Abstract
Background
Increased cardiometabolic risk (CMR) is documented in obese and non-obese adolescents with low muscular fitness. However, the association of low muscle mass (LMM) with CMR, independent of weight status, has not been examined. We analyzed the relationship of LMM with CMR in adolescents, regardless of their weight status.
Methods
Observational study in 660 adolescents. BMI, waist circumference (WC), arterial blood pressures (ABP) were measured. Total fat mass (TFM), total lean tissue (TLT) and appendicular skeletal muscle mass (ASM) were estimated (DXA). Fasting lipid profile, glucose, and insulin were measured. HOMA-IR was estimated. Metabolic Syndrome (MetS) was diagnosed (AHA/NHLBI/IDF). ROC analysis was performed to find the optimal cutoffs of TLT percentage for MetS diagnosis. Values below these cutoffs defined LMM. ANCOVA examined the association of LMM with selected cardiometabolic biomarkers.
Results
In both sexes, TLT showed better sensitivity and specificity than ASM for MetS diagnosis. In males and females, TLT of 66.1% and 56.3%, respectively, were the optimal cutoff for MetS diagnosis. In the sample, 17.3% of males and 23.7% of females had LMM. In both sexes, adolescents with LMM had significantly higher values of WC, ABP, TG, TC/HDL, HOMA-IR and MetS z-score than non-LMM participants. Adolescents with LMM, regardless nutritional status, had significantly increased values of MetS z-score, ABP, TG, TC/HDL-chol and HOMA-IR than non-obese non-LMM adolescents. Adolescents having both obesity and LMM had the unhealthiest CMR profile.
Conclusion
In adolescents, LMM was associated with higher CMR, regardless of nutritional status. In obese adolescents, LMM increased obesity-associated CMR.
Keywords: low lean mass, obesity, cardiometabolic risk, metabolic syndrome, adolescents
Introduction
Metabolic syndrome (MetS) in children and adolescents, which includes abdominal obesity, high blood pressure, triglycerides, low HDL-chol and fasting hyperglycemia, is associated with greater risk of type-2 diabetes mellitus (T2DM) and ischemic heart disease later in life [1-3] MetS is more prevalent in overweight children and adolescents (29.2%) compared to the overall population (3.3%) [4], partly because abdominal obesity is a requirement for MetS diagnosis according to the AHA/NHLBI/IDF definition [5]. However, not all obese children and adolescents suffer from MetS and, according to some evidence, leg fat mass would be protective against cardio-metabolic risk and insulin resistance (IR) [6]. Similarly, increased cardiovascular and metabolic risk has been documented in obese and non-obese adolescents with low muscular fitness [4,7]. In young adults, low lean mass (LMM) has been associated with IR and MetS, regardless of weight status [8,9].
The key role of muscle mass in health and disease has been widely recognized [10,11]. Lean mass plays a pivotal role in the maintenance of metabolic homeostasis. Skeletal muscle is the largest insulin-sensitive and primary tissue for glucose and triglyceride metabolism. It also has a role in inflammation, contributing to energy homeostasis and the pathogenesis of obesity, T2DM, and other diseases [11]. Additionally, muscle mass is the primary reservoir for amino acids to maintain protein synthesis in vital tissues and organs [12]. Last, muscular strength has been recognized in the pathogenesis and prevention of chronic diseases, due to inverse association with adiposity gains as well as risk of hypertension, T2DM and prevalence and incidence of MetS [13].
In younger populations, muscular fitness has been inversely related to IR, clustered cardiometabolic risk and pro-inflammatory proteins [7,14]. Furthermore, physical fitness and scheduled exercise have been found to be associated with improved glycemic control in children with type-1 diabetes. (15). Knowing the importance of muscular fitness in the maintenance of metabolic homeostasis, the International Society for Pediatric and Adolescent Diabetes (ISPAD), launched the Clinical Practice Consensus Guidelines 2014, with precise indications for the practice of both muscular and aerobic exercise in children and adolescents with type-1 diabetes mellitus (16).
In healthy male and female adolescents from Chile, relative sarcopenia showed a strong positive association with the risk of MetS, independent of other influences [17]. According to the 2015 physical activity evaluation part of the System for the Assessment of Educational Quality (SIMCE), a national standardized test administered to all 8th grade Chilean students, 81% of females and 76% of males had impaired muscle functioning [18]. Consistent with these numbers, the 2012 Global School-based Student Health Survey showed that the percentage of 13-15 and 16-17 year-old Chilean adolescents who were physically active for a total of at least 60 min/day on all seven days was 15% and 12%, respectively [19]. Physical inactivity is an important determinant of a poor muscle development.
This research aims to assess the relation of LMM with cardiometabolic risk (CMR) in apparently healthy adolescents, regardless of weight status. We first determined the optimal cutoff value of muscle mass for MetS diagnosis and, then, examined whether LMM assessed by using this cutoff related to a number of cardiometabolic biomarkers in this population. We hypothesized that LMM would be associated with increased cardiometabolic risk in adolescents with and without obesity.
Methods
Study design and population
We studied 678, 16- to 17-year-old adolescents living in Santiago, Chile, from low-to-middle socioeconomic status (SES), who were part of a follow-up study beginning in infancy. The infants, recruited at 4 months, were healthy, full-term singletons and weighing ≥3 kg at birth. They were assessed for developmental outcomes in infancy, 5, 10 and 16y [20]. At 16 years, they were also assessed for obesity risk and the presence of cardiovascular risk factors in a half-day evaluation that included body fat and lean mass measurement [17]. Of 678 participants who were assessed for obesity/cardiovascular risk, 660 had complete data and were eligible for this study. Ethical approval was obtained by the IRBs of the University of Michigan, Institute of Nutrition and Food Technology (University of Chile), and the University of California, San Diego. Informed and written consent was provided according to the norms for Human Experimentation, Code of Ethics of the World Medical Association (Declaration of Helsinki, 1995).
Measurements
A research physician used standardized procedures to measure, in duplicate, adolescent height (cm) to the nearest 0.1 cm, using a Holtain stadiometer, and weight (kg) to the nearest 0.1 kg, using a Seca scale (SECA 703, Seca GmbH & co. Hamburg, Germany). Waist circumference (WC) was measured with non-elastic flexible tape and recorded to 0.1 cm (Seca 201, Seca GmbH & co. Hamburg, Germany). Measurements were taken twice, with a third measurement if the difference between the first two exceeded 0.3 kg for weight, 0.5 cm for height and 1.0 cm for waist. Body-Mass Index, BMI (Kg/m2) was calculated and obesity status was evaluated according to 2007 WHO references (BMI ≥ 2.0 SD). Pubertal maturation was assessed by using Marshall and Tanner criteria for breast and genital stage in females and males, respectively. Total lean tissue (TLT) and appendicular skeletal muscle mass (ASM) and total fat mass (TFM) were determined on dual X-ray absorptiometry (DXA) apparatus, Lunar Prodigy Corp., Madison, WI, USA, and software Lunar iDXA ENCORE 2011, Version 13.60.033 Copyright © 1998-2010.
After 15 minutes at rest and before other physical evaluations, systolic and diastolic blood pressures (SBP and DBP) were measured three times on the non-dominant arm using a standard mercury sphygmomanometer; the average value was used for analyses. Fasting serum total glucose (Gli), total cholesterol (TC), triglycerides (TG), high-density lipoprotein (HDL), and insulin levels were assessed after a 12-hour overnight fast. Radioimmunoassay (RIA DCP Diagnostic Products Corporation LA, USA) was used for insulin determination. Gli was measured with enzymatic-colorimetric test (QCA SA, Amposta, Spain) and cholesterol profile was determined with dry analytical methodology (Vitros, Johnson & Johnson, Clinical Diagnostics Inc).
Definition of low lean mass
Total lean tissue (TLT) was expressed as the percentage of muscle mass relative to the sum of muscle and fat mass {100 × muscle mass (kg)/[muscle mass (kg) + fat mass (kg)]} [21]. ASM was calculated by sum of muscle mass in arms and legs (kg) and expressed as percentage of body weight (ASM/Weight). ROC analysis was performed to find the optimal cutoff values of TLT ASM percentage for MetS diagnosis in males and females. The muscle indicator with the highest sensitivity and specificity for MetS diagnosing was used to evaluate LMM status of participants in the sample. The optimal cutoff value of TFM for MetS diagnosis was also estimated with ROC analysis.
Definition of cardiovascular risk and MetS
Atherogenic index (TC/ HDL-chol) and HOMA-IR (the product of fasting glucose (mmol/L) and insulin (μU/mL) divided by the constant 22.5) were estimated. HOMA-IR values ≥2.6 were considered IR, according to a previous work [22]. MetS was diagnosed based on the 2009 AHA/NHLBI/IDF Joint Interim Statement [5]. These criteria include having three of the following cardiometabolic biomarkers: abdominal obesity (WC ≥ 80 and 90 cm in females and males, respectively), high blood arterial pressure (SBP ≥130 mmHg, DBP ≥85 mmHg), hypertriglyceridemia (TG ≥150 mg/dL), low HDL (≤50 and ≤40 mg/dL in females and males, respectively), and fasting hyperglycaemia (Gli ≥100 mg/dl). A continuous score representing a composite cardio-metabolic risk factor profile was computed with gender-specific z-scores of WC, SBP, Gli, TG and HDL, according to previous work. [23]. A lower composite metabolic risk score denotes a healthier cardio-metabolic profile.
Other covariates
Information on breastfeeding (BF) duration was gathered in infancy by maternal self-report. BF as the sole source of milk for less than 90 days or 3 mo, was considered short BF duration. Anthropometric assessments in infancy early childhood (1y and 5y) were performed with standardized procedures [20]. Obesity status in infancy was determined according to the WHO 2006 references. Participants diagnosed with obesity at 1y and 5y were regarded as having early onset obesity.
Statistical analysis
All variables were checked for distribution normality (Shapiro-Wilk test) before the analysis. WC, SBP, DBP, HOMA-IR, TG, HDL and TC/HDL were normalized by natural logarithm transformation. Statistical analysis was conducted using transformed data, but untransformed data are presented here for ease of interpretation. Student's t test was used for comparison of mean values of anthropometric and cardio-metabolic variables according to LMM status in both sexes. Pearson Chi-square analysis assessed differences in the prevalence of CMR factors and MetS in the overall sample, according to weight- and LMM status. Participants were divided into four groups according to the presence of obesity and LMM: non-obese non-LMM, non-obese with LMM, obese non-LMM and obese with LMM. To examine the association of obesity, LMM and obesity with LMM (obesity*LMM) with selected biomarkers of cardio-metabolic risk including MetS, we conducted analysis of covariance (ANCOVA) after adjusting for sex (model 1). A second model additionally controlled for early onset obesity and breastfeeding duration to examine whether the relation of obesity, LMM and obesity*LMM with the cardio-metabolic profile of apparently health adolescents was independent of these influences. Post hoc analyses were conducted with Bonferroni correction to assess further differences between groups. A P value of <0.05 denoted statistical significance. Data were analyzed using Stata for Windows version 13.0 (Lakeway Drive College Station, TX, US).
Results
Of 678 adolescents enrolled in the obesity and cardiovascular study, 660 had complete data and entered the analysis. When comparisons of characteristics were made between these two groups, no differences were found in age, BMI and cardiometabolic biomarkers (data available from corresponding author).
Our sample included 52.2% male and 47.8% female adolescents with completed pubertal development (Tanner 5), who were on average 16.8 (SD 0.3) years old. The prevalence of obesity was 16.4% and 9.7% met criteria for MetS.
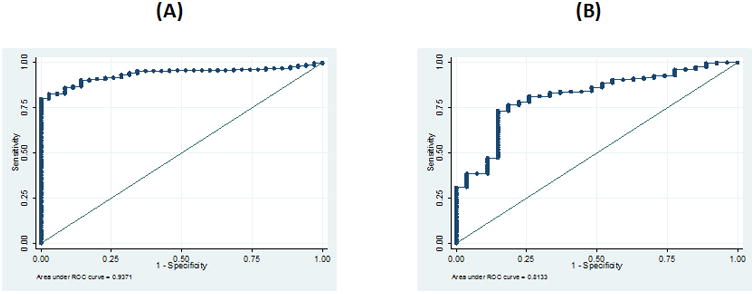
In both sexes, TLT showed better AUC and LHR+ than ASM and TFM for MetS diagnosis (Table 1), suggesting that the likelihood of finding adolescents with MetS is higher in adolescents with low TLT compared to adolescents with low ASM or high TFM. In males, a TLT value of 66.1% showed the highest sensitivity (85.4%) and specificity (96.7%) for diagnosing MetS (AUC: 0.941; correctly classified: 86.4%; LR+: 25.6) (Figure 1A). In females, a TLT of 56.3% had the best sensitivity (73.4%) and specificity (85.2%) for MetS diagnosis (AUC: 0.813; correctly classified: 74.4%; LR+: 5.0) (Figure 1B). Based on these cutoff limits, 17.3% of males and 23.7% of females in our sample had LMM.
Table 1. Sensitivity and specificity of Total Lean Tissue, Appendicular Skeletal Muscle and Total Fat Mass for Metabolic Syndrome diagnosis.
| Cut-off | Sensitivity | Specificity | Correctly Classified (%) | LHR+ | ROC Area | 95% CI | |
|---|---|---|---|---|---|---|---|
| Males | |||||||
| TLT (%) | 66.1 | 85.4 | 96.7 | 86.4 | 25.6 | 0.941 | 0.92-0.97 |
| ASM (%) | 31.2 | 86.2 | 80.0 | 85.6 | 4.3 | 0.919 | 0.87-0.95 |
| TFM (%) | 28.9 | 100.0 | 81.7 | 83.0 | 5.5 | 0.940 | 0.92-0.97 |
| Females | |||||||
| TLT (%) | 56.3 | 73.4 | 85.2 | 74.4 | 5.0 | 0.813 | 0.73-0.89 |
| ASM (%) | 25.2 | 76.9 | 81.5 | 77.3 | 4.2 | 0.809 | 0.73 -0.88 |
| TFM (%) | 40.9 | 85.2 | 76.6 | 77.3 | 3.6 | 0.810 | 0.74-0.89 |
TLM: Total Lean Mass. ASM: Appendicular Skeletal Muscle. TFM: Total Fat Mass.
Fig 1. ROC curve to determine the optimal value of lean tissue (%) for Metabolic Syndrome diagnosis in male (A) and female (B) adolescents.
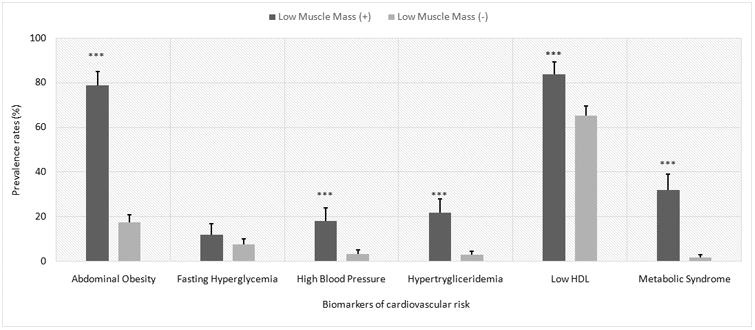
As shown in Table 2, in both sexes, adolescents with LMM had significantly higher values of BMI z-score, WC, SBP, DBP, TG, TC/HDL-chol, insulin, HOMA-IR and MetS z-score. Likewise, they had significantly lower mean values of HDL. Prevalence of abdominal obesity, high BP, hypertriglyceridemia, low HDL-chol and MetS was significantly higher in male and female adolescents with LMM (Figure 2).
Table 2. Anthropometric and cardiometabolic profile, by sex and muscle mass status.
| Male adolescents | Female adolescents | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| Variable | LMM (-) (n=271) | LMM (+) (n=76) | P value | LMM (-) (n=214) | LMM (+) (n=99) | P value |
|
|
||||||
| Age (years) | 16.8 ± 0.3a | 16.8 ± 0.3 | .96 | 16.8 ± 0.3a | 16.8 ± 0.3 | |
| Body Mass Index (z-score) | 0.14 ± 0.94 | 2.11± 0.74 | <.001 | 0.18 ± 0.90 | 1.91± 0.76 | <.001 |
| Body Mass Index (kg/m2) | 21.8±2.7 | 29.6±4.1 | <.001 | 21.9±2.8 | 29.3±4.5 | <.001 |
| Fat Mass (%) | 18.8 ± 5.9 | 35.5 ± 3.6 | <.001 | 32.3 ± 5.2 | 44.9 ± 3.4 | <.001 |
| Total Lean Mass (%) | 78.2 ± 6.0 | 61.2 ± 3.7 | <.001 | 63.9 ± 5.1 | 51.2 ± 3.4 | <.001 |
| Waist circumference (cm) | 77.0±6.5 | 96.3±11.0 | <.001 | 75.9±8.0 | 92.4±11.0 | <.001 |
| Systolic Blood Pressure (mmHg) | 113.7±9.5 | 121.3±11.3 | <.001 | 106.2±8.2 | 114.3±10.5 | <.001 |
| Diastolic Blood Pressure (mmHg) | 70.2±6.9 | 73.9±7.0 | <.001 | 66.3±6.4 | 70.3±6.5 | <.001 |
| Triglycerides G (mg/dl) | 76.4±38.3 | 128.8±68.6 | <.001 | 81.9±41.2 | 100.6±57.0 | .001 |
| High Density Lipoprotein (mg/dl) | 39.4±10.2 | 32.9±8.0 | <.001 | 43.4±11.1 | 40.3±9.5 | .019 |
| Atherogenic Index | 3.9±1.1 | 5.1±1.7 | <.001 | 3.8±1.1 | 4.2±1.3 | .006 |
| Glucose (mg/dl) | 89.9±8.5 | 92.5±12.6 | .04 | 86.1±8.7 | 87.3±9.6 | .28 |
| Insulin (μUI/ml) | 6.5±3.7 | 12.8±8.3 | <.001 | 7.2±3.8 | 11.0±7.0 | <.001 |
| HOMA-IR | 1.4±0.9 | 3.0±2.1 | <.001 | 1.5±0.9 | 2.4±1.7 | <.001 |
| Metabolic Syndrome (z score) | -0.09±0.4 | 0.56±0.4 | <.001 | -0.15±0.5 | 0.33±0.5 | <.001 |
| Early onset obesity (≤5y) | 29(10.7%)b | 42(55.3%) | <.001 | 14(6.5%) | 36(36.4%) | <.001 |
| Breast feeding ≤3 mo | 123(45.4%) | 33(43.4%) | NS | 75(35.1%) | 46.(46.5%) | .046 |
Mean ± SD.
Number (%).
LMM: Low muscle mass
Fig 2. Prevalence rates of cardiovascular risk factors and MetS by presence of low muscle mass in both sexes.
When LMM status was cross-tabulated with obesity status, we found that 72% of participants were non-obese non-LMM, 11.7% were non-obese with LMM, 1.5% had obesity without LMM, and 15.9% had both LMM and obesity. Differences for sex were observed among non-obese participants only.
Table 3 shows the association of obesity, LMM and obesity*LMM with several measures of cardiovascular risk. After accounting for the effect of female sex, early obesity and short breastfeeding, we found that non-obese adolescents with LMM had higher CMR than non-obese non-LMM participants (reference group). They had significantly higher values of WC (+9.3 cm), SBP (+3.4 mmHg), TG (+14.6 mg/dL), TC/HDL (+0.41), HOMA-IR (+0.32) and MetS z-score (+0.37 SD). Compared to the reference group, participants having obesity without LMM had significantly higher values of WC (+12.8) and MetS z-score (+0.42 DE) only. Other CMR factors were increased in this group, but the association was not significant. Last, adolescents with both obesity and LMM showed the greatest deterioration in their cardiovascular profile. Compared to the reference category, they had significantly increased values of WC (+21.1 cm), SBP (+10.3 mmHg), DBP (+4.24 mmHg), TG (+51.6 mg/dL), TC/HDL (+0.99), HOMA-IR (+1.7) and MetS z-score (+0.72 SD), along with significantly lower values of HDL (-5.8 mg/dL).
Table 3. Estimated regression coefficients examining the association of obesity, LMM and obesity with LMM with cardiovascular risk.
| Non-obese non-LMM§ | Non-obese with LMM | Obese without LMM | Obese with LMM | |||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Intercept | P | Coeff. | P | Coeff. | P | Coeff. | P | |
|
|
||||||||
| Model 1a | ||||||||
|
|
||||||||
| MetS (z score) | -0.089 | <.001 | 0.344 | <.001 | 0.524 | <.001 | 0.771 | <.001 |
| WC (cm) | 76.68 | <.001 | 10.45 | <.001 | 16.49 | <.001 | 24.15 | <.001 |
| SBP (mm Hg) | 113.45 | <.001 | 3.72 | <.001 | 4.22 | .16 | 11.24 | <.001 |
| DBP (mm Hg) | 69.92 | <.001 | 1.69 | .04 | 3.93 | .06 | 4.81 | <.001 |
| TG (mg/dL) | 79.38 | <.001 | 14.36 | .01 | 17.48 | .24 | 50.59 | <.001 |
| HDL (m/dL) | 39.19 | <.001 | -2.66 | .04 | -5.71 | .08 | -6.38 | <.001 |
| TC/HDL | 3.97 | <.001 | 0.46 | .002 | 0.62 | .11 | 1.07 | <.001 |
| HOMA-IR | 1.48 | <.001 | 0.41 | .004 | 0.70 | .06 | 1.77 | <.001 |
|
|
||||||||
| Model 2b | ||||||||
|
|
||||||||
| MetS (z score) | -0.109 | <.001 | 0.32 | <.001 | 0.46 | .001 | 0.72 | <.001 |
| WC (cm) | 75.68 | <.001 | 9.32 | <.001 | 12.82 | <.001 | 21.11 | <.001 |
| SBP (mm Hg) | 113.39 | <.001 | 3.44 | <.001 | 3.22 | .29 | 10.33 | <.001 |
| DBP (mm Hg) | 69.87 | <.001 | 1.50 | .13 | 3.30 | 0.07 | 4.24 | <.001 |
| TG (mg/dL) | 78.84 | <.001 | 14.57 | .013 | 18.46 | .23 | 51.59 | <.001 |
| HDL (m/dL) | 40.28 | <.001 | -2.23 | .08 | -4.64 | .16 | -5.78 | <.001 |
| TC/HDL | 3.83 | <.001 | 0.41 | .007 | 0.47 | .23 | 0.99 | <.001 |
| HOMA-IR | 1.42 | <.001 | 0.37 | .01 | 0.58 | .13 | 1.69 | <.001 |
Obesity: BMI z > 2 SD for age and sex. Sarcopenia: Total Lean Mass (TLM) ≤ 66.1% in males and ≤ 56.3% in females.
Model 1 adjusted for sex (female=1).
Model 2 adjusted for sex, early onset obesity (≤5y), and short maternal breast feeding (≤3 mo).
Non-obese non-sarcopenic participants are the reference group. Coefficients are the mean difference between a given category and the reference group.
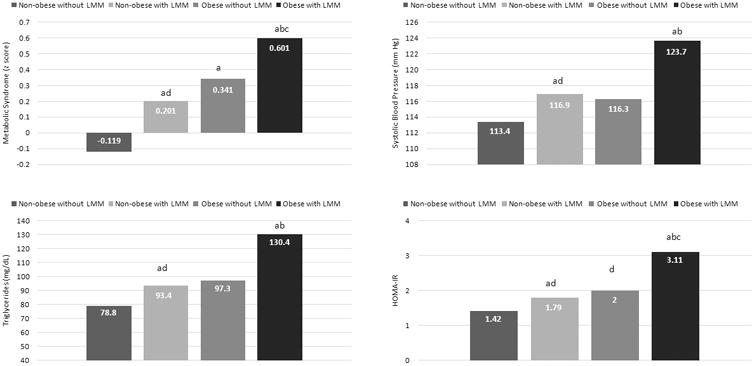
A comparison of the cardiometabolic profile between categories showed that adolescents with LMM, regardless of obesity status, had significantly higher values of MetS z-score, SBP, TG, and HOMA-IR (Figures 3) compared with the reference group. Second, non-obese participants with LMM did not differ from participants having obesity without LMM in terms of CMR. Third, adolescents having both obesity and LMM were the group with the unhealthiest CMR profile.
Fig 3. Association of obesity and low lean mass (LMM) with cardiovascular risk in 16 year-old adolescents (n=660).
Discussion
This study analyzed the optimal cutoff value of lean tissue for MetS diagnosis in adolescents of both sexes, in order to obtain a definition of low lean mass based on biological risk. TLT showed higher sensitivity and specificity than ASM for Mets diagnosis, especially in male adolescents. The optimal cutoff was 66.1% in males and 56.3% in females.
We further examined the association of LMM with the cardiometabolic profile of apparently healthy male and female adolescents, including: CMR factors, HOMA-IR and MetS. A positive and significant association was found between LMM and most of these parameters. Very few studies have approached the link of LMM and cardiovascular risk in children and adolescents, and their results are consistent with ours. In a representative U.S. sample aged 8 to 20 years (NHANES 1999-2004), a negative and significant relationship between muscle mass and seven CMR factors was reported [21]. In this study, as in ours, muscle mass was evaluated as a percentage of muscle mass relative to the sum of muscle and fat mass, using DXA. Values at or below 64.3% (those into the 1st quartile) defined sarcopenia in both males and females. Although Kim and Valdez [21] used the same cutoff limit for sarcopenia diagnosis in both sexes and this cutoff was estimated based on statistical criteria, the prevalence of low lean mass in US children and adolescents was similar to that observed in our sample. Another study in young males from South Korea, the prevalence of MetS, high waist circumference, high triglycerides, and high blood pressure was significantly greater in the low muscle mass group than in the high muscle mass group [10]. In US adolescents from the Third National Health and Nutrition Examination Survey (NHANES III), the prevalence of MetS in non-Hispanic blacks was significantly lower (2.5%) compared to Mexican-Americans (12.9%) and non-Hispanic whites (10.9%) [24]. In this population of adolescents, the Lean Mass/Height2 Index was higher in non-Hispanic blacks than Non-Hispanic white and Mexican-Americans [25]. These numbers are consistent with those found in adults from the NHANES III, and support the idea of a negative relationship between muscle mass and the prevalence of MetS [26].
Finally we assessed the association of LMM, obesity without LMM and obesity with LMM with MetS and its components. In our sample, LMM (with and without obesity) was significantly associated with higher MetS z-score and higher values of WC, SBP, DBP, TG, TC/HDL and HOMA-IR. Also with lower values of HDL-chol. It is worth noting that, adolescents with LMM, regardless of obesity status, had increased values of MetS z-score, SBP, TG, TC/HDL-chol and HOMA-IR. Other studies have reported similar results. In non-obese Korean youths, the prevalence of MetS and cardiovascular risk factors was significantly greater in the group with LMM compared to the group with high muscle mass [9]. Among the non-obese subjects, the risk of having MetS was 3.6 times higher in adolescents with LMM compared to adolescents with high muscle mass. However, in obese participants, no significant associations of LMM with MetS and its components was found in females, whereas in males the association was lost after adjusting for WC. In this study, ASM as percentage of total body weight was used for assessment of lean mass. This methodology might be less sensitive to estimate muscle tissue in obese participants. Another study conducted in an adult US population (NANHES III) showed that reduced muscle mass (referred to as sarcopenia by the authors) is associated with IR and adverse glucose metabolism, independent of obesity status [8]. The association was stronger in the group < 60 years of age, which suggests that: (1) LMM might be an early predictor of diabetes susceptibility; and (2) reduced muscle mass might exacerbate obesity-associated IR and dysglycemia.
All this evidence should be of no surprise, since inadequate muscle function influences the genesis of both obesity and IR. Myokines, the hormones secreted by the skeletal muscle, mediates the communications of muscle with liver, adipose tissue, the brain and other organs. It also has profound effects on glucose and lipid metabolism and inflammation, contributing to energy homeostasis and the pathogenesis of obesity, diabetes and other diseases [11]. Reduced muscle mass is associated to impaired insulin signaling and action in the skeletal muscle, contributing to the expansion of adipose tissue, increasing the levels of inflammatory adipokines, enhancing the activity of the renin-angiotensin-aldosterone system (RAAS) activity, increasing the accumulation of intramuscular lipids and reactive oxygen species, and decreasing the muscle mitochondrial oxidative capacity [27]. While greater muscle mass optimizes metabolic function, increasing fat oxidation and glucose uptake and, thereby, the total energy expenditure rate, reduced muscle mass decreases these metabolic functions, favoring IR and the imbalance between caloric intake and expenditure [9]. Furthermore, the impact of low muscle mass on metabolic risk in young subjects is also expected. Type II muscle fibers, which have less of an effect on metabolic actions of insulin than type I muscle fibers, are mostly lost as a result of age-related muscle atrophy [28]. However, type I muscle fibers, which exert a recognized metabolic action that increases with exercise, are significantly reduced with physical inactivity, regardless of age [29,30]. In all age groups physical inactivity levels are growing fast in both developed and developing countries. In this sample, a previous study showed that 39.7% of adolescents had <2 h/week of scheduled exercise (Physical Education and extracurricular sport activities) [22]. Adolescence is a period in which physical activity shows a great decline and this has been associated with higher rates of overweight/obesity [31-33]. In Chile, sedentary behaviors seem to be the rule among adolescents. Population-based study carried out in high schoolers from the Santiago Metropolitan Region reported that 80% of students devote less than 2 h/week to scheduled exercise [34,35]. Another study in 16-year-old Chilean adolescents found that males and females in the lowest quartile of physical activity had higher risk of obesity, abdominal obesity, and MetS [36].
Limitations and strengths
This research has some limitations that should be considered when interpreting results. Our sample is not representative of the Chilean adolescent population, as it was made up of adolescents from low to middle SES only. However, our findings may be equally relevant for a number of reasons. According to the Chilean National Health Survey, the prevalence of obesity, physical inactivity, T2DM, and cardiovascular risk factors is significantly higher in individuals from low- to middle SES [19]. Second, low- to middle SES Chilean adolescents are highly exposed to risk factors that increase the risk of MetS and non-communicable chronic diseases, such as obesity, reduced allocation of time to regular exercise, impaired muscle functioning and low aerobic capacity [17,19,34]. A further limitation is the cross-sectional nature of the study, which limits the ability to draw conclusions related to the temporality of these associations. Future studies should aim to longitudinally explore the link between muscle mass and CMR, which may become clearer over time. In spite of these limitations, this study makes two important contributions. First, it provides a definition of LMM in adolescents based on biological risk criteria. Second, these results confirm that reduced muscle mass should be considered a relevant risk factor for MetS in young populations regardless of obesity status.
Conclusion
In apparently healthy adolescents, LMM was associated with higher CMR regardless nutritional status. Furthermore, LMM increased cardiovascular risk associated with obesity. Especially in males, lean body mass might be a good predictor of MetS. Preserving muscle strength in younger age populations, through the practice of repetitive and planned exercise, may improve metabolic function and, thus, prevent early onset of degenerative chronic diseases.
Acknowledgments
Funding: This research was carried out with financial support from the National Heart Lung and Blood Institute (NHLBI), National Institutes of Health (USA), under grant R01HL088530-2980925 (PI: Gahagan). Dr. Correa-Burrows was sponsored by the Advanced Human Capital Program (grant code: 79140003), from the National Council for Scientific Research and Technology (CONICYT) (Chile).
Abbreviations
- CMR
Cardiometabolic risk
- LMM
Low muscle mass
- BMI
Body-mass Index
- WC
Waist Circumference
- SBP
Systolic Blood Pressure
- DBP
Diastolic Blood Pressure
- TFM
Total Fat Mass
- TLT
Total Lean Tissue
- ASM
Appendicular Skeletal Muscle
- MetS
Metabolic Syndrome
- T2DM
Type-2 Diabetes Mellitus
- Gli
Glucose
- TC
Total Cholesterol
- TG
Triglycerides
- HDL
High-density lipoprotein
Footnotes
Disclosures: The authors declared no conflict of interest.
Competing interests: The authors declare that they have no competing interests.
Authors' contributions: Conceived and designed the study: RB, SG. Performed the field study: RB, MR. Analyzed the data: RB, PC. Wrote the paper: RB, PC. Critical analysis of manuscript draft: SG, CA, EB, MR. Language editing: EB, SG. All authors read and approved the final manuscript.
References
- 1.Mattsson N, Rönnemaa T, Juonala M, Viikari JS, Raitakari O. Childhood predictors of the metabolic syndrome in adulthood. The Cardiovascular Risk in Young Finns Study. Ann Med. 2008;40(7):542–552. doi: 10.1080/07853890802307709. [DOI] [PubMed] [Google Scholar]
- 2.Bao W, Srinivasan SR, Wattigney WA, Berenson GS. Persistence of multiple cardiovascular risk clustering related to syndrome X from childhood to young adulthood. The Bogalusa Heart Study. Arch Intern Med. 1994;154(16):1842–1847. [PubMed] [Google Scholar]
- 3.Morrison JA, Friedman LA, Gray-McGuire C. Metabolic Syndrome In Childhood Predicts Adult Cardiovascular Disease 25 Years Later: The Princeton Lipid Research Clinics Follow-Up Study. Pediatrics. 2007;120(2):340–345. doi: 10.1542/peds.2006-1699. [DOI] [PubMed] [Google Scholar]
- 4.Friend A, Craig L, Turner S. The Prevalence of Metabolic Syndrome in Children: A Systematic Review of the Literature. Metab Synd Relat Dis. 2013;11(2):71–80. doi: 10.1089/met.2012.0122. [DOI] [PubMed] [Google Scholar]
- 5.Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 6.Samouda H, De Beaufort C, Stranges S, et al. Cardiometabolic risk: leg fat is protective during childhood. Pediatr Diabetes. 2016;17(4):300–308. doi: 10.1111/pedi.12292. [DOI] [PubMed] [Google Scholar]
- 7.Artero EG, Ruiz JR, Ortega FB, et al. Muscular and cardiorespiratory fitness are independently associated with metabolic risk in adolescents: the HELENA study. Pediatr Diabetes. 2011;12(8):704–712. doi: 10.1111/j.1399-5448.2011.00769.x. [DOI] [PubMed] [Google Scholar]
- 8.Srikanthan P, Hevener AL, Karlamangla AS. Sarcopenia Exacerbates Obesity-Associated Insulin Resistance and Dysglycemia: Findings from the National Health and Nutrition Examination Survey III. PLoSOne. 2010;5(5):e10805. doi: 10.1371/journal.pone.0010805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim BC, Kim MK, Han K, et al. Low muscle mass is associated with metabolic syndrome only in non-obese young adults: the Korea National Health and Nutrition Examination Survey 2008-2010. Nutr Res. 2015;35(12):1070–1078. doi: 10.1016/j.nutres.2015.09.020. [DOI] [PubMed] [Google Scholar]
- 10.Wolfe R. The underappreciated role of muscle in health and disease. Am J Clin Nutr. 2006;84(3):475–82. doi: 10.1093/ajcn/84.3.475. [DOI] [PubMed] [Google Scholar]
- 11.Ahima RS, Park HK. Connecting Myokines and Metabolism. Endocrinol Metab. 2015;30(3):235–45. doi: 10.3803/EnM.2015.30.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Biolo G, Zhang XJ, Wolfe RR. Role of membrane transport in interorgan amino acid flow between muscle and small intestine. Metabolism. 1995;44(6):719–24. doi: 10.1016/0026-0495(95)90183-3. [DOI] [PubMed] [Google Scholar]
- 13.Artero E, Lee D, Lavie C, et al. Effects of Muscular Strength on Cardiovascular Risk Factors and Prognosis. J Cardiopulm Rehabil Prev. 2012;32(6):351–358. doi: 10.1097/HCR.0b013e3182642688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia-Artero E, Ortega FB, Ruiz JR, et al. Lipid and metabolic profiles in adolescents are affected more by physical fitness than physical activity (AVENA Study) Rev Esp Cardiol. 2007;60(6):581–588. doi: 10.1157/13107114. [DOI] [PubMed] [Google Scholar]
- 15.Nguyen T, Obeid J, Walker RG, et al. Fitness and physical activity in youth with type 1 diabetes mellitus in good or poor glycemic control. Pediatr Diabetes. 2015;16(1):48–57. doi: 10.1111/pedi.12117. [DOI] [PubMed] [Google Scholar]
- 16.Robertson K, Riddell MC, Guinhouya BC, Adolfsson P, Hanas R. Exercise in children and adolescents with diabetes. Pediatric Diabetes. 2014;15(Suppl. 20):203–223. doi: 10.1111/pedi.12176. [DOI] [PubMed] [Google Scholar]
- 17.Burrows R, Correa P, Reyes M, Blanco E, Albala C, Gahagan S. High cardiometabolic risk in healthy Chilean adolescents: Association with anthropometric, biological and life style factors. Public Health Nutr. 2016;19(3):486–93. doi: 10.1017/S1368980015001585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ministry of Education. Informe de Resultados Nacionales de Educación Física 2015. Santiago de Chile, Chile: Ministry of Education; 2016. [Google Scholar]
- 19.Ministry of Health. Global Health School-based Survey. Department of Epidemiology; Santiago de Chile: Ministry of Health; 2013. Retrieved from: http://www.who.int/chp/gshs/2013_Chile_GSHS_fact_sheet.pdf. [Google Scholar]
- 20.Lozoff B, Castillo M, Clark K, Smith J, Sturza J. Iron supplementation in infancy contributes to more adaptive behavior at 10 years of age. J Nutr. 2014;144(6):838–45. doi: 10.3945/jn.113.182048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim S, Valdez R. Metabolic risk factors in U.S. youth with low relative muscle mass. Obes Res Clin Pract. 2015;9(2):125–132. doi: 10.1016/j.orcp.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burrows R, Correa P, Reyes M, Blanco E, Albala C, Gahagan S. Healthy Chilean adolescents with HOMA-IR ≥ 2.6 have increased cardio-metabolic risk: Association with genetic, biological and environmental factors. J Diab Res. 2015 doi: 10.1155/2015/783296. Article ID 783296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khuc K, Blanco E, Burrows R, et al. Adolescent metabolic syndrome risk is increased with higher infancy weight gain and decreased with longer breast feeding. Int J Pediatr. 2012;2012:478610. doi: 10.1155/2012/478610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Ferranti S, Gauvreau K, Ludwig D, Neufeld E, Newburger J, Rifai N. Prevalence of the metabolic syndrome in American adolescents. Circulation. 2004;110:2494–7. doi: 10.1161/01.CIR.0000145117.40114.C7. [DOI] [PubMed] [Google Scholar]
- 25.Kelly TL, Wilson KE, Heymsfield SB. Dual Energy X-Ray Absorptiometry Body Composition Reference Values from NHANES. PLoS ONE. 2009;4(9):e7038. doi: 10.1371/.0007038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 27.Stump CS, Henriksen EJ, Wei Y, Sowers JR. The metabolic syndrome: Role of skeletal muscle metabolism. Ann Med. 2006;38:389–402. doi: 10.1080/07853890600888413. [DOI] [PubMed] [Google Scholar]
- 28.Liu S, Jemiolo B, Lavin M, Lester B, Trappe S, Trappe T. Prostaglandin E2/cyclooxygenase pathway in human skeletal muscle: Influence of muscle fiber type and age. J App Physiol. 2015;120(5):546–51. doi: 10.1152/00396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nader G, Esser K. Intracellular signaling specificity in skeletal muscle in response to different modes of exercise. J Appl Physiol. 2001;90(5):1936–1942. doi: 10.1152/jappl.2001.90.5.1936. [DOI] [PubMed] [Google Scholar]
- 30.Luna MV, Daikoku E, Ono F. Slow skeletal muscles across vertebrate species. Cell Biosci. 2015;5:62. doi: 10.1186/s13578-015-0054-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koezuka N, Koo M, Allison K, et al. The Relationship between sedentary activities and physical inactivity among adolescents: results from the Canadian community health survey. J Adol Health. 2006;39(4):515–522. doi: 10.1016/j.jadohealth.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 32.Kahn JA, Huang B, Gillman MW, et al. Patterns and determinants of physical activity in US adolescents. J Adol Health. 2008;42(4):369–377. doi: 10.1016/j.jadohealth.2007.11.143. [DOI] [PubMed] [Google Scholar]
- 33.Kong IG, Lee HJ, Kim SY, Sim S, Choi HG. Physical Activity, Study Sitting Time, Leisure Sitting Time, and Sleep Time Are Differently Associated With Obesity in Korean Adolescents: A Population-Based Study. Medicine. 2015;94(44):e1965. doi: 10.1097/MD.0000000000001965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burrows R, Montoya A, Sciaraffia V, Gattas V, Lera L. Dietary intake and physical activity in school age children. Rev Med Chile. 2008;136(1):53–63. [PubMed] [Google Scholar]
- 35.Correa-Burrows P, Burrows R, Ibaceta C, Orellana Y, Ivanovic D. Physically active Chilean school kids perform better in language and mathematics. Health Promot Int. 2014 doi: 10.1093/heapro/dau010. [DOI] [PubMed] [Google Scholar]
- 36.Correa-Burrows P, Burrows R. Physical inactivity and obesity in Chilean youth: Individual costs in health-related choices. Health Educ J. 2013;73(6):657–667. [Google Scholar]


