Abstract
While epidemiological studies show that alcohol abuse is often comorbid with affective disorders, the causal direction of this association is unclear. We examined this relationship using mouse models including social defeat stress (SDS), social interaction (SI), and voluntary alcohol consumption. C57BL6/J mice exposed to SDS segregate into two subpopulations, those that express depressive-like phenotypes (“susceptible”), and those that do not (“resilient”). First, we stratified SDS-exposed mice and measured their voluntary alcohol consumption. Next, we determined if SI behavior in alcohol-naïve mice could predict alcohol intake. Finally, we assessed the effect of binge-like alcohol exposure on sensitivity to SDS. We quantified Tacr1 (neurokinin-1 receptor gene) and Avp (vasopressin peptide gene) mRNA in brain regions involved in depression, addiction, and social behavior. We found that susceptible mice consumed more alcohol compared to resilient mice suggesting that depression-like phenotypes associate with increased alcohol intake. Interestingly, we observed a negative correlation between SI and alcohol intake in stress- and alcohol-naïve mice, suggesting that individual differences in SI associate with alcohol preference. Finally, alcohol pretreatment increased sensitivity to SDS, indicating that alcohol exposure alters sensitivity to social stress. Quantification of mRNA revealed that increased expression of Tacr1 and Avp generally associated with decreased SI and increased alcohol intake. C57BL6/J mice are an inbred strain; thus it is likely that individual differences in behavior and gene expression are driven by epigenetic factors. Collectively, these results support a bidirectional relationship between alcohol exposure and susceptibility to stress that is associated with variations in neuropeptide expression.
Keywords: stress, alcohol, neurokinin-1 receptor, vasopressin, amygdala, nucleus accumbens
Introduction
Several epidemiological studies have found a significant comorbidity between alcohol abuse and affective disorders such as depression and anxiety (Boden & Fergusson, 2011; Grant & Harford, 1995). However, it is currently unclear if preexisting depression increases risk of alcohol dependence, if ongoing alcohol abuse increases the susceptibility to depression, or if the relationship between these two conditions is bidirectional. Additionally, depression and addiction are heterogeneous conditions, and the development of these disorders is attributed to the interaction of several factors, including an individual’s social environment, stress exposure, and underlying neurobiological predispositions (Boden & Fergusson, 2011). Within the human population, the relationship between these factors is difficult to assess as depression or alcohol abuse often goes undiagnosed for years prior to seeking treatment. Therefore it is difficult to pinpoint to what degree either condition precipitates the other and identify the environmental conditions or genetic factors that mediate this relationship.
Depression can be reliably modeled in preclinical research via the chronic social defeat stress (SDS) paradigm. This protocol induces depressive-like phenotypes in rodents, such as social avoidance and anhedonia (Golden, Covington, Berton, & Russo, 2011). These behavioral responses can be ameliorated with chronic, but not acute, antidepressant treatment, providing predictive validity (Berton et al., 2006). Similar to people that experience stress, not all animals that are exposed to SDS develop depressive phenotypes. Specifically, mice exposed to SDS segregate into two subpopulations, those that express depressive-like behaviors (“susceptible”) and those that are resistant to these effects (“resilient”; (Golden, et al., 2011)). Although social defeat experiments have been previously carried out in C57BL6/J mice, and this model has been heavily validated including the consistent observation of distinct subpopulations described above, this is an inbred strain and therefore behavioral variation in these mice is likely due to epigenetic alterations as opposed to genetic polymorphisms.
Although mice susceptible to SDS display increased cocaine intake compared to resilient mice (Bruchas et al., 2011; Covington et al., 2005; Covington & Miczek, 2005; Sun et al., 2016), no current studies have addressed these distinct phenotypes in relation to alcohol consumption. Indeed, the effect of SDS exposure on alcohol consumption is not entirely clear. Previous studies have observed an increase (Caldwell & Riccio, 2010; Croft, Brooks, Cole, & Little, 2005; Dong et al., 2011; Hwa, Holly, DeBold, & Miczek, 2016; Karlsson et al., 2016; Molander et al., 2012; Norman et al., 2015; Rodriguez-Arias et al., 2016), decrease (Funk, Harding, Juzytsch, & Le, 2005; Lopez, Anderson, & Becker, 2016; Norman, et al., 2015; van Erp & Miczek, 2001), or no change (Sillaber et al., 2002) in alcohol consumption after SDS exposure. The effect of SDS on alcohol intake may be influenced by a complex set of variables including duration/intensity of stress (Funk, et al., 2005; Karlsson, et al., 2016), procedure for measuring alcohol consumption (Croft, et al., 2005; Rodriguez-Arias, et al., 2016), age/species/strain/sex of animal (Rodriguez-Arias, et al., 2016; van Erp & Miczek, 2001), and time between SDS exposure and alcohol availability (Caldwell & Riccio, 2010; Norman, et al., 2015) . All studies to date have combined SDS-exposed mice into a single “stressed” group and have not stratified the subjects into susceptible or resilient subpopulations. In the experiments presented here we considered this distinction, which provides an opportunity to examine differences between resilient and susceptible individuals in response to social stress and propensity to consume alcohol. Furthermore, while the studies mentioned above assess the impact of social stress on subsequent alcohol consumption, the effect of prior alcohol exposure on SDS sensitivity has not been examined. This is an additional relationship that we will explore in these experiments.
Although much preclinical research has focused on the effects of stressors on depression and alcohol consumption, fewer studies have focused on identifying behavioral markers that predict sensitivity to these phenotypes. In our studies we found that social avoidance in stress-naïve mice correlated with alcohol consumption. These data suggest a behavioral marker that is predictive of future alcohol consumption. In turn, we observed that voluntary alcohol consumption positively predicted susceptibility to SDS. Specifically, mice that consumed more alcohol were more likely to be susceptible to defeat stress. Identifying underlying behaviors that predict alcohol consumption has great potential for guiding pharmacological studies in diverse populations of alcoholics that may differ in terms of psychiatric comorbidities. Furthermore, early identification of predisposing factors may allow for early intervention in susceptible individuals before alcohol dependence has taken root.
In addition to examining behavioral phenotypes underlying stress, depression, and alcohol abuse, we also aimed to identify molecular targets that facilitate the relationship between these factors. Two main targets of interest based on our preliminary findings were the neurokinin-1 receptor (NK1R) and the neuropeptide vasopressin (AVP). The NK1R is the primary endogenous target of the neuropeptide substance P and plays a prominent role in stress, depression, and alcohol consumption (Ebner & Singewald, 2006; Schank, 2014). For example, we have previously shown that the NK1R mediates stress-induced reinstatement of alcohol seeking via its effects in the nucleus accumbens (NAC; (Schank et al., 2014; Schank et al., 2015; Schank et al., 2011)), and that NK1R expression in the central nucleus of the amygdala is associated with excessive alcohol consumption in alcohol-preferring (P) rats (Schank et al., 2013). Although traditionally studied in relation to its effects in the periphery, recent research has indicated that AVP directly affects the brain and influences social, affective, and addictive behaviors (Dumais & Veenema, 2016; Litvin, Murakami, & Pfaff, 2011; Meyer-Lindenberg et al., 2009; Zhou, Leri, Cummins, Hoeschele, & Kreek, 2008). For example, AVP synthesis in the amygdala (AMG) and hypothalamus (HYP) regulates stress, anxiety, and depressive behaviors (Ebner, Wotjak, Landgraf, & Engelmann, 2002; Griebel et al., 2002; Salome, Stemmelin, Cohen, & Griebel, 2006; Wigger et al., 2004; Zhou et al., 2005). In relation to social stress specifically, increased hypothalamic AVP mediates the long-term enhancement of corticosterone release in mice after SDS (Keeney et al., 2006). The effects of AVP on drinking behavior have been studied for decades, and has shown that AVP inhibition decreases alcohol consumption in rodents (Edwards, Guerrero, Ghoneim, Roberts, & Koob, 2012; Rigter & Crabbe, 1985; Sanbe et al., 2008) as well as in humans, particularly in highly stressed alcoholics (Ryan et al., 2016). More recent data has identified amygdalar AVP as a candidate involved in alcoholism and depression. For example, vasopressin V1b receptor (V1bR) in the amygdala is involved in the transition to ethanol dependence in male Wistar rats (Edwards, et al., 2012), and similar to our own findings with the NK1R, P rats display increased AVP expression in the AMG (Zhou et al., 2011). The role of AVP in various affective disorders, including stress-induced disorders (Griebel, et al., 2002) makes this peptide an attractive candidate to examine for social stress and drinking-induced changes in behavior. In our studies, we assess the expression of these targets by measuring the level of transcript present. It is important to note that these studies utilized C57BL6/J mice, which are an inbred strain. Thus, changes in expression are not likely due to genetic polymorphisms, but rather more likely to be due to epigenetic alterations induced by early life experience.
The overall goal of these studies was to examine the relationship between alcohol consumption, social behavior, and stress, targeting underlying behavioral and molecular markers. We addressed these ideas by performing individual experiments examining each relationship including: 1) Determining the relationship between susceptibility to SDS and voluntary alcohol consumption; 2) Identifying behavioral factors in stress-naïve mice that predict future alcohol consumption; and 3) Examining the effects of prior binge-like alcohol treatment on susceptibility to subthreshold social defeat. For the majority of these experiments we examined the association of gene expression of Avp and Tacr1 (gene for NK1R) with behavioral phenotypes.
Materials and Methods
Animals
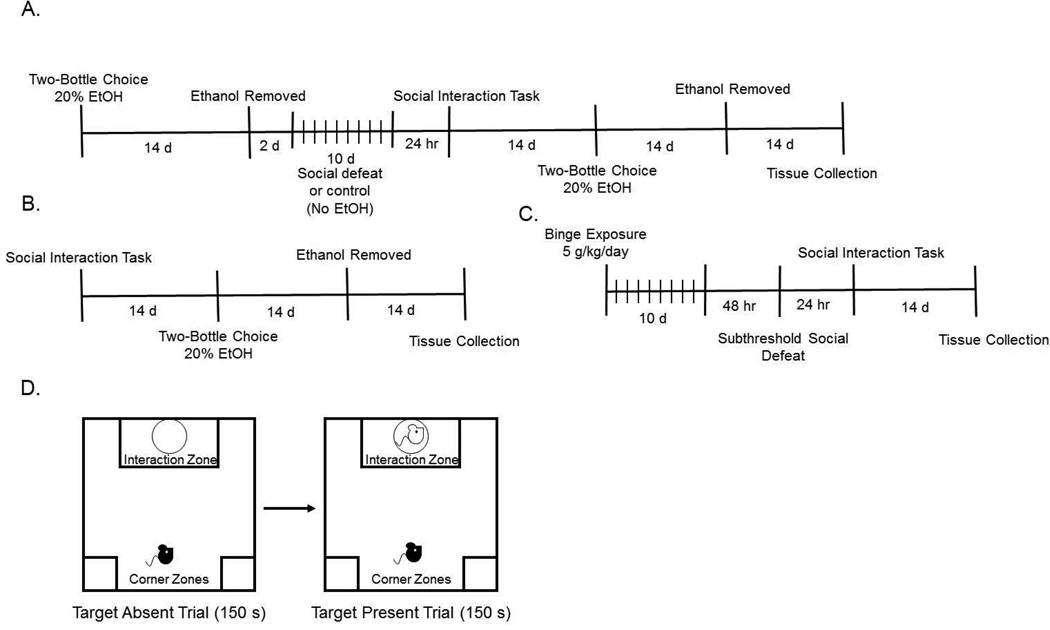
Male C57BL6/J mice (7–8 weeks of age, Jackson Laboratory, Bar Harbor ME) and retired breeder male CD-1 mice (4–5 months of age, Charles River, Wilmington MA) were allowed at least 1 week habituation before experimental procedures. Food and water were available ad libitum, except when stated. All procedures were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and were approved by the Institutional Animal Care and Use Committee of the University of Georgia. Experimental timelines are provided in Figure 1A–C and described in detail below.
Figure 1. Experimental timeline and diagram for the Social Interaction Task.
(A) Timeline for the first experiment examining the effects of social defeat on social interaction and drinking behaviors (B) Timeline for the second experiment examining how social interaction in stress-naïve mice affects drinking behaviors (C) Timeline for the third experiment examining the effects of prior-exposure to a binge drinking paradigm on susceptibility to a sub-threshold level of social defeat (D) Diagram depicting the protocol of the social interaction task used in all experiments.
SDS Protocol
The SDS protocol and social interaction (SI) test was carried out as previously described by Golden and colleagues (Golden, et al., 2011). CD-1 mice were first screened for aggressive behavior by placing a screener C57BL/6J mouse into the home cage of the CD-1 mouse for 180 seconds for 4 consecutive days. Aggressors were selected based on the following criteria: the CD-1 mouse must initiate an attack in at least 2 consecutive sessions and the latency to initial aggression must be less than 60 seconds. Once aggressor mice were identified, the 10-day SDS protocol began. CD-1 aggressor mice were then placed in a hamster cage with a perforated divider 24 hours before social defeat. C57BL/6J mice were exposed to physical defeat with an aggressor by placing them into the homecage of the CD1 mouse for 5 minutes. Afterwards, the defeated mice were placed on the opposite side of the partition for the remainder of the 24 hours. This exposes the mice to olfactory, visual, and auditory cues but no physical interaction with the aggressor mouse. This process was repeated for 10 consecutive days with the C57BL/6J mice encountering a novel aggressor each day. Control mice were placed in pairs in an identical defeat cage setup with a mouse on each side of the perforated divider. The controls were rotated each day.
SI Test
Immediately following the last defeat session, mice were singly housed with food and water provided ad libitum. The SI task was carried out 24 hours after the last defeat session during the dark cycle as previously described (Golden, et al., 2011). This task used a novel CD-1 mouse that had met aggressor criteria and consisted of two trials: target absent and target present. The defeated mouse (test mouse) was first placed in the rear center of an open field arena and allowed to explore the arena with an empty wire mesh enclosure in the interaction zone for 150 seconds (see figure 1D for diagram of testing procedure and arena). The test mouse was then removed for 30 seconds and the novel aggressor mouse (target mouse) was placed into a wire-mesh enclosure within the social interaction zone of the arena. The test mouse was then placed back into the arena for 150 seconds. Following the SI test, video recordings were scored for the amount of time spent in the interaction zone and the corner zones by an experimenter blind to treatment. Time spent in the interaction zone or corner zone was classified when all paws were in the respective areas of the arena. Included in time spent in the interaction zone for both trials was time when the mouse was able to climb the wire mesh enclosure within the interaction zone. SI ratios were calculated as time spent in the interaction zone when the target mouse was present divided by time spent in the interaction zone when the target mouse was absent. Defeated mice with SI ratios greater than 1 were classified as resilient and mice with SI ratios less than 1 were classified as susceptible. While the social interaction data is generally not bimodal, dividing the animals using an SI score threshold of 1.0 is congruent with most other social defeat studies (Chaudhury et al., 2013; Donahue, Muschamp, Russo, Nestler, & Carlezon, 2014; Friedman et al., 2014; Hodes et al., 2014; Iniguez et al., 2010; Sun, et al., 2016). In particular, a study by Krishnan and colleagues goes into detail describing the rationale of using 1.0 as a cutoff between resilient and susceptible subpopulations (Krishnan et al., 2007).
Two Bottle Choice
Before SDS, mice were given continuous access to alcohol in the two bottle choice paradigm where the animal is given two bottles in the homecage, one containing water and one containing 20% alcohol (v/v in water). This phase proceeded for approximately 2 weeks to establish baseline alcohol consumption. Bottles were weighed daily at the same time in order to calculate amount of alcohol consumed per day (g/kg/day). Two weeks after SDS, mice were given access to two-bottle choice for at least 14 days or until criteria for stability were met. In general, acute SDS reduces alcohol consumption while 1–3 week delay between stress and drinking increases alcohol consumption (see references above). Thus, we allowed a 2 week delay between SDS exposure and alcohol availability.
Quantitative Polymerase Chain Reaction (qPCR)
After baseline criteria were met, alcohol was removed for 2 weeks before sacrifice to allow for alcohol washout. We allowed this washout period because we were interested in the long-term effects of stress, social interaction behavior, and prior-binge drinking exposure. Therefore, we did not want any short-term effects of ethanol consumption to mask these effects. Briefly, mice were decapitated, and brains were removed and snap frozen in isopentane. Thick (200 µm) sections were obtained using a freezing cryostat and transferred to RNAse free slides. The following brain regions were microdissected using 1–2 mm biopsy punches: NAC, AMG, HYP, and caudate/putamen (CPU). Total RNA was extracted and reverse-transcribed using a first-strand cDNA synthesis kit (Invitrogen, Carlsbad CA). qPCR reactions were run in triplicate using specific FAM labeled TaqMan probes (Gapdh Mm99999915_g1, Tacr1 Mm00436892_g1, Tac1 Mm00197498_cn, Avp Mm00437761_g1; Applied Biosystems, Foster City CA), and run on an Applied Biosystems QuantStudio6 machine. Reactions were normalized to Gapdh, an endogenous control. Although Tac1 expression was measured for all experiments, there were no significant correlations and these data are not included in the manuscript. Three samples were excluded from the prescreening experiment due to insufficient sample quality to conduct qPCR.
SI Prescreening
The SI test was carried out as described above in mice that were naïve to SDS and alcohol. Two weeks after SI testing, mice were exposed to the two-bottle choice paradigm as described above for approximately 2 weeks or until criteria for stability of alcohol consumption were met.
Binge Alcohol Exposure
Mice were treated with 5 g/kg alcohol (20% in water, intragastric gavage) or water once per day for 10 days. This method of alcohol exposure was chosen because it allows for tight experimenter control over exposure levels, and because it induces very high blood alcohol levels, comparable to what would be observed in human alcohol abusers. Two days after the last intragastric gavage, mice underwent the subthreshold defeat paradigm. Mice were exposed to 1 day of social defeat (3 exposures of 5 minutes of defeat, with 5 minute intervals between each exposure). Twenty four hours after defeat, the SI task was performed as previously described. Because this subthreshold defeat exposure does not typically induce depressive like phenotypes in control mice and because 50–70% of mice exposed to the full 10 day SDS protocol exhibit susceptibility, subthreshold defeat is the preferred method for assessing potential increases in sensitivity to SDS.
Statistical Analyses
SI ratio and alcohol consumption were analyzed by one way ANOVA. Comparison of corner time between target absent and target present phases was performed using two way ANOVA with factors of test phase (within subjects) and group (susceptible, resilient, control; between subjects). Bonferroni posthoc tests were used to probe group differences following corresponding main effect or interaction. Linear regressions were used to correlate alcohol consumption and SI ratio. Gene expression was analyzed via unpaired t-test.
Results
Figure 1 shows the experimental timelines (1A–C) as well as the social interaction task (1D) used in these experiments.
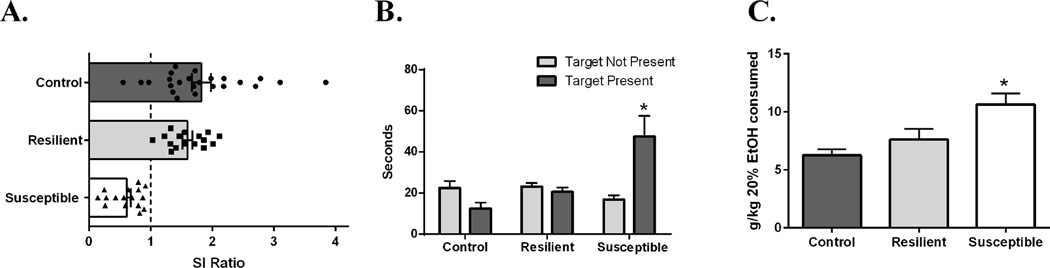
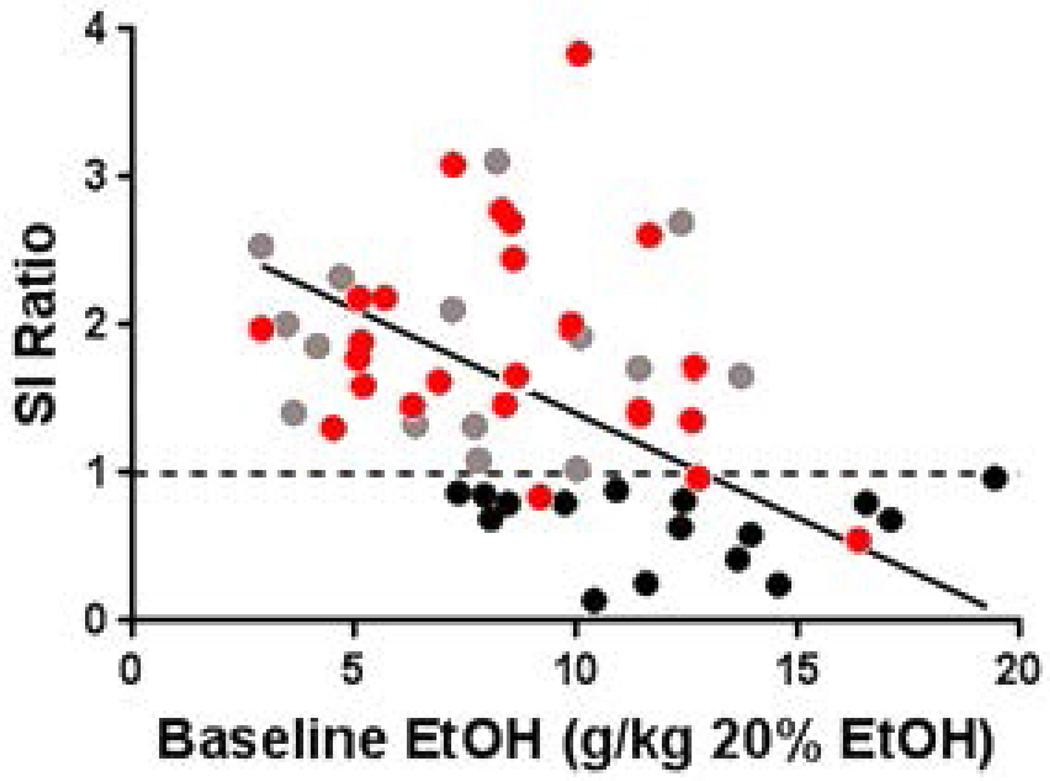
The goal of the first experiment was to test the hypothesis that resiliency or susceptibility to SDS influences subsequent alcohol consumption. Analysis of SI ratio in susceptible (N=16), resilient (N=15), and control (N=24; unstressed) mice revealed a main effect of group (F(2,54)=9.1, p=0.007). Post hoc tests indicated a significantly lower SI score in mice that were classified as susceptible when compared to resilient (p=0.004) and control mice (p<0.001; Figure 2A). Analysis of time spent in the corner zone revealed a main effect of group (F(2, 52)= 16.8776, p<0.001), a main effect of test phase (F(1, 52) = 15.7663, p<0.001), and a group x test phase interaction (F(2, 52) = 60.05, p<0.001). Posthoc tests indicated that susceptible mice spent more time in the corner zone only when the target mouse was present, indicating social avoidance (p<0.001; See Figure 2B). This also indicates that baseline anxiety is not different between resilient and susceptible mice, which would have been evidenced by differences in corner time when the target mouse was absent. Analysis of alcohol consumption revealed a main effect of group (F(2,54)=9.1, p=0.0004; Figure 2C). Post hoc test indicated significantly higher alcohol consumption in susceptible mice when compared to resilient mice (p=0.032) and control mice (p=0.0003). Linear regression analysis revealed that SI ratio negatively correlated with baseline (before SDS exposure) alcohol consumption (r2=0.1747, p=0.0125; Figure 3). This suggests that pre-stress alcohol consumption could predict later sensitivity to SDS and SI behavior. It is important to note that SDS-exposure did have an effect on SI as would be expected, because very few non-stressed control mice exhibited SI ratios less than 1.
Figure 2. SI, corner time, and alcohol consumption in SDS-exposed mice.
(A) One way ANOVA revealed a main effect for SI score (F(2,54)=9.1,p=0.007). Susceptible mice had lower SI scores compared to all other groups (p<0.05). Dotted line indicates threshold criteria for susceptibility. (B) Average time spent (s) in the corner during pretest and test phases (±SEM). There was a significant main effect of group (F(1,54) = 4.2, P = .03). Posthoc Bonferroni tests were performed. (p<0.05 vs. all groups). (C) Average alcohol consumption (g/kg; ±SEM) over the last three days of two-bottle choice after animals had met criteria of baseline. One way ANOVA revealed a main effect for alcohol consumption after SDS exposure (F(2,54)=9.1, p=0.0004). Susceptible mice consumed more alcohol than all other groups (p<0.05). N=7–20/group.
Figure 3. Baseline alcohol consumption correlates with post-SDS SI score.
Control mice are represented as red individual data points (N = 24), susceptible mice are indicated by individual black data points (N=16), and resilient mice are indicated by grey individual data points (N=15). The x axis represents the average of the last three days of pre-defeat ethanol consumption when mice had met baseline criteria. The dotted line indicates the threshold used to separate stressed mice into resilient and susceptible subpopulations.
This correlational analysis presented at least two possibilities. First, alcohol preference and SI behavior could be behavioral phenotypes that are expressed together and driven by common neurobiological factors. However, mice had different levels of alcohol exposure prior to SDS due to their baseline alcohol preference, and this could in turn affect SDS sensitivity. As such, another possibility is that increased voluntary alcohol consumption could influence subsequent social behavior (and SDS sensitivity) by virtue of neuroadaptations induced by chronic alcohol exposure. To assess the former, we performed SI testing in alcohol- and stress-naïve mice and measured subsequent alcohol consumption. To address the latter, we exposed mice to experimenter-delivered, binge level alcohol concentrations and assessed the resulting sensitivity to SDS.
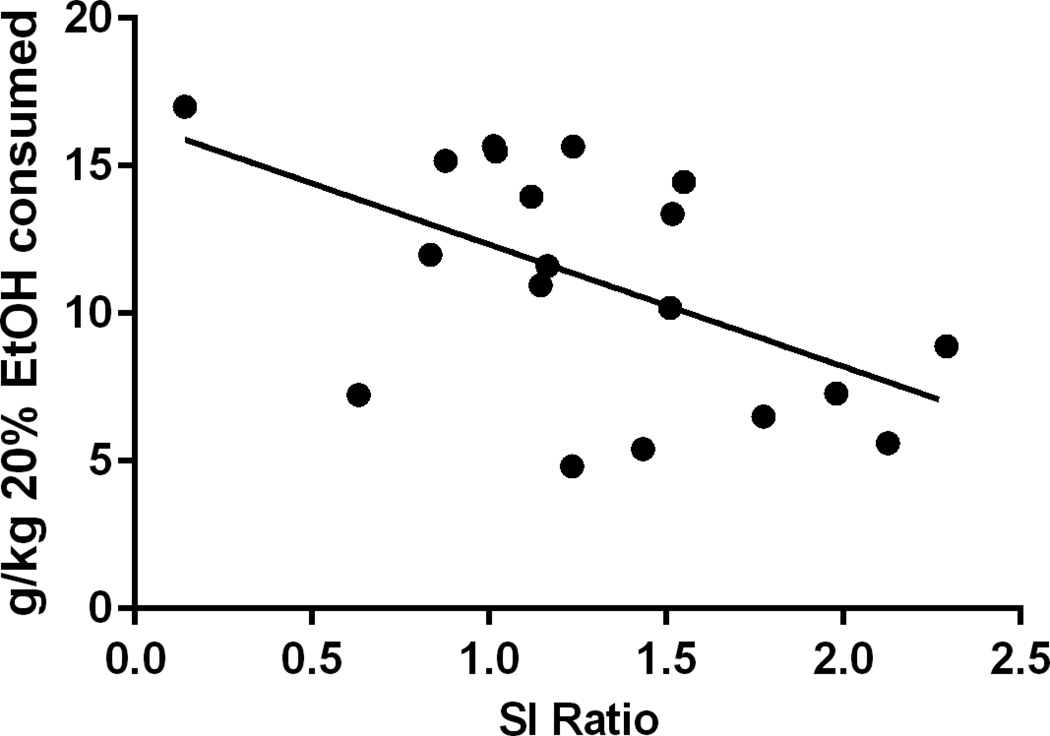
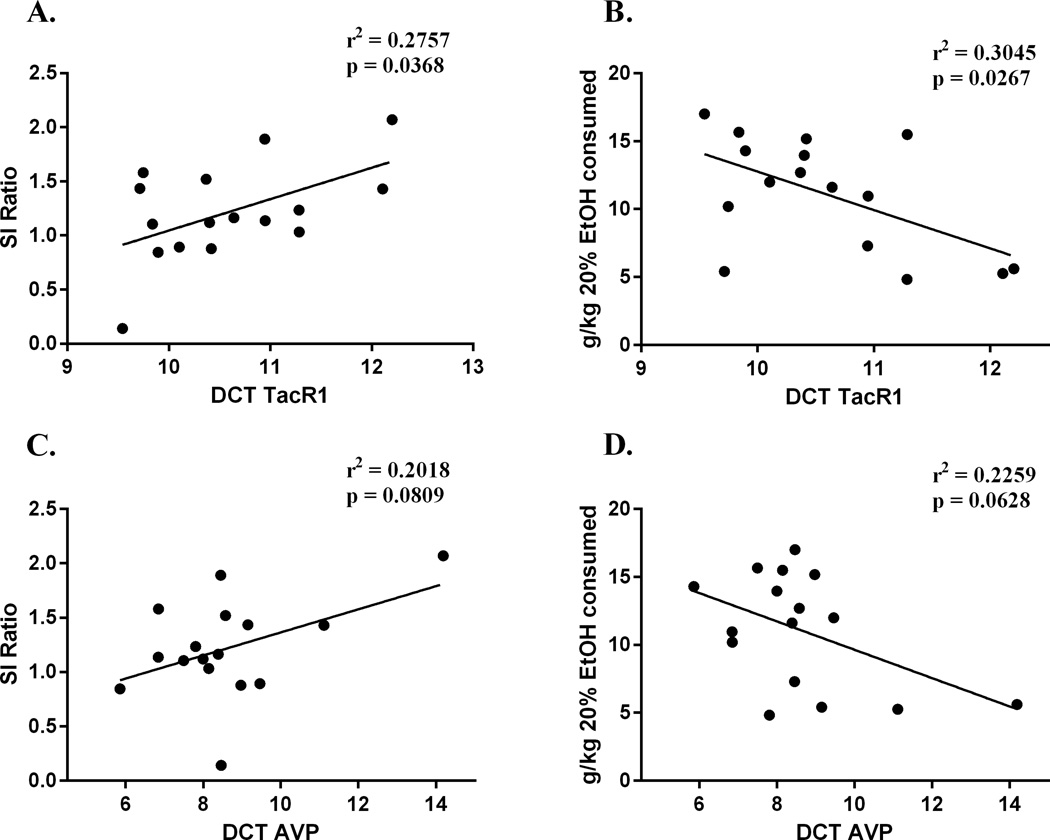
As stated above, the goal of the next experiment was to test the hypothesis that SI behavior correlates with alcohol consumption in stress- and alcohol-naïve mice, and that these behaviors are associated with common neurobiological factors. Analysis of SI ratio in stress- and alcohol- naïve mice (N=19) revealed a negative correlation to future alcohol consumption, indicating that mice that displayed increased social avoidance behavior also consumed more alcohol (r2=0.2863, p=0.0182; Figure 4). There was no correlation between corner time when the target mouse was absent and future alcohol consumption (r2=0.0594, p=0.3630), indicating that baseline anxiety does not correlate with future alcohol consumption. Tacr1, Tac1 and Avp expression was then measured in mice (n=16) from this SI prescreening experiment. Tacr1 expression in the AMG negatively correlated to SI ratios (r2=0.2757, p=0.0368; Figure 5A) and positively correlated to alcohol consumption (r2=0.3045, p=0.0267; Figure 4B). Additionally, Avp expression in the amygdala showed a trend toward a negative correlation to SI ratios (r2=0.2018, p=0.0809; Figure 5C) and a trend toward a positive correlation to alcohol consumption (r2=0.2259, p=0.0628; Figure 5D). There was a positive correlation of Tacr1 and SI ratios in the CPU (r2=0.2569, p=0.0451), however there was no correlation between Tacr1 and alcohol consumption in this region (r2=0.1793, p=0.1022). There was no correlation between SI ratio and Tacr1 in the NAC (r2=0.03707, p=0.5096), or HYP (r2=0.08375, p=0.2770). There was no correlation between SI ratio and Avp in the HYP (r2=0.1614, p=0.225). Avp expression was not assessed in the NAC or CPU, as there is no evidence for local expression of the peptide in these regions.
Figure 4. Initial SI score in alcohol naïve mice correlates with subsequent alcohol consumption.
Social interaction tested at 8–10 weeks of age and alcohol access in two bottle choice paradigm began 2 weeks later. (N=19)
Figure 5. Tacr1 (A,B) and Avp (C,D) expression are negatively correlated with SI Ratio and positively correlated to alcohol consumption in the AMG.
Alcohol naïve mice were prescreened at 8–10 weeks of age and then allowed access to alcohol 2 weeks later. DCT: delta cycle threshold; relative difference in cycle threshold compared to GAPDH expression (housekeeping gene). Note: lower DCT value indicates greater expression level. N=16.
Given these results, and our previous findings that NK1R activity in the NAC and AMG can influence stress-induced alcohol seeking (Schank, et al., 2015; Schank, et al., 2013), we measured the expression of Tacr1 and Avp in the AMG and NAC of SDS-exposed mice. We found that susceptible mice (N=8) had increased expression of Avp in the AMG (t13=2.753, p=0.0165; Figure 6A) and Tacr1 in the NAC (t14=2.953, p=0.0105; Figure 6B) compared to resilient mice (N=7). These data suggest that Tacr1 expression could potentially have a role in drinking behavior associated with variations in social behavior as well as increased alcohol consumption induced by SDS exposure, however there may be a dissociation between the brain regions where these effects occur.
Figure 6. Susceptible mice have increased Avp expression in the AMG and increased Tacr1 expression in the NAC compared to resilient mice.
(A) T-test revealed that susceptible mice had increased expression of Avp in the AMG (t=2.914, p=0.0165) (B) T-test revealed that susceptible mice had increased expression of Tacr1 in the NAC (t=2.953, p=0.0105). DCT: delta cycle threshold; relative difference in cycle threshold compared to housekeeping gene. Note: lower DCT value indicates greater expression level. N=7–8/group.
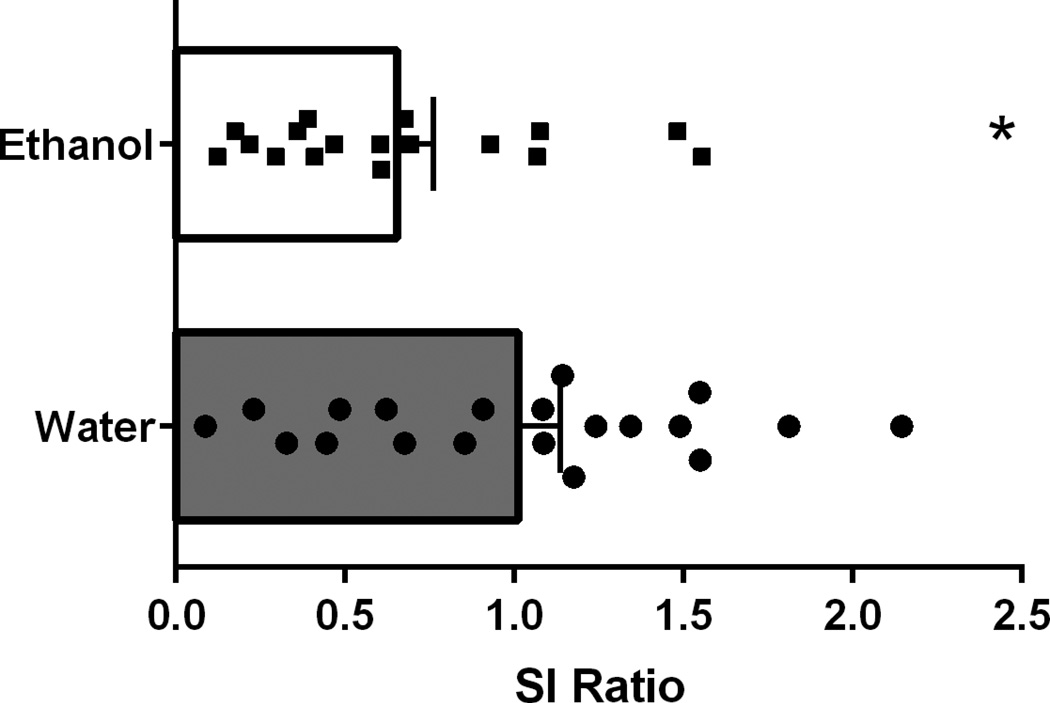
The goal of the final experiment was to test the hypothesis that prior alcohol exposure increases vulnerability to social stress. T-test of SI scores following exposure to subthreshold defeat revealed a main effect of treatment on SI ratio (n=17–20/group; t35=2.164, p=0.0374, Figure 7), indicating that binge-like alcohol exposure increases sensitivity to subthreshold defeat. It is of note that overall SI ratios in mice gavaged with water are slightly lower than average SI expected from an untreated control group. This is likely a result of mild stress induced by repeated intragastric gavage procedures and subthreshold social stress. No differences were seen in Tacr1 gene expression in the NAC (t14=0.3498, p=0.7318) or AMG (t14=0.03205, p=0.9749) in animals that were given alcohol treatment (N=8) when compared to water treatment (N=8). No differences were seen in Avp gene expression in the AMG (t14=0.1664, p=0.8702) in animals that were given alcohol treatment (N=8) compared to water treatment (N=8). Taken together, these results suggest that prior binge alcohol exposure can increase sensitivity to social stress; however, this is not likely to be mediated by AVP or the NK1R in the AMG or NAC. This increase in sensitivity is either influenced by alternative brain regions or different signaling systems.
Figure 7. Effect of chronic alcohol exposure on behavioral response to subthreshold social defeat.
Mice were treated with 5 g/kg alcohol (20% in water, intragastric gavage) once per day for 10 days. T-test revealed that this pretreatment increased the number of mice that were classified as susceptible and induced lower SI scores compared to water treated controls (t=2.164, p=0.0374). N=17–20/group.
Discussion
Our findings support the hypothesis that there is a bidirectional relationship between alcohol intake and sensitivity to social stress. This is evidenced by our data indicating higher alcohol consumption in mice susceptible to SDS as well as data showing that prior alcohol exposure increases sensitivity to subthreshold social defeat. Furthermore, we found that social behavior associates with subsequent alcohol intake and that these behaviors may be influenced by common neurobiological factors. This also suggests that social avoidance could serve as a behavioral marker of increased alcohol preference, and that baseline alcohol intake can in turn predict sensitivity to SDS. It is particularly intriguing that these behaviors may be related to Tacr1 expression, as this gene has been found to associate with increased risk of alcoholism and increased sensitivity to alcohol paired cues in human studies (Blaine, Claus, Harlaar, & Hutchison, 2013; Seneviratne et al., 2009).
To date, the findings concerning the effect of SDS on subsequent alcohol consumption have not been entirely consistent. While protocols used in previous studies have been rather varied, the one that is most similar to the procedure used here was our own previous work (Karlsson, et al., 2016). In this study we observed an early onset, short duration increase in alcohol consumption, although we did not stratify for susceptible/resilient, and it is unknown if these phenotypes influenced drinking behavior. In either case, an early increase in drinking could be independent of specific SDS-induced phenotypes, as some stress responses such as corticosterone release are observed in both susceptible and resilient animals (Krishnan, et al., 2007). Furthermore, we do not know if a second, delayed increase in alcohol consumption would have been observed in the SDS-exposed animals from this previous experiment, or if this would be influenced by susceptibility, as we did not perform drinking measurements beyond 4–5 days. In general, the results presented here are consistent with a subset of the previously reported findings: that SDS exposure induces greater alcohol intake relative to non-stressed controls that has a late (1–3 week) onset (Caldwell & Riccio, 2010; Croft, et al., 2005; Dong, et al., 2011; Funk, et al., 2005; Funk, Li, & Le, 2006; Hwa, et al., 2016; Norman, et al., 2015). However, our current findings advance previous reports in that we found higher alcohol consumption to be specific to susceptible mice, with resilient mice showing no change in drinking relative to unstressed controls. Although many studies examining alcohol consumption and social defeat have required an “incubation” period before finding differences in alcohol consumption between stressed and unstressed mice, the exact neurobiological cause of this delay is unknown and should warrant further investigation. One possibility is that social defeat affects gene and protein expression differently depending on length of time after defeat. For example, defeat-stress induced alterations in BDNF protein expression take up to 4 weeks to emerge in some brain regions (Fanous, Hammer, & Nikulina, 2010).
One finding from our initial SDS experiment was that baseline alcohol intake, prior to SDS, correlated with post-SDS SI scores. In other words, animals that drank more alcohol initially were more likely to be identified as susceptible. This raised at least two possibilities that are not mutually exclusive. First, baseline alcohol intake and social interaction/SDS susceptibility could be behavioral phenotypes that are expressed together and are potentially mediated by common neurobiological factors. A second possibility is that mice that drank more alcohol were more susceptible to SDS as a result of increased alcohol exposure. We addressed the first possibility by determining if SI behavior in alcohol- and stress-naïve mice correlated with later alcohol consumption, which it did. This provides support for the interpretation that social avoidance could be a behavioral marker that predicts future alcohol intake, and that alcohol consumption could in turn predict susceptibility to SDS. Based on our experiments, it is clear to us that elevated drinking relative to controls after defeat depends on two conditions: 1. high baseline consumption, and 2. exposure to social defeat. It is very important to note that control and defeated groups were matched by consumption prior to the start of social defeat, and the control mice were treated in an identical fashion as the defeated mice (with the exception of defeat stress exposure), yet only susceptible mice from the defeated group showed increased alcohol consumption relative to controls. To examine the second possibility above, we controlled the level of pre-SDS alcohol exposure by delivering intragastric alcohol at highly intoxicating levels for 10 days prior to stress and found that this treatment increased sensitivity to subthreshold defeat. This suggests that the magnitude of previous alcohol exposure can also influence sensitivity to SDS. This has an interesting link to the literature concerning the transcription factor nuclear factor kappa B (NFkB), suggesting a mechanism for alcohol-induced increase in sensitivity to defeat stress. Specifically, NFkB activity is upregulated by the same alcohol exposure protocol used here (Qin et al., 2008), NFkB has been shown to regulate defeat stress sensitivity (Christoffel et al., 2011; Christoffel et al., 2012), and inhibition of NFkB activity attenuates alcohol intake (Truitt et al., 2016). Taken together, these results suggest a complex and multidirectional relationship between alcohol exposure, social avoidance, and stress sensitivity.
Here we show that Tacr1 and Avp expression in the AMG associate with SI behavior and alcohol consumption. Additionally, we show that Tacr1 expression is increased in the NAC of susceptible mice, while Avp expression is increased in the AMG of these animals. As stated in the methods section, we assessed transcript levels in these mice after a 2 week washout. As such, we believe that we are getting an accurate readout of stress-induced changes and underlying individual differences in these signaling systems and are not detecting alterations induced by the pharmacological effects of alcohol. It is unclear what results would be obtained if samples were taken during alcohol access. Importantly, it has been found in some studies that alcohol consumption can normalize baseline differences in gene expression in some alcohol preferring rat models (Hansson, Cippitelli, Sommer, Ciccocioppo, & Heilig, 2007). Thus, AVP levels in the AMG appear to be a common factor that contributes to SI behavior, alcohol consumption, and SDS-induced susceptibility. These results are consistent with previous research that identifies amygdalar AVP and AVP receptors as regulators of depression (Litvin, et al., 2011) and addiction (Zhou, et al., 2005; Zhou, et al., 2011). Although we were not able to measure gene expression in subregions of the amygdala, it is likely that the increase in AVP expression is driven by the medial amygdala (Rood & De Vries, 2011). This region contains AVP producing cells that receive innervation from the olfactory system, which is hypothesized to help facilitate social memories and appropriate social behavioral in various situations (Keller, Baum, Brock, Brennan, & Bakker, 2009). For NK1R, this receptor appears to play a consistent role in social stress and alcohol consumption, but there is a distinction between the brain regions that involved in specific relationships, with AMG Tacr1 expression associating with the social avoidance/drinking relationship and NAC Tacr1 expression being altered in susceptible mice. While not entirely analogous to these conditions, it is interesting to note that in our rat studies we have observed that Tacr1 expression is increased specifically in the central amygdala of genetically selected P rats (Schank, et al., 2013), and that local infusion of a neurokinin-1 receptor antagonist to this region specifically decreases alcohol consumption. However, there may also be a role for the medial amygdala here as well (Ayanwuyi et al., 2015). On the other hand, NK1R activation in the NAC has been shown to regulate alcohol seeking induced by acute footshock stress (Schank, et al., 2015). Chronic alcohol exposure, which we found to increase sensitivity to SDS, did not alter Tacr1 or Avp expression in these regions. This suggests that, at least in the NAC and AMG, chronic alcohol exposure does not alter Tacr1 or Avp expression, and that these transmitters do not contribute to alcohol-induced increase in sensitivity to social stress.
It is well known that alcohol abuse is comorbid with depression. While many assume that depression precedes and influences the risk of alcoholism, this has not been demonstrated definitively. An alternative possibility is that chronic alcohol exposure influences stress sensitivity, and this could in turn mediate the risk of major depression. The preclinical findings that we present here support a bidirectional relationship, with the onset of one condition increasing the risk of the other. In addition, we identify expression of NK1R and AVP as potential neurobiological factors that contribute to this effect. As such, future research should focus on agents that target this receptor and peptide for the treatment of comorbid alcoholism and affective disorders. Interestingly, clinical work has shown that antagonism of the NK1R can attenuate alcohol cravings, including both spontaneous cravings as well as those that are induced by a combination social stress/cue exposure (George et al., 2008). While expression level of the NK1R was not assessed in this study, the subjects were selected for psychiatric comorbidity. Thus, it is tempting to speculate that this may have also associated with variations in NK1R expression, or at the least, that a behavioral marker exists that could predict responsivity to NK1R antagonist treatment.
Acknowledgments
This work funded by the University of Georgia and NIH grant R00AA021805.
Footnotes
Authors Contribution
JRS and BSN conceived of the experiments, analyzed data, and wrote the manuscript. MKS contributed to collection of experimental data.
References
- Ayanwuyi LO, Stopponi S, Ubaldi M, Cippitelli A, Nasuti C, Damadzic R, Ciccocioppo R. Neurokinin 1 receptor blockade in the medial amygdala attenuates alcohol drinking in rats with innate anxiety but not in wistar rats. Br J Pharmacol. 2015 doi: 10.1111/bph.13280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, Nestler EJ. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311(5762):864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- Blaine S, Claus E, Harlaar N, Hutchison K. TACR1 genotypes predict fMRI response to alcohol cues and level of alcohol dependence. Alcohol Clin Exp Res. 2013;(37 Suppl 1):E125–E130. doi: 10.1111/j.1530-0277.2012.01923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boden JM, Fergusson DM. Alcohol and depression. Addiction. 2011;106(5):906–914. doi: 10.1111/j.1360-0443.2010.03351.x. [DOI] [PubMed] [Google Scholar]
- Bruchas MR, Schindler AG, Shankar H, Messinger DI, Miyatake M, Land BB, Chavkin C. Selective p38alpha MAPK deletion in serotonergic neurons produces stress resilience in models of depression and addiction. Neuron. 2011;71(3):498–511. doi: 10.1016/j.neuron.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell EE, Riccio DC. Alcohol self-administration in rats: Modulation by temporal parameters related to repeated mild social defeat stress. Alcohol. 2010;44(3):265–274. doi: 10.1016/j.alcohol.2010.02.012. [DOI] [PubMed] [Google Scholar]
- Chaudhury D, Walsh JJ, Friedman AK, Juarez B, Ku SM, Koo JW, Han MH. Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature. 2013;493(7433):532–536. doi: 10.1038/nature11713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoffel DJ, Golden SA, Dumitriu D, Robison AJ, Janssen WG, Ahn HF, Russo SJ. IkappaB kinase regulates social defeat stress-induced synaptic and behavioral plasticity. J Neurosci. 2011;31(1):314–321. doi: 10.1523/JNEUROSCI.4763-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoffel DJ, Golden SA, Heshmati M, Graham A, Birnbaum S, Neve RL, Russo SJ. Effects of inhibitor of kappaB kinase activity in the nucleus accumbens on emotional behavior. Neuropsychopharmacology. 2012;37(12):2615–2623. doi: 10.1038/npp.2012.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covington HE, 3rd, Kikusui T, Goodhue J, Nikulina EM, Hammer RP, Jr, Miczek KA. Brief social defeat stress: long lasting effects on cocaine taking during a binge and zif268 mRNA expression in the amygdala and prefrontal cortex. Neuropsychopharmacology. 2005;30(2):310–321. doi: 10.1038/sj.npp.1300587. [DOI] [PubMed] [Google Scholar]
- Covington HE, 3rd, Miczek KA. Intense cocaine self-administration after episodic social defeat stress, but not after aggressive behavior: dissociation from corticosterone activation. Psychopharmacology (Berl) 2005;183(3):331–340. doi: 10.1007/s00213-005-0190-5. [DOI] [PubMed] [Google Scholar]
- Croft AP, Brooks SP, Cole J, Little HJ. Social defeat increases alcohol preference of C57BL/10 strain mice; effect prevented by a CCKB antagonist. Psychopharmacology (Berl) 2005;183(2):163–170. doi: 10.1007/s00213-005-0165-6. [DOI] [PubMed] [Google Scholar]
- Donahue RJ, Muschamp JW, Russo SJ, Nestler EJ, Carlezon WA., Jr Effects of striatal DeltaFosB overexpression and ketamine on social defeat stress-induced anhedonia in mice. Biol Psychiatry. 2014;76(7):550–558. doi: 10.1016/j.biopsych.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong L, Bilbao A, Laucht M, Henriksson R, Yakovleva T, Ridinger M, Schumann G. Effects of the circadian rhythm gene period 1 (per1) on psychosocial stress-induced alcohol drinking. Am J Psychiatry. 2011;168(10):1090–1098. doi: 10.1176/appi.ajp.2011.10111579. [DOI] [PubMed] [Google Scholar]
- Dumais KM, Veenema AH. Vasopressin and oxytocin receptor systems in the brain: Sex differences and sex-specific regulation of social behavior. Front Neuroendocrinol. 2016;40:1–23. doi: 10.1016/j.yfrne.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner K, Singewald N. The role of substance P in stress and anxiety responses. Amino Acids. 2006;31(3):251–272. doi: 10.1007/s00726-006-0335-9. [DOI] [PubMed] [Google Scholar]
- Ebner K, Wotjak CT, Landgraf R, Engelmann M. Forced swimming triggers vasopressin release within the amygdala to modulate stress-coping strategies in rats. Eur J Neurosci. 2002;15(2):384–388. doi: 10.1046/j.0953-816x.2001.01869.x. [DOI] [PubMed] [Google Scholar]
- Edwards S, Guerrero M, Ghoneim OM, Roberts E, Koob GF. Evidence that vasopressin V1b receptors mediate the transition to excessive drinking in ethanol-dependent rats. Addict Biol. 2012;17(1):76–85. doi: 10.1111/j.1369-1600.2010.00291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanous S, Hammer RP, Jr, Nikulina EM. Short- and long-term effects of intermittent social defeat stress on brain-derived neurotrophic factor expression in mesocorticolimbic brain regions. Neuroscience. 2010;167(3):598–607. doi: 10.1016/j.neuroscience.2010.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman AK, Walsh JJ, Juarez B, Ku SM, Chaudhury D, Wang J, Han MH. Enhancing depression mechanisms in midbrain dopamine neurons achieves homeostatic resilience. Science. 2014;344(6181):313–319. doi: 10.1126/science.1249240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk D, Harding S, Juzytsch W, Le AD. Effects of unconditioned and conditioned social defeat on alcohol self-administration and reinstatement of alcohol seeking in rats. Psychopharmacology (Berl) 2005;183(3):341–349. doi: 10.1007/s00213-005-0194-1. [DOI] [PubMed] [Google Scholar]
- Funk D, Li Z, Le AD. Effects of environmental and pharmacological stressors on c-fos and corticotropin-releasing factor mRNA in rat brain: Relationship to the reinstatement of alcohol seeking. Neuroscience. 2006;138(1):235–243. doi: 10.1016/j.neuroscience.2005.10.062. [DOI] [PubMed] [Google Scholar]
- George DT, Gilman J, Hersh J, Thorsell A, Herion D, Geyer C, Heilig M. Neurokinin 1 receptor antagonism as a possible therapy for alcoholism. Science. 2008;319(5869):1536–1539. doi: 10.1126/science.1153813. [DOI] [PubMed] [Google Scholar]
- Golden SA, Covington HE, 3rd, Berton O, Russo SJ. A standardized protocol for repeated social defeat stress in mice. Nat Protoc. 2011;6(8):1183–1191. doi: 10.1038/nprot.2011.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Harford TC. Comorbidity between DSM-IV alcohol use disorders and major depression: results of a national survey. Drug Alcohol Depend. 1995;39(3):197–206. doi: 10.1016/0376-8716(95)01160-4. [DOI] [PubMed] [Google Scholar]
- Griebel G, Simiand J, Serradeil-Le Gal C, Wagnon J, Pascal M, Scatton B, Soubrie P. Anxiolytic- and antidepressant-like effects of the non-peptide vasopressin V1b receptor antagonist, SSR149415, suggest an innovative approach for the treatment of stress-related disorders. Proc Natl Acad Sci U S A. 2002;99(9):6370–6375. doi: 10.1073/pnas.092012099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson AC, Cippitelli A, Sommer WH, Ciccocioppo R, Heilig M. Region-specific down-regulation of Crhr1 gene expression in alcohol-preferring msP rats following ad lib access to alcohol. Addict Biol. 2007;12(1):30–34. doi: 10.1111/j.1369-1600.2007.00050.x. [DOI] [PubMed] [Google Scholar]
- Hodes GE, Pfau ML, Leboeuf M, Golden SA, Christoffel DJ, Bregman D, Russo SJ. Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proc Natl Acad Sci U S A. 2014;111(45):16136–16141. doi: 10.1073/pnas.1415191111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwa LS, Holly EN, DeBold JF, Miczek KA. Social stress-escalated intermittent alcohol drinking: modulation by CRF-R1 in the ventral tegmental area and accumbal dopamine in mice. Psychopharmacology (Berl) 2016;233(4):681–690. doi: 10.1007/s00213-015-4144-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iniguez SD, Vialou V, Warren BL, Cao JL, Alcantara LF, Davis LC, Bolanos-Guzman CA. Extracellular signal-regulated kinase-2 within the ventral tegmental area regulates responses to stress. J Neurosci. 2010;30(22):7652–7663. doi: 10.1523/JNEUROSCI.0951-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson C, Schank JR, Rehman F, Stojakovic A, Bjork K, Barbier E, Heilig M. Proinflammatory signaling regulates voluntary alcohol intake and stress-induced consumption after exposure to social defeat stress in mice. Addict Biol. 2016 doi: 10.1111/adb.12416. [DOI] [PubMed] [Google Scholar]
- Keeney A, Jessop DS, Harbuz MS, Marsden CA, Hogg S, Blackburn-Munro RE. Differential effects of acute and chronic social defeat stress on hypothalamic-pituitary-adrenal axis function and hippocampal serotonin release in mice. J Neuroendocrinol. 2006;18(5):330–338. doi: 10.1111/j.1365-2826.2006.01422.x. [DOI] [PubMed] [Google Scholar]
- Keller M, Baum MJ, Brock O, Brennan PA, Bakker J. The main and the accessory olfactory systems interact in the control of mate recognition and sexual behavior. Behav Brain Res. 2009;200(2):268–276. doi: 10.1016/j.bbr.2009.01.020. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, Nestler EJ. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131(2):391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Litvin Y, Murakami G, Pfaff DW. Effects of chronic social defeat on behavioral and neural correlates of sociality: Vasopressin, oxytocin and the vasopressinergic V1b receptor. Physiol Behav. 2011;103(3–4):393–403. doi: 10.1016/j.physbeh.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Lopez MF, Anderson RI, Becker HC. Effect of different stressors on voluntary ethanol intake in ethanol-dependent and nondependent C57BL/6J mice. Alcohol. 2016;51:17–23. doi: 10.1016/j.alcohol.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Kolachana B, Gold B, Olsh A, Nicodemus KK, Mattay V, Weinberger DR. Genetic variants in AVPR1A linked to autism predict amygdala activation and personality traits in healthy humans. Mol Psychiatry. 2009;14(10):968–975. doi: 10.1038/mp.2008.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molander A, Vengeliene V, Heilig M, Wurst W, Deussing JM, Spanagel R. Brain-specific inactivation of the Crhr1 gene inhibits post-dependent and stress-induced alcohol intake, but does not affect relapse-like drinking. Neuropsychopharmacology. 2012;37(4):1047–1056. doi: 10.1038/npp.2011.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman KJ, Seiden JA, Klickstein JA, Han X, Hwa LS, DeBold JF, Miczek KA. Social stress and escalated drug self-administration in mice I. Alcohol and corticosterone. Psychopharmacology (Berl) 2015;232(6):991–1001. doi: 10.1007/s00213-014-3733-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L, He J, Hanes RN, Pluzarev O, Hong JS, Crews FT. Increased systemic and brain cytokine production and neuroinflammation by endotoxin following ethanol treatment. J Neuroinflammation. 2008;5:10. doi: 10.1186/1742-2094-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigter H, Crabbe JC. Vasopressin and ethanol preference. I. Effects of vasopressin and the fragment DGAVP on altered ethanol preference in Brattleboro diabetes insipidus rats. Peptides. 1985;6(4):669–676. doi: 10.1016/0196-9781(85)90170-6. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Arias M, Navarrete F, Blanco-Gandia MC, Arenas MC, Bartoll-Andres A, Aguilar MA, Manzanares J. Social defeat in adolescent mice increases vulnerability to alcohol consumption. Addict Biol. 2016;21(1):87–97. doi: 10.1111/adb.12184. [DOI] [PubMed] [Google Scholar]
- Rood BD, De Vries GJ. Vasopressin innervation of the mouse (Mus musculus) brain and spinal cord. J Comp Neurol. 2011;519(12):2434–2474. doi: 10.1002/cne.22635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan ML, Falk DE, Fertig JB, Rendenbach-Mueller B, Katz DA, Tracy KA, Litten RZ. A Phase 2, Double-Blind, Placebo-Controlled Randomized Trial Assessing the Efficacy of ABT-436, a Novel V1b Receptor Antagonist, for Alcohol Dependence. Neuropsychopharmacology. 2016 doi: 10.1038/npp.2016.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salome N, Stemmelin J, Cohen C, Griebel G. Differential roles of amygdaloid nuclei in the anxiolytic- and antidepressant-like effects of the V1b receptor antagonist, SSR149415, in rats. Psychopharmacology (Berl) 2006;187(2):237–244. doi: 10.1007/s00213-006-0424-1. [DOI] [PubMed] [Google Scholar]
- Sanbe A, Takagi N, Fujiwara Y, Yamauchi J, Endo T, Mizutani R, Tanoue A. Alcohol preference in mice lacking the Avpr1a vasopressin receptor. Am J Physiol Regul Integr Comp Physiol. 2008;294(5):R1482–R1490. doi: 10.1152/ajpregu.00708.2007. [DOI] [PubMed] [Google Scholar]
- Schank JR. The neurokinin-1 receptor in addictive processes. J Pharmacol Exp Ther. 2014;351(1):2–8. doi: 10.1124/jpet.113.210799. [DOI] [PubMed] [Google Scholar]
- Schank JR, King CE, Sun H, Cheng K, Rice KC, Heilig M, Schroeder JP. The role of the neurokinin-1 receptor in stress-induced reinstatement of alcohol and cocaine seeking. Neuropsychopharmacology. 2014;39(5):1093–1101. doi: 10.1038/npp.2013.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schank JR, Nelson BS, Damadzic R, Tapocik JD, Yao M, King CE, Heilig M. Neurokinin-1 receptor antagonism attenuates neuronal activity triggered by stress-induced reinstatement of alcohol seeking. Neuropharmacology. 2015;99:106–114. doi: 10.1016/j.neuropharm.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schank JR, Pickens CL, Rowe KE, Cheng K, Thorsell A, Rice KC, Heilig M. Stress-induced reinstatement of alcohol-seeking in rats is selectively suppressed by the neurokinin 1 (NK1) antagonist L822429. Psychopharmacology (Berl) 2011;218(1):111–119. doi: 10.1007/s00213-011-2201-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schank JR, Tapocik JD, Barbier E, Damadzic R, Eskay RL, Sun H, Heilig M. Tacr1 gene variation and neurokinin 1 receptor expression is associated with antagonist efficacy in genetically selected alcohol-preferring rats. Biol Psychiatry. 2013;73(8):774–781. doi: 10.1016/j.biopsych.2012.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seneviratne C, Ait-Daoud N, Ma JZ, Chen G, Johnson BA, Li MD. Susceptibility locus in neurokinin-1 receptor gene associated with alcohol dependence. Neuropsychopharmacology. 2009;34(11):2442–2449. doi: 10.1038/npp.2009.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillaber I, Rammes G, Zimmermann S, Mahal B, Zieglgansberger W, Wurst W, Spanagel R. Enhanced and delayed stress-induced alcohol drinking in mice lacking functional CRH1 receptors. Science. 2002;296(5569):931–933. doi: 10.1126/science.1069836. [DOI] [PubMed] [Google Scholar]
- Sun H, Martin JA, Werner CT, Wang ZJ, Damez-Werno DM, Scobie KN, Nestler EJ. BAZ1B in Nucleus Accumbens Regulates Reward-Related Behaviors in Response to Distinct Emotional Stimuli. J Neurosci. 2016;36(14):3954–3961. doi: 10.1523/JNEUROSCI.3254-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truitt JM, Blednov YA, Benavidez JM, Black M, Ponomareva O, Law J, Mayfield RD. Inhibition of IKKbeta Reduces Ethanol Consumption in C57BL/6J Mice. eNeuro. 2016;3(5) doi: 10.1523/ENEURO.0256-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Erp AM, Miczek KA. Persistent suppression of ethanol self-administration by brief social stress in rats and increased startle response as index of withdrawal. Physiol Behav. 2001;73(3):301–311. doi: 10.1016/s0031-9384(01)00458-9. [DOI] [PubMed] [Google Scholar]
- Wigger A, Sanchez MM, Mathys KC, Ebner K, Frank E, Liu D, Landgraf R. Alterations in central neuropeptide expression, release, and receptor binding in rats bred for high anxiety: critical role of vasopressin. Neuropsychopharmacology. 2004;29(1):1–14. doi: 10.1038/sj.npp.1300290. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Bendor JT, Yuferov V, Schlussman SD, Ho A, Kreek MJ. Amygdalar vasopressin mRNA increases in acute cocaine withdrawal: evidence for opioid receptor modulation. Neuroscience. 2005;134(4):1391–1397. doi: 10.1016/j.neuroscience.2005.05.032. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Colombo G, Carai MA, Ho A, Gessa GL, Kreek MJ. Involvement of arginine vasopressin and V1b receptor in alcohol drinking in Sardinian alcohol-preferring rats. Alcohol Clin Exp Res. 2011;35(10):1876–1883. doi: 10.1111/j.1530-0277.2011.01532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Leri F, Cummins E, Hoeschele M, Kreek MJ. Involvement of arginine vasopressin and V1b receptor in heroin withdrawal and heroin seeking precipitated by stress and by heroin. Neuropsychopharmacology. 2008;33(2):226–236. doi: 10.1038/sj.npp.1301419. [DOI] [PubMed] [Google Scholar]