Abstract
Objective
Recent data show significant phenotypic and genetic associations between ovarian hormones and binge eating in adulthood. Theories of hormonal risk focus on puberty and the possibility that hormone activation induces changes in genetic effects that then lead to differential risk for binge eating in post-puberty and adulthood. Although this theory is difficult to test in humans, an indirect test is to examine whether genetic influences on binge eating increase during the pubertal period in girls. Prior work has shown pubertal increases in genetic influences on overall disordered eating symptoms, but no study to date has examined binge eating. The present study was the first to examine these increases for binge eating.
Methods
Participants included 1,568 female twins (ages 8–25 years) from the Michigan State University Twin Registry. Binge eating and pubertal development were assessed with self-report questionnaires.
Results
Twin moderation models showed significant linear increases in genetic effects from pre-puberty (5%) to post-puberty (42%), even after controlling for the effects of age and body mass index.
Discussion
Results provide critical support for increased genetic influences on binge eating during puberty. Additional studies are needed to identify hormonal mechanisms and fully test contemporary models of ovarian hormone risk.
Recent etiologic theories focus on the role of ovarian hormones in risk for binge eating in females (Klump, Culbert, & Sisk, 2017). These theories rest on animal and human data showing significant associations between binge eating and estrogen and progesterone in adulthood (Klump et al., 2017). Proposed mechanisms center on the genomic effects of hormones and their role in altering gene transcription in neurobiological systems known to be disrupted in binge eating (e.g., dopamine; Klump et al., 2017).
A key aspect of these theories is the belief that ovarian hormone activation during puberty induces changes in genetic effects that then lead to increased rates of binge eating in adulthood (Klump, 2013; Klump et al., 2017). To date, no study has examined these developmental pathways, likely due to difficulties in directly examining developmental changes in neural gene expression in humans. However, an indirect method is to examine differences in the degree of genetic influence on binge eating across pubertal development. Given that ovarian hormones drive pubertal development in girls, increasing genetic effects across puberty would provide indirect support for an effect of ovarian hormones on genetic risk. Importantly, increasing genetic effects across puberty have been observed for overall disordered eating symptoms (i.e., omnibus measures that include binge eating and body dissatisfaction, weight preoccupation, compensatory behaviors; see Culbert, Burt, McGue, Iacono, & Klump, 2009), but have never been examined for binge eating. The aim of this study was to examine differences in genetic influences on binge eating across puberty in a large and developmentally diverse twin sample that has never been investigated in twin studies of puberty.
Methods
Participants
The sample consisted of 1,568 (MZ = 814 (52%); DZ = 754 (48%)) female twins ages 8 to 25 (M(SD) =14.19 (3.57); see Table 1 in Supplemental Material for more sample descriptives) from the Michigan State University Twin Registry (MSUTR; Burt & Klump, 2012; Klump & Burt, 2006). The MSUTR is a population-based twin registry that recruits twins (ages 3–50 years) using birth records in collaboration with the Michigan Department of Health and Human Services (see Klump & Burt, 2006 and Burt & Klump, 2012 for recruitment details). The current study included archival data from two studies within the MSUTR: (1) the Twin Study of Mood, Behavior, and Hormones during Puberty (see O’Connor, Burt, VanHuysse, & Klump, 2016), and (2) the Twin Study of Hormones and Behavior across the Menstrual Cycle (Klump et al., 2013, 2014, 2016). Twins had to meet several inclusion/exclusion criteria for these studies (e.g., no current medication use) but were nevertheless representative of MSUTR twins who did not meet these criteria in terms of disordered eating symptoms (d’s = .07–.11) and racial/ethnic composition (White (81.3%), Black (10.7%), Asian (0.7%), American Indian/Alaskan Native (0.3%), multiracial (7.2%), Hispanic (6.6%)). The Michigan State University Institutional Review Board approved these studies, and all participants provided informed consent/assent and (for minors) parental consent.
Measures
Zygosity was determined using a well-validated physical similarity questionnaire that has been shown to be over 95% accurate when compared to genotyping (Lykken, Bouchard, McGue, & Tellegen, 1990; Peeters, Van Gestel, Vlietinck, Derom, & Derom, 1998). Trained research assistants independently completed the questionnaire for all twins. All twins over the age of 15 also completed a self-report version, while twins under the age of 18 had a parent (i.e., the mother in over 95% of cases) complete the questionnaire as well. Reports were compared amongst raters, and discrepancies were resolved using questionnaire responses, review of photographs of the twins by one of the principal investigators (KLK), and/or twin concordance across (Burt & Klump, 2012).
Binge eating was assessed using the binge eating scale (assessing binge episodes, thinking about binge eating) from the Minnesota Eating Behavior Survey (von Ranson, Klump, Iacono, & McGue, 2005).1 The MEBS is appropriate for use in pre-pubertal children (Luo, Donnellan, Burt, & Klump, 2016) and shows adequate internal consistency in past (alpha = .65–.69) (von Ranson et al., 2005) and current (alpha = .68) samples. Women with bulimia nervosa (BN) also score significantly higher on the this scale than controls (von Ranson et al., 2005).
Pubertal development was measured with the self-report Pubertal Development Scale (Petersen, Crockett, Richards, & Boxer, 1988) that assesses secondary sex characteristics (e.g., breast development) using a 4-point scale (i.e., development (1) has not yet begun, (2) has barely started, (3) is definitely underway, or (4) seems complete) that is summed across items and averaged. Maternal reports on the PDS were used for a subset of twins (n = 16; 1% of sample) who were missing PDS scores. PDS scores correlate with physician ratings (r = .61–.67) and exhibit good internal consistency in past (alphas = .76–.83) (Petersen et al., 1988) and current (alpha = .82) samples.
Body mass index (BMI) was calculated (weight in kg/height in m2) using height and weight measured with a wall-mounted ruler and digital scale, respectively. Similar to previous developmental twin studies (Culbert et al., 2013; Culbert et al., 2009; Culbert, Burt, Sisk, Nigg, & Klump, 2014; Klump et al., 2012, 2003; Klump, Burt, McGue, & Iacono, 2007; Klump, Burt, McGue, Iacono, & Wade, 2010; Klump, Holly, Iacono, McGue, & Willson, 2000; Klump, Keel, Sisk, & Burt, 2010), we used raw (instead of age- and sex-adjusted) BMI scores in analyses, although the two types of BMIs were very highly correlated in our sample (r > .99) with minimal mean differences (Mean difference = .038, SD = .03).
Statistical Analyses
Binge eating and PDS scores were log transformed prior to analysis to account for positive skew. Twin age and BMI were regressed out of each twin’s binge eating score to ensure that differences across puberty were not due to these potentially confounding variables.
Twin correlations provided initial indications of pubertal differences in genetic effects. Although continuous PDS scores were used in model-fitting (see below), categorical groups were developed for the correlations based on previous twin studies (pre-pubertal: PDS < 2.5, N = 593; pubertal: PDS = 2.5–3.9, N = 317, post-pubertal: PDS = 4.0; N = 658; see Culbert et al., 2009). Only pairs concordant on pubertal status (e.g., both co-twins pre-pubertal) were included in correlations, although concordant and discordant co-twins were included in model-fitting. The number of discordant pairs was small (n = 57; 7% of sample), and twins from concordant/discordant pairs did not differ significantly on binge eating (d = .12; p = .18).
We used extended univariate twin moderation models (see Supplemental Figure 1) (van der Sluis, Posthuma, & Dolan, 2012) to examine differences in additive genetic (A; effects that add across genes), shared environmental (C; factors that are common to siblings and contribute to similarity), and nonshared environmental (E; factors that are unique to siblings and contribute to differences, including measurement error) influences on binge eating across pubertal status. These models estimate: 1) path coefficients assessing genetic/environmental influences at the lowest level of pubertal development; 2) linear moderators assessing linear increases/decreases in etiologic influences across puberty; and 3) quadratic moderators assessing non-linear increases/decreases.2
We first fit the “full” model that included all parameters. We then directly tested our hypotheses by comparing the fit of this model to one that constrained all genetic moderators (linear and non-linear) to 0. This model provided a poor fit to the data (see below), and thus we fit other nested models that differentially constrained the moderators. Because of the large number of submodels that could be fit, we used parameter estimates from the full model to identify appropriate submodels. This approach allowed us to test relevant submodels without unduly increasing the number of tests.
Models were fit to the raw data using the maximum likelihood option in Mx (Neale, Boker, Xie, & Maes, 2003). Fit comparisons were made by taking the difference in minus twice the log-likelihood (−2lnL) between the full and nested models, which is chi-squared distributed under the null hypothesis implied by the reduced model. Large (statistically significant) differences led to a rejection of the nested model. Akaike’s information criterion (AIC), Bayesian information criterion (BIC), sample-size adjusted BIC, and deviance information criterion (DIC) were also used to select the best fitting model, where models that minimized these scores were preferred.
Prior to model-fitting, binge eating scores were standardized and minimum PDS scores were “floored” to 0. Unstandardized parameter estimates are reported in figures, as they more accurately depict absolute differences in etiologic effects than standardized estimates that represent differences as proportions of the total variance. However, we report standardized estimates in the text, as these estimates allow for a direct comparison of our findings to previous studies.
Results
Twin correlations provided initial support for study hypotheses. Genetic influences were significant during puberty/post-puberty, as the MZ twin correlations (rMZ = .44/.39) were ~2x higher than the DZ twin correlations (rDZ = .27/.15) (z tests of equality = 1.72 and 3.00, p’s = .08 and .001; effect size (q) = .20–.26). By contrast, in pre-puberty, the MZ/DZ twin correlations were very similar (rMZ = .37; rDZ = .35) and not significantly different (z test of equality = .28, p = .78, q = .02), suggesting a lack of genetic effects.
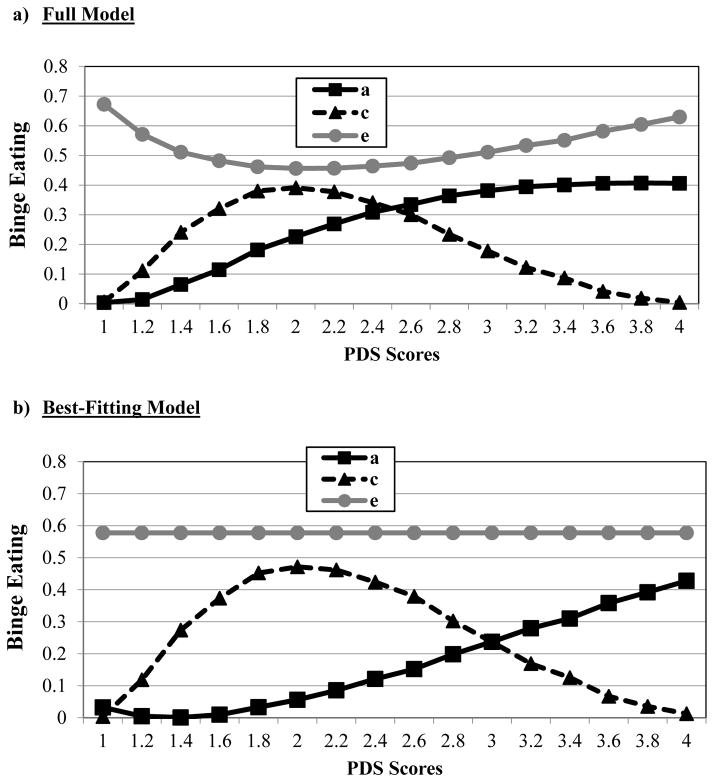
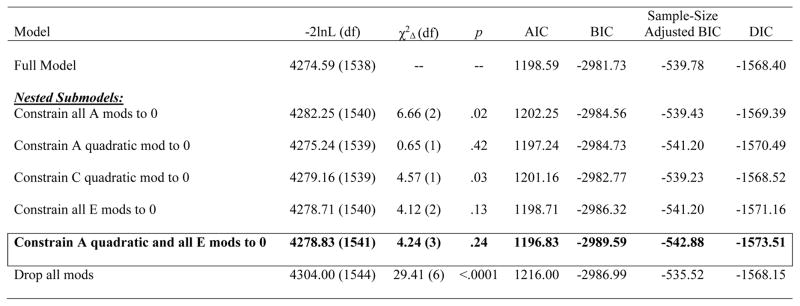
Unstandardized estimates from the full model (see Figure 1) showed continuous increases in genetic effects across pubertal development. Model fit comparisons indicated that the model constraining all genetic moderators to 0 provided a poor fit to the data (i.e., significant chi-square change test; see Table 1). Submodels were subsequently fit based on full model estimates that seemed to show: 1) linear increases in genetic effects, 2) non-linear increases in shared environmental effects; and 3) minimal changes in nonshared environmental influences. The best-fitting submodel constrained the quadratic genetic moderators and all of the nonshared environmental moderators to 0 (see Table 1). Unstandardized (see Figure 2) and standardized (see parentheses) estimates from this model showed significant linear increases in genetic effects from pre- (5%) to post- (42%) puberty, increases (from 1%–42%) and then decreases (to 1%) in shared environmental effects, and no differences in nonshared environmental influences (51–82%)3 across pubertal development.4
Figure 1. Unstandardized Estimates of Additive Genetic (a), Shared Environmental (c), and Nonshared Environmental (e) Influences from the a) Full Model and b) Best-Fitting Model across Pubertal Development Scale (PDS) Scores.
Although log-transformed PDS scores that were floored to 0 were used in all models (see Methods), raw PDS scores are depicted here for ease of interpretation. The best-fitting model constrained the quadratic genetic moderator and all of the nonshared environmental moderators to 0.
Table 1.
Model Fit Comparisons.
Note. −2lnL = minus twice the log-likelihood; χ2Δ = chi-square change; AIC = Akaike information criterion; BIC = Bayesian information criterion; DIC = deviance information criterion; Full Model = model with paths, linear, and quadratic moderators; A = additive genetic effects; C = shared environmental effects; E = nonshared environmental effects; mod(s) = moderator(s); quadratic = non-linear increases/decreases in effects; Constrain all A mods to 0 = model in which both the linear and quadratic (i.e., nonlinear) additive genetic moderators were constrained to 0; Constrain A quadratic mod to 0 = model in which just the quadratic (i.e., non-linear) additive genetic moderator was constrained to 0; Constrain all C mods to 0 = model in which both the linear and quadratic (i.e., non-linear) shared environmental moderators were constrained to 0; Constrain all E mods to 0 = model in which both the linear and quadratic (i.e., non-linear) nonshared environmental moderators were constrained to 0; Constrain A quadratic and all E mods to 0 = model in which the quadratic (i.e., non-linear) additive genetic moderator and all of the nonshared environmental moderators (i.e., linear and quadratic) were constrained to 0; Drop all mods = model in which the linear and quadratic moderators for additive genetic, shared environmental, and nonshared environmental influences were constrained to 0. Each nested submodel is compared to the full model when calculating the χ2Δ and degrees of freedom. The best-fitting model, as determined by a non-significant chi-square change test and the lowest AIC, BIC, sample-adjusted BIC, and DIC values, is noted by cell borders and bolded text. Unstandardized estimates (95% confidence intervals) from the best-fitting model is included here, with significant effects noted in bolded text: additive genetic path (a) = −.17 (−.49, .36), shared environment path (c) = .06 (−.28, .31), nonshared environment path (e) = −.76 (−.81, −.72), linear genetic moderator (βX) = 1.39 (.47, 1.98), linear shared environment moderator (βY) = 4.09 (2.06, 5.92), linear nonshared environment moderator (βZ) = 0, quadratic genetic moderator (βX2) = 0, quadratic shared environment moderator (βY2) = −6.67 (−9.84, −3.45), quadratic nonshared environment moderator (βZ2) = 0.
Discussion
This was the first study to examine pubertal differences in genetic effects on binge eating. Results showed substantial increases in genetic influences from pre-puberty to post-puberty that were linear in nature and became noticeably pronounced around mid-puberty (i.e., PDS score ≥ 2.5).
Although findings are similar to those for overall disordered eating (see Culbert et al., 2009; Klump et al., 2007), findings conflict somewhat with studies showing no significant age differences in genetic influences on binge eating in twins aged 11 versus aged 17 (Klump, McGue, & Iacono, 2000). This past study did not examine puberty, and given the diverse ages in our pre-pubertal (range = 8–15 years) and pubertal (range = 9–16 years) groups, differing results are likely due to the imprecise nature of age as a proxy for pubertal development. To confirm this, we conducted post hoc age models and found no significant differences in genetic effects across age in our study (i.e., the “no age difference model” fit the data best – comparisons with the full model: χ2Δ(6) = 11.29, p = .08). These data suggest that developmental differences for binge eating may be more closely tied to pubertal development than age and provide additional, albeit indirect, support for significant hormone/binge eating associations. Findings from pilot work showing increased genetic effects on binge eating at high (versus low) estradiol levels during puberty (Klump, Keel, et al., 2010) further support these associations and the need for studies examining hormonal mechanisms underlying puberty’s effects.
Although not a specific focus of this brief report, there were significant differences in shared environmental influences on binge eating across puberty as well. The quadratic shared environmental moderator was significant (see bolded estimates in the Table 1 Note), and unstandardized parameter estimates (see Figure 1) indicated an initial increase during pre-early puberty (from 0–42% standardized estimates) and then a pretty dramatic decrease (from 42-1% standardized estimates) from mid-puberty into post-puberty. Decreasing shared environmental influences across puberty are similar to past work for overall levels of disordered eating (Klump, Perkins, et al., 2007), although the nature of the effects differ – past work showed linear decreases (Klump, Perkins, et al., 2007), while the current study showed non-linear increases then decreases. Discrepant results may reflect true differences in the nature of shared environmental influences on overall eating pathology versus binge eating, or they could reflect sample differences. Because we included much younger twins (i.e., ages 8 and up; Klump, Perkins, et al. (2007) included ages 13 and up) and a substantially larger sample of pre-early puberty twins in the current study, we likely had much more power to detect quadratic/non-linear effects during pre-early puberty than previous work. Regardless of similarities/differences, however, our current results suggest that shared environmental factors that are specific to the early pubertal period (e.g., changing overall pressures for thinness) rather than common across development (e.g., general family factors, e.g., divorce) might be the most important types of shared environmental risk factors for binge eating.
Before ending, we should note two key limitations. We relied on a self-report rather than interview measure of binge eating. Although self-reports might over-estimate binge eating (Berg, Peterson, Frazier, & Crow, 2011), the percentage of our twins (3.4%; see Supplemental Table 1) scoring above the binge eating scale cut-off for women with BN (von Ranson et al., 2005) is on par with what would be expected. Moreover, our measure performs well across the broad ages examined (Luo et al., 2016) and it reliably distinguishes between individuals with BN versus controls (von Ranson et al., 2005). We focused on binge eating rather than binge-related disorders. Samples sizes for disorders were too small for analysis, but binge eating appears to be dimensional rather than categorical in nature (Luo et al., 2016), and heritabilities of binge eating and binge-related disorders are similar (e.g., Bulik, Sullivan, & Kendler, 1998). Clearly, more studies of clinical disorders with interview-based measures are needed, but initial data suggest that results might extend to these disorders and measures as well.
Supplementary Material
Acknowledgments
This work was supported by grants awarded to KLK and SAB by the National Institute of Mental Health (NIMH) (R01 MH092377, R01 MH082054). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIMH.
Footnotes
The Minnesota Eating Behavior Survey (MEBS; previously known as the Minnesota Eating Disorder Inventory [M-EDI]) was adapted and reproduced by special permission of Psychological Assessment Resources, Inc., 16204 North Florida Avenue, Lutz, FL 33549, from the Eating Disorder Inventory (collectively, EDI and EDI-2) by Garner, Olmstead, and Polivy (1983) by the Psychological Assessment Resources, Inc. Further reproduction of the MEBS is prohibited without prior permission from Psychological Assessment Resources, Inc.
Twin moderation models rest on the assumption that the moderator (puberty) is genetically independent of the dependent variable (binge eating). Without independence, genetic mediation (i.e., gene-environment correlations (rGE)) could “masquerade” as genetic moderation. We ruled out mediation effects by fitting “GxE in the presence of rGE” models (Purcell, 2002) and finding that the parameter testing rGE (i.e., the moderation of the covariance path) was non-significant and could be constrained to 0 (comparison with full model: χ2Δ(3) = 5.38, p = .15).
Percentages are not identical because they are standardized estimates that are proportional to the total variance.
We were concerned that inclusion of twins ages 16–25 may have unduly influenced our results. We re-fit all models in twins ages 8–15 only and found identical results (see Supplemental Table 2 and Figure 2).
References
- Baker JH, Maes HM, Lissner L, Aggen SH, Lichtenstein P, Kendler K. Genetic risk factors for disordered eating in adolescent males and females. Journal of Abnormal Psychology. 2009;118(3):576–586. doi: 10.1037/a0016314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg KC, Peterson CB, Frazier P, Crow SJ. Convergence of scores on the interview and questionnaire versions of the eating disorder examination: A meta-analytic review. Psychological Assessment. 2011;23(3):714–724. doi: 10.1037/a0023246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulik CM, Sullivan PF, Kendler KS. Heritability of binge-eating and broadly defined bulimia nervosa. Biological Psychiatry. 1998;44(12):1210–1218. doi: 10.1016/s0006-3223(98)00280-7. [DOI] [PubMed] [Google Scholar]
- Burt SA, Klump KL. The Michigan State University Twin Registry (MSUTR): An Update. Twin Research and Human Genetics. 2012;16(1):344–350. doi: 10.1017/thg.2012.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culbert KM, Breedlove SM, Nigg JT, Sisk CL, Burt SA, Klump KL. The emergence of sex differences in risk for disordered eating attitudes during puberty: A role for prenatal tesosterone exposure. Journal of Abnormal Psychology. 2013;122(2):420–432. doi: 10.1037/a0031791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culbert KM, Burt SA, McGue M, Iacono WG, Klump KL. Puberty and the genetic diathesis of disordered eating attitudes and behaviors. Journal of Abnormal Psychology. 2009;118(4):788–796. doi: 10.1037/a0017207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culbert KM, Burt SA, Sisk CL, Nigg JT, Klump KL. The effects of circulating testosterone and pubertal maturation on risk for disordered eating symptoms in adolescent males. Psychological Medicine. 2014;44(11):2271–2286. doi: 10.1017/S0033291713003073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klump KL. Puberty as a critical risk period for eating disorders: A review of human and animal studies. Hormones and Behavior. 2013;64:399–410. doi: 10.1016/j.yhbeh.2013.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klump KL, Burt SA. The Michigan State University Twin Registry (MSUTR): Genetic, environmental and neurobiological influences on behavior across development. Twin Research and Human Genetics. 2006;9(6):971–977. doi: 10.1375/183242706779462868. [DOI] [PubMed] [Google Scholar]
- Klump KL, Burt SA, McGue M, Iacono WG. Changes in genetic and environmental influences on disordered eating across adolescence: A longitudinal twin study. Archives of General Psychiatry. 2007;64(12):1409–1415. doi: 10.1001/archpsyc.64.12.1409. [DOI] [PubMed] [Google Scholar]
- Klump KL, Burt SA, McGue M, Iacono WG, Wade TM. Age differences in genetic and environmental influences on weight and shape concerns. International Journal of Eating Disorders. 2010;43(8):679–688. doi: 10.1002/eat.20772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klump KL, Culbert KM, Sisk CL. Sex differences in binge eating: Gonadal hormone effects across development. Annual Review of Clinical Psychology. 2017;13 doi: 10.1146/annurev-clinpsy-032816-045309. [DOI] [PubMed] [Google Scholar]
- Klump KL, Culbert KM, Slane JD, Burt SA, Sisk CL, Nigg JT. The effects of puberty on genetic risk for disordered eating: Evidence for sex difference. Psychological Medicine. 2012;42(3):627–638. doi: 10.1017/S0033291711001541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klump KL, Holly A, Iacono WG, McGue M, Willson L. Physical similarity and twin resemblance for eating attitudes and behaviors: A test of the Equal Environments Assumption. Behavior Genetics. 2000;30:51–58. doi: 10.1023/a:1002038610763. [DOI] [PubMed] [Google Scholar]
- Klump KL, Keel PK, Racine SE, Burt SA, Neale M, Sisk CL, Boker S, Hu JY. The interactive effects of estrogen and progesterone on changes in emotional eating across the menstrual cycle. Journal of Abnormal Psychology. 2013;122(1):131–137. doi: 10.1037/a0029524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klump KL, Keel PK, Sisk CL, Burt SA. Preliminary evidence that estradiol moderates genetic influences on disordered eating attitudes and behaviors during puberty. Psychological Medicine. 2010;40(10):1745–1753. doi: 10.1017/S0033291709992236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klump KL, McGue M, Iacono WG. Age differences in genetic and environmental influences on eating attitudes and behaviors in female adolescent twins. Journal of Abnormal Psychology. 2000;109(2):239–251. [PubMed] [Google Scholar]
- Klump KL, McGue M, Iacono WG. Differential heritability of eating attitudes and behaviors in prepubertal versus pubertal twins. International Journal of Eating Disorders. 2003;33(3):287–292. doi: 10.1002/eat.10151. [DOI] [PubMed] [Google Scholar]
- Klump KL, O’Connor SAM, Hildebrandt BA, Keel PK, Neale M, Sisk CL, Boker S, Burt SA. Differential effects of estrogen and progesterone on genetic and environmental risk for emotional eating in women. Clinical Psychological Science. 2016;4(5):895–908. doi: 10.1177/2167702616641637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klump KL, Perkins P, Burt SA, McGue M, Iacono WG. Puberty moderates genetic influences on disordered eating. Psychological Medicine. 2007;37(5):627–634. doi: 10.1017/S0033291707000189. [DOI] [PubMed] [Google Scholar]
- Klump KL, Racine SE, Hildebrandt B, Burt SA, Neale M, Sisk CL, Boker S, Keel PK. Ovarian hormone influences on dysregulated eating: A comparison of associations in women with versus without binge episodes. Clinical Psychological Science. 2014;2(5):545–559. doi: 10.1177/2167702614521794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X, Donnellan MB, Burt SA, Klump KL. The dimensional nature of eating pathology: Evidence from a direct comparison of categorical, dimensional, and hybrid models. Journal of Abnormal Psychology. 2016;125(5):715–726. doi: 10.1037/abn0000174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lykken DT, Bouchard TJ, McGue M, Tellegen A. The Minnesota Twin Family Registry: Some initial findings. Acta Geneticae Medicae et Gemellologiae. 1990;39(1):35–70. doi: 10.1017/s0001566000005572. [DOI] [PubMed] [Google Scholar]
- Neale M, Boker S, Xie G, Maes H. Mx: Statistical Modeling. Richmond, VA: 2003. [Google Scholar]
- O’Connor SM, Burt SA, VanHuysse JL, Klump KL. What drives the association between weight-conscious peer groups and disordered eating? Disentangling genetic and environmental selection from pure socialization effects. Journal of Abnormal Psychology. 2016;125(3):356–368. doi: 10.1037/abn0000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters H, Van Gestel S, Vlietinck R, Derom C, Derom R. Validation of telephone zygosity questionnaire in twins of known zygosity. Behavior Genetics. 1998;28(3):159–161. doi: 10.1023/a:1021416112215. [DOI] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence. 1988;17(2):117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Purcell S. Variance components models for gene-environment interaction in twin analysis. Twin Research. 2002;5(6):554–571. doi: 10.1375/136905202762342026. [DOI] [PubMed] [Google Scholar]
- Reichborn-Kjennerud T, Bulik CM, Kendler K, Roysamb E, Maes HHM, Tambs K. Gender differences in binge-eating: A population-based twin study. Acta Psychiatrica Scandinavica. 2003;108(3):196–202. doi: 10.1034/j.1600-0447.2003.00106.x. [DOI] [PubMed] [Google Scholar]
- van der Sluis S, Posthuma D, Dolan CV. A note on false positives and power in G x E modelling of twin data. Behavior Genetics. 2012;42(1):170–186. doi: 10.1007/s10519-011-9480-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Ranson KM, Klump KL, Iacono WG, McGue M. The Minnesota Eating Behaviors Survey: A brief measure of disordered eating attitudes and behaviors. Eating Behaviors. 2005;6(4):373–392. doi: 10.1016/j.eatbeh.2004.12.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.