Abstract
The endocannabinoid system plays an important role in reward and addiction. One of the two main endocannabinoid neurotransmitters, anandamide, is metabolized by fatty acid amide hydrolase, an enzyme with a functional genetic polymorphism (FAAH Pro129Thr, rs324420). The Thr129 allele has been linked to problem drug and alcohol use, but the association has not been widely replicated and may be stronger for clinical measures of severity rather than categorical diagnosis. In the present study, we sought to determine whether the Thr129 allele was associated with both alcohol dependence (AD) diagnosis and severity in a sample of 1434 European American and African American individuals, 952 of whom were diagnosed with lifetime AD. Participants were genotyped for FAAH rs324420 and ancestry was determined via a genome-wide panel of ancestry informative markers. Subjects participated in Structured Clinical Interviews for psychiatric disorders and 90-day Timeline Followback interviews to assess recent alcohol use. European American participants with current AD had a higher Thr129 allele frequency than non-dependent controls. In European Americans with lifetime AD, there were significantly different distributions of drinking days and binge drinking days between the two genotype groups, with Thr129 carriers reporting a median of 10 fewer abstinent days and 13 more binge drinking days than Pro129/Pro129 homozygotes. In African American participants, there were no significant differences between Thr129 allele frequency in cases and controls and no significant differences in measures of AD severity by genotype. These findings provide evidence that the Pro129Thr missense variant is associated with AD severity in European Americans.
Keywords: Alcohol Use Disorder, Endocannabinoids, Genetics, Polymorphism, rs324420
Introduction
The endocannabinoid system modulates brain reward signaling and related behaviors and may therefore influence addiction vulnerability (Parsons & Hurd, 2015). The two best characterized endocannabinoids are 2-arachidonoylglycerol and anandamide (Devane et al., 1992; Stella, Schweitzer & Piomelli, 1997), which predominantly exert their effects in the central nervous system by activating the CB1 receptor (Matsuda et al., 1990). Anandamide signaling is terminated by the integral membrane enzyme Fatty Acid Amide Hydrolase (FAAH) through conversion to arachidonic acid (Cravatt et al., 2001; Cravatt et al., 1996). FAAH has a common functional missense variant, Pro129Thr (rs324420), which decreases FAAH activity and results in higher anandamide levels (Chiang et al., 2004; Dincheva et al., 2015). By increasing anandamide signaling, the Thr129 allele may directly influence the reinforcing properties of addictive substances.
The FAAH polymorphism may have particularly important implications for alcohol use disorder as there is substantial evidence that manipulation of the endocannabinoid system affects alcohol intake in rodents. CB1 agonists have been shown to increase rodent alcohol consumption (Colombo et al., 2002; Gallate et al., 1999) whereas CB1 antagonists have the opposite effect (Arnone et al., 1997; Colombo et al., 1998; Femenia, Garcia-Gutierrez & Manzanares, 2010; Wang et al., 2003). Both knockout mice lacking the FAAH gene and wild-type mice given specific inhibitors of FAAH demonstrate increased preference for alcohol and greater voluntary alcohol consumption (Blednov et al., 2007; Vinod et al., 2008). Alcohol-preferring rats have been shown to have decreased FAAH expression in the prefrontal cortex as compared to non-alcohol preferring rats and alcohol self-administration can be increased in non-selected rats by local injection of a FAAH inhibitor (Hansson et al., 2007). Collectively, these studies suggest that increased CB1 activity and decreased FAAH activity increase rodent alcohol consumption whereas decreased CB1 activity has the opposite effect. Genetic alteration of FAAH activity in humans could therefore influence alcohol dependence vulnerability and severity.
The FAAH Pro129Thr polymorphism has been implicated in substance use disorders, but the findings are conflicting. Both European and mixed ancestry Thr129 carriers were found to have increased odds of problem drug or alcohol use and substance dependence diagnoses (Flanagan et al., 2006; Sipe et al., 2002), results that parallel the rodent literature. However, other studies have failed to replicate these associations in samples of European (Buhler et al., 2014; Tyndale et al., 2007) and East Asian ancestry (Iwasaki et al., 2007; Morita et al., 2005). These association studies have several important limitations. Except for the Iwasaki et al. study, prior studies had small substance misuse samples ranging from 80 to 249 cases. Small samples not only reduce the power to detect real associations but also increase the probability of false positive findings (Button et al., 2013). Furthermore, all but one of these human studies examined substance misuse as a dichotomous outcome (problem drug or alcohol use versus no problem drug or alcohol use, substance use disorder versus no substance use disorder) which fails to account for the fact that substance use disorders have a continuum of severity. These methodological limitations and conflicting findings demonstrate the need for more thorough analyses in a larger sample.
In the present study, data were analyzed from a sample of European American and African American participants who were genotyped for FAAH rs324420 and underwent a comprehensive assessment for alcohol use disorders. By using a sample that included approximately one thousand individuals with alcohol dependence, we aimed to have sufficient power to reduce the likelihood of false positive or negative findings, although we did not conduct formal power calculations as the heterogeneity of the substance use disorders and ancestries analyzed in previous association studies make it difficult to reliably estimate an effect size for the present analysis. We also used standardized assessments to analyze alcohol dependence severity as a continuous measure in addition to dichotomizing subjects as alcohol dependent cases and controls. We hypothesized that Thr129 carrier status would be associated with both alcohol dependence diagnosis and greater alcohol dependence severity. To the best of our knowledge, the association between the Pro129Thr polymorphism and alcohol dependence severity has not yet been investigated.
Materials and Methods
Participants
1612 participants were recruited under two NIH Institutional Review Board-approved screening and evaluation protocols and were comprehensively assessed at the National Institutes of Health Clinical Center (Bethesda, Maryland, USA). All participants provided written informed consent. Participants were assessed using Structured Clinical Interviews for DSM-IV axis I disorders (SCID-IV). To avoid potential confounds from population stratification, the analysis was limited to individuals of European or African ancestry, the two major ancestries included in the sample. Without reference to FAAH genotype or clinical status, 95 participants of other ancestries were excluded from the analysis. Subjects with missing SCID data were also excluded (n = 83). The final sample size was 1434 participants. The participants were divided into those who had never met criteria for alcohol dependence when assessed at our center (control group, n = 482, 70.5% European ancestry) and participants who had met alcohol dependence criteria on at least one assessment (lifetime alcohol dependence group, n = 952, 56.4% European ancestry).
Genotyping
Genotyping was performed at the National Institute on Alcohol Abuse and Alcoholism Laboratory of Neurogenetics. Genomic DNA was extracted from whole blood using standard protocols and run on an Illumina OmniExpress BeadChip array (Illumina San Diego, California, USA). FAAH Pro129Thr (rs324420) genotype as well as independent Ancestry Informative Markers (AIMs) were obtained from the array data. The average genotype reproducibility was more than 0.99994. Ancestry informative markers (n = 2500) were extracted from the Illumina array to calculate ancestral proportions for all study participants. Using methods described previously (Hodgkinson et al., 2008), ancestry assessment identified six ethnic factors: Africa, Europe, Asia, Far East Asia, Oceania, and Americas. Only individuals who were of European (European American) and African (African American) ancestry were included in our analysis.
Outcome Measures
Alcohol Dependence Diagnosis
Alcohol dependence was assessed using the Structured Clinical Interview for DSM-IV axis I disorders (First et al., 1995). This structured interview has high inter-rater reliability for alcohol dependence or abuse (Martin et al., 2000). Individuals who were diagnosed with alcohol dependence at any prior visit or the most recent visit to our center were defined as having lifetime alcohol dependence whereas only those who were diagnosed at the most recent assessment were defined as having current alcohol dependence.
Timeline Followback (TLFB) Interviews
Alcohol TLFB interviews are a method of retrospectively estimating daily drinking using a calendar and memory aids to enhance recall (Sobell & Sobell, 1992). We used an Alcohol TLFB interview in which subjects recalled their drinking over the previous 90 days. These interviews have been found to have high test-retest reliability (Sobell et al., 1996) and are widely used as measures of alcohol consumption in clinical studies. We looked at three outcome measures: total drinks, total number of drinking days (days in which participants consumed at least one drink), and total number of binge drinking days over the 90-day period. We used the National Institute on Alcohol Abuse and Alcoholism’s definition of a binge: four or more drinks for women and five or more drinks for men in a given day (NIAAA, 2004). TLFB interviews were conducted with the majority of participants (n = 1260).
Alcohol Use Disorders Identification Test (AUDIT)
The AUDIT is a 10-item questionnaire that assesses problematic alcohol use in the past year (Saunders et al., 1993). Responses to each question are scored from 0–4 with a total score that can range from 0–40, with greater scores indicating increasing severity. The AUDIT can also be divided into three subscales, a consumption (hazardous alcohol use) subscale based on questions 1–3 (AUDIT-C), a dependence symptom subscale based on questions 4–6 (AUDIT-D), and a harmful alcohol use subscale based on questions 7–10 (AUDIT-H). The AUDIT demonstrates good test-retest reliability and internal consistency (Reinert & Allen, 2007). AUDIT scores were obtained for 41.9% of the participants in our sample (n = 601).
Statistical Analysis
Due to the relationship between ancestry and FAAH Pro129Thr genotype distribution in our sample (χ2(2) = 91.599, p < 0.001), all comparisons were made between individuals of the same ancestry or were controlled for ancestry. Previous reports have demonstrated that both FAAH Thr129/Pro129 heterozygotes and Thr129/Thr129 homozygotes produce FAAH enzymes with decreased activity resulting in higher anandamide levels (Chiang et al., 2004; Dincheva et al., 2015; Sipe et al., 2002). Furthermore, there were only a small number of Thr129/Thr129 homozygotes in our sample (41 European Americans and 81 African Americans) which limited our power to detect differences between Thr129/Thr129 homozygotes and other genotype groups. Thr129 carriers (Thr129/Thr129 homozygotes and Thr129/Pro129 heterozygotes) were therefore pooled and compared with Pro129/Pro129 homozygotes for all analyses (see Tables S1, S2, and S3, for demographic and outcome data for all three genotype groups).
The relationship between AD diagnosis and genotype was analyzed using two-proportion Z-tests to compare Thr129 allele frequency between cases and controls. We then performed a whole sample (n = 1434) binary logistic regression to assess the effect of genotype on the probability of AD diagnosis while controlling for gender, ancestry, and the most common psychiatric comorbidities in our sample (cocaine and cannabis dependence or abuse, any anxiety disorder, and major depressive disorder). As a measure of diagnostic severity, we compared AD symptom count at the most recent assessment between genotype groups. We used the Kendall rank correlation coefficient (Kendall’s Tau-b) to determine whether associations were present between genotype and symptom count in each ancestry. We then used binary logistic regression to compare whether Thr129 carriers had increased probability of having a more severe symptom count (6–7 DSM-IV AD symptoms) as compared to a mild-moderate symptom count (2–5 DSM-IV AD symptoms) while controlling for the same confounding variables as in the AD diagnosis analysis. Cut-offs were based on DSM-5 severity specifiers for Alcohol Use Disorder (APA, 2013), although it should be noted that these cut-offs were not designed to be used for DSM-IV Alcohol Dependence which has less total criteria than DSM-5 Alcohol Use Disorder and does not have defined severity specifiers. We only had symptom count data for a subset of the sample (n = 632) and in the binary logistic regression analyses we also excluded those who met less than two criteria from the analysis (n = 65), therefore the sample sizes for these analyses were smaller than the previous analysis (n = 632 for the correlation analysis and n = 567 participants for the binary logistic regression analysis).
To further assess the impact of FAAH genotype on AD severity, we compared TLFB measures and AUDIT scores between genotype groups among those with lifetime AD. Mann-Whitney U tests were performed for data which were not normally distributed and independent samples t-tests were performed for normally distributed data. Prior to each analysis, the following confounding variables were analyzed between genotype groups: age, gender, years of education, cigarette pack-years, Fagerström Test for Nicotine Dependence score (Heatherton et al., 1991), Childhood Trauma Questionnaire score (Bernstein & Fink, 1998), and the most common comorbid substance use and psychiatric diagnoses in our sample (major depressive disorder, any anxiety disorder, cannabis abuse or dependence, and cocaine abuse or dependence). Confounding variables for each group were compared using chi-square tests of independence for categorical variables and Mann-Whitney U tests for continuous variables and subgroup analyses were performed if there were differences between genotype groups.
Rank-biserial correlation coefficients (rrb), Cohen’s d, and odds ratios are reported as measures of effect size for Mann-Whitney U tests, independent samples t-tests, and logistic regression analyses respectively. Given the strong evidence that correcting for multiple comparisons reduces false positive results in candidate gene studies (Sullivan, 2007), we used an experiment-wise Benjamini-Hochberg false discovery rate (FDR)(Benjamini & Hochberg, 1995) which excluded the logistic regression analyses and AUDIT subscale analyses (given that AUDIT subscales were highly correlated with total AUDIT score: Spearman’s rho 0.62 – 0.88, p < 0.001 for all correlations). Uncorrected p-values are reported along with whether significant tests no longer remain significant after FDR correction.
Results
Demographics
The final sample used for analysis (n = 1434) was predominantly male (67.2%) with a mean age of 39.0 years (SD = 11.7). 877 participants were of European ancestry and 557 participants were of African ancestry (for overall sample demographics by genotype and ancestry, see Table 1). The lifetime alcohol dependent group (n = 952) was also predominantly male (71.1%) with a mean age of 42.6 (SD = 10.5). 537 participants were of European ancestry and 415 participants were of African ancestry (for demographics by genotype and ancestry in lifetime AD participants with TLFB interview data, see Table S4).
Table 1.
Demographics, Full Sample
| Variable | European American Pro129/Pro129 (n = 549) | European American Thr129‡ (n = 328) | Test Statistic | African American Pro129/Pro129 (n = 217) | African American Thr129‡ (n=340) | Test Statistic |
|---|---|---|---|---|---|---|
| Age, mean (SD), years | 37.2 (12.2) | 38.3 (12.4) | Zu = −1.0 | 41.2 (10.2) | 41.1 (10.4) | Zu = −0.3 |
| Female sex, % | 33.0 | 36.0 | χ2 = 0.8 | 28.6 | 32.1 | χ2 = 0.8 |
| Median Income Bracket, USD† | 30 000 – 39 999 | 30 000 – 39 999 | NA | 20 000 – 29 999 | 10 000 – 19 999 | NA |
| Education, mean (SD), years† | 15.0 (2.7) | 15.1 (2.7) | Zu = −0.6 | 13.2 (2.5) | 13.2 (2.8) | Zu = −0.3 |
| Major Depressive Disorder, % | 6.4 | 7.3 | χ2 = 0.3 | 6.5 | 6.5 | χ2 < 0.1 |
| Anxiety Disorder, % | 20.0 | 24.1 | χ2 = 2.0 | 22.6 | 25.3 | χ2 = 0.5 |
| Cigarette Pack-Years, mean (SD)† | 7.2 (12.6) | 7.9 (14.7) | Zu = −0.5 | 6.6 (11.8) | 6.3 (10.2) | Zu = −0.6 |
| FTND Score, mean (SD)† | 1.7 (2.6) | 1.8 (2.7) | Zu = −0.8 | 2.0 (2.5) | 1.9 (2.4) | Zu = −0.2 |
| Cannabis Abuse or Dependence, % | 9.5 | 10.4 | χ2 = 0.2 | 15.2 | 9.4 | χ2 = 4.3* |
| Cocaine Abuse or Dependence, % | 7.1 | 8.5 | χ2 = 0.6 | 17.1 | 13.8 | χ2 = 1.1 |
| CTQ Score, mean (SD)† | 36.5 (13.7) | 38.2 (15.6) | Zu = −0.9 | 41.7 (16.6) | 41.7 (17.5) | Zu = −0.7 |
Abbreviations: FTND, Fagerström Test for Nicotine Dependence; CTQ, Childhood Trauma Questionnaire; NA, not applicable; Zu, Mann-Whitney U-Test Statistic.
European American Thr129 Carriers: 41 Thr129/Thr129 homozygotes, 287 Thr129/Pro129 heterozygotes; African American Thr129 Carriers: 81 Thr129/Thr129 homozygotes, 259 Thr129/Pro129 heterozygotes.
p<0.05.
Data only available for a subset of participants:
Income Bracket: European Americans: Pro129/Pro129 n = 293, Thr129 n = 193, African Americans: Pro129/Pro129 n = 150, African American Thr129 n = 241;
Education: European Americans: Pro129/Pro129 n = 447, Thr129 n = 283, African Americans: Pro129/Pro129 n = 197, Thr129 n = 304;
Pack-Cigarette Pack-Years: European Americans: Pro129/Pro129 n = 466, Thr129 n = 278, African Americans: Pro129/Pro129 n = 183, Thr129 n = 295;
Fagerström Test for Nicotine Dependence Score: European Americans: Pro129/Pro129 n = 491, Thr129 n = 296, African Americans: Pro129/Pro129 n = 191, Thr129 n = 305;
CTQ Score: European Americans: Pro129/Pro129 n = 321, Thr129 n = 210, African Americans: Pro129/Pro129 n = 160, Thr129 n =249.
FAAH Genotype Distribution
In European Americans, FAAH genotype distribution was 62.6% Pro129/Pro129 (n = 549), 32.7% Thr129/Pro129 (n = 287), and 4.7% Thr129/Thr129 (n = 41). In African Americans, FAAH genotype distribution was 39.0% Pro129/Pro129 (n = 217), 46.5% Thr129/Pro129 (n = 259), and 14.5% Thr129/Thr129 (n = 81). Neither group’s allele distributions significantly deviated from Hardy-Weinberg equilibrium (χ2(2) = 0.081, p = 0.960; χ2(2) = 0.045, p = 0.978). We used data from the 1000 Genomes Project Consortium (Abecasis et al., 2010) to compare the allele frequency of our sample to that of the general population. European American FAAH Thr129 allele frequency was 0.210, which did not differ significantly from the population frequency of 0.211 reported by The 1000 Genomes Project Consortium (Z = 0.02, p = 0.982; data accessed via dbSNP, NCBI). African American Thr129 allele frequency was 0.378, which also did not differ significantly from the population frequency of 0.368 reported by The 1000 Genomes Project Consortium (Z = 0.52, p = 0.601; data accessed via dbSNP, NCBI).
In the overall group, there were no significant differences by genotype and ancestry in confounding variables with the exception of cannabis abuse or dependence diagnosis, which was lower in African American Thr129 carriers as compared to Pro129/Pro129 homozygotes (Table 1). In the lifetime AD group, there were no significant differences by genotype and ancestry in confounding variables for TLFB interview analyses and AUDIT analyses (Tables S4 and S5).
FAAH Genotype and Alcohol Dependence Diagnosis
Participants were divided into cases (n = 952, 56.4% European American) and controls (n = 482, 70.5% European American) based on lifetime diagnosis of alcohol dependence. A secondary analysis was also performed. This analysis only included individuals who met criteria for alcohol dependence at their most recent assessment as cases (current AD group, 892 participants, 56.7% European American) as participants who had recovered from a previous AD diagnosis by their most recent assessment (n = 60) drank significantly less than current AD cases (246 versus 824 median total drinks during the 90 day period; U(57,831) = 7596.5, p < 0.001) and had consumption patterns closer to those of controls.
Thr129 allele frequencies in European Americans with and without lifetime AD were 0.226 and 0.185 respectively. A two-proportion Z-test revealed that the Thr129 allele was found at a greater frequency in European Americans with lifetime AD (Z = 2.02, p = 0.037, 95% CI 0.002 to 0.079), but this difference was not significant after correction for multiple comparisons. When only those with current AD and more severe drinking patterns were used as cases, Thr129 allele frequency was significantly higher in cases than controls after correction for multiple comparisons (0.234 for cases and 0.178 for controls, Z = 2.91, p = 0.004, 95% CI 0.018 to 0.094). In contrast, African Americans showed no difference in Thr129 allele frequency between those with and without both lifetime AD (0.377 cases, 0.380 controls, Z = −0.10, p = 0.944, 95% CI −0.069 to 0.062) and current AD (0.380 cases, 0.374 controls, Z = 0.17, p = 0.867, 95% CI −0.055 to 0.067, Figure 1). As diagnosis of cannabis abuse or dependence was found to be higher in African American Pro129/Pro129 homozygotes compared to Thr129 carriers (Table 1), we repeated these analyses using only African American participants without a DSM-IV diagnosis of cannabis abuse, which did not significantly alter these findings.
Figure 1.
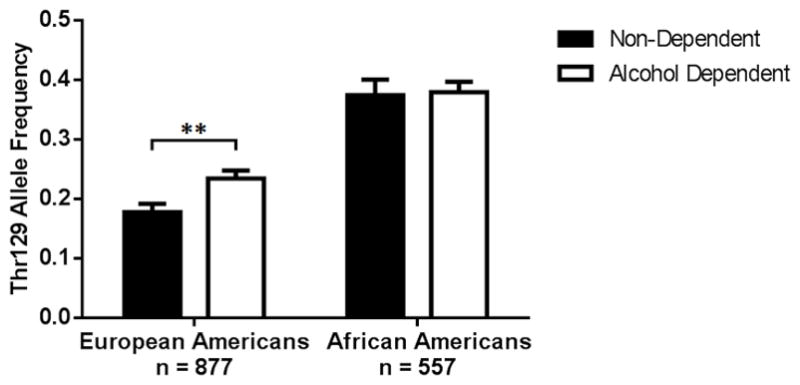
FAAH rs324420 Thr129 allele frequency in participants with and without a diagnosis of alcohol dependence at their most recent assessment (alcohol dependent cases and non-dependent controls). Standard error is indicated by the error bars. European American Thr129 allele frequency was significantly higher in participants with current alcohol dependence than in controls. European Americans: cases n = 506, controls n = 371, African Americans: cases n = 386, controls n = 171. **p<0.01
Logistic regression was used to investigate the effect of Thr129 carrier status on the probability of lifetime and current AD diagnosis after controlling for gender, ancestry, and comorbid DSM-IV diagnoses including cannabis and cocaine abuse and dependence, major depressive disorder, and any anxiety disorder. An interaction term for FAAH genotype by ancestry was initially included in the lifetime AD and current AD analyses, but was dropped as it was not significantly contributing to either model (χ2(1) = 0.185, p = 0.667, lifetime AD analysis; χ2(1) = 1.32, p = 0.251, current AD analysis). FAAH Pro129Thr was not a significant predictor of lifetime AD after controlling for the other variables (OR 1.20, 95% CI 0.93 to 1.55, p = 0.168); the results were unchanged after removing an outlier that influenced goodness of fit (OR 1.19, 95% CI 0.92 to 1.54, p = 0.180, Table S6). When current AD was used as the outcome, FAAH Pro129Thr genotype was a significant predictor of diagnosis after controlling for the other variables, with Thr129 allele carriers demonstrating higher odds of current AD compared to Pro129/Pro129 homozygotes (OR 1.35, 95% CI 1.05 to 1.74, p = 0.021). The results were unchanged after removing an outlier that influenced goodness of fit (OR 1.35, 95% CI 1.04 to 1.74, p = 0.023, Table S7).
FAAH Genotype and Alcohol Dependence Symptom Count
FAAH genotype was correlated with AD symptom count in European Americans (Kendall’s Tau-b = 0.126, p = 0.013) but not African Americans (Kendall’s Tau-b = −0.040, p = 0.430). A binary logistic regression was performed using the same covariates as the AD diagnosis analysis to determine whether Thr129 carriers were more likely to have a severe symptom count (6–7 criteria) than a mild to moderate symptom count (2–5 criteria) when controlling for confounding variables. In this model, there was a significant main effect of Thr129 genotype (OR = 1.87, 95% CI 1.01 to 3.48 p = 0.047) and a significant interaction between Thr129 genotype and ancestry (OR = 0.39, 95% CI 0.17 to 0.88, p = 0.024) on AD severity after controlling for other covariates. When the sample was split by ancestry, Thr129 carriers had a marginally significant higher probability of being diagnosed with severe AD compared to Pro129/Pro129 homozygotes in European Americans (OR = 1.86, 95% 1.00 to 3.48, p = 0.051) but not African Americans (OR = 0.77, 95% CI 0.45 to 1.31, p = 0.332) after controlling for other covariates.
FAAH Genotype and Alcohol Consumption
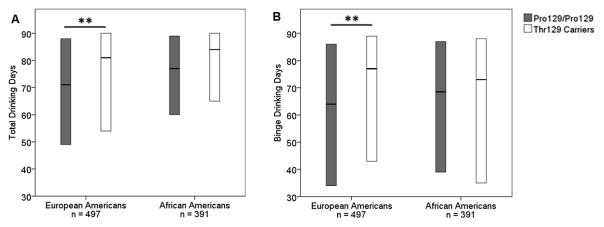
Timeline Followback interviews were conducted with 888 of our participants with lifetime alcohol dependence (Table S4). European American Thr129 carriers had a higher median number of drinking and binge drinking days compared to non-carriers in the 90 days prior to assessment: 81 versus 71 drinking days and 77 versus 64 binge drinking days. The distribution of drinking and binge drinking days was significantly different between genotype groups (U(298,199) = 25 352, p = 0.006, rrb = 0.206 for number of drinking days, U(298,199) = 25 055.5, p = 0.003, rrb = 0.155 for number of binge drinking days, Figure 2). European American Thr129 carriers also consumed a higher median number of total drinks during the 90-day period than non-carriers, with a difference in distribution of total drinks between genotype groups marginally exceeding the 0.021 threshold for statistical significance after correction for multiple comparisons (860 versus 753 median total drinks, U(298,199) = 26 060, p = 0.022, rrb = 0.121). In African Americans, there were no significant differences in the distributions of any of the TLFB interview outcomes between genotype groups (U(150,241) = 17 654.5, p = 0.699, rrb = 0.023 for total drinks, U(150,241) = 16 018, p = 0.056, rrb = 0.114 for number of drinking days, and U(150,241) = 17 842.5, p = 0.830, rrb = 0.013 for number of binge drinking days, Figure 2).
Figure 2.
Boxplots of (A) drinking days and (B) binge drinking days over the past 90 days as assessed by Timeline Followback interview in participants with a diagnosis of alcohol dependence at any assessment. The horizontal line in the middle of each box indicates the median, while the bottom and top borders of the box represent the 25th and 75th percentile values respectively. The distribution of drinking and binge drinking days was significantly different between European American genotype groups. European Americans: Thr129/Thr129 n = 30, Thr129/Pro129 n = 169, Pro129/Pro129 n = 298, African Americans: Thr129/Thr129 n = 56, Thr129/Pro129 n = 185, Pro129/Pro129 n = 150. **p<0.01
AUDIT scores were analyzed to confirm our TLFB interview findings and were available for 207 of our participants with lifetime alcohol dependence (Table S5). In European Americans, the mean AUDIT score was significantly higher in Thr129 carriers relative to non-carriers (mean AUDIT scores: 22.3 Thr129 carriers, 15.4 Pro129/Pro129 homozygotes; t(61) = 2.872, p = 0.006, 95% CI 2.1 to 11.7, Cohen’s d = 0.74, Figure 3). There was no difference in AUDIT scores between the two genotype groups in African Americans (mean AUDIT scores: 19.9 Thr129 carriers, 17.9 Pro129/Pro129 homozygotes; t(142) = 1.536, p = 0.127, 95% CI −0.6 to 4.8, Cohen’s d = 0.26, Figure 3).
Figure 3.
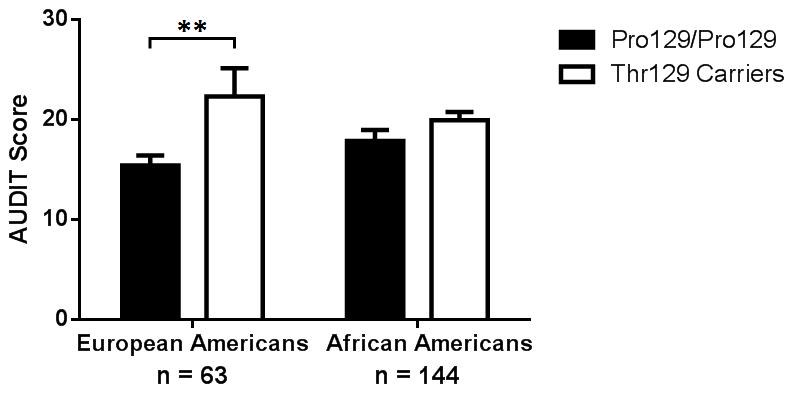
Mean AUDIT score ± SEM in participants with a diagnosis of alcohol dependence at any assessment. Mean AUDIT score was significantly higher in European American Thr129 carriers versus Pro129/Pro129 homozygotes. European Americans: Thr129/Thr129 n = 3, Thr129/Pro129 n = 10, Pro129/Pro129 n = 50, African Americans: Thr129/Thr129 n = 19, Thr129/Pro129 n = 74, Pro129/Pro129 n = 51. **p<0.01
To determine whether the differences in AUDIT scores between European Americans Thr129 carriers and non-carriers were being driven solely by the AUDIT’s alcohol consumption questions, we performed an additional analysis comparing each AUDIT subscale between genotype groups. In Thr129 carriers, median scores were higher for all three subscales with significantly different distributions for the consumption (hazardous alcohol use) and dependence symptom subscales (AUDIT-C Thr129 carrier median = 9.0, Pro129/Pro129 median = 8.0, U(50,13) = 456.5, p = 0.024, rrb = 0.405; AUDIT-D Thr129 carrier median = 3.0, Pro129/Pro129 median = 2.0, U(50,13) = 439.0, p = 0.050, rrb = 0.351; AUDIT-H Thr129 carrier median = 7.0, Pro129/Pro129 median = 5.5, U(50,13) = 421.0, p = 0.101, rrb = 0.295).
Discussion
Our results provide preliminary evidence of an association between the FAAH Pro129Thr polymorphism and both probability and severity of alcohol dependence. Alcohol dependent subjects were found to have a higher Thr129 allele frequency than controls. Logistic regression analyses demonstrated that Thr129 carriers retained a higher probability of current AD after controlling for multiple confounding variables. Thr129 carriers were found to have greater AD severity than non-carriers using AD symptom counts, TLFB interview measures, and AUDIT scores. Allele frequency findings and associations between Thr129 carrier status and AD severity were restricted to European Americans.
Thr129 allele frequency was higher in European Americans with a current or lifetime alcohol dependence diagnosis than in controls, but only the former comparison remained significant after FDR correction. Given that we’ve shown that Thr129 carriers in our sample had more severe AD in terms of symptom count, alcohol consumption, and AUDIT score, it is possible that Pro129/Pro129 homozygotes were more likely to recover over time which would explain why the association between Thr129 and AD diagnosis was strengthened when recovered AD individuals were grouped with controls. Future studies should seek to determine whether Pro129/Pro129 homozygotes are indeed more likely to attain sustained remission and whether FAAH genotype could be a clinically useful prognostic factor for alcohol dependence.
Our findings add to a growing body of literature suggesting that altered FAAH activity affects alcohol consumption. Knock-in models show that Thr129/Thr129 mice have both higher levels of anandamide and greater alcohol intake than their Pro129/Pro129 counterparts (Dincheva et al., 2015; Zhou et al., 2016). This accords with studies showing increased anandamide levels and ethanol intake when FAAH is pharmacologically inhibited (Blednov et al., 2007; Hansson et al., 2007; Kathuria et al., 2003; Vinod et al., 2008). Alcohol consumption also affects anandamide levels. Chronic alcohol administration decreases anandamide uptake into neurons which in turn leads to increased extracellular anandamide and decreased CB1 receptor density in rodents chronically exposed to alcohol (Basavarajappa et al., 2003; Vinod et al., 2006). It is therefore possible that chronic alcohol consumption further increases anandamide levels in the human brain thus accentuating the effects of the Thr129 allele on alcohol consumption, although this remains to be empirically studied. Increased alcohol consumption in Thr129 carriers may be mediated by endocannabinoid-induced alterations in striatal circuits involved in habit formation and reward. Retrograde anandamide signaling is implicated in long-term depression (LTD) in the dorsal striatum, an area thought to play a crucial role in habit learning (Gerdeman, Ronesi & Lovinger, 2002). Habit formation in mice is prevented by deletion of CB1 receptors from cortical projections to the dorsal striatum (Gremel et al., 2016), demonstrating the behavioral importance of endocannabinoid-induced LTD. Endocannabinoids also affect reward signaling in the nucleus accumbens. Anandamide administration in rodents increases extracellular dopamine levels in the nucleus accumbens shell (Solinas et al., 2006) whereas CB1 receptor knockout mice lack ethanol-induced acccumbal dopamine release (Hungund et al., 2003). Thr129 carriers may therefore have alterations in striatal circuits that enhance reward sensitivity and promote the development of habitual behaviors, both of which could increase alcohol dependence vulnerability and severity.
Neuroimaging studies in humans confirm that Thr129 carriers have distinct neurobiological differences compared to Pro129/Pro129 homozygotes. Thr129 carriers demonstrate increased reward-related ventral striatal reactivity which correlates with a behavioral preference for smaller immediate over larger delayed rewards (Hariri et al., 2009), suggesting that differences in striatal reactivity could help explain the increased levels of alcohol consumption observed in these individuals. There is also evidence that the neurobiological differences in Thr129 carriers extend beyond the realm of reward and addiction. Thr129 carriers show decreased amygdala response and faster amygdala-mediated habituation to angry or fearful faces (Gunduz-Cinar et al., 2013; Hariri et al., 2009). Resting-state functional analyses have demonstrated increased connectivity between the ventromedial prefrontal cortex and the amygdala in Thr129 carriers, results which parallel connectivity findings in knock-in mice homozygous for the Thr129 allele (Dincheva et al., 2015). Thr129 carriers also demonstrate decreased placebo-induced mu-opioid neurotransmission accompanied by lower placebo-induced analgesia (Pecina et al., 2014). In addition to the effects on neuroimaging outcomes, Thr129 carriers with AD and comorbid posttraumatic stress disorder have higher basal serum anandamide levels and lower subjective anxiety responses during stress challenge (Spagnolo et al., In Press). Whether the effects of the Thr129 allele on stress reactivity, fear extinction, and pain perception also play a role in increasing alcohol dependence vulnerability and severity remains to be demonstrated.
Despite evidence that altered FAAH activity and endocannabinoid signaling affect both addictive behavior and related neural circuitry, results from genetic association studies have been mixed. Our findings offer potential explanations for these discrepancies. The odds ratios and rank-biserial correlations obtained from our analyses suggest that the magnitude of the Pro129Thr polymorphism’s effect on both probability and severity of alcohol dependence is small. This could explain why this locus was not found to be associated with alcohol dependence in the largest genome-wide association study to date (Gelernter et al., 2014), which primarily confirmed associations for well-known alcohol-metabolizing enzyme genes that are thought to have larger effects (Bierut et al., 2012; Hart & Kranzler, 2015; Li, Zhao & Gelernter, 2011). Larger GWAS samples will be needed to achieve the statistical power necessary to identify loci with smaller effects such as FAAH Pro129Thr (Hart & Kranzler, 2015). Our results also indicate that this polymorphism has ancestry-specific effects, which suggests that findings will vary depending on the ancestry of the sample being studied. This provides a possible explanation for results demonstrating the absence of this genetic association in Japanese samples (Iwasaki et al., 2007; Morita et al., 2005). In our study, the lack of an association between the Thr129 allele and both AD diagnosis and severity in African Americans could be due to a larger role for socioeconomic factors in this group thus diminishing the contribution of this specific polymorphism to the AD phenotype. In support of this, our African American participants had a markedly lower median income bracket than our European American participants. Alternately, ancestry-specific variations in gene expression or specific gene-gene interactions could explain these discrepancies. Future studies should seek to confirm whether the FAAH Pro129Thr polymorphism’s effect is ancestry-specific and if so, should seek to determine which factors are responsible for its differential effects. Finally, our study only examined the association between the Thr129 allele and alcohol-related phenotypes, whereas prior studies examined associations with both alcohol and various other drugs of abuse. This raises the possibility that the Thr129 allele may predispose individuals to specific substance use disorders and may even be protective for others. In support of this, in our demographic analyses we found that African American Thr129 carriers had lower rates of cannabis abuse or dependence, which parallels a previous finding in individuals of European ancestry (Tyndale et al., 2007). However, this finding should be approached with some discretion as (1) it was not an a priori hypothesis of our study, (2) our study was designed to recruit alcohol dependent individuals and therefore had relatively low numbers of individuals with cannabis abuse or dependence, and (3) we did not apply the same stringent statistical corrections for multiple comparisons in these demographic analyses as we did for our alcohol-related analyses.
As this is a candidate polymorphism study, our results should be interpreted with caution. Association studies tend to overestimate the predisposition conferred by a genetic polymorphism and are often not replicated in subsequent studies (Ioannidis et al., 2001). To avoid reporting spurious associations, we limited our analysis to one single nucleotide polymorphism with known neurochemical and behavioral effects. Furthermore, we used a Benjamini-Hochberg false discovery rate to adjust for experiment-wise multiple comparisons as there is strong evidence that using a more conservative significance threshold reduces false positive rates in candidate gene studies (Sullivan, 2007). Nevertheless, findings from our study should be considered tentative until these results are replicated in other large alcohol dependent samples. Supportive results should be obtained from large genome-wide association studies (GWAS) or meta-analytic approaches before definitive conclusions can be made.
We have demonstrated an association between the FAAH Pro129Thr polymorphism and alcohol dependence risk and severity, but it remains to be shown whether there is a Thr129 allele dose-response effect. Although we lacked a sufficient number of Thr129/Thr129 homozygotes to examine this in our study, we continue to collect data and will hopefully be adequately powered for such analyses in the future. Given that CB1 agonist administration leads to a dose-dependent increase in ethanol intake in rodents (Colombo et al., 2002), it is possible that increasing Thr129 allele dose could lead to greater increases in anandamide levels and subsequent ethanol consumption. The development of FAAH inhibitors for therapeutic use in humans (Bisogno & Maccarrone, 2013) provides an exciting opportunity to further resolve these questions as it allows for the direct manipulation of FAAH activity in vivo to determine how varying levels of activity influence alcohol consumption.
Supplementary Material
Acknowledgments
This work was supported by the Division of Intramural Clinical and Biological Research of the National Institute on Alcohol Abuse and Alcoholism (Z1A AA000466). The authors thank Markus Heilig, David T. George, Nancy Diazgranados, Mary Lee, Reza Momenan, Lorenzo Leggio, Cheryl Jones, Betsy Davis and Monte Phillips for clinical protocol support. The authors also thank Peter Sloan and Jenna Gale for their valuable comments during manuscript preparation. The authors declare no conflicts of interest.
Footnotes
Author Contributions
MES and VAR were responsible for the study concept and design. MES conducted the data analysis and drafted the manuscript. JLG assisted with data analysis and revised the manuscript. JY and MLS were involved with data collection and preparation and revised the manuscript. PAS revised the manuscript for important intellectual content. HS and CAH performed genotyping and extracted ancestry informative markers. VAR and DG assisted with development of the protocol, provided support with data collection, and provided critical review of the manuscript. All authors critically reviewed content and approved the final version for publication.
References
- Abecasis GR, Altshuler D, Auton A, Brooks LD, Durbin RM, Gibbs RA, Hurles ME, McVean GA. A map of human genome variation from population-scale sequencing. Nature. 2010;467:1061–1073. doi: 10.1038/nature09534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- APA. DSM 5. American Psychiatric Association; 2013. [Google Scholar]
- Arnone M, Maruani J, Chaperon F, Thiebot MH, Poncelet M, Soubrie P, Le Fur G. Selective inhibition of sucrose and ethanol intake by SR 141716, an antagonist of central cannabinoid (CB1) receptors. Psychopharmacology (Berl) 1997;132:104–106. doi: 10.1007/s002130050326. [DOI] [PubMed] [Google Scholar]
- Basavarajappa BS, Saito M, Cooper TB, Hungund BL. Chronic ethanol inhibits the anandamide transport and increases extracellular anandamide levels in cerebellar granule neurons. Eur J Pharmacol. 2003;466:73–83. doi: 10.1016/s0014-2999(03)01557-7. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the royal statistical society Series B (Methodological) 1995:289–300. [Google Scholar]
- Bernstein DP, Fink L. Childhood trauma questionnaire: A retrospective self-report: Manual. Psychological Corporation; 1998. [Google Scholar]
- Bierut LJ, Goate AM, Breslau N, Johnson EO, Bertelsen S, Fox L, Agrawal A, Bucholz KK, Grucza R, Hesselbrock V, Kramer J, Kuperman S, Nurnberger J, Porjesz B, Saccone NL, Schuckit M, Tischfield J, Wang JC, Foroud T, Rice JP, Edenberg HJ. ADH1B is associated with alcohol dependence and alcohol consumption in populations of European and African ancestry. Mol Psychiatry. 2012;17:445–450. doi: 10.1038/mp.2011.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisogno T, Maccarrone M. Latest advances in the discovery of fatty acid amide hydrolase inhibitors. Expert Opin Drug Discov. 2013;8:509–522. doi: 10.1517/17460441.2013.780021. [DOI] [PubMed] [Google Scholar]
- Blednov YA, Cravatt BF, Boehm SL, 2nd, Walker D, Harris RA. Role of endocannabinoids in alcohol consumption and intoxication: studies of mice lacking fatty acid amide hydrolase. Neuropsychopharmacology. 2007;32:1570–1582. doi: 10.1038/sj.npp.1301274. [DOI] [PubMed] [Google Scholar]
- Buhler KM, Huertas E, Echeverry-Alzate V, Gine E, Molto E, Montoliu L, Lopez-Moreno JA. Risky alcohol consumption in young people is associated with the fatty acid amide hydrolase gene polymorphism C385A and affective rating of drug pictures. Mol Genet Genomics. 2014;289:279–289. doi: 10.1007/s00438-013-0809-x. [DOI] [PubMed] [Google Scholar]
- Button KS, Ioannidis JP, Mokrysz C, Nosek BA, Flint J, Robinson ES, Munafo MR. Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci. 2013;14:365–376. doi: 10.1038/nrn3475. [DOI] [PubMed] [Google Scholar]
- Chiang KP, Gerber AL, Sipe JC, Cravatt BF. Reduced cellular expression and activity of the P129T mutant of human fatty acid amide hydrolase: evidence for a link between defects in the endocannabinoid system and problem drug use. Hum Mol Genet. 2004;13:2113–2119. doi: 10.1093/hmg/ddh216. [DOI] [PubMed] [Google Scholar]
- Colombo G, Agabio R, Fa M, Guano L, Lobina C, Loche A, Reali R, Gessa GL. Reduction of voluntary ethanol intake in ethanol-preferring sP rats by the cannabinoid antagonist SR-141716. Alcohol Alcohol. 1998;33:126–130. doi: 10.1093/oxfordjournals.alcalc.a008368. [DOI] [PubMed] [Google Scholar]
- Colombo G, Serra S, Brunetti G, Gomez R, Melis S, Vacca G, Carai MM, Gessa L. Stimulation of voluntary ethanol intake by cannabinoid receptor agonists in ethanol-preferring sP rats. Psychopharmacology (Berl) 2002;159:181–187. doi: 10.1007/s002130100887. [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Demarest K, Patricelli MP, Bracey MH, Giang DK, Martin BR, Lichtman AH. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc Natl Acad Sci U S A. 2001;98:9371–9376. doi: 10.1073/pnas.161191698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384:83–87. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- Dincheva I, Drysdale AT, Hartley CA, Johnson DC, Jing D, King EC, Ra S, Gray JM, Yang R, DeGruccio AM, Huang C, Cravatt BF, Glatt CE, Hill MN, Casey BJ, Lee FS. FAAH genetic variation enhances fronto-amygdala function in mouse and human. Nat Commun. 2015;6:6395. doi: 10.1038/ncomms7395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Femenia T, Garcia-Gutierrez MS, Manzanares J. CB1 receptor blockade decreases ethanol intake and associated neurochemical changes in fawn-hooded rats. Alcohol Clin Exp Res. 2010;34:131–141. doi: 10.1111/j.1530-0277.2009.01074.x. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured clinical interview for DSM-IV axis I disorders-patient edition (SCID-I/P, Version 2.0) New York: Biometrics Research Department, New York State Psychiatric Institute; 1995. p. 722. [Google Scholar]
- Flanagan JM, Gerber AL, Cadet JL, Beutler E, Sipe JC. The fatty acid amide hydrolase 385 A/A (P129T) variant: haplotype analysis of an ancient missense mutation and validation of risk for drug addiction. Hum Genet. 2006;120:581–588. doi: 10.1007/s00439-006-0250-x. [DOI] [PubMed] [Google Scholar]
- Gallate JE, Saharov T, Mallet PE, McGregor IS. Increased motivation for beer in rats following administration of a cannabinoid CB1 receptor agonist. Eur J Pharmacol. 1999;370:233–240. doi: 10.1016/s0014-2999(99)00170-3. [DOI] [PubMed] [Google Scholar]
- Gelernter J, Kranzler H, Sherva R, Almasy L, Koesterer R, Smith A, Anton R, Preuss U, Ridinger M, Rujescu D. Genome-wide association study of alcohol dependence: significant findings in African-and European-Americans including novel risk loci. Molecular psychiatry. 2014;19:41–49. doi: 10.1038/mp.2013.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdeman GL, Ronesi J, Lovinger DM. Postsynaptic endocannabinoid release is critical to long-term depression in the striatum. Nat Neurosci. 2002;5:446–451. doi: 10.1038/nn832. [DOI] [PubMed] [Google Scholar]
- Gremel CM, Chancey JH, Atwood BK, Luo G, Neve R, Ramakrishnan C, Deisseroth K, Lovinger DM, Costa RM. Endocannabinoid Modulation of Orbitostriatal Circuits Gates Habit Formation. Neuron. 2016;90:1312–1324. doi: 10.1016/j.neuron.2016.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunduz-Cinar O, MacPherson KP, Cinar R, Gamble-George J, Sugden K, Williams B, Godlewski G, Ramikie TS, Gorka AX, Alapafuja SO, Nikas SP, Makriyannis A, Poulton R, Patel S, Hariri AR, Caspi A, Moffitt TE, Kunos G, Holmes A. Convergent translational evidence of a role for anandamide in amygdala-mediated fear extinction, threat processing and stress-reactivity. Mol Psychiatry. 2013;18:813–823. doi: 10.1038/mp.2012.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson AC, Bermudez-Silva FJ, Malinen H, Hyytia P, Sanchez-Vera I, Rimondini R, Rodriguez de Fonseca F, Kunos G, Sommer WH, Heilig M. Genetic impairment of frontocortical endocannabinoid degradation and high alcohol preference. Neuropsychopharmacology. 2007;32:117–126. doi: 10.1038/sj.npp.1301034. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Gorka A, Hyde LW, Kimak M, Halder I, Ducci F, Ferrell RE, Goldman D, Manuck SB. Divergent effects of genetic variation in endocannabinoid signaling on human threat- and reward-related brain function. Biol Psychiatry. 2009;66:9–16. doi: 10.1016/j.biopsych.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart AB, Kranzler HR. Alcohol Dependence Genetics: Lessons Learned From Genome-Wide Association Studies (GWAS) and Post-GWAS Analyses. Alcohol Clin Exp Res. 2015;39:1312–1327. doi: 10.1111/acer.12792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hodgkinson CA, Yuan Q, Xu K, Shen PH, Heinz E, Lobos EA, Binder EB, Cubells J, Ehlers CL, Gelernter J, Mann J, Riley B, Roy A, Tabakoff B, Todd RD, Zhou Z, Goldman D. Addictions biology: haplotype-based analysis for 130 candidate genes on a single array. Alcohol Alcohol. 2008;43:505–515. doi: 10.1093/alcalc/agn032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hungund BL, Szakall I, Adam A, Basavarajappa BS, Vadasz C. Cannabinoid CB1 receptor knockout mice exhibit markedly reduced voluntary alcohol consumption and lack alcohol-induced dopamine release in the nucleus accumbens. J Neurochem. 2003;84:698–704. doi: 10.1046/j.1471-4159.2003.01576.x. [DOI] [PubMed] [Google Scholar]
- Ioannidis JP, Ntzani EE, Trikalinos TA, Contopoulos-Ioannidis DG. Replication validity of genetic association studies. Nat Genet. 2001;29:306–309. doi: 10.1038/ng749. [DOI] [PubMed] [Google Scholar]
- Iwasaki S, Ishiguro H, Higuchi S, Onaivi ES, Arinami T. Association study between alcoholism and endocannabinoid metabolic enzyme genes encoding fatty acid amide hydrolase and monoglyceride lipase in a Japanese population. Psychiatr Genet. 2007;17:215–220. doi: 10.1097/YPG.0b013e32809913d8. [DOI] [PubMed] [Google Scholar]
- Kathuria S, Gaetani S, Fegley D, Valino F, Duranti A, Tontini A, Mor M, Tarzia G, La Rana G, Calignano A, Giustino A, Tattoli M, Palmery M, Cuomo V, Piomelli D. Modulation of anxiety through blockade of anandamide hydrolysis. Nat Med. 2003;9:76–81. doi: 10.1038/nm803. [DOI] [PubMed] [Google Scholar]
- Li D, Zhao H, Gelernter J. Strong association of the alcohol dehydrogenase 1B gene (ADH1B) with alcohol dependence and alcohol-induced medical diseases. Biol Psychiatry. 2011;70:504–512. doi: 10.1016/j.biopsych.2011.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CS, Pollock NK, Bukstein OG, Lynch KG. Inter-rater reliability of the SCID alcohol and substance use disorders section among adolescents. Drug Alcohol Depend. 2000;59:173–176. doi: 10.1016/s0376-8716(99)00119-2. [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- Morita Y, Ujike H, Tanaka Y, Uchida N, Nomura A, Ohtani K, Kishimoto M, Morio A, Imamura T, Sakai A, Inada T, Harano M, Komiyama T, Yamada M, Sekine Y, Iwata N, Iyo M, Sora I, Ozaki N, Kuroda S. A nonsynonymous polymorphism in the human fatty acid amide hydrolase gene did not associate with either methamphetamine dependence or schizophrenia. Neurosci Lett. 2005;376:182–187. doi: 10.1016/j.neulet.2004.11.050. [DOI] [PubMed] [Google Scholar]
- NIAAA. NIAAA council approves definition of binge drinking. NIAAA newsletter. 2004:3. [Google Scholar]
- Parsons LH, Hurd YL. Endocannabinoid signalling in reward and addiction. Nat Rev Neurosci. 2015;16:579–594. doi: 10.1038/nrn4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecina M, Martinez-Jauand M, Hodgkinson C, Stohler CS, Goldman D, Zubieta JK. FAAH selectively influences placebo effects. Mol Psychiatry. 2014;19:385–391. doi: 10.1038/mp.2013.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinert DF, Allen JP. The alcohol use disorders identification test: an update of research findings. Alcohol Clin Exp Res. 2007;31:185–199. doi: 10.1111/j.1530-0277.2006.00295.x. [DOI] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption--II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- Sipe JC, Chiang K, Gerber AL, Beutler E, Cravatt BF. A missense mutation in human fatty acid amide hydrolase associated with problem drug use. Proc Natl Acad Sci U S A. 2002;99:8394–8399. doi: 10.1073/pnas.082235799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell L, Sobell M. Timeline Followback: A Technique for Assessing Self Reported Ethanol Consumption. Vol. 17. Totowa, NJ: Humana Press; 1992. [Google Scholar]
- Sobell LC, Brown J, Leo GI, Sobell MB. The reliability of the Alcohol Timeline Followback when administered by telephone and by computer. Drug Alcohol Depend. 1996;42:49–54. doi: 10.1016/0376-8716(96)01263-x. [DOI] [PubMed] [Google Scholar]
- Solinas M, Justinova Z, Goldberg SR, Tanda G. Anandamide administration alone and after inhibition of fatty acid amide hydrolase (FAAH) increases dopamine levels in the nucleus accumbens shell in rats. J Neurochem. 2006;98:408–419. doi: 10.1111/j.1471-4159.2006.03880.x. [DOI] [PubMed] [Google Scholar]
- Spagnolo PA, Ramchandani VA, Schwandt ML, Kwako LE, George DT, Mayo LM, Hillard CJ, Heilig M. FAAH gene variation moderates stress response and symptom severity in patients with post-traumatic stress disorder and comorbid alcohol dependence. Alcoholism: Clinical and Experimental Research. doi: 10.1111/acer.13210. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stella N, Schweitzer P, Piomelli D. A second endogenous cannabinoid that modulates long-term potentiation. Nature. 1997;388:773–778. doi: 10.1038/42015. [DOI] [PubMed] [Google Scholar]
- Sullivan PF. Spurious genetic associations. Biol Psychiatry. 2007;61:1121–1126. doi: 10.1016/j.biopsych.2006.11.010. [DOI] [PubMed] [Google Scholar]
- Tyndale RF, Payne JI, Gerber AL, Sipe JC. The fatty acid amide hydrolase C385A (P129T) missense variant in cannabis users: studies of drug use and dependence in Caucasians. Am J Med Genet B Neuropsychiatr Genet. 2007;144b:660–666. doi: 10.1002/ajmg.b.30491. [DOI] [PubMed] [Google Scholar]
- Vinod KY, Sanguino E, Yalamanchili R, Manzanares J, Hungund BL. Manipulation of fatty acid amide hydrolase functional activity alters sensitivity and dependence to ethanol. J Neurochem. 2008;104:233–243. doi: 10.1111/j.1471-4159.2007.04956.x. [DOI] [PubMed] [Google Scholar]
- Vinod KY, Yalamanchili R, Xie S, Cooper TB, Hungund BL. Effect of chronic ethanol exposure and its withdrawal on the endocannabinoid system. Neurochem Int. 2006;49:619–625. doi: 10.1016/j.neuint.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Wang L, Liu J, Harvey-White J, Zimmer A, Kunos G. Endocannabinoid signaling via cannabinoid receptor 1 is involved in ethanol preference and its age-dependent decline in mice. Proc Natl Acad Sci U S A. 2003;100:1393–1398. doi: 10.1073/pnas.0336351100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Huang T, Lee F, Kreek MJ. Involvement of Endocannabinoids in Alcohol “Binge” Drinking: Studies of Mice with Human Fatty Acid Amide Hydrolase Genetic Variation and After CB1 Receptor Antagonists. Alcohol Clin Exp Res. 2016;40:467–473. doi: 10.1111/acer.12989. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.