Abstract
Background Context
Non-steroidal anti-inflammatory (NSAID) agents are a widely utilized treatment for low back pain (LBP). Literature on NSAID use in articular cartilage has shown detrimental effects; however, minimal data exist to detail the effects of NSAIDs in intervertebral disc degeneration (IDD). As IDD is a major cause of LBP, we explored the effects of indomethacin, a commonly used NSAID, on disc matrix homeostasis in an animal model of IDD.
Purpose
To determine the effects of oral indomethacin administration on IDD in an in vivo rabbit model. This study hypothesized that indomethacin use would accelerate the progression of IDD based upon serial imaging and tissue outcomes.
Study Design/Setting
This was a laboratory-based, controlled, in vivo evaluation of the effects of oral indomethacin administration on rabbit intervertebral discs.
Methods
Six skeletally mature New Zealand White rabbits were divided into two groups: disc puncture alone to induce IDD (Puncture group) and disc puncture plus indomethacin (Punc+Ind group). The Punc+Ind group received daily administration of 6mg/kg oral indomethacin. Serial magnetic resonance imaging (MRI) were obtained at 0, 4, 8, and 12 weeks. MRI index and nucleus pulposus (NP) area were calculated. Discs were harvested at 12 weeks for determination of disc glycosaminoglycan (GAG) content, relative gene expression measured by real time polymerase chain reaction, and histological analyses.
Results
MRI index and NP area of punctured discs in the Punc+Ind group demonstrated no worsening of degeneration compared to the Puncture group. Histological analysis was consistent with less severe disc degeneration in the Punc+Ind group. Minimal differences in gene expression of matrix genes were observed between Puncture and Punc+Ind groups. GAG content was higher in animals receiving indomethacin in both annulus fibrosus (AF) and NP at adjacent uninjured discs.
Conclusions
Oral indomethacin administration did not result in acceleration of IDD in an in vivo rabbit model. Future research is needed to ascertain long-term effects of indomethacin and other NSAIDs on disc matrix homeostasis.
Introduction
Low back pain (LBP) is one of the most common clinical reasons for morbidity in the United States. LBP places an enormous burden on the health care system as it results in a high amount of patient disability and loss of productivity [1]. While LBP is often multifactorial, intervertebral disc degeneration (IDD) has been cited as a major potential source of pain generation [2]. Commonly the initial clinical treatment for uncomplicated LBP includes administration of non-steroidal anti-inflammatory (NSAID) agents [3, [4]. NSAIDs are widely used anti-inflammatory and pain-relieving agents that function by blocking the actions of cyclooxygenases (COX-1 and COX-2) to inhibit the production of the inflammatory mediators. NSAIDs function well in providing short-term pain relief for many patients, but little is known regarding their effect regarding the natural history of intervertebral disc pathology and disc matrix homeostasis [5, [6]. One study suggested that use of indomethacin, a common NSAID used in low back pain, was associated with improvement in glycosaminoglycan (GAG) content based upon improved DNA and water content from both the annulus fibrosus (AF) and nucleus pulposus (NP), however they did not evaluate the disc matrix itself [7]. Additionally, the effects of indomethacin on IDD using outcome measures such as gene expression, histology and clinical imaging modalities such as magnetic resonance imaging (MRI) are yet unknown.
Previous findings in the intervertebral disc require further confirmation in light of the mixed results observed in indomethacin use in articular cartilage studies [8]. NSAIDs have been shown to have differing capacities for blocking COX-1 and 2 in human chondrocytes; indomethacin was one of the strongest inhibitors [9]. Some studies suggest that blocking the production of prostaglandin E2 (PGE2) through COX-1 and 2 may have beneficial effect on articular cartilage as PGE2 has been shown to result in matrix degradation through increased catabolism [10]. NSAIDs may also alter the catabolic profile of chondrocytes; some studies suggest a decrease in matrix metalloproteinase (MMP) 1 activity with NSAIDs [11]. However, many in vitro studies investigating NSAID use showed decreased proteoglycan and collagen production in articular chondrocytes [11, [12, [13, [14]. Clinically there is a concern that NSAID use may increase progression of osteoarthritis (OA) over time [15, [16, [17, [18].
Currently the effect of NSAIDs on IDD matrix homeostasis is yet to be determined. The goal of this study was to determine the effects of oral NSAID administration, specifically indomethacin, on disc histology, matrix composition, and gene expression in an in vivo rabbit model for IDD. Indomethacin was chosen as the representative NSAID agent as it has been shown to be one of the strongest inhibitors of COX-1 and 2 [9]; additionally it has been shown to have deleterious effects on articular cartilage in a rat knee OA model based on worsening histological morphology [19]. This study hypothesized that over a 12 week timeline rabbits with IDD treated with indomethacin (Punc+Ind) would demonstrate more severe disc degeneration characteristics than the IDD control (Puncture) group based on MRI characteristics, GAG analysis, histological appearance, and gene expression.
Materials and Methods
Animal model
This study was approved by the Institutional Animal Care and Use Committee (IACUC) at the institution of the principle investigator under protocol 1201569A-1. Six female, skeletally mature New Zealand white rabbits at approximately one year of age and weighing 4–5 kg were randomly assigned to one of two groups (n=3 each): disc puncture alone (Puncture) or disc puncture plus indomethacin (Punc+Ind). Rabbits in both groups underwent a previously described and validated method of annular puncture to induce IDD [20]. Prior to surgery rabbits were sedated, endotracheal intubation was performed, and they were placed under general anesthesia. The rabbits were positioned in the lateral decubitus position and the spine was approached via an anterolateral retroperitoneal approach. Fluoroscopy was utilized to confirm anatomic positioning of desired discs. Annular puncture was performed at L2-3, L3-4, and L4-5 discs with a 16g needle to a depth of 5mm. Careful attention was taken to ensure that the needle was inserted through the AF pointed directly towards the center of the NP with the needle oriented parallel to the vertebral end plates. Surgical incisions were closed and standard postoperative care was provided to the animals, with the only change being that no post-operative NSAIDs were given to animals in the Puncture group. Oral indomethacin was chosen to simulate oral NSAID administration by human patients. Starting one day after the disc puncture procedure, the animals in the Punc+Ind group were given 6mg/kg oral indomethacin (Heiber’s Compounding Pharmacy, Pittsburgh, PA) once daily via oral solution. This dosage was chosen given that it is the metabolic equivalent to a human dose of 75mg three times per day of indomethacin [21]. This treatment was continued daily for a period of 12 weeks (Figure 1).
Figure 1.
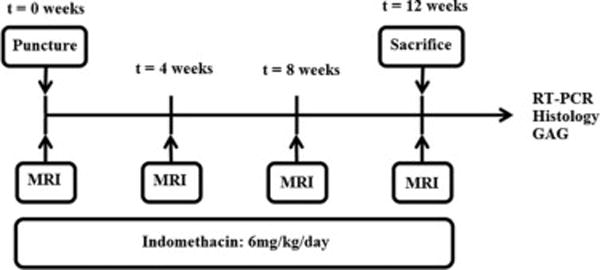
Animals in both Puncture (n=3) and Punc+Ind (n=3) group both received the annular puncture procedure at week 0. All animals underwent baseline MRIs immediately prior to this procedure and subsequently at weeks 4, 8 and 12. Rabbits were sacrificed at week 12, at which time spines were harvested and additional outcome measures were performed.
MRI Evaluation
MRI scans were performed using a 3.0 Tesla Trio magnet (Siemens Medical Solutions, Malvern, PA) as previously described [22]. Rabbits were sedated and placed supine with the lumbar region centered over a 5-inch diameter circular surface coil. Sagittal T2-weighted MRI images of the rabbit lumbar spines (3mm slices) were obtained pre-operatively at week 0 to obtain a baseline. MRI imaging was repeated at week 4, week 8, and week 12. The mid-sagittal cut was identified and used to select and highlight the NP in the discs of interest (L2-3, L3-4 and L4-5). The degree of disc degeneration was quantified by outlining the NP area of each disc using a previously validated semi-automated contour segmentation method using MATLAB software (MATLAB, Natick, MA) [23]. This method normalizes the signal intensity for each individual MRI at each time point. MRI index was calculated as the signal intensity of the NP multiplied by the NP area, as previously described [20]. This metric was utilized as a determinate for the degree of disc degeneration as it provides information regarding the NP architecture (area) and T2-weighted signal intensity. MRI index and NP area for each rabbit at the injured discs (L2-3, L3-4, and L4-5) were normalized to a week 0 (baseline) values and reported as percent change from baseline. Values were averaged for the discs of interest for rabbits in each group.
Histological analysis
Animals were sacrificed after the 12 week MRI and their spines were harvested en bloc. For animals in both the Punc+Ind and the Puncture groups, one of the discs that had previously underwent the puncture procedure (L4-5) was utilized for histology. The L4-5 functional spinal unit was removed at the time of sacrifice, decalcified using Decalcifier I (Surgipath, Leica Biosystems, Buffalo Grove, IL) for 4 weeks, and embedded in paraffin using Tissue Tek processor (Sakura, Torrance, CA) and Leica embedding solution (Leica) before sectioning. Discs were then sectioned to a thickness of 5 μm in the sagittal plane and mounted on glass slides. Tissues were then stained with Hematoxylin and Eosin (Fisher Scientific, Pittsburgh, PA) and Safranin O (Fisher Scientific) staining [24]. Images were then qualitatively analyzed (Nikon Ts100, Nikon Inc., Melville, NY) under 20–200× magnification with images assessed for changes in the NP and AF architecture, cellularity, and morphology.
Gene Expression
For animals in both the Punc+Ind and the Puncture groups, two discs that had previously undergone the puncture procedure (L2-3, L3-4) were extracted and AF and NP tissues were separately isolated and stored at −80°C. To isolate RNA, tissues were minced, homogenized by bead milling, and extracted using Qiazol Lysis Reagent (Qiagen, Germantown, MD) and 24:1 chloroform:isoamyl alcohol (Sigma-Aldrich, St. Louis, MO). RNA was then purified using the RNeasy Universal Tissue Kit (Qiagen). Real-time reverse-transcriptase polymerase chain reaction (RT-PCR) was performed using an iQ5 real-time thermal cycler (BioRad, Hercules, CA) with SYBR green and previously validated rabbit primers [25, [26, [27] for collagen 1 (COL-1), collagen 2 (COL-2), aggrecan (AGC), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Table 1). Relative gene expression was determined after normalizing to GAPDH, a housekeeping gene, by calculating the average ΔCt for each group and calculating relative gene expression between groups using the 2−ΔΔCt method [28].
Table 1.
Custom-designed primer sequences used for real-time reverse transcriptase polymerase chain reaction (RT-PCR). Abbreviations: glyceraldehyde-3-phosphate dehydrogenase (GAPDH), collagen 1 (COL-1), collagen 2 (COL-2), and aggrecan (AGC).
| Gene: | Sequence: |
|---|---|
| GAPDH | Reverse (Rev): 5′-GCTGAGATGATGACCCTTTTGG-3′ |
| Forward (For): 5′-GATGCTGGTGCCGAGTAC-3′ | |
| COL-1 | Rev: 5′-GCCATCGACAAGAACAGTGTAAGT-3′ |
| For: 5′-ATGGATGAGGAAACTGGCAACT-3′ | |
| COL-2 | Rev: 5′-CAGTCCCCGTGTCACAGACA-3′ |
| For: 5′-AAGAGGTATAATGATAAGGATGTGTGGAA-3′ | |
| AGC | Rev: 5′-CGTAAAAGACCTCACCCTCCAT-3′ |
| For: 5′-GCTACGGAGACAAGGATGAGTTC-3′ |
Glycosaminoglycan analysis
For animals in both the Punc+Ind and the Puncture groups, non-annular puncture discs T11-12 and T12-L1 discs were dissected. AF and NP were isolated and separated and stored at −80°C. These disc levels were selected to assess the impact of indomethacin on total GAG content in previously uninjured adjacent discs. The tissue was digested in papain (Sigma-Aldrich) under standard methods [29]. Samples were prepared in 1,9 dimethylmethylene blue buffer (DMMB, Sigma-Aldrich) and the optical density was read at 540nm and compared with established standards for chondroitin sulfate (C-8529, Sigma-Aldrich) [22]. GAG content was normalized based upon DNA concentration of each sample; this was measured using the Picogreen dsDNA quantitation kit (Thermo-Fisher, Eugene, OR).
Statistical analysis
To evaluate MRI outcome measures (MRI index and NP area), mixed-measures ANOVA with a post-hoc Mann-Whitney U test was performed to assess effects of time and treatment. For total GAG content, 95% confidence interval was calculated to determine differences between groups based on the t distribution due to the small sample size. Statistical analysis was not performed for gene expression or histological analysis. For all findings, statistical significance was defined as p< 0.05. Computations were performed using Excel (Microsoft, Redmond, WA) and SPSS (v 21.0, IBM, Armonk, NY).
Results
MRI Evaluation
Representative MRI images are shown in Figure 2. The average % Disc Area (Figure 3) for the Punc+Ind group vs Puncture group was 71% vs 40% (p=0.161) at week 4, 44% vs 47% (p=0.666) at week 8, and 47% vs 47% (p=0.546) at week 12 (Figure 3). The average % MRI index for Punc+Ind group vs Puncture group was 61% vs 22% (p=0.190) at week 4, 35% vs 35% (p=0.605) at week 8, and 35% vs 32% (p=0.605) at week 12 (Figure 3). Both the Puncture and Punc+Ind groups showed a steady decline in MRI index and NP area and were not significantly different at week 4, 8, or 12.
Figure 2.
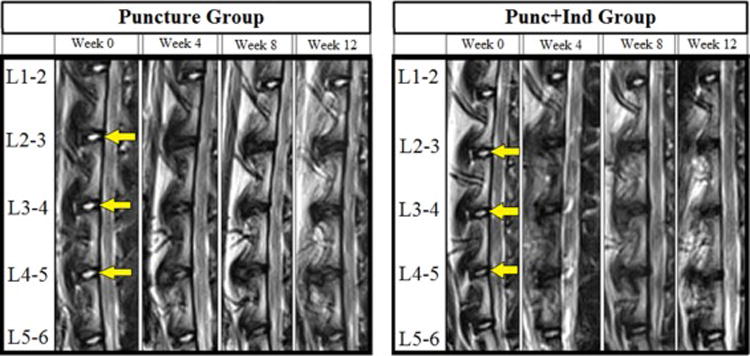
Representative mid-sagittal T2-weighted magnetic resonance imaging (MRI) slices of animals in Puncture and Punc+Ind groups at each timepoint: Week 0, Week 4, Week 8, Week 12. Yellow arrow indicates levels at which annular puncture procedure was performed (L2-3, L3-4, and L4-5).
Figure 3.
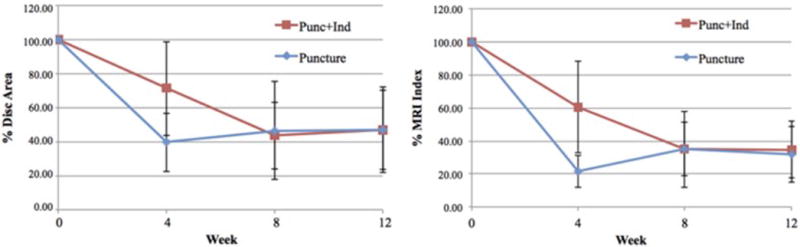
Disc Area (A) and Magnetic resonance imaging (MRI) Index (B) values calculated from MRI data. Values calculated at each timepoint for both the Puncture and Punc+Ind groups. Values displayed are percentage change from baseline (week 0) MRI. Error bars represent standard error.
Histological Analysis
Histologic analysis of midsagittal cuts of L4-5 disc (Figure 4) on hematoxylin and eosin and Safranin O staining demonstrated an overall preserved matrix organization and cellularity, deceased fibrous content in the AF and NP, and more intact NP structure in the Punc+Ind group compared to the Puncture group.
Figure 4.
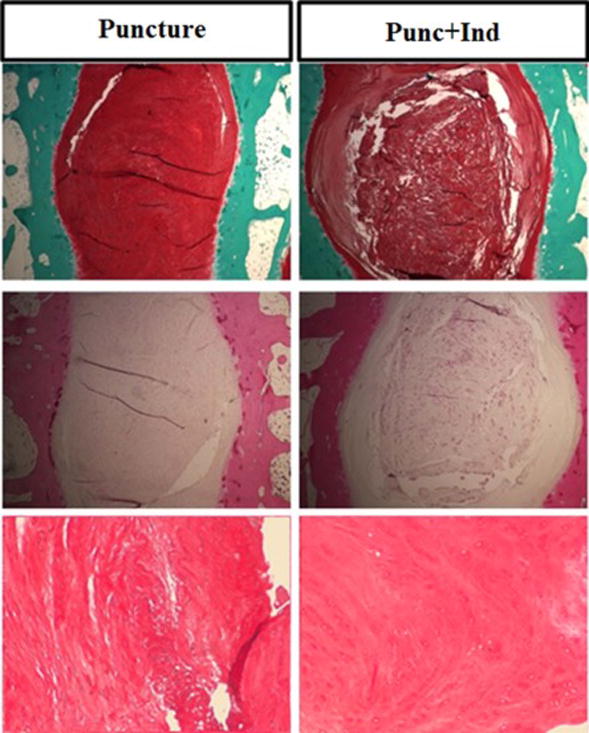
A) Safranin-O staining of representative discs from Puncture and Punc+Ind groups at 20X, B) Hematoxylin and Eosin staining of representative discs from Puncture and Punc+Ind groups groups at 20X, C) Hematoxylin and Eosin staining of representative nucleus pulposus tissue from Puncture and Punc+Ind groups at 100X.
Gene Expression Analysis
In the AF, the Punc+Ind group demonstrated relative gene expression of collagen I (0.93×), collagen II (0.85×), and Aggrecan (0.91×) relative to the Puncture group (Figure 5). In the NP, the Punc+Ind group demonstrated relative gene expression of aggrecan (1.49×), collagen I (1.02×), and collagen II (1.72×) relative to the Puncture group. No significant differences between groups were noted in any genes tested.
Figure 5.
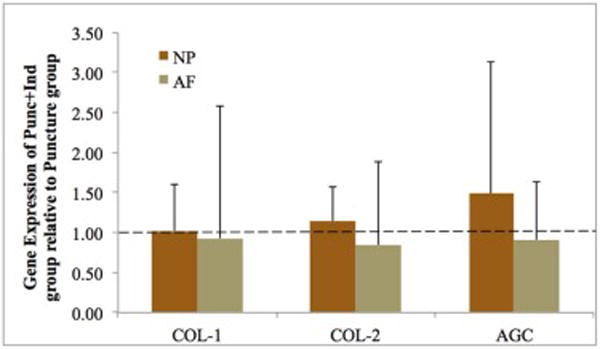
Relative gene expression (RGE) for nucleus pulposus (NP) and annulus fibrosus (AF) tissue in the Punc+Ind group. Values displayed are fold change in gene expression in Punc+Ind group relative to Puncture group. Abbreviations: collagen 1 and 2 (COL-1 and COL-2) and aggrecan (AGC). Error bars represent standard error.
GAG analysis
Non-injured discs T11-12 and T12-L1 in the Punc+Ind group demonstrated significantly higher GAG content in both the AF and NP (Figure 6). Total GAG content (measured as μg GAG/ng DNA) in AF tissues was 30.45 in the Punc+Ind group and 20.91 in the Puncture group. The confidence interval for the difference between the two groups was 1.9 – 17.1. Total GAG content (μg GAG/ng DNA) in NP tissues was 35.65 in the Punc+Ind group and 28.03 in the Puncture group. The confidence interval for the difference between the two groups was 0.5 – 14.9.
Figure 6.
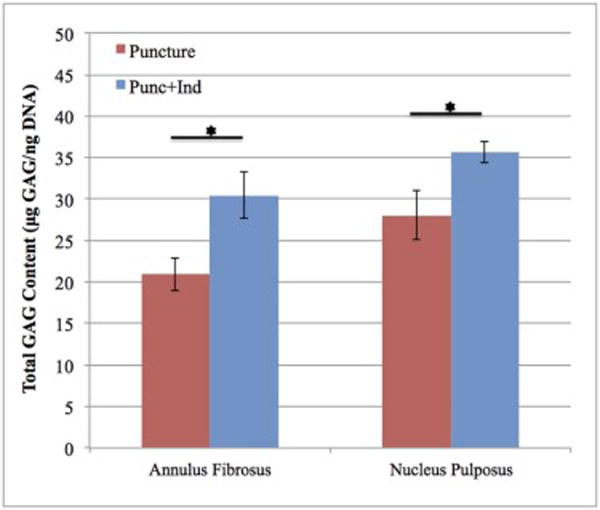
Total glycosaminoglycan (GAG) content relative to DNA content (μg GAG/ng DNA) for both annulus fibrosus (AF) and nucleus pulposus (NP) tissues in the Puncture and Punc+Ind groups. Significant differences (p<0.05) between groups denoted with a *. Error bars represent standard error.
Discussion
The findings in this study did not support our hypothesis that indomethacin administration would result in worsening of IDD. Conversely, the cumulative results in MRI analysis, GAG analysis, histological appearance, and gene expression suggest that indomethacin shows no worsening of degeneration and may have a net positive effect on adjacent uninjured discs. Indeed, a previous study found a possible beneficial action of indomethacin on GAG content by observing increased DNA and water content following indomethacin administration in an in vivo porcine model for IDD [7]. To our knowledge, our study is the first to evaluate indomethacin effects comprehensively based upon a broad array of relevant outcomes—MRI changes, quantitative GAG content, gene expression and histological appearance of discs—in an in vivo animal model for IDD.
MRI analysis was selected as an outcome measure because of its non-invasive nature and ability to quantitatively measure disc morphology temporally. Previous studies have suggested validated MRI protocols as the most valuable tool to evaluate NSAID use in OA models [15]. Intervertebral discs in both groups ultimately developed degeneration by the end of the study period. However, a trend towards delayed degeneration was observed in the Punc+Ind group with less degeneration at week 4, though this finding was not statistically significant. This finding argues for a temporary beneficial effect of indomethacin, especially in the acute phase after traumatic injury to the disc. This is consistent with clinical findings of NSAID use in LBP as frequently NSAID use is effective in the first few weeks but does not confer a benefit in chronic LBP patients [4].
Histological sections demonstrated less severe disc degeneration in the Punc+Ind group. Safranin O dye within the Punc+Ind group demonstrated preserved matrix architecture, indicating less structural damage that would be seen with degeneration. Hematoxylin and eosin staining pointed towards increased cellularity and decreased fibrous content in the Punc+Ind group. An apparent contradiction exists as the body of evidence regarding indomethacin use in articular cartilage showed detrimental effects on GAG synthesis and collagen metabolism [11, [12, [13, [14, [15, [19, [25] while the single study on indomethacin use in IDD reported a possible beneficial action on GAG content [7]. Our results of improved GAG content are consistent with prior evidence in IDD and differ from findings in articular cartilage, which may be explained by differences in matrix homeostasis between articular cartilage and the intervertebral disc [30, [31].
In our study, evaluation of GAG content by DMMB assay was chosen to be performed on adjacent discs T11-12 and T12-L1 to provide insight into indomethacin’s effects on the matrix of healthy discs. Non-punctured discs at T11-12 and T12-L1 showed significant increases in GAG content in indomethacin treated animals in both AF and NP, suggesting matrix upregulation. One previous study showed no change in GAG content in articular cartilage with administration of indomethacin in an in vitro model for OA [32]. In the intervertebral disc, prostaglandins have been shown to have a negative effect on matrix metabolism in human NP cells [33]. It has been suggested that NSAID blockage of PGE2 yields a net anabolic action for matrix components in the disc [33], and this possible explanation should be explored in future studies.
The findings in this study must be viewed in light of several limitations. The main limitations of this study revolve around the use of an in vivo animal model. We attempted to minimize this limitation by utilizing an animal model that has been previously validated in evaluating IDD [34]. A small sample size limited the strength of our findings. While we were able to perform statistical analysis for some of our outcomes, our study was underpowered which reduces the likelihood that a statistically significant result reflects a true effect. This limitation of a small sample size has been observed in several published studies using similar methods [35, [36, [37]. Another limitation is that different NSAIDs have varying effects on articular cartilage [8]. Choosing to examine only indomethacin limits the generalizability of this study to comment on the effects of other NSAIDs on IDD. We chose indomethacin as it has been shown to have the most deleterious effects on articular cartilage [19]; our intention was to choose the NSAID most likely to show harmful effects with administration. Additionally, as the intervertebral disc contains multiple cell populations that are themselves distinct from articular chondrocytes investigated in previous literature, these disc cells may exhibit a different sensitivity to NSAIDs.
This in vivo study investigated short-term indomethacin use in an IDD model. Indomethacin may alter the natural history of early disc degeneration and perhaps delay the course of degeneration in the acute phase; future studies are needed to elucidate the long-term effects of NSAIDs in IDD. The large-scale utilization of NSAID medications for back pain despite the lack of conclusive evidence as to how the unique environment of the disc is altered with NSAID treatment makes this study relevant clinically. It is important to identify any harmful treatment modalities for back pain in order to avoid them, as well as to identify and utilize treatments that do not promote progression of the disease. Future studies are needed to investigate further effects of NSAIDs on healthy intervertebral discs; the purpose of this study was to look at IDD specifically. This study suggests that indomethacin use in IDD does not cause a worsening or acceleration of degeneration in the short-term.
Acknowledgments
Financial support for this project was provided by the Cervical Spine Research Society (CSRS) (awarded to GS), the Orthopaedic Research Education Fund (OREF) (awarded to GS), and the NIH/NCCAM K08AT004718-02 Grant (awarded to GS). We thank the staff of the Ferguson Laboratory for Orthopaedic and Spine Research for their support and assistance in completing this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Study Approval: This experimental protocol was approved by the Institutional Animal Care and Use Committee (IACUC) at our institution under protocol 1201569A-1.
References
- 1.Dagenais S, Caro J, Haldeman S. A systematic review of low back pain cost of illness studies in the United States and internationally. Spine J. 2008;8(1):8–20. doi: 10.1016/j.spinee.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 2.Luoma K, Riihimaki H, Luukkonen R, Raininko R, Viikari-Juntura E, Lamminen A. Low back pain in relation to lumbar disc degeneration. Spine (Phila Pa 1976) 2000;25(4):487–92. doi: 10.1097/00007632-200002150-00016. [DOI] [PubMed] [Google Scholar]
- 3.Roelofs PD, Deyo RA, Koes BW, Scholten RJ, van Tulder MW. Nonsteroidal anti-inflammatory drugs for low back pain: an updated Cochrane review. Spine (Phila Pa 1976) 2008;33(16):1766–74. doi: 10.1097/BRS.0b013e31817e69d3. [DOI] [PubMed] [Google Scholar]
- 4.Henschke N, Maher CG, Refshauge KM, et al. Prognosis in patients with recent onset low back pain in Australian primary care: inception cohort study. BMJ. 2008;337:a171. doi: 10.1136/bmj.a171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Madigan L, Vaccaro AR, Spector LR, Milam RA. Management of symptomatic lumbar degenerative disk disease. J Am Acad Orthop Surg. 2009;17(2):102–11. doi: 10.5435/00124635-200902000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Kadow T, Sowa G, Vo N, Kang JD. Molecular basis of intervertebral disc degeneration and herniations: what are the important translational questions? Clin Orthop Relat Res. 2015;473(6):1903–12. doi: 10.1007/s11999-014-3774-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karppinen J, Inkinen RI, Kaapa E, et al. Effects of tiaprofenic acid and indomethacin on proteoglycans in the degenerating porcine intervertebral disc. Spine (Phila Pa 1976) 1995;20(10):1170–7. doi: 10.1097/00007632-199505150-00012. [DOI] [PubMed] [Google Scholar]
- 8.Dingle JT. The effects of NSAID on the matrix of human articular cartilages. Z Rheumatol. 1999;58(3):125–9. doi: 10.1007/s003930050161. [DOI] [PubMed] [Google Scholar]
- 9.Blanco FJ, Guitian R, Moreno J, de Toro FJ, Galdo F. Effect of antiinflammatory drugs on COX-1 and COX-2 activity in human articular chondrocytes. J Rheumatol. 1999;26(6):1366–73. [PubMed] [Google Scholar]
- 10.Attur M, Al-Mussawir HE, Patel J, et al. Prostaglandin E2 exerts catabolic effects in osteoarthritis cartilage: evidence for signaling via the EP4 receptor. J Immunol. 2008;181(7):5082–8. doi: 10.4049/jimmunol.181.7.5082. [DOI] [PubMed] [Google Scholar]
- 11.Sadowski T, Steinmeyer J. Effects of non-steroidal antiinflammatory drugs and dexamethasone on the activity and expression of matrix metalloproteinase-1, matrix metalloproteinase-3 and tissue inhibitor of metalloproteinases-1 by bovine articular chondrocytes. Osteoarthritis Cartilage. 2001;9(5):407–15. doi: 10.1053/joca.2000.0406. [DOI] [PubMed] [Google Scholar]
- 12.Ou YS, Tan C, An H, et al. The effects of NSAIDs on types I, II, and III collagen metabolism in a rat osteoarthritis model. Rheumatol Int. 2012;32(8):2401–5. doi: 10.1007/s00296-011-1978-8. [DOI] [PubMed] [Google Scholar]
- 13.Mastbergen SC, Jansen NW, Bijlsma JW, Lafeber FP. Differential direct effects of cyclo-oxygenase-1/2 inhibition on proteoglycan turnover of human osteoarthritic cartilage: an in vitro study. Arthritis Res Ther. 2006;8(1):R2. doi: 10.1186/ar1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shield MJ. Anti-inflammatory drugs and their effects on cartilage synthesis and renal function. Eur J Rheumatol Inflamm. 1993;13(1):7–16. [PubMed] [Google Scholar]
- 15.Ding C. Do NSAIDs affect the progression of osteoarthritis? Inflammation. 2002;26(3):139–42. doi: 10.1023/a:1015504632021. [DOI] [PubMed] [Google Scholar]
- 16.Huskisson EC, Berry H, Gishen P, Jubb RW, Whitehead J. Effects of antiinflammatory drugs on the progression of osteoarthritis of the knee. LINK Study Group. Longitudinal Investigation of Nonsteroidal Antiinflammatory Drugs in Knee Osteoarthritis. J Rheumatol. 1995;22(10):1941–6. [PubMed] [Google Scholar]
- 17.Lapane KL, Yang S, Driban JB, et al. Effects of prescription nonsteroidal antiinflammatory drugs on symptoms and disease progression among patients with knee osteoarthritis. Arthritis Rheumatol. 2015;67(3):724–32. doi: 10.1002/art.38933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reijman M, Bierma-Zeinstra SM, Pols HA, Koes BW, Stricker BH, Hazes JM. Is there an association between the use of different types of nonsteroidal antiinflammatory drugs and radiologic progression of osteoarthritis? The Rotterdam Study. Arthritis Rheum. 2005;52(10):3137–42. doi: 10.1002/art.21357. [DOI] [PubMed] [Google Scholar]
- 19.Gencosmanoglu BE, Eryavuz M, Dervisoglu S. Effects of some nonsteroidal anti-inflammatory drugs on articular cartilage of rats in an experimental model of osteoarthritis. Res Exp Med (Berl) 2001;200(3):215–26. [PubMed] [Google Scholar]
- 20.Sobajima S, Kompel JF, Kim JS, et al. A slowly progressive and reproducible animal model of intervertebral disc degeneration characterized by MRI, X-ray, and histology. Spine (Phila Pa 1976) 2005;30(1):15–24. doi: 10.1097/01.brs.0000148048.15348.9b. [DOI] [PubMed] [Google Scholar]
- 21.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22(3):659–61. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 22.Jacobs L, Vo N, Coelho JP, et al. Glucosamine supplementation demonstrates a negative effect on intervertebral disc matrix in an animal model of disc degeneration. Spine (Phila Pa 1976) 2013;38(12):984–90. doi: 10.1097/BRS.0b013e318286b31e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bechara BP, Leckie SK, Bowman BW, et al. Application of a semiautomated contour segmentation tool to identify the intervertebral nucleus pulposus in MR images. AJNR Am J Neuroradiol. 2010;31(9):1640–4. doi: 10.3174/ajnr.A2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenberg L. Chemical basis for the histological use of safranin O in the study of articular cartilage. J Bone Joint Surg Am. 1971;53(1):69–82. [PubMed] [Google Scholar]
- 25.Sowa GA, Coelho JP, Bell KM, et al. Alterations in gene expression in response to compression of nucleus pulposus cells. Spine J. 2011;11(1):36–43. doi: 10.1016/j.spinee.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sowa G, Coelho P, Vo N, et al. Determination of annulus fibrosus cell response to tensile strain as a function of duration, magnitude, and frequency. J Orthop Res. 2011;29(8):1275–83. doi: 10.1002/jor.21388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sowa GA, Coelho JP, Jacobs LJ, et al. The effects of glucosamine sulfate on intervertebral disc annulus fibrosus cells in vitro. Spine J. 2015;15(6):1339–46. doi: 10.1016/j.spinee.2013.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yuan JS, Reed A, Chen F, Stewart CN., Jr Statistical analysis of real-time PCR data. BMC Bioinformatics. 2006;7:85. doi: 10.1186/1471-2105-7-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883(2):173–7. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 30.Minogue BM, Richardson SM, Zeef LA, Freemont AJ, Hoyland JA. Characterization of the human nucleus pulposus cell phenotype and evaluation of novel marker gene expression to define adult stem cell differentiation. Arthritis Rheum. 2010;62(12):3695–705. doi: 10.1002/art.27710. [DOI] [PubMed] [Google Scholar]
- 31.Lee CR, Sakai D, Nakai T, et al. A phenotypic comparison of intervertebral disc and articular cartilage cells in the rat. Eur Spine J. 2007;16(12):2174–85. doi: 10.1007/s00586-007-0475-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Srinivas GR, Chichester CO, Barrach HJ, Matoney AL. Effects of certain antiarthritic agents on the synthesis of type II collagen and glycosaminoglycans in rat chondrosarcoma cultures. Agents Actions. 1994;41(3–4):193–9. doi: 10.1007/BF02001916. [DOI] [PubMed] [Google Scholar]
- 33.Vo NV, Sowa GA, Kang JD, Seidel C, Studer RK. Prostaglandin E2 and prostaglandin F2alpha differentially modulate matrix metabolism of human nucleus pulposus cells. J Orthop Res. 2010;28(10):1259–66. doi: 10.1002/jor.21157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sobajima S, Shimer AL, Chadderdon RC, et al. Quantitative analysis of gene expression in a rabbit model of intervertebral disc degeneration by real-time polymerase chain reaction. Spine J. 2005;5(1):14–23. doi: 10.1016/j.spinee.2004.05.251. [DOI] [PubMed] [Google Scholar]
- 35.Elliott DM, Yerramalli CS, Beckstein JC, Boxberger JI, Johannessen W, Vresilovic EJ. The effect of relative needle diameter in puncture and sham injection animal models of degeneration. Spine (Phila Pa 1976) 2008;33(6):588–96. doi: 10.1097/BRS.0b013e318166e0a2. [DOI] [PubMed] [Google Scholar]
- 36.Kim KS, Yoon ST, Li J, Park JS, Hutton WC. Disc degeneration in the rabbit: a biochemical and radiological comparison between four disc injury models. Spine (Phila Pa 1976) 2005;30(1):33–7. doi: 10.1097/01.brs.0000149191.02304.9b. [DOI] [PubMed] [Google Scholar]
- 37.Abbushi A, Endres M, Cabraja M, et al. Regeneration of intervertebral disc tissue by resorbable cell-free polyglycolic acid-based implants in a rabbit model of disc degeneration. Spine (Phila Pa 1976) 2008;33(14):1527–32. doi: 10.1097/BRS.0b013e3181788760. [DOI] [PubMed] [Google Scholar]


