ANSD (73.3%) and non-ANSD subjects (87.8%) achieved open-set speech perception, and there was no difference between the groups (“N-1” Chi-squared, χ= 3.3, p=0.07). Figure 4 shows the relationships between these outcomes and CAP, ANN and nerve scores. The ANSD subjects with no detectable CAP or ANN (score <1), or those with a low nerve score (<3), failed to achieve open-set speech perception more frequently than those with ECochGs demonstrating a better functioning neural substrate. However, some individuals with strong evidence of functional neural substrate (e.g., nerve score>3) failed to achieve open-set speech perception.
Figure 4.
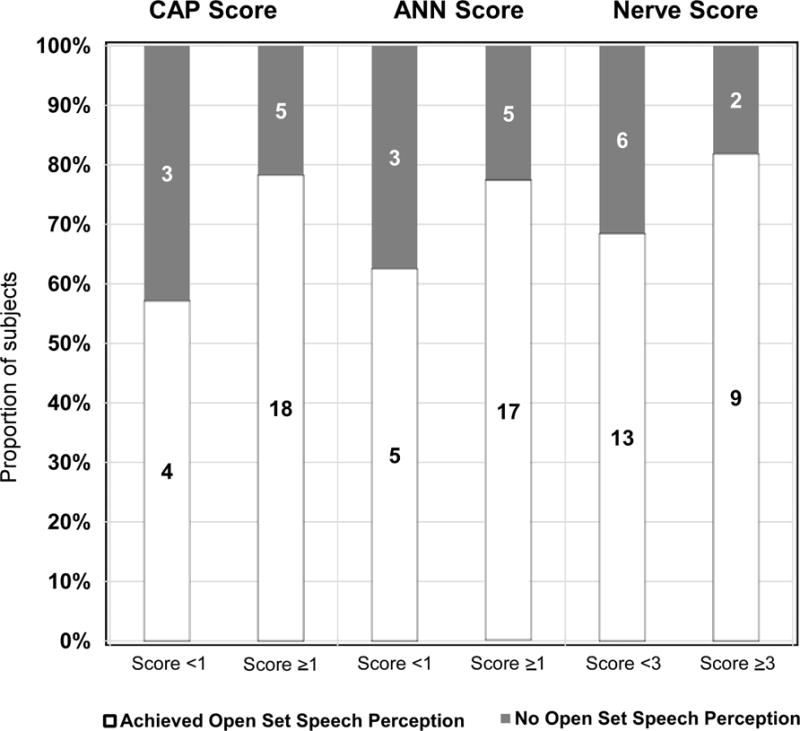
Proportion of ANSD CI recipients who achieve testable speech perception (white) and those who did not (grey) depending on their CAP, ANN and the Nerve score.
CAP (A) and ANN (B) Scores of at least 1 and Nerve score (C) of at least 3.
The sensitivities, specificities, and positive and negative predictive values (PPV and NPV) of the CAP, ANN and nerve score for detecting lack of progression to open-set speech perception testing were calculated (Table 2). In both populations, the CAP score as well as the ANN score each had higher specificity for failure to progress to open-set speech perception compared to the composite nerve score. The more stringent standard for strong evidence of neural activity used for the nerve score (>3) led to a high sensitivity. The NPVs were high for all three metrics.
Table 2.
Diagnostic features of the CAP, ANN and Nerve score in identifying CI recipients at risk for not achieving speech perception.
| Sensitivity | Specificity | PPV | NPV | |
|---|---|---|---|---|
| ANSD Subjects | ||||
| CAP score | 37.5% (95% CI: 0.10–0.74) |
81.8% (95% CI: 0.59–0.94) |
42.9% (95% CI: 0.12–0.80) |
78.3% (95% CI: 0.56–0.92) |
| ANN score | 37.5% (95% CI: 0.10–0.74) |
77.3% (95% CI: 0.54–0.91) |
37.5% (95% CI: 0.10–0.74) |
77.3% (95% CI: 0.54–0.91) |
| Nerve score | 75.0% (95% CI: 0.36–0.96) |
40.9% (95% CI: 0.21–0.63) |
31.6% (95% CI: 0.14–0.57) |
81.8% (95% CI: 0.58–0.97) |
| Non-ANSD Subjects | ||||
| CAP score | 50.0% (95% CI: 0.14–0.86) |
60.4% (95% CI: 0.46–0.73) |
12.5% (95% CI: 0.03–0.33) |
91.4% (95% CI: 0.76–0.98) |
| ANN score | 66.7% (95% CI: 0.24–0.94) |
75.5% (95% CI: 0.61–0.86) |
23.5% (95% CI: 0.08–0.50) |
95.2% (95% CI: 0.83–0.99) |
| Nerve score | 66.7% (95% CI: 0.24–0.94) |
45.3% (95% CI: 0.32–0.60) |
12.1% (95% CI: 0.04–0.29) |
92.3% (95% CI: 0.73–0.99) |
CI: confidence interval, PPV: positive predictive value, NPV: negative predictive value
3.4 Clinical factors and outcomes
Table 3 contains results of the Pearson Chi-square analysis of the clinical factors associated with ANSD and comorbidities. The analysis showed significant negative association between anatomical abnormalities of the temporal bone, CNS abnormalities and prelingual deafness with acquisition of open-set speech perception. Conversely, prematurity had significant positive association with open-set speech perception among ANSD subjects.
Table 3.
Risk factors for lack of speech perception acquisition in ANSD CI recipients.
| ANSD associated risk factor | φ | p |
|---|---|---|
| Hyperbilirubinemia | −0.1 | 0.957 |
| Premature birth | 0.375 | 0.040* |
| NICU (with mechanical ventilation) | 0.023 | 0.901 |
| Temporal bone abnormalities | −0.56 | 0.002* |
| Comorbidity | φ | p |
| CNS abnormalities | −0.45 | 0.013* |
| Developmental Delay | −0.21 | 0.257 |
| Prelingually Deafened | −0.46 | 0.012* |
4. DISCUSSION
The ECochG waveforms of children with ANSD receiving CIs have markedly larger amplitudes compared to their non-ANSD peers. However, there was not a significant difference in the prevalence of neural activity in the two patient populations. The correlation of TR with speech perception outcomes was significant in both subject groups but the relationship was stronger in the ANSD subjects. Surprisingly PTA exceeded TR in strength of the predictive relationship in the ANSD group, but had no role in the non-ANSD group. The degree of neural activity provided significant but not conclusive information regarding subjects’ likelihood of achieving open-set speech perception.
4.1. The ANSD and non-ANSD groups
The ANSD group included CI recipients whose ABR demonstrated a small or absent wave V and a present CM, while the non-ANSD group included all CI recipients that did not meet this diagnostic criteria. These clinical definitions of ANSD and the non-ANSD groups encompass a multitude of underlying conditions and risk factors (see Table 1). Due to the presence of multiple causative factors within these groups, a clear conclusion regarding outcomes should not necessarily be expected. However, because the hearing deficits of the ANSD subjects are attributed to lesions of the auditory neural pathway, we expected that the role of neural responses could be different in these populations. We found that while the TR was larger in ANSD subjects, there were no significant differences in levels of neural activity between the two groups, and the diagnostic value neural activity and speech perception outcomes was relatively small. A recent, more detailed study of frequency-specific ECochG responses in these groups found that the large magnitude of the responses in ANSD was primarily due to larger to hair cell responses from high frequency parts of the cochlea, while, just as in the present study, the neural responses of the ANSD and non-ANSD subjects were similar [39]. Thus, the hair cell responses from the basal cochlea are responsible for the large magnitude of ECochG responses as well as the presence of CM in ABRs of subjects with ANSD, while the similarity of the ECochG responses to low frequencies helps to explain apparent lack of disparity in the outcomes in these broadly defined etiologic groups. As the number of study subjects grows, analyses examining ECochG waveform features of specific pathophysiologies of ANSD and non-ANSD hearing loss and their role in outcomes can be appropriately powered and may prove to have better prognostic value.
4.2 ECochG responses to tones as markers for site-of-lesion in CI subjects
In previous reports, etiologies with lesions distal to the myelinated portion of auditory nerve were associated with more favorable CI outcomes in subjects with ANSD [1, 7, 19, 23]. The CAP, SP and dendritic potentials in ECochG have been used as potential markers of distal sites of neural injury [1, 28]. The ANN has not previously been considered. The presence and morphology of these potentials depend strongly on the stimuli used. Most previous studies that focused on ANSD subjects have used clicks or high frequency tone-pips which contain limited energy to low frequencies [1, 28, 40, 41]. In CI recipients, however, ECochG responses are most reliably elicited by low frequency stimuli [25, 26, 31, 32]. In contrast, in ECochG waveforms to tones, the CAP occurs transiently at the onset and offset while CM and ANN reach a steady-state during the tone presentation.
Nearly all subjects at the upper end of the TR distribution were in the ANSD group. The TR is primarily composed of the CM, so this result is consistent with the identification of these subjects by the presence of a CM in scalp recordings during ABR. A wide range of neural activity in the form of CAP and ANN was present in the ANSD and non-ANSD subject groups. In theory, evidence of neural activity in ECochG localizes the site-of-lesion proximal to the inner hair cell-auditory nerve synapse suggesting that these individuals should have favorable outcomes [1]. In practice, however, the presence of a functional neural substrate does not necessarily indicate its utility for processing complex auditory stimuli. In addition, postsynaptic causes of ANSD produce highly variable outcomes, so the presence of a CAP may not be a universally positive sign [12, 15, 42–44].
4.3 The TR, PTA and open-set speech perception
In ANSD pediatric CI recipients, the TR accounted for 33.4% of the variability in speech perception outcomes, but the PTA accounted for 69%. The primacy of PTA over TR in ANSD subjects reflects the predominance of outer hair cell activity in the ECochG waveforms, such that the CM magnitudes are so large that they apparently do not closely reflect of the proportion of underlying neural elements that survive. In these subjects, the PTA appears to be a better indicator of the surviving neural elements. In contrast, for non-ANSD subjects, the PTA likely underestimates the amount of remaining neural substrate leaving TR as the better predictor of speech perception outcomes.
Previous reports indicate that approximately 25–30% of ANSD subjects do not achieve open-set speech perception with their CIs, similar to the 26% found in the current study [22, 45]. Either a CAP or an ANN and often both were present in majority of ANSD subjects; these markers had relatively high specificities identifying subjects at low risk for failing to achieve open-set speech perception. Neither the absence of the CAP nor the ANN was very sensitive for predicting lack of progress with CI. In contrast, the composite neural score was sensitive for identifying subjects at high risk of failure to progress but not as specific as the individual neural markers.
4.4 External factors: comorbidities, device issues, social issues
Several risk factors associated with ANSD and other comorbidities were represented in this cohort. Anatomical abnormalities of the temporal bone, including CND and enlarged vestibular aqueduct, were negatively associated with achievement of open-set speech perception. Conversely, the association between premature birth and achievement of open-set speech perception was positive. Additional disabilities may affect speech perception outcomes of pediatric CI recipients regardless of etiology of hearing loss [8, 12, 18, 19, 46–49]. The present study indicates that prelingual deafness and CNS abnormalities had a negative association with the outcome. The lack of association between the diagnosis of developmental delay and outcomes may be related to low frequency of the diagnosis in the present cohort.
Main limitations this prospective observational study is the inherent variability of the PB-k test timing dictated by age and developmental stage of the child at implantation. The developmental effects play a significant role in the youngest subjects who may not have the full grasp of the test words presented to them. The MLV presentation of PB-k word list also introduces inter-speaker variability into the results that is limited by the use of the recorded presentation.
4.5 Conclusion
There is a large spectrum of baseline physiology, neural substrate, and additional co-morbidities, associated with the wide range of speech perception outcomes in the ANSD CI recipient population. In general, ECochG waveforms of subjects with ANSD and non-ANSD demonstrate similar levels of neural activity. The absence of a CAP and ANN in the ECochG signal appears to be the first distinct identifiable risk factor from ECochG to tones for failure to achieve open-set speech perception in ANSD and non-ANSD CI recipients.
Acknowledgments
This study was supported by NIH T32 grant through National Institute on Deafness and Other Communication Disorders (# 5T32DC005360-12).
Douglas C. Fitzpatrick: Contractual research support from MED-EL Corporation and research grant support from the NIH-NIDCD.
Kevin Brown: Contractual research support from Cochlear Corporation and MED-EL Corporation.
Craig A. Buchman: Contractual research support from Cochlear Corporation and MED-EL Corporation. Consultant for Advanced Bionics and Cochlear Corporations.
Oliver F. Adunka: Contractual research support from Advanced Bionics, Cochlear Corporation, and MED-EL Corporation. Consultant for MED-EL Corporation and Cochlear Corporation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contents of this manuscript were presented at the annual meeting of American Society for Pediatric Otolaryngology at Combined Sections Meeting, Chicago, IL, USA. May 18–22nd 2016.
Conflicts of Interest:
The remaining authors have no conflict of interest to disclose.
References
- 1.Santarelli R, Starr A, Michalewski HJ, Arslan E. Clin Neurophysiol 2008. 2008;119:1028–1041. doi: 10.1016/j.clinph.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 2.Rance G, Starr A. Brain 2015. 2015;138:3141–3158. doi: 10.1093/brain/awv270. [DOI] [PubMed] [Google Scholar]
- 3.Zeng FG, Oba S, Garde S, Sininger Y, Starr A. Neuroreport 1999. 1999;10:3429–3435. doi: 10.1097/00001756-199911080-00031. [DOI] [PubMed] [Google Scholar]
- 4.Zeng FG, Kong YY, Michalewski HJ, Starr A. J Neurophysiol 2005. 2005;93:3050–3063. doi: 10.1152/jn.00985.2004. [DOI] [PubMed] [Google Scholar]
- 5.Starr A, Picton TW, Sininger Y, Hood LJ, Berlin CI. Brain 1996. 1996;119(Pt 3):741–753. doi: 10.1093/brain/119.3.741. [DOI] [PubMed] [Google Scholar]
- 6.Hang AX, Kim GG, Zdanski CJ. Curr Opin Otolaryngol Head Neck Surg 2012. 2012;20:507–517. doi: 10.1097/MOO.0b013e328359eea4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rance G, Beer DE, Cone-Wesson B, Shepherd RK, Dowell RC, King AM, Rickards FW, Clark GM. Ear Hear 1999. 1999;20:238–252. doi: 10.1097/00003446-199906000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Teagle HF, Roush PA, Woodard JS, Hatch DR, Zdanski CJ, Buss E, Buchman CA. Ear Hear 2010. 2010;31:325–335. doi: 10.1097/AUD.0b013e3181ce693b. [DOI] [PubMed] [Google Scholar]
- 9.Boudewyns A, Declau F, van den Ende J, Hofkens A, Dirckx S, Van de Heyning P. Eur J Pediatr 2016. 2016;175:993–1000. doi: 10.1007/s00431-016-2735-5. [DOI] [PubMed] [Google Scholar]
- 10.Roush P, Frymark T, Venediktov R, Wang B. Am J Audiol 2011. 2011;20:159–170. doi: 10.1044/1059-0889(2011/10-0032). [DOI] [PubMed] [Google Scholar]
- 11.Santarelli R. Genome Med 2010. 2010;2:91. doi: 10.1186/gm212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rance G, Barker EJ. Otol Neurotol 2008. 2008;29:179–182. doi: 10.1097/mao.0b013e31815e92fd. [DOI] [PubMed] [Google Scholar]
- 13.Buchman CA, Teagle HFB, Roush PA, Park LR, Hatch D, Woodard J, Zdanski C, Adunka OF. Laryngoscope 2011. 2011;121:1979–1988. doi: 10.1002/lary.22032. [DOI] [PubMed] [Google Scholar]
- 14.Olds C, Oghalai JS. Clin Perinatol 2016. 2016;43:313–323. doi: 10.1016/j.clp.2016.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buchman CA, Roush PA, Teagle HF, Brown CJ, Zdanski CJ, Grose JH. Ear Hear 2006. 2006;27:399–408. doi: 10.1097/01.aud.0000224100.30525.ab. [DOI] [PubMed] [Google Scholar]
- 16.Stuermer KJ, Beutner D, Streicher B, Foerst A, Felsch M, Lang-Roth R, Walger M. Int J Audiol 2015. 2015;55:412–418. doi: 10.3109/14992027.2016.1172392. [DOI] [PubMed] [Google Scholar]
- 17.Budenz CL, Starr K, Arnedt C, Telian SA, Arts HA, El-Kashlan HK, Zwolan TA. Otol Neurotol 2013. 2013;34:1615–1621. doi: 10.1097/MAO.0b013e3182a1ab5b. [DOI] [PubMed] [Google Scholar]
- 18.Ching TY, Day J, Dillon H, Gardner-Berry K, Hou S, Seeto M, Wong A, Zhang V. Int J Audiol 2013. 2013;52(Suppl 2):S55–64. doi: 10.3109/14992027.2013.796532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Breneman AI, Gifford RH, Dejong MD. J Am Acad Audiol 2012. 2012;23:5–17. doi: 10.3766/jaaa.23.1.2. [DOI] [PubMed] [Google Scholar]
- 20.Humphriss R, Hall A, Maddocks J, Macleod J, Sawaya K, Midgley E. Int J Audiol 2013. 2013;52:442–454. doi: 10.3109/14992027.2013.786190. [DOI] [PubMed] [Google Scholar]
- 21.Rodriguez-Ballesteros M, Reynoso R, Olarte M, Villamar M, Morera C, Santarelli R, Arslan E, Meda C, Curet C, Volter C, Sainz-Quevedo M, Castorina P, Ambrosetti U, Berrettini S, Frei K, Tedin S, Smith J, Cruz Tapia M, Cavalle L, Gelvez N, Primignani P, Gomez-Rosas E, Martin M, Moreno-Pelayo MA, Tamayo M, Moreno-Barral J, Moreno F, del Castillo I. Hum Mutat 2008. 2008;29:823–831. doi: 10.1002/humu.20708. [DOI] [PubMed] [Google Scholar]
- 22.Gibson WP, Sanli H. Ear Hear 2007. 2007;28:102S–106S. doi: 10.1097/AUD.0b013e3180315392. [DOI] [PubMed] [Google Scholar]
- 23.Santarelli R, Rossi R, Scimemi P, Cama E, Valentino ML, La Morgia C, Caporali L, Liguori R, Magnavita V, Monteleone A, Biscaro A, Arslan E, Carelli V. Brain 2015. 2015;138:563–576. doi: 10.1093/brain/awu378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang T, Santarelli R, Starr A. Brain Res 2009. 2009;1300:97–104. doi: 10.1016/j.brainres.2009.08.083. [DOI] [PubMed] [Google Scholar]
- 25.Formeister EJ, McClellan JH, Merwin WH, 3rd, Iseli CE, Calloway NH, Teagle HF, Buchman CA, Adunka OF, Fitzpatrick DC. Ear Hear 2015. 2015;36:249–260. doi: 10.1097/AUD.0000000000000106. [DOI] [PubMed] [Google Scholar]
- 26.Fitzpatrick DC, Campbell AT, Choudhury B, Dillon MP, Forgues M, Buchman CA, Adunka OF. Otol Neurotol 2014. 2014;35:64–71. doi: 10.1097/MAO.0000000000000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santarelli R, Del Castillo I, Rodriguez-Ballesteros M, Scimemi P, Cama E, Arslan E, Starr A. J Assoc Res Otolaryngol 2009. 2009;10:545–556. doi: 10.1007/s10162-009-0181-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McMahon CM, Patuzzi RB, Gibson WP, Sanli H. Ear Hear 2008. 2008;29:314–325. doi: 10.1097/AUD.0b013e3181662c2a. [DOI] [PubMed] [Google Scholar]
- 29.Dallos P. The Auditory Periphery Biophysics and Physiology. City: Academic Press, Inc; 1973. [Google Scholar]
- 30.Snyder RL, Schreiner CE. Hear Res 1984. 1984;15:261–280. doi: 10.1016/0378-5955(84)90033-9. [DOI] [PubMed] [Google Scholar]
- 31.Choudhury B, Fitzpatrick DC, Buchman CA, Wei BP, Dillon MT, He S, Adunka OF. Otol Neurotol 2012. 2012;33:1507–1515. doi: 10.1097/MAO.0b013e31826dbc80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McClellan JH, Formeister EJ, Merwin WH, 3rd, Dillon MT, Calloway N, Iseli C, Buchman CA, Fitzpatrick DC, Adunka OF. Otol Neurotol 2014. 2014;35:e245–252. doi: 10.1097/MAO.0000000000000557. [DOI] [PubMed] [Google Scholar]
- 33.Scott WC, Giardina CK, Pappa AK, Fontenot TE, Anderson ML, Dillon MT, Brown KD, Pillsbury HC, Adunka OF, Buchman CA, Fitzpatrick DC. Otol Neurotol 2016. 2016;37:1654–1661. doi: 10.1097/MAO.0000000000001224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang NY, Eisenberg LS, Johnson KC, Fink NE, Tobey EA, Quittner AL, Niparko JK, Team CDI. Otol Neurotol 2008. 2008;29:240–245. doi: 10.1097/MAO.0b013e3181627a37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eisenberg LS, Johnson KC, Martinez AS, Cokely CG, Tobey EA, Quittner AL, Fink NE, Wang NY, Niparko JK. Audiol Neuro-Otol 2006. 2006;11:259–268. doi: 10.1159/000093302. [DOI] [PubMed] [Google Scholar]
- 36.Kirk KI, Choi S. In: Clinical investigations of cochlear implant performance. Niparko JK, editor. City: Lippincott Williams & Wilkins; 2009. pp. 191–222. [Google Scholar]
- 37.Haskins H. Masters Thesis. Northwestern University; Evanston, IL: 1949. A phonetically balanced test of speech discrimitation for children. [Google Scholar]
- 38.Ahmad FI, Demason CE, HF BT, Henderson L, Adunka OF, Buchman CA. Laryngoscope 2012. 2012 doi: 10.1002/lary.23362. [DOI] [PubMed] [Google Scholar]
- 39.Riggs WJ, Roche JR, Giardina CK, Harris MS, Bastian ZJ, Fontenot TE, Buchman CA, Brown KD, Adunka OF, Fitzpatrick DC. In preparation for publication in Frontiers Neuroscience. The Ohio State University; Columbus OH: 2017. Intraoperative Electrocochleographic Characteristics of Auditory Neuropathy Spectrum Disorder In Cochlear Implant Subjects. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berlin CI, Bordelon J, St John P, Wilensky D, Hurley A, Kluka E, Hood LJ. Ear Hear 1998. 1998;19:37–47. doi: 10.1097/00003446-199802000-00002. [DOI] [PubMed] [Google Scholar]
- 41.Yiu EM, Tai G, Peverill RE, Lee KJ, Croft KD, Mori TA, Scheiber-Mojdehkar B, Sturm B, Praschberger M, Vogel AP, Rance G, Stephenson SE, Sarsero JP, Stockley C, Lee CY, Churchyard A, Evans-Galea MV, Ryan MM, Lockhart PJ, Corben LA, Delatycki MB. J Neurol 2015. 2015;262:1344–1353. doi: 10.1007/s00415-015-7719-2. [DOI] [PubMed] [Google Scholar]
- 42.Jeong SW, Kim LS, Kim BY, Bae WY, Kim JR. Acta Otolaryngol Suppl. 2007;2007:36–43. doi: 10.1080/03655230701624848. [DOI] [PubMed] [Google Scholar]
- 43.Rance G, Barker EJ. Int J Audiol 2009. 2009;48:313–320. doi: 10.1080/14992020802665959. [DOI] [PubMed] [Google Scholar]
- 44.Brookes JT, Kanis AB, Tan LY, Tranebjaerg L, Vore A, Smith RJ. International journal of pediatric otorhinolaryngology 2008. 2008;72:121–126. doi: 10.1016/j.ijporl.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 45.Teagle HFB, Roush PA, Woodard JS, Hatch DR, Zdanski CJ, Buss E, Buchman CA. Ear and Hearing 2010. 2010;31:325–335. doi: 10.1097/AUD.0b013e3181ce693b. [DOI] [PubMed] [Google Scholar]
- 46.Birman CS, Elliott EJ, Gibson WP. Otol Neurotol 2012. 2012;33:1347–1352. doi: 10.1097/MAO.0b013e31826939cc. [DOI] [PubMed] [Google Scholar]
- 47.Boons T, Brokx JP, Dhooge I, Frijns JH, Peeraer L, Vermeulen A, Wouters J, van Wieringen A. Ear Hear 2012. 2012;33:617–639. doi: 10.1097/AUD.0b013e3182503e47. [DOI] [PubMed] [Google Scholar]
- 48.Ching TYC, Dillon H, Marnane V, Hou SN, Day J, Seeto M, Crowe K, Street L, Thomson J, Van Buynder P, Zhang V, Wong A, Burns L, Flynn C, Cupples L, Cowan RSC, Leigh G, Sjahalam-King J, Yeh A. Ear and Hearing 2013. 2013;34:535–552. doi: 10.1097/AUD.0b013e3182857718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Budenz CL, Telian SA, Arnedt C, Starr K, Arts HA, El-Kashlan HK, Zwolan TA. Otol Neurotol 2013. 2013;34:477–483. doi: 10.1097/MAO.0b013e3182877741. [DOI] [PubMed] [Google Scholar]


