Abstract
Differences of Sex Development (DSD) includes a wide spectrum of etiologies and phenotypes. A subset of individuals with DSDs are predisposed to gonadal germ cell tumor (GCT). In this setting, GCT risk varies widely, depending on the DSD molecular etiology and penetrance. Prognostication based on molecular diagnosis remains challenging, as natural history data specific to recently identified molecular causes of DSD is lacking. In this review, we provide a framework for the clinical geneticist to consider GCT tumor risk in the patient with DSD. We discuss germ cell development and etiology of GCT growth, along with parameters to consider when recommending prophylactic gonadectomy including fertility, hormonal output, and malignant GTC treatment outcomes. Shortly after the 2006 reorganization of DSD nomenclature, literature reviews of natural history publications stratified GCT risk by a chromosomal, pathological, and hormonal taxonomy. Our 2017 literature review reveals a larger body of publications. However, the broad DSD GCT risk stratification within the 2006 taxonomy remains stable. We discuss precise GCT risk assessment for specific diagnoses, including androgen insensitivity, Smith-Lemli-Opitz, and 46,XY with MAP3K1 mutations and gonadal dysgenesis, as examples. We also examine the GCT risk in non-DSD syndromes, in addition to the cancer risks in DSD patients with dimorphic gonads and genitalia. This review is intended to provide a nuanced assessment of relative germ cell tumor risk in the DSD patient, including modern precise molecular diagnosis, for use by the clinical geneticist.
Keywords: Difference of Sex Development, Germ Cell Tumor, Gonadal Development, Gonadoblastoma, Intersex
Introduction
Differences of Sex Development (DSD) encompass a diverse set of congenital conditions in which the development of chromosomal, gonadal, or anatomic sex is atypical. Although the term ‘DSD’ is commonly used interchangeably with ‘ambiguous genitalia’, only a subset of DSD patients has non-dimorphic (i.e. atypical) genitalia. Reports dating to the turn of the last century recognized the increased risk of gonadal tumors in some individuals with DSD [Abel, 1891]. Despite advances in genetic diagnosis, determining the personalized risk of gonadal germ cell tumor (GCT) for an individual with DSD remains a daunting task, with limited attempts to stratify risk by precise molecular etiology. As interpretation becomes increasingly complicated with the rapid identification of novel genetic causes of DSD [Achermann et al., 2015], the clinical geneticist has a critical role to play, participating as a member of a clinical team attempting to estimate the GCT risk for an individual patient with DSD.
The DSD consensus statement of 2006 established the standardized term DSD, and reorganized diagnoses into broad groups, subdivided primarily by specific chromosomal etiology, and secondarily by gonadal pathology and hormonal findings [Hughes et al., 2006]. With advances in genetic/molecular diagnostic technology, a more precise taxonomy is possible, and genetic testing can become the primary driver of categorization. The detection of a canonical mutation that causes a known DSD disorder with no minimal GCT risk, for example loss of function in CYP21A, the most common form of congenital adrenal hyperplasia (CAH), mitigates the need for additional clinical investigation including gonadal biopsy and pathologic analysis. In addition, the number of known causative genes and mutations for DSD is ever-increasing, resulting in entities that push the boundaries of the 2006 classification [Baxter et al., 2015; Eggers et al., 2016]. Twenty-five (25) single-gene causes of DSD were listed in the 2006 consensus statement; Baxter and colleagues evaluated 64 single-gene causes of 46,XY DSD alone in 2015, and in the tenth anniversary update of the 2006 consensus paper Lee and colleagues note “it is assumed that many DSD-causative genes remain to be identified…” [Baxter et al., 2015; Lee et al., 2016, 2006]. Eventually, elucidating the genotype-risk correlation may enable the development of GCT biomarkers and screening tools, providing a personalized risk assessment. Meanwhile, risk assessment is dependent on understanding the natural history of specific disease entities. This natural history is challenging to quantify because of: 1) high rate of prophylactic gonadectomy obscuring the natural history; 2) reporting bias of tumor cases; 3) imprecise DSD classification and molecular diagnoses; and 4) challenging histopathology leading to heterogeneous classification of GCT neoplasia.
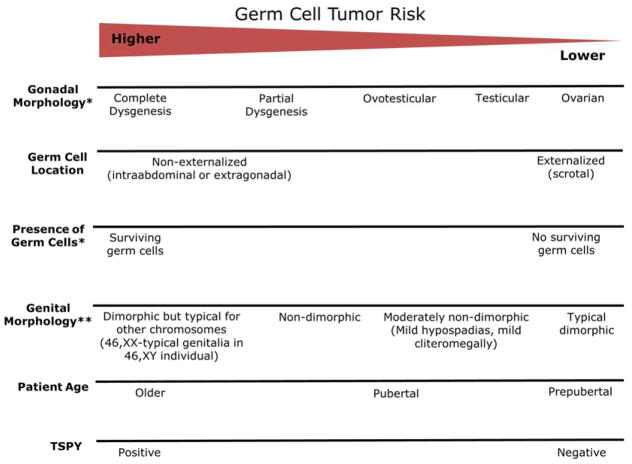
The decision to pursue gonadectomy in the patient with DSD is based on multiple parameters, including gender identity, gonadal GCT risk, and desire for fertility. Ideally, patient-specific recommendations are made by a formalized interdisciplinary team including minimally geneticists, endocrinologists, behavioral health specialists, and urologists [Palmer et al., 2012]. The benefit of gonadectomy includes both reduced gonadal GCT risk, and reduction of endogenous sex hormone production, if discordant with gender identity. Benefits of gonad retention can include endogenous hormone production congruent with gender identity, preservation of possible future fertility, and delay of the decision until the patient is the age of assent/consent. The parameters used to assign risk level include chromosomal compliment, particularly inclusion of Y-material; gonadal pathology (which requires surgical biopsy or gonadectomy to ascertain); phenotype of the genitals and reproductive organs; the endocrine milieu; and in some cases, the potential risk of syndrome-related complicating medical comorbidities such as adrenal insufficiency or cancer of other organs (Figure 1). Connecting these parameters with molecular etiology and GCT requires a knowledge of germ cell biology.
Figure 1.
Germ Cell Cancer Development
Germ cell development is a highly complex process requiring alignment spatially, temporally, and genetically (recently reviewed by [Tang et al., 2016; Hersmus et al., 2017]). The development of the human germ cell starts with specification of the primordial germ cells (PGC) from other somatic cells around the time of gastrulation (developmental week 2). Specification is driven by BLIMP1 and SOX17 [Irie et al., 2015] in humans, and PRDM14 in mice [Grabole et al., 2013]. The divergent primary drivers of human and mouse specification are emblematic of broader inter-species sex development variation, which presents challenges to accurate laboratory modeling. PGCs migrate in a cluster along the midline from the proximal epiblast into the genital ridge, guided by KIT/KITLG signaling, and reach their destination by weeks 5 to 6. Cells that do not reach the genital ridge apoptose. During migration PCGs undergo “licensing” (global demethylation) in preparation for imprinting, and after arrival at the undifferentiated gonad (in the genital ridge) PGCs are referred to as gonocytes (testicular) or oogonia (ovarian). PGCs until this time express several markers that are also histopathological markers of GCT, including OCT 3/4 and PLAP. Next, a cascade of signaling drives sex determination and assigns the developing gonad as future testicle or ovary. SRY and SOX9 are the primary pro-testicular drivers, working through multiple transcription factors including NR5A1 and ZFPM2 [Nikolova and Vilain, 2006]. Mutations in all four genes have been shown to cause DSD. Ovarian development is both the non-SRY default and proactively driven by signaling pathways, downstream of WNT4/β-catenin, FOXL2, and RSPO1 [Parma et al., 2006; Uhlenhaut et al., 2009; Mandel et al., 2008]. After the fate of the gonad is determined (testicular, ovarian, or atypical/non-dimorphic), the gonad becomes the driver of sex differentiation. (Gonads not morphologically typical for an ovary or testis are referred to in this review as ‘non-dimorphic’.) By week 9, Leydig and Sertoli cells of the developing typical testicle have differentiated and are producing testosterone, INSL3, and AMH, and by Week 11 the ovary is developing Theca and Granulosa cells.
At each step in this process, there is opportunity for maldevelopment of the germ cell. By definition, germ cells are the most pluripotent cells in the human body after embryogenesis; their hypomethylation and gene expression are most similar to embryonic stem cells and iPS cells [Hersmus et al., 2017; Van Der Zwan et al., 2013]. Any developmental derangement that has a sufficient impact on germ cell location or milieu, but permits cell survival, contributes to oncogenic potential. In contrast, environments that are sufficiently hostile as to induce germ cell death protect against GCT development, including complete androgen insensitivity and some variants of Turner syndrome (Figure 2). Due to the pluripotency of germ cells, GCTs form either germ cell-like uniform tumors (termed seminoma, dysgerminoma, or germinoma, based on the location: testicular, ovarian/non-dimorphic gonad, or extra-gonadal respectively), or more mixed embryonal lineages (non-seminoma, non-dysgerminoma, or non-germinoma) variably composed of choriocarcinoma, yolk sac tumor, immature teratoma, and embryonal carcinoma (Figure 3). Despite the variety of cell types, most GCTs share genetic signatures, including widespread hypomethylation and 12p amplification. Often a GCT precursor lesion can be identified, either gonadoblastoma (GB) in setting of an ovary or a non-dimorphic gonad, or germ cell neoplasia in situ (GCNIS) in the testicle. We will refer to these lesions as GCNIS/GB. The current testicular germ cell tumor (TGCT) oncogenesis model proposes that GCNIS is present from birth as a defect of fetal development, and is 100% predestined to eventually development into a malignant tumor (mean age 20–35yo) [Hoei-Hansen et al., 2005]. In contrast, it appears that GB can either be present at birth or develop later in life and only 50% will develop into malignant tumors [Cools et al., 2006a]. However, GCNIS/GB cannot be histologically distinguished from delayed gonocyte development prior to six, or even twelve, months of age [Cools et al., 2014]. Gonadal development can be delayed in a number of genetic disorders, including both DSDs and non-DSD syndromes, such as trisomy 21; this prolonged state of gonocyte immaturity increases the risk of oncologic transformation [Cools et al., 2006b]. Gonadal biopsy exclusively for the purposes of GCT risk assessment should therefore be delayed beyond one year of age.
Figure 2.
Figure 3.
Risk-Benefit Analysis of Gonadectomy
The decision to recommend gonadectomy is individualized when psychosocial factors are considered; however, concurrently, it lacks scientific precision based on genetic etiology. Attempts have been made to formalize risk factors including age, gonadal location, positive testing for TSPY, and genitalia phenotype, into clinical decision-making tools, but have not been universally adopted [van der Zwan et al., 2015]. As more DSD-causing genes are identified, there is a unique opportunity for clinical geneticists to follow the molecular diagnosis with rational gene-specific guidelines for malignant GCT screening and prevention. Creating such guidelines is a challenging venture, as much work remains to be done in understanding the relationship between genotype and phenotype in DSDs. For example, there is a high degree of variability in gonadal morphology and GCT risk levels, even within family members. Thus, the relationship between genotype and GCT-risk phenotype is not direct.
The risk/benefit analysis for prophylactic gonadectomy requires consideration of the success of an alternative screening and early treatment strategy. For those DSD patients retaining their gonads, proactive surveillance for malignant GCT is challenging. Imaging of intra-abdominal gonads has proven insufficient for detection of GCT [Wünsch et al., 2012; Nakhal et al., 2013; Alaniz et al., 2016; Hartigan and Tasian, 2014]. Alternative options have been proposed, including relocating gonads adjacent to biopsy laparoscopy port sites or the inguinal canal, to more easily examine and image them. Screening of the non-abdominal and accessible (scrotal) gonad is easily accomplished through trained self-exams in the adherent patient.
Data on treatment outcomes on malignant GCT related to specific etiologies of DSD are minimal due to rarity of patients. However, we can extrapolate treatment and survival risk from the testicular and ovarian germ cell tumors. Testicular GCT is relatively rare (lifetime risk 0.4%), and ten-year survival for testicular cancer survival is in excess of 95% [Fosså et al., 2011; Howlader et al.]. The high survival rates are, in part, secondary to GCT primordial differentiation state and exquisite sensitivity to platinum-based agents. Ovarian GCTs (OCGT) are much more rare (lifetime risk 0.07%) and also have a high survival rate (95% at five years) when treated with platinum-based therapy, despite the difficulty in self-detection of an intra-abdominal tumor [Solheim et al., 2014]. Post-treatment complications of GCT treatment include cardiovascular disease and metabolic syndrome, infertility, and need for hormonal supplementation [Travis et al., 2010]. From testicular and ovarian GCT, we can infer that gonadal GCT is responsive to treatment and survival is high. Infertility and hormonal treatment are often pre-existing conditions for the DSD patient, but cardiovascular disease is a survivorship risk. Appropriately diagnosed DSD patients have the added of advantage over individuals with dimorphic gonads of knowing they are at risk of cancer, although that may prefer to resolve the concern with gonadectomy.
GCT Risk Stratification by 2006 Taxonomy
Risk analyses since 2006 of for GCT in DSD individuals have been based upon categorization put forth in the DSD Consensus Nomenclature, which stratifies first by karyotype, followed by gonadal pathology and hormonal milieu, when available. In 2007, Looijenga and colleagues reviewed studies of GCT risk in various DSD diagnoses known at that time, and developed risk categories based on the consensus nomenclature [Looijenga et al., 2007], which have been incrementally refined since that time. GCT risk is extrapolated from the rate of GCNIS/GB identified on prophylactic gonadectomy or biopsy, although the frequency of GCNIS/GB transformation into invasive tumor is not known. Presence of the testes-specific protein Y-linked region (TSPY) is required for GCT risk in patients with DSD. However it is important to note that 46,XX women can develop invasive ovarian GCT [Lau et al., 2009]. The risk of GCNIS/GB among TSPY-positive individuals with WT1 mutations (Frasier or Denys-Drash syndromes) is ~50%. Other conditions that result in gonadal dysgenesis carry a risk variably reported between 12–40%. Importantly, these risks are often quoted as lifetime risks, but these data are largely gathered from individuals from birth to ~25 years, and the true natural history is not known. Gonadal dysgenesis may develop secondary to defects of any step of gonadal development including sex determination, e.g. changes in SRY), decreased androgen/defects of androgen sensing (sex differentiation, e.g. partial androgen insensitivity [PAIS] with intra-abdominal gonads), or generalized sex chromosome aneuploidy (e.g. constitutional 45,X, or 45,X/46XY mosaicism (Table I). Complete androgen insensitivity (CAIS), Turner syndrome without Y-material, and conditions with ovotestes (in which fully differentiated ovarian and testicular tissue are both present) have the lowest GCT risk. Molecular data can further risk stratify these groups. As one such example, individuals with an external CAIS phenotype who have a genotype consistent with residual androgen receptor activity (PAIS) are in fact at higher risk of GCT [Şimşek et al., 2016].
Table I.
DSD Classification based on 2006 Consensus Nomenclature
| Sex Chromosome | 46,XY | 46,XX |
|---|---|---|
| 45,X Turner | Disorder of Sex Determination | Disorder of Sex Determination |
| 47,XXY Klinefelter | - loss of function in testicular determination pathway | - gain of function in testicular determination pathway |
| 47,XXX Trisomy X | ||
| 45,X/46,XY & other 45,X with Y | Disorder of Sex Differentiation | Disorder of Sex Differentiation |
| 46,XX/46,XY | - loss of androgen synthesis or action | - excess androgen production |
| - loss of AMH signaling | ||
| Anatomical | Anatomical | |
| - cloacal extrophy | - cloacal extrophy | |
| - MRKH/MURCS | ||
| - vaginal atresia |
To survey the literature for newer reports of DSD patients and GCNIS/GB rates, we conducted a PubMed search for “disorder of sex development” and “cancer”, from January 2006 to January 2017 (Figure 4). The search identified 96 publications which described GCNIS/GB in the setting of DSD, 171 publications reporting non-GCT cancers in DSD, and twelve general publications on GCT with no mention of DSD. Five publications were large retrospective series of greater than 24 patients. The term DSD was inconsistently applied throughout the literature; some authors used DSD to indicate only the subset of patients with non-dimorphic genitalia. Ages, phenotypes, and gonadal histology were variably reported in the case reports (under five patients), and less than five of the case series (five patients or more) detailed all three variables along with molecular etiology. Despite these limitations, newer publications suggested no major change to the gonadal tumor risk stratification previously proposed by Loojenga et al., although the estimated level of risk varied from the original publication (Table II). Further searches for specific DSD diagnoses by more precise names (e.g. AIS) revealed additional publications not captured in Figure 4. Both approaches were using in the following sections, which review the invasive GCT risk for some illustrative causes of DSD.
Figure 4.
Table II.
Lifetime risk of GCNIS/GB in various DSD types (updated)
| DSD Type | % lifetime GCNIS/GB risk |
|---|---|
| TSPY positive with WT1 Mutations (Frasier and Denys-Drash syndromes) | 40–60 |
| TSPY positive gonadal dysgenesis | 12–40 |
| PAIS | 15–20 |
| CAIS | 0.8–15 |
| Ovotesticular DSD | 2.6 |
Androgen Receptor Loss of Function – A Well Described DSD Etiology
Androgen insensitivity (AIS) is a well described cause of DSD resulting from androgen receptor (AR) loss of function mutations (review [Hughes et al., 2012]). Mutations in 46,XY individuals result in normal testicular determination, however androgen-responsive sex differentiation is diminished. Truncating and other severe mutations (complete androgen insensitivity [CAIS]) result in testicular morphology with paucity of germ cells, but some germ cells survive in patients with hypomorphic mutations (PAIS) [Szafran et al., 2009; Batch et al., 1993]. The risk of GCT in AIS echoes the mutation severity, but inversely. Full loss of AR function reduces GCT risk secondary to loss of germ cells (Figure 5). For women with CAIS, the pre-pubertal risks of malignancy are estimated to be 0.8–2% [Cools et al., 2006a], whereas risks for adult women may be as high as 15% (0–22%) [Deans et al., 2012]. Gonadectomy is sometimes deferred until time of puberty and age of assent, in part to allow for endogenous androgen production, which is peripherally aromatized into estrogen [Deans et al., 2012; Mouriquand et al., 2016]. In contrast, individuals with PAIS are more likely to have surviving germ cells than CAIS, and thus have a higher risk of GCT [Nakhal et al., 2013]. Older studies quote a GCNIS/GB risk of approximately 15% in PAIS, consistent with a more recent paper reporting, of women 16–34 years old, 3/18 patients with GCNIS and none with malignant cancer [Pleskacova et al., 2010; Liu et al., 2014]. In children that are assigned male gender, the gonads are often put into the scrotum which mitigates the cancer risk. The external genitalia phenotype usually echoes the severity of the mutations, with complete AR loss of function resulting in typical female genitalia. However, this association in not absolute [Hughes et al., 2012], and it is important that a diagnosis of complete versus partial AIS not be assigned based on phenotype alone, prior to mutation identification.
Figure 5.
Mutations in MAP3K1 – A “New” Cause of 46,XY Gonadal Dysgenesis
Mutations in MAP3K1 have been recently identified as a cause of DSD, accounting for 13–18% of patients with 46,XY gonadal dysgenesis [Ostrer, 2014]. 46,XX individuals appear to have no gonadal abnormalities, whereas gonadal morphology in 46,XY individuals includes gonadal dysgenesis, streak gonads, and absence of testicular tissue [Das et al., 2013; Eggers et al., 2016; Lee et al., 2015]. Within the same family, phenotypes can range from mild hypospadias and retained male fertility to clitoral hypertrophy and complete gonadal dysgenesis [Pearlman et al., 2010]. MAP3K1 was first identified as mutated in association with DSD in a family of 11, of which four were 46,XY males with no signs of tumor, and seven were 46,XY females, two of which had dysgerminoma (including the study proband at 7 years old) [Jawaheer et al., 2003]. Human variants subtly alter the balance of pro-testicular (SOX9/FGF9) and pro-ovarian (WNT4/β-catenin) signaling in vitro via gain-of-function mutations [Loke et al., 2014]. Upregulation of the MAPK signaling pathway are well described across multiple tumor types (particularly breast cancer) secondary to MAP3K1 loss-of-function mutations [Pham et al., 2013]. Given the involvement of mutations in MAP3K1 in other cancer types, and a single report of a seven-year-old with malignant GCT, MAP3K1 mutations should be regarded with concern in the patient in intra-abdominal gonads. MAP3K1 genotype/phenotype correlations will require additional clinical reports with detailed information including age, genital phenotype, intrafamilial phenotype variability, and gonadal histology.
Smith-Lemli-Opitz - A Classical Malformation and Metabolic Syndrome
DSD is associated with other congenital anomalies in 25% of cases, including classical malformation syndromes such as Smith-Lemli-Opitz (SLO) [Cox et al., 2014]. Genital anomalies are found in 74% of patients with SLO, and 65% of those with biochemically confirmed SLO (elevated 7-dehydrocholesterol secondary to defects of DHCR7). SLO external genitalia in 46,XY individuals include typical male, typical female, and the full non-dimorphic genital spectrum between [Kelley and Hennekam, 2000]. Normal testes, absent gonads, and ovotestes have been reported [Lachman et al., 1991; Kohler, 1983]. Based on genital morphology and karyotype alone, these patients would be classified as 46,XY DSD with unknown gonad status, and may have been assumed to have a high risk of GCT. However, there is are only two reports of GCT in SLO, which may represent coincidental association. A 19 year old 46,XX female without reported molecular/biochemical confirmation of SLO presented with a mixed malignant germ cell tumor of the ovary [Patsner et al., 1989]. An additional patient, a 14-year-old 46,XY male with genetically confirmed SLO, presented with a malignant germinoma of the brain [Oslejskova et al., 2008]. It is intriguing to consider why loss of androgen signaling in the setting of androgen insensitivity increases gonadal GCT risk in the 46,XY individual but, apparently, loss of androgen signaling via decreased androgen synthesis (as in SLO) does not.
Y-Containing Turner Syndrome
Turner Syndrome patients with Y-containing material, specifically the TSPY region have a 12–40% risk of GCNIS/GB [Looijenga et al., 2007; Lau et al., 2009]. In most studies, patients with Turner Syndrome with multiple Y-variants are grouped in a single cohort. This grouping means that stratification based on levels of mosaicism, inclusion/exclusion of TSPY, translocated portions of the Y, rings and other Y chromosome variants, is lacking. Although non-TSPY containing Turner Syndrome is technically considered a DSD, these patients are at negligible risk of GCT. Review of publications contained within the above search (Figure 4) for those including ten or more patients with Y-positive Turner Syndrome provided seven reports since 2006 in addition to the UK national cohort study (Table III). These publications describe 99 patients, including 26 cases of GCNIS/GB (26.2%), and three patients with dysgerminoma (3.0%). In contrast, a UK cohort study published within the same time frame including 212 patients described five (2.4%) with GCNIS/GB and two (1.0%) with dysgerminoma [Schoemaker et al., 2008]. The differing rates are likely due to reporting or lead-time bias. This high level of discrepancy highlights the difficulty in providing precise guidelines for patients. Individuals with dysgerminoma ranged in age from 13 to 24 years old, concordant with the model where-in malignant GCT risk increases with age, and gonadectomy should be considered at puberty. However, our center has identified dysgerminoma in Y-positive Turner patients as young as six and ten years old (unpublished cases). Cools and colleagues reported a correlation between genital phenotype (external masculinization score) and the presence of GCNIS/GB, but other groups were unable to replicate the finding [Cools et al., 2011; Lindhardt Johansen et al., 2012]. A Y-positive Turner Syndrome patient with externalized scrotal gonads (versus abdominal) has relatively reduced GCT risk, but otherwise, level of genital non-dimorphism is not a reliable predictor of gonadal morphology. This rule carries over to other non-dimorphic DSD conditions, with the exception of specific molecular diagnoses that are extremely well studied, such as androgen receptor loss of function (above).
Table III.
GCT reported in Series of Y-positive Turner Syndrome Since 2006
| Population | Patients | Age at Gonadectomy | GCNIS/GB | Dysgerminoma (malignant GCT) | Source |
|---|---|---|---|---|---|
| 45,X with Y-material | 20 | 17 weeks – 20 years | 3 | 1 | [Cools et al., 2011] |
| 45,X/46XY mosaic | 15 | 0–20 years | 2 | 0 | [Lindhardt Johansen et al., 2012] |
| 45,X with Y-material | 11 | 8 months – 55 years | 2 | 0 | [Bianco et al., 2009] |
| 45,X with Y-material | 16 | 8 – 18 years | 4 | ? | [Barros et al., 2011] |
| 45,X with Y-material | 17 | 6 | 1 | [Zelaya et al., 2015] | |
| 45,X/46XY mosaic | 14 | 5 months – 13 years | 4 | 0 | [Coyle et al., 2016] |
| 45,X with Y-material | 6 | 7 – 18 years | 5 | 1 | [Silveri et al., 2015] |
46,XX DSDs
46,XX patients who are not mosaic for the TSPY-region are thought to have no increased GCT risk. Guidelines suggest testing for Y-material in the otherwise unremarkable female with OGCT [Stewart et al., 2007; Colombo et al., 2012]. It is important to note, 46,XX individuals can present with OGCT in the absence of TSPY, demonstrating that TSPY does not need to be constitutionally present for GCT to develop.
Congenital adrenal hyperplasia (CAH) secondary to loss of function mutations in CYP21A is the most common DSD in individuals with a 46,XX karyotype. CAH is associated with increased risk of non-GCT tumors of the gonad, particularly benign gonadal adrenal rest tumors. Despite the high prevalence of CYP21A-associated CAH (>1/1000 individuals) GCNIS/GB has never been reported [Claahsen-van der Grinten et al., 2009]. There are individual reports of GCT among the other, more rare causes of CAH, including mutations in CYP17A1 and HSD17B3. Two separate reports describe 17 year old 46,XX patients with CYP17A1 loss of function mutations, one found to have GCNIS/GB within streak gonads, and the other presented with malignant GCT [Deeb et al., 2015; Brooke et al., 2006]. These reports most likely represent coincidence, but may represent the rare finding indicating that 46,XX individuals with increased androgen are at increased risk of gonadal GCT.
GCT in non-DSD Genetic Diagnoses
Increased rates of germ cell tumor have been associated with various non-DSD genetic syndrome. The strongest association is with trisomy 21 (Table IV). 46,XY individuals with trisomy 21 have decreased risk of solid tumors, with the notable exception of TGCT (SIR 2.9; 95% CI 1.6–4.8) [Hasle et al., 2016]. Evaluation of infants with trisomy 21 revealed constitutional delay of germ cell maturation that may be akin to the delayed primordial gonocyte development identified in non-syndromic DSD, and explain the association between trisomy 21 and TGCT [Cools et al., 2006b]. An association has been hypothesized between Russell-Silver Syndrome and GCT on the basis of two case reports. However, it has not been confirmed with larger population-based studies [Funada et al., 2016; Weiss and Garnick, 1981]. Schinzel-Giedion syndrome is classically associated with GCT, but specifically with extragonadal mature neonatal sacrocoxygeal teratoma; however, in one report a Schinzel-Giedion associated teratoma contained components of non-germinoma [Robin et al., 1993; Kishimoto et al., 2015]. Overall, the GCT risk associated with Russell-Silver Syndrome is unconvincing, but the GCT risk in trisomy 21 is significant, and presents a research opportunity to better appreciate chromosome 21 influence on sex development in the 46,XY individual.
Table IV.
Risk of GCT in non-DSD syndromes
| Non-DSD Syndromes with GCT Risk | Evidence |
|---|---|
| Trisomy 21 | SIR 2.9; 95% CI 1.6–4.8 in large population |
| Russell-Silver Syndrome | 2 case reports |
| Schinzel-Giedion | strong association, 12p-negative GCT |
Extragonadal Cancer Predisposition in Dimorphic DSDs
Klinefelter Syndrome (47,XXY) is associated with a specific risk of mediastinal GCT. Extragonadal GCTs arise from germ cells that both failed to migrate appropriately to the genital ridge, and failed to apoptose; along with shared underlying disease origin, mediastinal and gondadal GCTs also share genetic signatures including hypomethylation and 12p copy number increases [Oosterhuis et al., 2007]. Unlike gonadal GCTs, mediastinal GCTs have an increased rate of platinum-based therapy resistance secondary to high expression of TP53, resulting in survival of only ~50% [Bagrodia et al., 2016]. Approximately 8% of men with mediastinal GCT have Klinefelter syndrome [De Sanctis et al., 2013], and it is important to screen cases of mediastinal GCT for covert Klinefelter. In addition, individuals with Klinefelter syndrome have cancer risks thought to be associated with the additional X-chromosome, including a 19–58-fold risk of breast cancer versus 46,XY men [Ferzoco and Ruddy, 2016; Brinton, 2011].
In contrast, Turner Syndrome without TSPY is associated with an increased risk of a number of non-GCT cancers. The UK national cohort study evaluated 3425 patients with 45,X and a variety of chromosome variants. Although, no overall increased risk of cancer (SIR 0.9 [0.7–1.2]) was found, increased risks for specific rare cancers including CNS (SIR 4.3 [2.3–7.4]), particularly meningeal (SIR 12.0 [4.8–24.8]), eye (SIR 10.5 [1.3–37.9]), and bladder/urethra (SIR 4.0 [1.3–9.2]) [Schoemaker et al., 2008]. The risk to individuals with Turner Syndrome and other sex chromosome aneuploidies of CNS cancer in particular is a focus of on-going investigation.
Among DSDs without chromosomal abnormalities, individuals with Mullerian remnants secondary to Mayer-Rokitansky-Kuster-Hauser (MRKH) or Persistent Mullerian Duct Syndrome (PMDS) are at increased risk of uterine leiomyomas, although these findings are confined to case reports, and are not considered indication for prophylactic resection of remnants [Kovachev et al., 2014]. All of these cancers are relatively rare, even within these rare populations, but are important to include in genetic counseling and genetic cancer predisposition teaching for patients with these diagnoses.
Conclusions
Determining the GCT risk for an individual with a DSD requires precise taxonomical categorization based on karyotype, molecular data, hormonal milieu, external phenotype and gonadal morphology. More-over, psychosocial factors must be taken into account, including patient comfort with a risk of GCT and self-determination over gonadectomy decisions. These psychosocial factors therefore become particularly challenging in the pediatric setting, where the patient is not yet at age of assent. An interdisciplinary DSD team including specialists from endocrinology, psychology, urology, genetics, and for some discussions, oncology, provides the ideal context for collection, interpretation, and patient counseling on DSD GCT predisposition.
Molecular diagnosis for DSD conditions is becoming more specific; however, lack of natural history data inhibits the clinician’s ability to assign GCT risk level based on genotype. Large, multi-institutional, prospective studies are required to better quantify the relationship between genetic etiology and development of GCNIS/GB. But given the frequency of early prophylactic gonadectomy, empirical data is limited, and we must develop alternate approaches. Individual patient mutations can be modeled in the research setting to examine their effects on germ cell biology and appraise personalized GCT risk. Novel screening tools for GCT, including peripheral blood miRNA testing and non-invasive labeled imaging of gonadal tissue, are in development and have the potential to radically transform screening and surgical guidelines for DSD. Pending availability of these technologies, the clinical geneticist is the primary resource for timely and rational genetic testing, with expert interpretation. Such data supports the multidisciplinary team’s effort to employ more conservative hormonal and surgical approaches while assessing GCT risk.
It is widely held in the dimorphic testicular and ovarian literature that there is no single high-penetrance gene predisposing to GCT. In fact, many of the genes causing DSD are just that, highly penetrant causes of GCT. Any genetic variation in germ cell development significant enough to strongly predispose to GCT will also, by definition, also cause differences in sex development. The well-elucidated breast cancer predisposition genes are analogous, including low-to-high penetrance variants, and a broad spectrum of presentation within a single family. The genetic continuum of GCT predisposition reflects the complexity of sex and germ cell development, a process involving multiple pathways and developmentally-dependent changes in signaling. A similar variety of predisposing loci have been identified in testicular GCT, with the addition of several non-DSD oncogenes [Pyle and Nathanson, 2016]. Variations in these oncogenes may prove additional modifiers to GCT penetrance in the DSD setting, and support yet more patient-specific GCT risk stratification in the future.
Acknowledgments
This work was supported by National Institutes of Health grants CA164947 (KLN), CA114478 (KLN), and T32GM008638 (LCP) and the Abramson Cancer Center at the University of Pennsylvania. Dr. Pyle and Dr. Nathanson have no conflicts of interest to declare. We wish to thank our colleagues Nicholas Cost, MD (Children’s Hospital Colorado) for insight and literature referrals, Rebecca Ganetzky, MD (Children’s Hospital of Philadelphia) for review and editing of the manuscript, and Christopher Ross-Gill for figure graphics support.
Biographies
Louise C. Pyle, M.D., Ph.D. is an Attending Physician in the Division of Human Genetics at the Children’s Hospital of Philadelphia, and Research Fellow in the Division of Translational Medicine and Human Genetics in the Department of Medicine at the Perelman School of Medicine at the University of Pennsylvania in Philadelphia, PA. She sees patients with Differences of Sex Development (DSD) at the Children’s Hospital of Philadelphia, and has a special interest in improving DSD patient care through identifying genetic/genomic changes predisposing to germ cell tumor risk.
Katherine L. Nathanson, M.D. is Professor of Medicine in the Division of Translational Medicine and Human Genetics in the Department of Medicine at the Perelman School of Medicine of the University of Pennsylvania. She also is Associate Director for Population Sciences at the Abramson Cancer Center. Her research focuses on inherited and somatic genetic/genomic changes in cancer, specifically in testicular germ cell tumors, and how discoveries in this area can be applied to improve patient care.
References
- Abel Ein Fall von Hermaphroditismus mit sarkomatöser Cryptorchis sinistra. Virchows Arch f pathol Anat u Physiol. 1891;126:420. [Google Scholar]
- Achermann JC, Domenice S, Bachega TASS, Nishi MY, Mendonca BB. Disorders of sex development: effect of molecular diagnostics. Nat Rev Endocrinol. 2015;11:478–488. doi: 10.1038/nrendo.2015.69. [DOI] [PubMed] [Google Scholar]
- Alaniz VI, Kobernik EK, Dillman J, Quint EH. Utility of Ultrasound and Magnetic Resonance Imaging in Patients with Disorders of Sex Development Who Undergo Prophylactic Gonadectomy. J Pediatr Adolesc Gynecol. 2016;29:577–581. doi: 10.1016/j.jpag.2016.03.007. [DOI] [PubMed] [Google Scholar]
- Bagrodia A, Lee BH, Lee W, Cha EK, Sfakianos JP, Iyer G, Pietzak EJ, Gao SP, Zabor EC, Ostrovnaya I, Kaffenberger SD, Syed A, Arcila ME, Chaganti RS, Kundra R, Eng J, Hreiki J, Vacic V, Arora K, Oschwald DM, Berger MF, Bajorin DF, Bains MS, Schultz N, Reuter VE, Sheinfeld J, Bosl GJ, Al-Ahmadie HA, Solit DB, Feldman DR. Genetic Determinants of Cisplatin Resistance in Patients With Advanced Germ Cell Tumors. J Clin Oncol. 2016;34:4000–4007. doi: 10.1200/JCO.2016.68.7798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batch JA, Davies HR, Evans BA, Hughes IA, Patterson MN. Phenotypic variation and detection of carrier status in the partial androgen insensitivity syndrome. Arch Dis Child. 1993;68:453–7. doi: 10.1136/adc.68.4.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter RM, Arboleda VA, Lee H, Barseghyan H, Adam MP, Fechner PY, Bargman R, Keegan C, Travers S, Schelley S, Hudgins L, Mathew RP, Stalker HJ, Zori R, Gordon OK, Ramos-Platt L, Pawlikowska-Haddal A, Eskin A, Nelson SF, Délot E, Vilain E. Exome sequencing for the diagnosis of 46,XY disorders of sex development. J Clin Endocrinol Metab. 2015;100:E333–44. doi: 10.1210/jc.2014-2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton LA. Breast cancer risk among patients with Klinefelter syndrome. Acta Paediatr. 2011;100:814–8. doi: 10.1111/j.1651-2227.2010.02131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooke AM, Taylor NF, Shepherd JH, Gore ME, Ahmad T, Lin L, Rumsby G, Papari-Zareei M, Auchus RJ, Achermann JC, Monson JP. A Novel Point Mutation in P450c17 (CYP17) Causing Combined 17α-Hydroxylase/17,20-Lyase Deficiency. J Clin Endocrinol Metab. 2006;91:2428–2431. doi: 10.1210/jc.2005-2653. [DOI] [PubMed] [Google Scholar]
- Claahsen-van der Grinten HL, Otten BJ, Stikkelbroeck MML, Sweep FCGJ, Hermus ARMM. Testicular adrenal rest tumours in congenital adrenal hyperplasia. Best Pract Res Clin Endocrinol Metab. 2009;23:209–220. doi: 10.1016/j.beem.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Colombo N, Peiretti M, Garbi A, Carinelli S, Marini C, Sessa C ESMO Guidelines Working Group. Non-epithelial ovarian cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23:vii20–vii26. doi: 10.1093/annonc/mds223. [DOI] [PubMed] [Google Scholar]
- Cools M, Drop SLS, Wolffenbuttel KP, Oosterhuis JW, Looijenga LHJ. Germ Cell Tumors in the Intersex Gonad: Old Paths, New Directions, Moving Frontiers. 2006a doi: 10.1210/er.2006-0005. http://dx.doi.org/10.1210/er.2006-0005. [DOI] [PubMed]
- Cools M, Honecker F, Stoop H, Veltman JD, de Krijger RR, Steyerberg E, Wolffenbuttel KP, Bokemeyer C, Lau Y-FC, Drop SLS, Looijenga LHJ. Maturation delay of germ cells in fetuses with trisomy 21 results in increased risk for the development of testicular germ cell tumors. Hum Pathol. 2006b;37:101–111. doi: 10.1016/j.humpath.2005.09.021. [DOI] [PubMed] [Google Scholar]
- Cools M, Looijenga LHJ, Wolffenbuttel KP, T’Sjoen G. Managing the Risk of Germ Cell Tumourigenesis in Disorders of Sex Development Patients. Endocrine development. 2014:185–196. doi: 10.1159/000363642. [DOI] [PubMed] [Google Scholar]
- Cools M, Pleskacova J, Stoop H, Hoebeke P, Van Laecke E, Drop SLS, Lebl J, Oosterhuis JW, Looijenga LHJ, Wolffenbuttel KP Mosaicism Collaborative Group. Gonadal Pathology and Tumor Risk in Relation to Clinical Characteristics in Patients with 45,X/46,XY Mosaicism. J Clin Endocrinol Metab. 2011;96:E1171–E1180. doi: 10.1210/jc.2011-0232. [DOI] [PubMed] [Google Scholar]
- Cox K, Bryce J, Jiang J, Rodie M, Sinnott R, Alkhawari M, Arlt W, Audi L, Balsamo A, Bertelloni S, Cools M, Darendeliler F, Drop S, Ellaithi M, Guran T, Hiort O, Holterhus P-M, Hughes I, Krone N, Lisa L, Morel Y, Soder O, Wieacker P, Ahmed SF. Novel Associations in Disorders of Sex Development: Findings From the I-DSD Registry. J Clin Endocrinol Metab. 2014;99:E348–E355. doi: 10.1210/jc.2013-2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das DK, Rahate SG, Mehta BP, Gawde HM, Tamhankar PM. Mutation analysis of mitogen activated protein kinase 1 gene in Indian cases of 46,XY disorder of sex development. Indian J Hum Genet. 2013;19:437–42. doi: 10.4103/0971-6866.124372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deans R, Creighton SM, Liao L-M, Conway GS. Timing of gonadectomy in adult women with complete androgen insensitivity syndrome (CAIS): patient preferences and clinical evidence. Clin Endocrinol (Oxf) 2012;76:894–898. doi: 10.1111/j.1365-2265.2012.04330.x. [DOI] [PubMed] [Google Scholar]
- Deeb A, Al Suwaidi H, Attia S, Al Ameri A. 17-hydroxylase/17,20-lyase deficiency due to a R96Q mutation causing hypertension and poor breast development. Endocrinol diabetes Metab case reports. 2015;2015:150069. doi: 10.1530/EDM-15-0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers S, Sadedin S, van den Bergen JA, Robevska G, Ohnesorg T, Hewitt J, Lambeth L, Bouty A, Knarston IM, Tan TY, Cameron F, Werther G, Hutson J, O’Connell M, Grover SR, Heloury Y, Zacharin M, Bergman P, Kimber C, Brown J, Webb N, Hunter MF, Srinivasan S, Titmuss A, Verge CF, Mowat D, Smith G, Smith J, Ewans L, Shalhoub C, Crock P, Cowell C, Leong GM, Ono M, Lafferty AR, Huynh T, Visser U, Choong CS, McKenzie F, Pachter N, Thompson EM, Couper J, Baxendale A, Gecz J, Wheeler BJ, Jefferies C, MacKenzie K, Hofman P, Carter P, King RI, Krausz C, van Ravenswaaij-Arts CMA, Looijenga L, Drop S, Riedl S, Cools M, Dawson A, Juniarto AZ, Khadilkar V, Khadilkar A, Bhatia V, Düng VC, Atta I, Raza J, Thi Diem Chi N, Hao TK, Harley V, Koopman P, Warne G, Faradz S, Oshlack A, Ayers KL, Sinclair AH. Disorders of sex development: insights from targeted gene sequencing of a large international patient cohort. Genome Biol. 2016;17:243. doi: 10.1186/s13059-016-1105-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferzoco RM, Ruddy KJ. The Epidemiology of Male Breast Cancer. Curr Oncol Rep. 2016;18:1. doi: 10.1007/s11912-015-0487-4. [DOI] [PubMed] [Google Scholar]
- Fosså SD, Cvancarova M, Chen L, Allan AL, Oldenburg J, Peterson DR, Travis LB. Adverse Prognostic Factors for Testicular Cancer–Specific Survival: A Population-Based Study of 27,948 Patients. J Clin Oncol. 2011;29:963–970. doi: 10.1200/JCO.2010.32.3204. [DOI] [PubMed] [Google Scholar]
- Funada S, Ikeuchi R, Yoshida T, Segawa T. Seminoma in a Man with Russell-Silver Syndrome Presenting with Testicular Torsion. Case Rep Urol. 2016;2016:1–3. doi: 10.1155/2016/6017636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabole N, Tischler J, Hackett JA, Kim S, Tang F, Leitch HG, Magnúsdóttir E, Surani MA. Prdm14 promotes germline fate and naive pluripotency by repressing FGF signalling and DNA methylation. EMBO Rep. 2013;14:629–37. doi: 10.1038/embor.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartigan S, Tasian GE. Unnecessary diagnostic imaging: a review of the literature on preoperative imaging for boys with undescended testes. Transl Androl Urol. 2014;3:359–64. doi: 10.3978/j.issn.2223-4683.2014.11.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasle H, Friedman JM, Olsen JH, Rasmussen SA. Low risk of solid tumors in persons with Down syndrome. Genet Med. 2016;18:1151–1157. doi: 10.1038/gim.2016.23. [DOI] [PubMed] [Google Scholar]
- Hersmus R, van Bever Y, Wolffenbuttel KP, Biermann K, Cools M, Looijenga LHJ. The biology of germ cell tumors in disorders of sex development. Clin Genet. 2017;91:292–301. doi: 10.1111/cge.12882. [DOI] [PubMed] [Google Scholar]
- Hoei-Hansen CE, Rajpert-De Meyts E, Daugaard G, Skakkebaek NE. Carcinoma in situ testis, the progenitor of testicular germ cell tumours: a clinical review. Ann Oncol. 2005;16:863–8. doi: 10.1093/annonc/mdi175. [DOI] [PubMed] [Google Scholar]
- Howlader N, Noone A, Krapcho M, Miller D, Bishop K, Altekruse S, Kosary C, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis D, Chen H, Feuer E, Cronin K, editors. SEER Cancer Statistics Review, 1975–2013. National Cancer Institute; Bethesda, MD: Apr, 2016. http://seer.cancer.gov/csr/1975_2013/ based on November 2015 SEER data submission, posted to the SEER web site. [Google Scholar]
- Hughes IA, Houk C, Ahmed SF, Lee PA LWPES Consensus Group, ESPE Consensus Group. Consensus statement on management of intersex disorders. Arch Dis Child. 2006;91:554–63. doi: 10.1136/adc.2006.098319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes I, Werner R, Bunch T, Hiort O. Androgen Insensitivity Syndrome. Semin Reprod Med. 2012;30:432–442. doi: 10.1055/s-0032-1324728. [DOI] [PubMed] [Google Scholar]
- Irie N, Weinberger L, Tang WWC, Kobayashi T, Viukov S, Manor YS, Dietmann S, Hanna JH, Surani MA. SOX17 is a critical specifier of human primordial germ cell fate. Cell. 2015;160:253–68. doi: 10.1016/j.cell.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jawaheer D, Juo S-HH, Le Caignec C, David A, Petit C, Gregersen P, Dowbak S, Damle A, McElreavey K, Ostrer H. Mapping a gene for 46,XY gonadal dysgenesis by linkage analysis. Clin Genet. 2003;63:530–5. doi: 10.1034/j.1399-0004.2003.00082.x. [DOI] [PubMed] [Google Scholar]
- Kelley RI, Hennekam RC. The Smith-Lemli-Opitz syndrome. J Med Genet. 2000;37:321–35. doi: 10.1136/jmg.37.5.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto K, Kobayashi R, Yonemaru N, Yamamoto H, Tsujioka T, Sano H, Suzuki D, Yasuda K, Suzuki M, Ando A, Tonoki H, Iizuka S, Uetake K, Kobayashi K. Refractory Sacrococcygeal Germ Cell Tumor in Schinzel-Giedion Syndrome. J Pediatr Hematol Oncol. 2015;37:e238–e241. doi: 10.1097/MPH.0000000000000236. [DOI] [PubMed] [Google Scholar]
- Kohler HG. Brief clinical report: familial neonatally lethal syndrome of hypoplastic left heart, absent pulmonary lobation, polydactyly, and talipes, probably Smith-Lemli-Opitz (RSH) syndrome. Am J Med Genet. 1983;14:423–8. doi: 10.1002/ajmg.1320140304. [DOI] [PubMed] [Google Scholar]
- Kovachev SM, Nikolov SD, Mihova AP. Uterine leiomyoma in a man with persistent Müllerian duct syndrome and seminoma. Isr Med Assoc J. 2014;16:735–7. [PubMed] [Google Scholar]
- Lachman MF, Wright Y, Whiteman DA, Herson V, Greenstein RM. Brief clinical report: a 46,XY phenotypic female with Smith-Lemli-Opitz syndrome. Clin Genet. 1991;39:136–41. doi: 10.1111/j.1399-0004.1991.tb03000.x. [DOI] [PubMed] [Google Scholar]
- Lau Y-FC, Li Y, Kido T. Gonadoblastoma locus and the TSPY gene on the human Y chromosome. Birth Defects Res Part C Embryo Today Rev. 2009;87:114–122. doi: 10.1002/bdrc.20144. [DOI] [PubMed] [Google Scholar]
- Lee J, Park JK, Kim DS, Lee HS, Choi SI, Cho YG. Detailed analysis of isodicentric Y in a case with azoospermia and 45,x/46,x,idic(Y) mosaicism. Ann Clin Lab Sci. 2015;45:206–8. [PubMed] [Google Scholar]
- Lee PA, Houk CP, Ahmed SF, Hughes IA International Consensus Conference on Intersex organized by the Lawson Wilkins Pediatric Endocrine Society and the European Society for Paediatric Endocrinology. Consensus Statement on Management of Intersex Disorders. Pediatrics. 2006;118:e488–e500. doi: 10.1542/peds.2006-0738. [DOI] [PubMed] [Google Scholar]
- Lee PA, Nordenström A, Houk CP, Ahmed SF, Auchus R, Baratz A, Baratz Dalke K, Liao L-M, Lin-Su K, Looijenga LHJ, Mazur T, Meyer-Bahlburg HFL, Mouriquand P, Quigley CA, Sandberg DE, Vilain E, Witchel S Global DSD Update Consortium and the GDU. Global Disorders of Sex Development Update since 2006: Perceptions, Approach and Care. Horm Res pædiatrics. 2016;85:158–80. doi: 10.1159/000442975. [DOI] [PubMed] [Google Scholar]
- Lindhardt Johansen M, Hagen CP, Rajpert-De Meyts E, Kjærgaard S, Petersen BL, Skakkebæk NE, Main KM, Juul A. 45,X/46,XY Mosaicism: Phenotypic Characteristics, Growth, and Reproductive Function—A Retrospective Longitudinal Study. J Clin Endocrinol Metab. 2012;97:E1540–E1549. doi: 10.1210/jc.2012-1388. [DOI] [PubMed] [Google Scholar]
- Liu A-X, Shi H-Y, Cai Z-J, Liu A, Zhang D, Huang H-F, Jin HM. Increased risk of gonadal malignancy and prophylactic gonadectomy: a study of 102 phenotypic female patients with Y chromosome or Y-derived sequences. Hum Reprod. 2014;29:1413–1419. doi: 10.1093/humrep/deu109. [DOI] [PubMed] [Google Scholar]
- Loke J, Pearlman A, Radi O, Zuffardi O, Giussani U, Pallotta R, Camerino G, Ostrer H. Mutations in MAP3K1 tilt the balance from SOX9/FGF9 to WNT/β-catenin signaling. Hum Mol Genet. 2014;23:1073–1083. doi: 10.1093/hmg/ddt502. [DOI] [PubMed] [Google Scholar]
- Looijenga LHJ, Hersmus R, Oosterhuis JW, Cools M, Drop SLS, Wolffenbuttel KP. Tumor risk in disorders of sex development (DSD) Best Pract Res Clin Endocrinol Metab. 2007;21:480–495. doi: 10.1016/j.beem.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Mandel H, Shemer R, Borochowitz ZU, Okopnik M, Knopf C, Indelman M, Drugan A, Tiosano D, Gershoni-Baruch R, Choder M, Sprecher E. SERKAL Syndrome: An Autosomal-Recessive Disorder Caused by a Loss-of-Function Mutation in WNT4. Am J Hum Genet. 2008;82:39–47. doi: 10.1016/j.ajhg.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouriquand PDE, Gorduza DB, Gay C-L, Meyer-Bahlburg HFL, Baker L, Baskin LS, Bouvattier C, Braga LH, Caldamone AC, Duranteau L, El Ghoneimi A, Hensle TW, Hoebeke P, Kaefer M, Kalfa N, Kolon TF, Manzoni G, Mure P-Y, Nordenskjöld A, Pippi Salle JL, Poppas DP, Ransley PG, Rink RC, Rodrigo R, Sann L, Schober J, Sibai H, Wisniewski A, Wolffenbuttel KP, Lee P. Surgery in disorders of sex development (DSD) with a gender issue: If (why), when, and how? J Pediatr Urol. 2016;12:139–149. doi: 10.1016/j.jpurol.2016.04.001. [DOI] [PubMed] [Google Scholar]
- Nakhal RS, Hall-Craggs M, Freeman A, Kirkham A, Conway GS, Arora R, Woodhouse CRJ, Wood DN, Creighton SM. Evaluation of retained testes in adolescent girls and women with complete androgen insensitivity syndrome. Radiology. 2013;268:153–60. doi: 10.1148/radiol.13121068. [DOI] [PubMed] [Google Scholar]
- Nikolova G, Vilain E. Mechanisms of Disease: transcription factors in sex determination-relevance to human disorders of sex development. Nat Clin Pract Endocrinol Metab. 2006;2:231–238. doi: 10.1038/ncpendmet0143. [DOI] [PubMed] [Google Scholar]
- Oosterhuis JW, Stoop H, Honecker F, Looijenga LHJ. Why human extragonadal germ cell tumours occur in the midline of the body: old concepts, new perspectives. 2007:30. doi: 10.1111/j.1365-2605.2007.00793.x. [DOI] [PubMed] [Google Scholar]
- Oslejskova H, Horinova V, Sterba J, Pavelka Z, Babovic-Vuksanovic D, Dubska L, Valik D. Malignant Intracranial Germinoma in Smith-Lemli-Opitz Syndrome: Cholesterol Homeostasis Possibly Connecting Morphogenesis and Cancer Development. J Pediatr Hematol Oncol. 2008;30:689–691. doi: 10.1097/MPH.0b013e318180bbde. [DOI] [PubMed] [Google Scholar]
- Ostrer H. Disorders of Sex Development (DSDs): An Update. J Clin Endocrinol Metab. 2014;99:1503–1509. doi: 10.1210/jc.2013-3690. [DOI] [PubMed] [Google Scholar]
- Palmer BW, Wisniewski AB, Schaeffer TL, Mallappa A, Tryggestad JB, Krishnan S, Chalmers LJ, Copeland K, Chernausek SD, Reiner WG, Kropp BP. A model of delivering multi-disciplinary care to people with 46 XY DSD. J Pediatr Urol. 2012;8:7–16. doi: 10.1016/j.jpurol.2011.08.013. [DOI] [PubMed] [Google Scholar]
- Parma P, Radi O, Vidal V, Chaboissier MC, Dellambra E, Valentini S, Guerra L, Schedl A, Camerino G. R-spondin1 is essential in sex determination, skin differentiation and malignancy. Nat Genet. 2006;38:1304–9. doi: 10.1038/ng1907. [DOI] [PubMed] [Google Scholar]
- Patsner B, Mann WJ, Chumas J. Malignant mixed germ cell tumor of the ovary in a young woman with Smith-Lemli-Opitz syndrome. Gynecol Oncol. 1989;33:386–8. doi: 10.1016/0090-8258(89)90534-9. [DOI] [PubMed] [Google Scholar]
- Pearlman A, Loke J, Le Caignec C, White S, Chin L, Friedman A, Warr N, Willan J, Brauer D, Farmer C, Brooks E, Oddoux C, Riley B, Shajahan S, Camerino G, Homfray T, Crosby AH, Couper J, David A, Greenfield A, Sinclair A, Ostrer H. Mutations in MAP3K1 Cause 46,XY Disorders of Sex Development and Implicate a Common Signal Transduction Pathway in Human Testis Determination. Am J Hum Genet. 2010;87:898–904. doi: 10.1016/j.ajhg.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham TT, Angus SP, Johnson GL. MAP3K1: Genomic Alterations in Cancer and Function in Promoting Cell Survival or Apoptosis. Genes Cancer. 2013;4:419–26. doi: 10.1177/1947601913513950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleskacova J, Hersmus R, Oosterhuis JW, Setyawati BA, Faradz SM, Cools M, Wolffenbuttel KP, Lebl J, Drop SL, Looijenga LH. Tumor risk in disorders of sex development. Sex Dev. 2010;4:259–69. doi: 10.1159/000314536. [DOI] [PubMed] [Google Scholar]
- Pyle LC, Nathanson KL. Genetic changes associated with testicular cancer susceptibility. Semin Oncol. 2016;43:575–581. doi: 10.1053/j.seminoncol.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robin NH, Grace K, DeSouza TG, McDonald-McGinn D, Zackai EH. New finding of Schinzel-Giedion syndrome: A case with a malignant sacrococcygeal teratoma. Am J Med Genet. 1993;47:852–856. doi: 10.1002/ajmg.1320470611. [DOI] [PubMed] [Google Scholar]
- De Sanctis V, Fiscina B, Soliman A, Giovannini M, Yassin M. Klinefelter syndrome and cancer: from childhood to adulthood. Pediatr Endocrinol Rev. 2013;11:44–50. [PubMed] [Google Scholar]
- Schoemaker MJ, Swerdlow AJ, Higgins CD, Wright AF, Jacobs PA UK Clinical Cytogenetics Group. Cancer incidence in women with Turner syndrome in Great Britain: a national cohort study. Lancet Oncol. 2008;9:239–246. doi: 10.1016/S1470-2045(08)70033-0. [DOI] [PubMed] [Google Scholar]
- Şimşek E, Binay Ç, Demiral M, Tokar B, Kabukçuoğlu S, Üstün M. Gonadoblastoma and Papillary Tubal Hyperplasia in Ovotesticular Disorder of Sexual Development. J Clin Res Pediatr Endocrinol. 2016;8:351–355. doi: 10.4274/jcrpe.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solheim O, Gershenson DM, Tropé CG, Rokkones E, Sun CC, Weedon-Fekjaer H, Fosså SD. Prognostic factors in malignant ovarian germ cell tumours (The Surveillance, Epidemiology and End Results experience 1978–2010) Eur J Cancer. 2014;50:1942–50. doi: 10.1016/j.ejca.2014.03.288. [DOI] [PubMed] [Google Scholar]
- Stewart C, JR, Baker E, Beaton C, Crook M, Peverall J, Wallace S. Detection of Y-chromosome in gonadal tumours using fluorescence in situ hybridization: diagnostic value in intersex conditions including older patients with clinically unsuspected androgen insensitivity syndrome. Histopathology. 2007;52:175–182. doi: 10.1111/j.1365-2559.2007.02927.x. [DOI] [PubMed] [Google Scholar]
- Szafran AT, Hartig S, Sun H, Uray IP, Szwarc M, Shen Y, Mediwala SN, Bell J, McPhaul MJ, Mancini MA, Marcelli M. Androgen receptor mutations associated with androgen insensitivity syndrome: a high content analysis approach leading to personalized medicine. PLoS One. 2009;4:e8179. doi: 10.1371/journal.pone.0008179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang WWC, Kobayashi T, Irie N, Dietmann S, Surani MA. Specification and epigenetic programming of the human germ line. Nat Rev Genet. 2016;17:585–600. doi: 10.1038/nrg.2016.88. [DOI] [PubMed] [Google Scholar]
- Travis LB, Beard C, Allan JM, Dahl AA, Feldman DR, Oldenburg J, Daugaard G, Kelly JL, Dolan ME, Hannigan R, Constine LS, Oeffinger KC, Okunieff P, Armstrong G, Wiljer D, Miller RC, Gietema JA, van Leeuwen FE, Williams JP, Nichols CR, Einhorn LH, Fossa SD. Testicular cancer survivorship: research strategies and recommendations. J Natl Cancer Inst. 2010;102:1114–30. doi: 10.1093/jnci/djq216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlenhaut NH, Jakob S, Anlag K, Eisenberger T, Sekido R, Kress J, Treier A-C, Klugmann C, Klasen C, Holter NI, Riethmacher D, Schütz G, Cooney AJ, Lovell-Badge R, Treier M. Somatic sex reprogramming of adult ovaries to testes by FOXL2 ablation. Cell. 2009;139:1130–42. doi: 10.1016/j.cell.2009.11.021. [DOI] [PubMed] [Google Scholar]
- Weiss GR, Garnick MB. Testicular cancer in a Russell-Silver dwarf. J Urol. 1981;126:836–7. doi: 10.1016/s0022-5347(17)54773-4. [DOI] [PubMed] [Google Scholar]
- Wünsch L, Holterhus PM, Wessel L, Hiort O. Patients with disorders of sex development (DSD) at risk of gonadal tumour development: management based on laparoscopic biopsy and molecular diagnosis. BJU Int. 2012;110:E958–65. doi: 10.1111/j.1464-410X.2012.11181.x. [DOI] [PubMed] [Google Scholar]
- van der Zwan YG, Biermann K, Wolffenbuttel KP, Cools M, Looijenga LHJ. Gonadal maldevelopment as risk factor for germ cell cancer: towards a clinical decision model. Eur Urol. 2015;67:692–701. doi: 10.1016/j.eururo.2014.07.011. [DOI] [PubMed] [Google Scholar]
- Van Der Zwan YG, Stoop H, Rossello F, White SJ, Looijenga LHJ. Role of epigenetics in the etiology of germ cell cancer. Int J Dev Biol. 2013;57:299–308. doi: 10.1387/ijdb.130017ll. [DOI] [PubMed] [Google Scholar]