Abstract
Background
Older patients can be especially susceptible to antipsychotic-induced side effects, and the pharmacodynamic mechanism underlying this phenomenon remains unclear. We hypothesized that age-related epigenetic alterations lead to decreased expression and functionality of the dopamine D2 receptor (D2R), contributing to this susceptibility.
Methods
In this study, we treated young (2–3 months old) and aged (22–24 months old) C57BL/6 mice with the D2R antagonist haloperidol (HAL) once a day for 14 days to evaluate HAL-induced motor side effects. In addition, we pretreated separate groups of young and aged mice with histone deacetylase (HDAC) inhibitors valproic acid (VPA) or entinostat (MS-275) and then administered HAL.
Results
Our results show that the motor side effects of HAL are exaggerated in aged mice as compared to young mice and that HDAC inhibitors are able to reverse the severity of these deficits. HAL-induced motor deficits in aged mice are associated with an age- and drug-dependent decrease in striatal D2R protein levels and functionality. Further, histone acetylation was reduced while histone trimethylation was increased at specific lysine residues of H3 and H4 within the Drd2 promoter in the striatum of aged mice. HDAC inhibitors, particularly VPA, restored striatal D2R protein levels and functionality and reversed age- and drug-related histone modifications at the Drd2 promoter.
Conclusions
These results suggest that epigenetic changes at the striatal Drd2 promoter drive age-related increases in antipsychotic side effect susceptibility, and HDAC inhibitors may be an effective adjunct treatment strategy to reduce side effects in aged populations.
Keywords: Antipsychotic drug, Motor side effects, Aging, Epigenetics, Histone deacetylase inhibitors, Dopamine D2 receptor, Striatum, Mice
INTRODUCTION
Antipsychotic drugs (APDs) are commonly prescribed to the elderly for the treatment of psychosis and for the Behavioral and Psychological Symptoms of Dementia (BPSD) (Agid et al. 2007; Aupperle 2006; Gareri et al. 2014a; Gareri et al. 2014b). However, the use of such drugs is problematic because of the increased severity and frequency of side effects in aged populations (Gareri et al. 2006; Singh and Hodgson 2010). The most common side effects induced by APDs in general are extrapyramidal side effects (EPS), including parkinsonism, bradykinesia, muscle rigidity and tremor, which have a higher occurrence and severity in elderly patients. Though pharmacokinetic changes could be partially responsible for this increase in side-effect occurrence and severity (Sweet and Pollock 1998), pharmacodynamic alterations during aging is likely a key factor (Graff-Guerrero et al. 2015; Uchida et al. 2009).
Almost all APDs have some degree of affinity for antagonizing the Dopamine 2 Receptor (D2R), as decreased activity of mesolimbic D2R is partially responsible for the therapeutic efficacy of these drugs (Miyamoto et al. 2004). However, off-target blockade of D2R in the nigrostriatal and striatopallidal pathways is thought to play a significant role in the manifestation of EPS (Greenbaum and Lerer 2015) Unsurprisingly then, nigrostriatal and striatopallidal D2Rs are known to be altered during aging (Kaasinen et al. 2000;Volkow et al. 1998; Wong et al. 1997), with decreases in D2R expression (Ishibashi et al. 2009) and ligand binding (Antonini et al. 1993; Rinne et al. 1993; Volkow et al. 1996) in older age. For example, in humans, age-related decreases in extrastriatal D2R have been reported in the frontal cortex (Kaasinen et al. 2002; Greenbaum and Lerer 2015). In rodents, striatal D2R ligand binding is reduced to 41% in aged compared to younger rats (Hoekzema et al. 2010; Suzuki et al. 2001). Recently, it was shown that the therapeutic dosing threshold predicted by D2R/3R occupancy was reduced from ~65 to 50% binding affinity in young versus aged patients (Graff-Guerrero et al. 2015), suggesting a potentially narrower therapeutic window in elderly patients. These age-related alterations in the expression and functionality of D2R underlie the increased sensitivity of older patients to these adverse effects (Shiroma et al. 2010).
Histone modifications have consistently been shown to regulate neural gene expression during development and in response to environmental stimuli (Graff and Tsai 2013), and unsurprisingly epigenetic alterations, specifically histone modifications, have been associated with aging (Maleszewska et al., 2016). In concert, epigenetic changes accumulate during aging and can affect brain function at the cellular and molecular levels (Graff and Tsai 2013; Peleg et al. 2010; Tang et al. 2011). Acetylation of histones at specific lysine residues are decreased in aged rats (Kozurkova et al. 1995), and increased histone deacetylases (HDAC) and decreased histone acetyltransferases (HAT) are evident in aged cells (Dagnas and Mons 2013; Graff and Tsai 2013; Willis-Martinez et al. 2010). HDAC inhibitors can restore memory function in aged mice through enhancement of LTP at hippocampal synapses (Castellano et al. 2012; Zeng et al. 2011). Importantly, our recent study showed that the HDAC inhibitors Valproic Acid (VPA) and MS-275 can increase the therapeutic effects of haloperidol (HAL) in aged mice, partially by modulaton of histone acetylation at the c-fos promoter in the shell of the nucleus accumbens and prefrontal cortex (Montalvo-Ortiz et al. 2014). Taken together, the changing epigenetic landscape in the aged brain may provide a mechanistic explanation for the profound deficits in pharmacotherapeutic success in older patients.
Though D2R expression and function are likely to affect a patient’s susceptibility to motor deficits, it is unclear if aged patients suffer greater side-effects due to this mechanism. In particular, it is unknown if the changing epigenetic landscape that is associated with aging may ultimately explain the increased side-effect occurrence in aged populations. It has been reported that epigenetic alterations in dopaminergic receptors accrue during chronic stress and drug administration (Aoyama et al. 2014; Moriam and Sobhani 2013; Nieratschker et al. 2014;), and similar epigenetic changes are associated with certain psychiatric disorders, including eating disorders (Abdolmaleky et al. 2008; Frieling et al. 2010; Vucetic et al. 2012). In this study, we hypothesized that one of the central mechanisms underlying increased APD-induced side effects in aged patients is driven by epigenetic changes accumulated during aging. To test our hypothesis, we demonstrate age-dependent differences in motor deficit severity with HAL administration between young and old mice and uncover an epigenetic mechanism for this difference. In particular, we show that age-related increases in motor side effects with HAL are associated with histone modifications at the Drd2 promoter leading to decreased D2R expression and functionality in the striatum. Further, we demonstrate that co-treatment with HDAC inhibitors reverse these age-related histone modifications and restore D2R expression and functionality in the striatum. Taken together, our results suggest an important mechanism driving age-related changes in APD-induced side effects and a possible translational approach to ameliorating these symptoms in the clinic.
METHODS AND MATERIALS
Animals
Young (2–3 months old) and aged (20–24 months old) C57BL/6 male mice (n=6–8/group for behavioral tests, n=4–5/group for biochemical analysis) from the Charles River Laboratories (Bar Harbor, Maine) were used for this study. We chose this strain because it is the most common mouse strain used for preclicinal studies. Animals were group housed on a 12 hour light/dark cycle and given food and water ad libitum. All procedures were performed according to NIH guidelines for the treatment of animal subjects and the Current Guide for the Care and Use of Laboratory Animals (2011, 8th edition) under a protocol approved by the Northwestern University Animal Care and Use Committee.
Drugs
All drugs used were purchased from Sigma (St. Louis, MO) and every dose was prepared on the day of administration. HAL (0.01, 0.1, 0.5 mg/kg) and MS-275 (10 mg/kg) were first dissolved in 50μL of glacial acetic acid and brought up to the final dose volume in 0.9% saline with pH adjusted to 5–6 and titrated with 0.1M NaOH. VPA (400 mg/kg) was dissolved in 0.9% saline with pH 7.3. All compounds and vehicles were administered intraperitoneally (i.p.) at a constant volume of 10 ml/kg of body weight once a day for 14 consecutive days. VPA (400mg/kg) or MS-275 (10mg/kg) was injected 30 minutes before HAL (0.01mg/kg) administration.
Behavioral Tests
For all behavioral experiments, mice were acclimated to a sound proof behavioral testing room for at least 30 minutes prior to testing, and assays were performed during the light part of the 12hr light/dark cycle. For a detailed description of the experimental design (drug paradigm, behavioral tests performed and tissue collection), refer to Supplemental Figure 1.
Catalepsy
To assess catalepsy, the Step Down assay was used. A plastic rod (1 cm diameter) was suspended 3.5 cm above a laboratory bench in a soundproof behavioral room. Thirty minutes after drug injection, the front paws of the animal were placed on the rod while the hind paws rested on the bench. The duration of a cataleptic episode was defined as the time to step off from the rod during a 300 sec trial (Fink-Jensen et al. 2011).
Motor Coordination
The TSE RotaRod System (Bad Homburg, Germany) was used to assess motor coordination after drug treatment. Mice were placed on an accelerating rod (4–40 rpm during the first 5 minutes) for 10 minutes, and the latency to fall from the rod was recorded. A total of four trials were conducted with a 10 minute inter-trial interval. The average latency to fall from the rod across the four trials was calculated (Kirschbaum et al. 2009) and used as the endpoint for this study.
Spontaneous Locomotor Activity
Locomotor activity was measured using the Open Field Test. An automated tracking system (Any-Maze) was used to track the locomotion of each subject in an open, plexiglass box (25 cm × 25 cm × 25 cm) that was evenly illuminated. Locomotion was defined as the distance travelled (cm) during the 10 minute trial (Viana et al. 2013).
Western Blot
Tissue samples were collected using 300 μm thick coronal cryosections of the striatum using a 1 mm microdissection punch and homogenized in 3% (w/v) ice-cold Tissue Protein Extraction Reagent (T-PER; Thermo Scientific) then centrifuged at 20,000 × g for 10 minutes at 4°C. Protein content was measured using the BCA protein assay kit (Thermo Scientific) according to the manufacturer’s instructions. Samples were transferred onto a nitrocellulose membrane (Invitrogen). Blots were immunostained overnight at 4°C with primary antibodies against D2R, H3ac, H4ac and H4K12ac (Millipore); H3K9ac (Abcam); H3K27ac (Active Motif). The membrane was then incubated with HRP-conjugated goat anti-mouse or goat anti-rabbit secondary antibodies for 2 hours at room temperature. Blotted protein was detected and quantified using the ChemiDoc™MC Imaging System (BioRad), and levels of target protein expression were normalized to β-actin, as described previously (Montalvo-Ortiz et al. 2014).
cAMP Assay
The cAMP-Glo Assay kit (Promega, WI) was used following manufacturer’s instructions with slight modifications. Brain tissue was diluted in induction buffer and heated at 70°C for 5 min. Samples were then incubated with DA (10uM) or forskolin (10uM) followed by quinpirole (starting dose of 250 uM) for 30 minutes. cAMP-Glo Lysis Buffer was then added to the samples, and the plate was incubated for 15 minutes. Samples were then incubated with cAMP-Glo detection solution containing cAMP-Glo reaction buffer for 20 minutes and incubated with Kinase-Glo reagent for 10 minutes. Luminescence (in RLU), which is inversely proportional to the intracellular cAMP concentrations, was measured by using a platereading luminometer. Responses by percent were then fitted by non-linear regression analysis using GraphPad Prism software (San Diego, CA). To quantify agonist potency, EC50 values (the concentration of agonists required to produce 50% of the maximum effect) were also calculated. Each concentration of quinpirole was tested five times in triplicate.
HAT and HDAC Activity Assay
Nuclear extracts from the striatum were prepared as previously described (Montalvo-Ortiz et al. 2014). HAT and HDAC activities were measured by using a colorimetric assay kit (Epigentek, NY) according to the manufacturer’s instructions and previously published protocols (Siuda et al. 2014; Toussirot et al. 2013). Briefly, this ELISA-based assay directly detects HAT/HDAC-converted deacetylated products, which is proportional to enzyme activity. The ratio of deacetylated products was measured by reading absorbance using a spectrophotometer at 450 nm. HAT or HDAC activities were expressed as OD/mg.
Chromatin Immunoprecipitation Assay (ChIP)
The commercially available Magna ChIP™ G Tissue Kit (17-20000, Millipore) was used, and the published protocol was followed (Montalvo-Ortiz et al. 2014). Fragmented chromatin lysate (400–600 bp size determined by agarose gel electrophoresis) was immunoprecipitated with 5μg of antibody directed against H3K27ac, H3K9ac, H4K12ac or H3K27me3. The DNA–histone complex was incubated with salmon sperm DNA/protein A-agarose beads for 1 hour to estimate non-specific binding. The DNA–histone complex was eluted from the beads and dissociated at 65°C for 4 hours under high-salt conditions. Proteins were digested using proteinase K treatment, and the associated DNA was precipitated with 100% ethanol and resuspended in 80 μl of PCR grade water.
Quantitative Real-Time PCR (qRT-PCR)
The levels of interaction between modified histones and the Drd2 gene promoter were determined by measuring the amount of histone-associated DNA, isolated via ChIP, using qRT-PCR. We used custom primers for the Drd2 promoter (forward: 5′-CTGGAGCCAAAAGCAGTCTG-3′, reverse: 5′-TCCTTCAGGTTTCCGACGCC-3′), and the β-actin promoter (forward: 5′-GCGTCCACCCGCGAGTACAA-3′, reverse: 5′-TCCATGGCGAACTGGTGGCG-3′) used as control. Input and immunoprecipitated DNA amplification reactions were run in triplicate in the presence of SYBR Green (Applied Biosystems) using an ABI Prism 7700 thermocycler (Applied Biosystems, Foster City, CA). Ct values from each sample were obtained using the Sequence Detector 1.1 software. Ct values were normalized to the endogenous gene, beta actin, to obtain a percent input. Fold differences (drug- treated versus control) were then determined using the ΔΔCt method.
Statistical Analysis
All statistical analyses were conducted using GraphPad Prism software (San Diego, CA). Data are expressed as mean ± standard error of the mean (S.E.M.). Two-way analysis of variance (ANOVA) was used to detect age and treatment effects followed by a multiple comparisons analysis using Bonferroni’s post-hoc method. For the cAMP accumulation analysis, results are presented as percentage of the maximal relative luminescence units (RLU), and the concentration-response curves were fitted by a nonlinear regression analysis. Significant differences are reported as p<0.05.
RESULTS
HDAC inhibitors decrease HAL-induced EPS-like behaviors in aged mice
To test whether epigenetic changes might underlie the increase in APD side effects in an aged population, we first assessed whether we could model this increase in side effects in mice. Because the classical antipsychotic HAL has been reliably shown to induce cataleptic behavior in mice (Campbell Baldessarini 1981), we first set out to establish the severity of HAL-induced catalepsy in young (2–3 months old) and aged (20–24 months old) cohorts. Using the Step Down assay, a well-validated assay of cataleptic behavior (Campbell and Baldessarini 1981), we assessed both cohorts’ motor function after daily administration of HAL i.p. for 14 days. Two-way ANOVA showed significant effects of age (F1,51 = 46.59, p<0.0001), treatment (F3,51 = 62.76, p<0.0001), and age × treatment interaction (F3,51 = 8.19, p<0.001) on the duration of cataleptic episodes in a dose-dependent manner. In young mice, post-hoc analysis revealed significant increases in the duration of cataleptic episodes in mice that received HAL at 0.1 (p<0.05) and 0.5 mg/kg (p<0.01) but not at 0.01mg/kg, as compared to age-matched control mice (Figure 1A). By contrast, aged mice showed significant increases in the duration of cataleptic episodes at all doses of HAL (p<0.01) compared to their age-matched controls. When comparing the young and aged mice, HAL administration increased the duration of cataleptic episodes in the aged mice at all three doses tested. This mouse model demonstrates that HAL-induced catalepsy increases with age and that this paradigm is useful to investigate the biochemical mechanisms underlying the increase in severity of APD-induced side effects associated with aging.
Figure 1. HDAC inhibitors decrease HAL-induced motor side effect severity in aged mice.
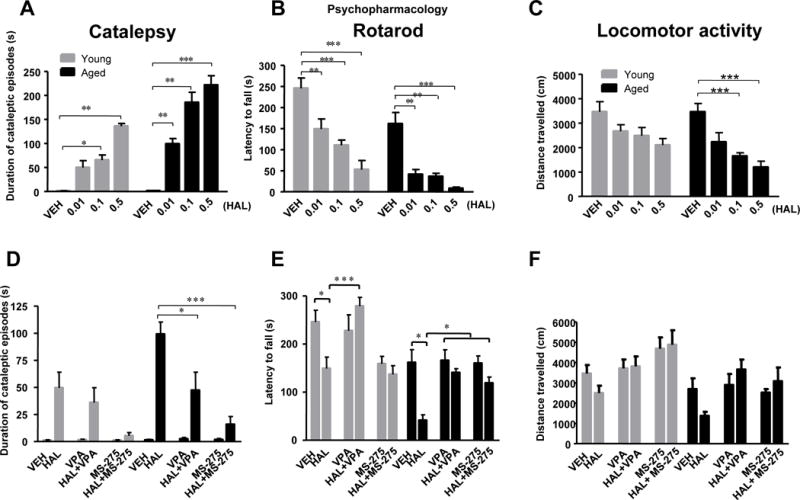
Panel A–C: HAL induces motor deficits in a dose-dependent manner, which are exaggerated in aged mice. Panel D–F: HDAC inhibitors VPA and MS-275 reverse motor-side effects induced by HAL. Catalepsy (A,D), measured as the duration of cataleptic episodes in seconds. Rotarod (B,E), measured as the latency to fall from the rod. Open field, measured as general motor movement (C,F). Data represent mean ± S.E.M. (n = 6–8/group). *p<0.05, **p<0.01, ***p<0.001.
To test our hypothesis that epigenetic changes might mediate the differences in cataleptic behavior, we assayed multiple cohorts of young and aged mice after being administered vehicle or HDAC inhibitors (VPA or MS-257) and subsequent vehicle or HAL daily for 14 days. Interestingly, two-way ANOVA revealed significant effects of age (F1,71 = 4.29, p<0.05) and treatment (F5,71 = 19.89, p<0.0001) after daily pre-treatment with VPA (400mg/kg) or MS-257 (10mg/kg). VPA or MS-275 significantly diminished the duration of HAL-induced cataleptic episodes in both young and aged mice with a much larger difference observed in the aged cohort. Post-hoc analysis revealed significant decreases in the duration of cataleptic episodes for aged mice given HAL+VPA (p<0.05) and HAL+MS-275 (p<0.001) treated groups as compared to HAL-treated age-matched mice (Figure 1D).
Because previous publications have shown APDs disrupt coordination and motor learning in an age-dependent manner (Kirschbaum et al., 2009; Chiu et al., 2011), we assayed both young and aged mice on the accelerating rotarod. Significant effects of age (F1,57 = 34.34, p<0.0001) and treatment (F3,57 = 30.24, p<0.001) were found on the latency to fall from the rotarod. HAL significantly impaired motor function by decreasing the latency to fall off the rotarod in a dose-dependent manner in both aged and young mice, and again, aged mice had the largest decrease in latency. (Figure 1B). Consistent with the catalepsy results, significant effects of age (F1,71 = 26.27, p<0.0001), treatment (F5,71 = 9.720, p<0.0001), and age × treatment interaction (F5,71 = 2.849, p<0.05) were observed when VPA or MS-275 pretreatment was combined with HAL administration on the latency to fall. Bonferroni’s post-hoc testing revealed a significant increase in the latency to fall in aged mice treated with HAL+VPA relative to aged mice treated with HAL alone (p<0.05). This effect was also found in young mice treated with HAL+VPA as compared to HAL alone (p<0.001) (Figure 1E). No significant differences were found between HAL and HAL+MS-275 in both young and aged group.
Locomotor activity was also assessed in the Open Field Test to evaluate whether HAL decreases locomotor activity differently in both young and aged mice. Two-way ANOVA showed significant effects of age (F1,33 = 5.21, p<0.05) and treatment (F3,33 = 11.49, p<0.0001) on the distance traveled in the open field. Significant decreases were found on the distance traveled of aged mice treated with 0.1 (p<0.001) and 0.5 (p<0.001) mg/kg but not 0.01mg/kg of HAL as compared to age-matched controls. Additionally no statistically significant differences in the young cohort were observed (Figure 1C). Again, when VPA or MS-275 pretreatment was combined with HAL administration, we observed significant effects of age (F1,54 = 14.17, p<0.001) and treatment (F5,54 = 3.931, p<0.01) in the distance traveled. In aged mice, VPA and MS-275 alone or combined with HAL demonstrated a trend towards increased locomotor activity as compared to HAL-only treated mice, but this comparison did not reach statistical significance (Figure 1F). Taken together, our results suggest that HAL induces increasingly severe motor side effects in older versus younger mice and that pretreatment with HDAC inhibitors is sufficient to reverse the severity of these side effects.
HDAC inhibitors restore D2R protein levels and functionality in the striatum of aged mice
Age-related alterations in the homeostasis of the dopaminergic system influence the action of APDs targeting dopamine receptors (Hoskins et al., 1991; Leon et al., 2010). One of the most important alterations is found in the activity of the striatal D2R, which mediates the severity of motor side effects induced by APDs. Because aged mice have an increase in the severity of APD-induced motor deficits, we examined whether D2R changes in the striatum of these mice. In addition, we examined whether daily treatment with HAL with or without HDAC inhibitors might change the levels of the D2R protein via Western Blot. Two-way ANOVA revealed significant effects of age (F1,22 = 25.57, p<0.0001) and treatment (F3,22 = 5.24, p<0.01) on the D2R protein levels. Bonferroni’s post-hoc analysis showed a significant age-related decrease in the striatal D2R protein of HAL-treated mice (0.01 mg/kg, p<0.05) compared to age-matched controls (Figure 2A–B). In addition, striatal D2R protein levels increase in both cohorts but was more apparent in the young mice. When comparing young and aged mice after pretreatment with HDAC inhibitors and HAL, significant effects of treatment (F5,36 = 8.55, p<0.001) on the D2R protein levels in the striatum were observed (Figure 2C). In aged mice, significant increases in D2R protein levels were found in HAL+VPA treated mice (p<0.01) and a trend of increased D2R protein levels is observed in the HAL+MS-275 treated aged mice as compared to vehicle or HAL treated aged mice. These results indicate that the impairment of aged mice to appropriately increase D2R protein levels upon HAL administration is consistent with the increased severity of motor side effects in the behavioral assay. Furthermore, the decrease in D2R protein levels in aged mice at baseline and after HAL-administration may be reversed by treatment with HDAC inhibitors, particularly with VPA, which implies the possibility that age-related differences in the severity of side effects observed upon HAL administration may be mediated by histone modifications.
Figure 2. Divergent effects of HAL and HDAC inhibitors on D2R protein levels between young and aged mice.
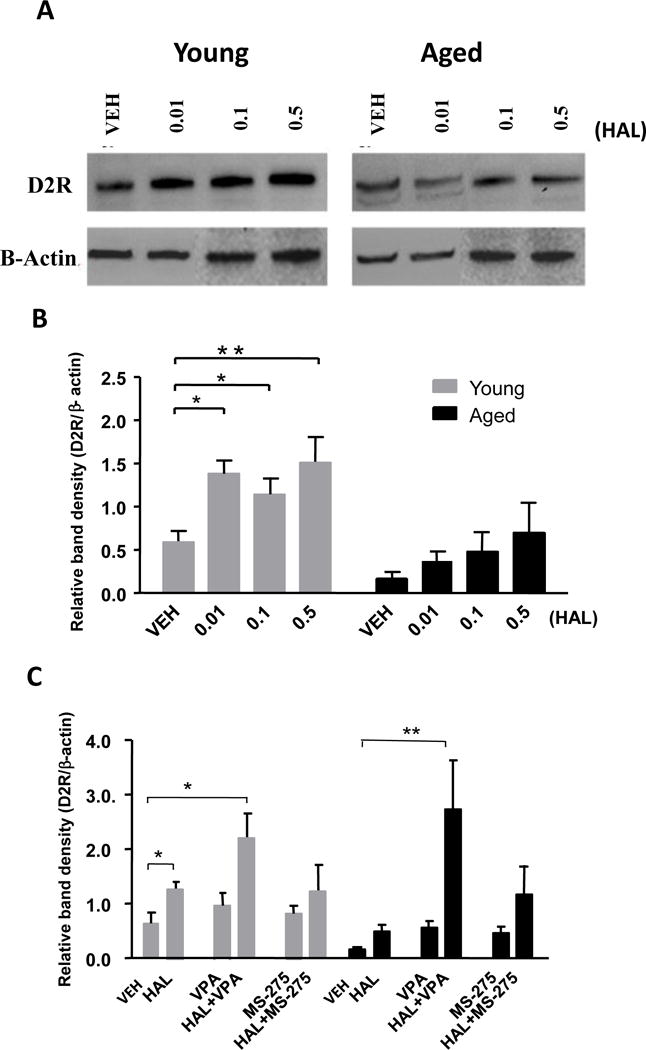
Panel A: Western blot images indicate D2R protein levels of young and aged mice treated with different doses (0.01, 0.1, 0.5 mg/kg) of HAL. β-actin was used as a control to normalize D2R signal to total protein loaded. Panel B: Quantitative analysis of western blot measurement representated in Panel A Panel C: HDAC inhibitors VPA and MS-275 increase D2R protein levels in aged mice in response to HAL stimulation (C). Data represent mean ± S.E.M. (n = 4 per group). *p<0.05, **p<0.01.
Though HDAC inhibition can reverse the decrease in D2R expression in aged mice, it is still unclear if D2R function is similarly affected, evident through D2R’s inhibition of adenylyl cyclase and cAMP production (Neve et al. 2004). To investigate D2R functionality, we measured the effects of dopamine (DA) (10uM) and quinpirole (D2R agonist) on intracellular cAMP concentrations of striatal tissue extracts from young and aged mice. Two-way ANOVA revealed significant effects of age (F1,12 = 505.42, p<0.0001), treatment (F1,12 = 288.87, p<0.0001), and age × treatment interaction (F1,12 = 124.28, p<0.0001) in striatal D2R function. Significant increases in D2R function were found in young mice after DA (10uM) incubation (p<0.0001) but not in aged mice (Figure 3A). We found that cAMP accumulation was significantly higher in the striatal tissues of aged mice (EC50: young=6.35×10−9, aged=1.41×10−6; F1,62 = 154.8, p<0.0001) (Figure 3B, D, F). VPA and MS-275 combined with HAL treatment significantly decreased cAMP accumulation after incubation with escalating doses of quinpirole in the striatal tissues of aged mice (Figure 3 D, F). No differences were observed among the different treatment groups of young mice (Figure 3 B, C, E). These findings suggest that HDAC inhibitors combined with HAL treatment restore D2R functionality in the striatum of aged mice.
Figure 3. Devergent effects of HAL and HDAC inhibitors on D2R receptor functionality in young and aged mice.
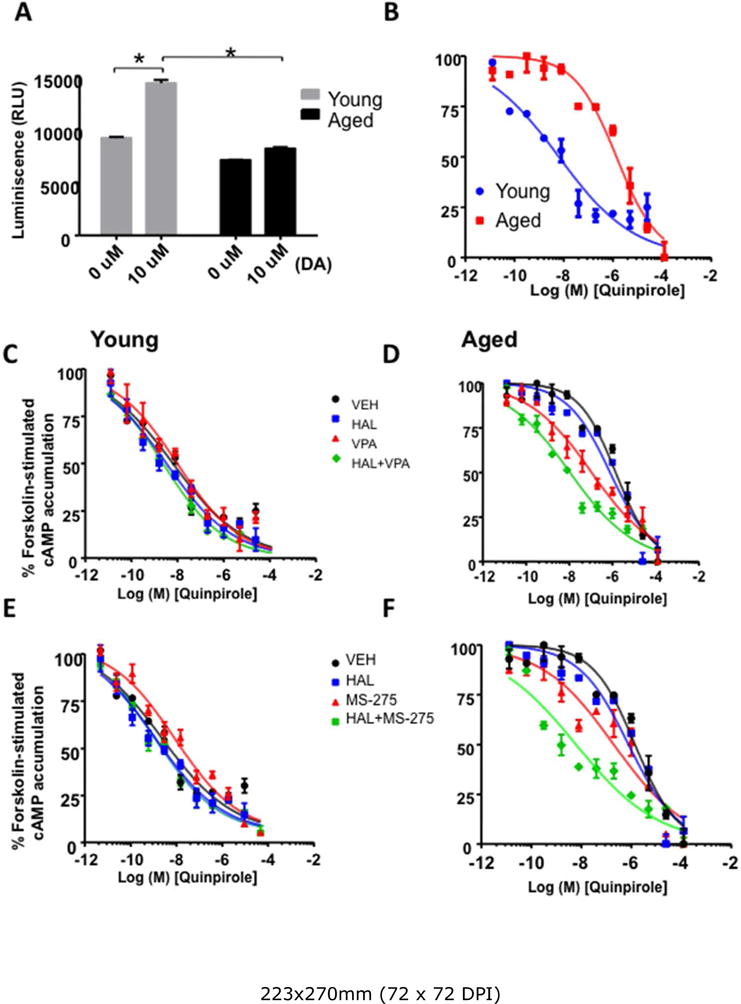
Panel A–B: Dopamine receptor functionality was examined as luminescence (in RLU) elicited by dopamine (DA, 10μm, Panel A) and as forskolin-induced cAMP accumulation (in percentages, Panel B) to quinpirole, a D2R agonist in young and aged mice. Aged mice showed a decreased response to DA and decreased response of D2R to quinpirole as demonstrated by increased cAMP accumulation. HDAC inhibitors VPA (Panel D) and MS-257 (Panel F) restored D2R function in aged mice as reflected by decreased cAMP accumulation, but there were no effects on D2R function in young mice (Panel C, E) Data represent mean ± S.E.M. (n = 4 per group).
HDAC inhibitors increase acetylation and decrease tri-methylation at the Drd2 promoter in the striatum of aged mice
To directly assay the changes in histone modifications influencing D2R protein levels and function, we conducted a ChIP assay at the Drd2 promoter followed by qRT-PCR to investigate the modifications in acetylation and methylation at specific H3 and H4 lysine residues. Results of the specific histone modifications in striatal tissues are found below:
H3K9 acetylation (H3K9ac)
Two-way ANOVA showed significant effects of treatment (F5,32 = 44.25, p<0.0001) and age × treatment interaction (F5,32 = 4.238, p<0.01) in H3K9ac binding at the Drd2 promoter. An age-related decrease in H3K9ac binding was found, and HAL significantly increased H3K9ac binding in the striatum of young but not aged mice. However, significant increases in H3K9ac binding were found in both groups of mice treated with VPA (p<0.01), HAL+VPA (p<0.0001), and HAL+MS-275 groups (p<0.05) relative to age-matched controls (Figure 4A).
Figure 4. Epigenetic alterations at the striatal DRD2 promoter after HAL and HDAC inhibitor administration in young and aged mice.
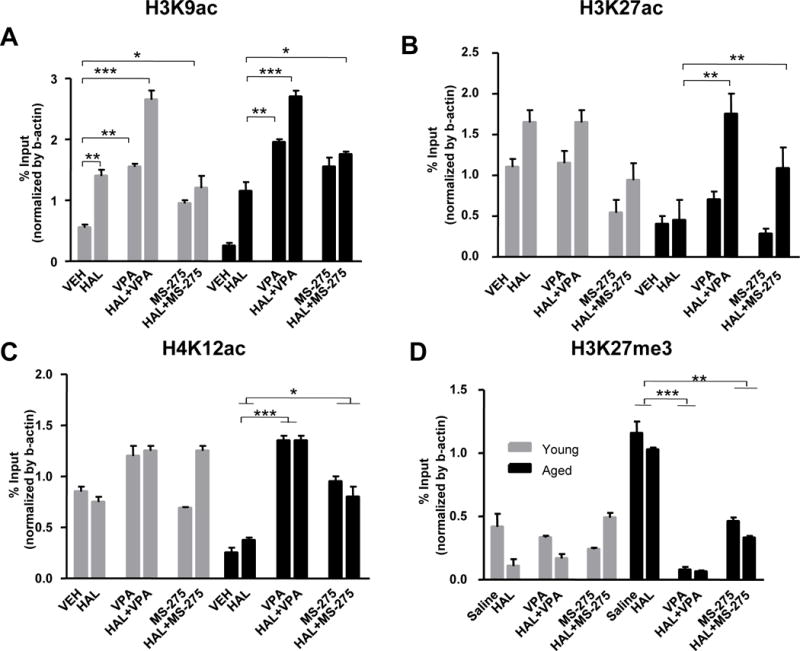
Histone acetylation H3K9ac, H3K27ac, and H4K12ac markers (Panel A–C), and histone mythylation H3K27me3 marker (Panel D) at the Drd2 promoter of the striatum in young (gray bars) and aged (black bars) mice were measured as the percentage of input with ChIP assay and RT-PCR. Results indicated a significant decrease in H3K9ac, H3K27ac and H4K12ac, and significant increase in H3K27me3 in VEH groups in aged mice as compared to young mice. VPA and MS-275 significantly increased H3K9ac, H3K27ac and H4K12ac at the Drd2 promoter of the striatum of aged mice. *p<0.05, **p<0.01, ***p<0.001. n=6–8/group.
H3K27 acetylation (H3K27ac)
Two-way ANOVA showed significant effects of age (F1,35 = 20.32, p<0.0001), treatment (F5,35 = 11.46, p<0.0001), and age × treatment interaction (F5,35 = 6.097, p<0.001) in H3K27ac binding at the Drd2 promoter of the striatum. H3K27ac binding was significantly decreased in aged mice treated with HAL relative to treatment-matched young mice (p<0.0001). However, a significant increase in H3K27ac binding was found in aged mice treated with HAL+VPA (p<0.001) and HAL+MS-275 (p<0.05) as compared to aged mice treated with vehicle. No significant changes in H3K27ac binding were observed in young mice treated with any of the HDAC inhibitors (Figure 4B). H4K12 acetylation (H4K12ac): Two-way ANOVA showed significant effects of age (F1,24 = 12.35, p=0.01), treatment (F5,24 = 40.06, p<0.0001), and an age × treatment interaction (F5,24 = 18.59, p<0.0001) in H4K12ac binding at the Drd2 promoter. We found significant decreases of H4K12ac binding in aged mice treated with vehicle (p<0.01) or HAL (p<0.05) as compared to their treatment-matched young mice. However, significant increases in H4K12ac binding were found in aged mice treated with VPA (p<0.001), HAL+VPA (p<0.001), MS-275 (p<0.05), and HAL+MS-275 (p<0.05) relative to age-matched control. Again, young mice did not show significantly different changes between any of the treatment groups (Figure 4C).
H3K27 tri-methylation (H3H27me3)
Two-way ANOVA showed significant effects of age (F1,35 =73.66, p<0.0001), treatment (F5,35 = 52.01, p<0.0001), and age × treatment interaction (F5,35 = 57.08, p<0.0001) in H3K27me3 at the Drd2 promoter of the striatum. H3K27me3 binding was significantly increased in aged mice treated with vehicle or HAL (p<0.0001) as compared to treatment-matched young mice. However, significant decreases of H3K27me3 binding were found in aged mice treated with HAL+VPA (p<0.0001) or HAL+MS-275 (p< 0.01) as compared to aged mice treated with vehicle or HAL. No significant changes were detected in the young mice at any treatment group (Figure 4D).
The protein levels of the H3 and H4 total and residual markers
Protein levels of these histone acetylation and methylation markers as well as bulk acetylated H3 (H3ac) and H4 (H4ac) were examined in the striatum of young and aged mice and are reported in the Supplementary materials (Supplementary Figure 2, 3). Our results indicate that there was no significant difference in the bulk acetylated H3ac and H4ac between young and aged mice with or without HAL or HDACi treatment (Fig S2). However, the protein levels of the individual histone acetylation (H3K27ac, H4K12ac and H3K9ac) and methylation (H3K27me3) markers were increased or decreased (Fig S3) in parallel with the acetylation and methylation level measured by the ChIP assay at the Drd2 promoter (Fig 4). In summary, these results suggest that there is a substantial increase in repressive histone marks and a decrease in permissive histone marks at the Drd2 locus in aged versus young mice. Further, these results show that while the epigenetic landscape of young mice at this promoter is relatively insensitive to HDAC inhibition, aged mice have a substantially altered epigenetic landscape at the Drd2 promoter with HDAC inhibitor treatment.
HDAC inhibitors decrease HDAC and increase HAT activities in the striatum of aged mice
Because our behavioral and biochemical data demonstrates that aged mice respond particularly strongly to HDAC inhibitors, we hypothesized that HDAC activity may be a driver of these age-related effects. To measure HDAC activity at baseline and after HAL-administration, we used an enzyme-based colorimetric assay with striatal tissue extracts. Two-way ANOVA revealed significant effects of age (F1,36 = 1304, p<0.0001), treatment (F5,36 = 92.88, p<0.0001), and age × treatment interaction (F5,36 = 69.66, p<0.0001) on total HDAC activity. Significant increases in HDAC activity were found in aged mice treated with vehicle and HAL as compared to either vehicle or HAL treated young mice (p<0.0001). However, the decrease in HDAC activity was significantly greater in aged mice treated with VPA (p<0.05), HAL+VPA (p<0.05), MS-275 (p<0.0001) or HAL+MS-275 (p<0.0001) as compared to their age-matched control or HAL-treated mice. Interestingly, there were no significant differences between treatment groups in the young mice nor any changes in either groups with HAL administrated alone (Figure 5A).
Figure 5. HDAC and HAT activity in the striatum after HAL and HADAC inhibitor administration in young and aged mice.
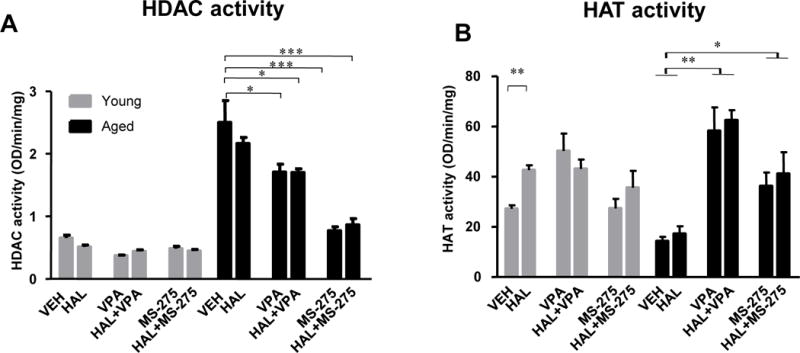
The total HDAC (OD/min/mg) (Panel A) and HAT activities (OD/min/mg) (Panel B) were examined in the striatal tissues of young and aged mice. Data was normalized to β-actin and presented as percentage of input. Data represent mean ± S.E.M. (n = 4–5 per group). *p<0.05, **p<0.01, ***p<0.001
As balances in gene expression are also controlled by active histone acetylation, we evaluated histone acetyl transferase (HAT) activity in both aged and young mice treated with vehicle or HAL and mice pretreated with HDAC inhibitors. Two-way ANOVA revealed significant effects of treatment (F5,36 = 15.96, p<0.0001) and age × treatment interaction (F5,36 = 6.26, p<0.001) on HAT activity in the striatum. HAT activity was significantly decreased in aged mice when compared to young mice at baseline and under HAL conditions (p<0.05). However, significantly greater increases in HAT activity were found in aged mice treated with VPA, HAL+VPA (p<0.01), MS-275, and HAL+MS-275 (p<0.05) as compared to age-matched controls or mice treated with HAL alone. Interestingly, HAL induced an increase in HAT activity in young mice but not in aged mice. However, similar to the activity of the HDAC inhibitors, there was no change in HAT activity under any treatment with VPA or MS-275 in the young mice (Figure 5B). This suggests that only aged mice show significant changes in HDAC or HAT activity upon HDAC inhibition. In addition, the increase in HAT activity after HAL in young but not aged mice may suggest that the lack of compensatory increase in D2R with HAL may be due to blunted HAT activity in aged populations.
DISCUSSION
We demonstrated that the severity of HAL-induced motor side effects differ between young and aged mice, and through detailed biochemical and pharmacological analyses, our data suggest that the epigenetic regulation of the Drd2 promoter is likely to underlie some of the observed behavioral differences. Our data provide strong evidence indicating that histone hypoacetylation and hypermethylation at certain histone residues associated with the Drd2 promoter in the striatum result in reduced D2R protein levels and subsequent dysfunction, thus contributing to the exacerbated motor side effects in aged mice. These histone modifications are likely caused by the increased HDAC activity and decreased HAT activity observed in the striatum of these aged mice. In addition, while young mice show a compensatory response in upregulating D2R after HAL administration, likely mediated through increased HAT activity, the aged mice lack this compensatory increase in both HAT activity and D2R expression. This blunted response is likely to be associated with the increased sensitivity of aged mice to the motor side effects induced by chronic HAL administration. Lastly, we show that pharmacological inhibition of HDACs, particularly VPA, diminished the severity of HAL-induced motor side effects in aged mice, most likely through the restoration of D2R function in the striatum. Overall, these data suggest that histone modification may be a novel adjuvant therapy to reduce APD-related side effects in elderly patients.
To examine the phenomenon of age-dependent increases in APD-related side effects, we chose to examine the robust and reproducible deficits associated with a classical APD, HAL. We found that HAL increased the duration of cataleptic episodes, an often used marker of APD side-effects (Ionov et al., 2013), in a dose-dependent manner in both young and aged mice. However, the severity of cataleptic episodes is increased in aged mice, consistent with previous reports (Barnes et al. 1990; Campbell et al. 1988; Wiley and Evans 2008). In addition, similar results were obtained using the rotarod, a test of motor dysfunction (Kirschbaum et al. 2009). Although age-related differences were not significant in locomotor activity in the open field, higher doses of HAL resulted in decreased locomotion in aged mice. These findings present strong evidence that our model of age-dependent increases in motor side effect severity is robust and helps to contextualize the subsequent pharmacological and biochemical analyses.
Multiple studies from the literature have suggested differences in APD pharmacokinetics and pharmacodynamics between younger and older patient populations (Uchida and Mamo 2009; Uchida et al. 2009), though it is unclear what exact mechanisms underlie these changes and what contributions each makes to the resulting increase in side effect severity. Consistent with the clinical literature, our previous work (Montalvo-Ortiz et al. 2014) also observed changes in both these processes. Pharmacokinetically, we demonstrated increased accumulation of HAL in the plasma and brain of aged mice compared to controls in a dose-dependent manner. Importantly, however, the plasma and brain concentrations at the lowest dose of HAL assayed (0.01mg/kg) were comparable in both groups. Because our behavioral and biochemical experiments used this dose of HAL, it is very likely that the changes in side effect severity measured by our assays are truly differences in the pharmacodynamic responses due to age (Montalvo-Ortiz et al. 2014).
Age-related changes in dopaminergic signaling are thought to mediate the increased severity of APD-induced side effects found in elderly patients, and this change may underlie the pharmacodynamic response changes associated with age. In line with this, we assessed the functionality of the D2R in the striatum by measuring adenylyl cyclase activity following stimulation with DA and quinpirole (selective D2R agonist). We found that D2R function was impaired in the striatum of aged mice as shown by the reduced ability of DA and quinpirole to decrease cAMP levels. Along with these findings, we observed an age-related decrease in striatal D2R protein upon HAL administration. These results suggested that D2R was uniquely altered during aging and that the decrease in available D2R might explain both the decreased cAMP catabolism and more severe D2R-mediated motor deficits apparent in elderly patients treated with APDs, though a more complex mechanism cannot be ruled out. A recent study looking at dose reduction and D2/3R occupancy in elderly patients concluded that the percentage binding of DA to the receptor is likely correlated with the manifestation of EPS (Graff-Guerrero et al. 2015). Though a specific therapeutic threshold was difficult to establish, the implication from this study and our own data is that the decreased D2R expression may make small changes in endogenous DA transmission more drastic in older patients, which may result in greater changes in the percentage of occupied D2R due to an age-related low baseline amount of the receptor. This narrowing therapeutic window with age may be explained by the decreased D2R and epigenetic modifications found in our study, though further investigation is necessary to confirm this in patients.
Previous rodent studies examining D2R after chronic HAL administration have shown a compensatory increase in striatal D2R (Cazorla et al. 2014), similar to the young cohort of mice in our study. Interestingly, the ability of the aged cohort of mice to upregulate D2R after HAL administration was absent or greatly reduced in a dose-dependent manner. These results suggest that not only is baseline D2R expression reduced in aged mice, but they lack the ability to sufficiently upregulate the receptor upon its antagonism. Consistent with this, young mice show an increase in HAT activity upon HAL administration while aged mice lack this increase. Taken together, this further supports that a more restrictive and less plastic epigenetic landscape exists in aged populations and that this epigenetic constraint may underlie the increase in side-effect severity.
Precedence for epigenetic changes as a strong driver of behavior in aging has been previously demonstrated in the aging-dependent cognitive decline associated with changes in histone modifications in the hippocampus of mice. Specifically, age-related memory impairment has been linked with decreased hippocampal acetylation of H4K12 (Peleg et al. 2010). More generally, increased di-methyl and tri-methyl H3K9, both repressive epigenetic marks, have been implicated in the aging processes (Sen 2014). In our study, we show changes in specific histone residues: decreased acetylation of H3K9, H3K27, and H4K12 (markers associated with transcriptional activation) and increased H3K27 tri-methylation, (a marker associated with transcriptional repression) at the striatal Drd2 promoter. Concordantly, we found that the enzymes that modulate these histone modifications by removing (HDACs) or adding (HATs) acetyl groups are altered in aged versus young mice during APD administration. Our results are consistent with previous reports where HDAC activity is increased (Singh and Thakur 2014) while HAT activity is decreased (Li et al. 2002) during aging, and also consistant with previously shown increases in striatal HDAC1 and HDAC2 with age (Willis-Martinez et al. 2010). This suggests the possibility that specific changes in these histones may represent a more generalizable mechanism behind the increase in all APD-induced side effects found in the elderly population, though this would need to be validated through much more rigorous experimentation with different behaviors and biochemical analyses of the brain regions that facilitate those behaviors.
Aberrant patterns of histone modifications that occur with aging can be pharmacologically corrected with HDAC inhibitors. In our previous work, we demonstrated that pretreatment with the HDAC inhibitors VPA and MS-275 can restore the impaired ability of aged mice to respond to HAL by increasing acetylation at the c-fos promoter in the nucleus accumbens shell and prefrontal cortex (Montalvo-Ortiz et al. 2014). Previous studies have indicated that VPA can have protective effects on dopaminergic neurons (Chen et al. 2006; Kidd and Schneider 2010), and recent findings suggested that VPA may directly affect D2R signaling by modulating histone acetylation at the Drd2 promoter (Lee et al. 2012). HDAC inhibition promotes dopaminergic neuron survival by enhancing H3 acetylation, decreasing inflammatory factors, and increasing apoptosis in microglia (van Heesbeen et al. 2013). Further, blockade of D2R signaling by APDs can induce histone H3 modifications in the striatum, including H3 phospho-acetylation, H3pS10-acK14, associated with transcriptional activation (Lee et al. 2004). Our study adds to evidence that HDAC inhibition may be beneficial to restoring proper D2R signaling and subsequently affected behavior. However, HDAC inhibitors are relatively broad spectrum and will influence the expression of many other genes other than Drd2. Therefore, the observed effects on the Drd2 promoter are likely only a subset of the effects precipitated by these drugs. Our previous report that HDAC inhibitors influence the c-fos promoter supports this hypothesis. Additional studies to investigate HDAC inhibitors’ effects on other genes influenced by APDs, such as Htr2a, will provide a more complete perspective underlying the behavior induced by HDAC inhibiton and the biochemical sequelea in the brain.
It should be noted that differences were observed between VPA and MS-275. VPA is more effective in restoring D2R in the striatum of aged mice, most likely due to a stronger effect of VPA in increasing histone acetylation, as evidenced by higher acetylated H3K9, H3K27, and H4K12 at the Drd2 promoter and lower HAT activity when compared with MS-275 under HAL conditions. The differences observed between these two HDAC inhibitors may be due to: 1) broader effect of VPA at histone acetylation markers, 2) de-methylating action of VPA (Dong et al. 2010), or 3) VPA’s direct potentiation of DR2 activity by enhancing prostate apoptosis response-4 (PAR-4), an intracellular binding partner of DR2 (Lee et al. 2012). The doses of VPA and MS-275 used in our study were selected based on previous publications that have examined both behavioral effects and brain-specific histone acetylation (Ren et al. 2004; Simonini et al. 2006; Tremolizzo et al. 2002). The dose of 0.01 mg/kg selected for HAL-induced motor side effect in aged mice in this study was equivalent to the recommended daily dosages of 2.5mg (2/3 lower than regular dose) for clinical use in aged patients (Markianos et al. 1999) Future studies are needed to identify the dose dependent effects of HDAC inhibitors and optimal dosing for translation of HDAC inhibitors to a clinical population.
Another limitation of our study is the sole investigation of HAL, a classical APD, since atypical APDs are now more commonly prescribed. Our rationale for investigating HAL instead of an atypical APD was to develop a clear preclinical model to associate the changes in side effect severity with epigenetic alterations. It will be of utmost importance to investigate the generalizability of our current epigenetic results to the effects of atypical APDs in aged populations, and ongoing studies in our lab are investigating the atypical APD risperidone in age-related side effect severity. Lastly, our tissue collection method for the biochemical analyses precluded evaluation of more specific subregions within the striatum, as the dorsal and ventral striatum have distinct functional roles in motor function and resultant EPS (Bressan et al. 2003). Future studies are needed to further characterize the regional selectivity of APD action in the striatum. In addition, further investigations of other brain regions, including the prefrontal cortex, will be helpful in understanding the changes in the epigenetic landscape with advanced age.
In summary, the results of our study suggest that hypoacetylation and hypermethylation at certain histone residues associated with the Drd2 promoter may mediate the decrease in the expression and functionality of D2R in the striatum of aged mice at baseline. Further, it is likely that the adaptability of this epigenetic landscape is impaired in aged compared to young mice, as evidenced by diminished compensation of D2R with HAL administration in aged mice. These age-related epigenetic changes are likely one of the mechanisms that contributes to increases in the sensitivity of older individuals to the motor-related side effects induced by HAL and other APDs. Importantly, HDAC inhibitors, particularly VPA, mitigate such adverse effects by restoring histone modifications in the striatum of aged mice and easing the epigenetic constraints on the Drd2 promoter. This mechanism of epigenetic regulation underlying APDs’ behavioral effects in aged mice together with the reversal of side-effects with HDAC inhibitors has significant clinical implications for the development of novel, adjuvant treatment strategies that couple HDAC inhibitors and APDs for better treatment results in aged populations.
Supplementary Material
Supplementary Figure 1. Timeline of the experimental procedures for HDAC inhibitor effect on HAL-induced side effects in aged mice.
Young and aged mice were treated with HAL or HAL+HDAC inhibitors for 14 days. Tissue was collected after the last behavioral test was completed.
Supplementary Figure 2 Divergent effects of HAL and HDAC inhibitors on protein levels of histone acetylation and methylation markers between young and aged mice
Protein levels of histone acetylation H3K9ac, H3K27ac, and H4K12ac markers (Panel A–C), and histone mythylation H3K27me3 marker (Panel D) in young and aged mice. β-actin was used as a control to normalize D2R signal to total protein loaded. Data indicate that HDAC inhibitors VPA and MS-275 increase histone acetylation and decrease histone methylation marker protein levels in aged mice. Data represent mean ± S.E.M. (n = 4 per group). **p<0.01, ***p<0.001, ****p<0.0001
Supplementary Figure 3. The total protein level of H3ac and H4ac
The protein level of H3ac (Panel A) and H4ac (Panel B) in the striatum of young and aged mice. The protein level of total H3ac and H4ac did not show a difference between young and aged mice. Additionally, HAL and HDAC inhibitors did not change H3ac or H4ac expression between groups. (n=4 per group)
Acknowledgments
This work has been funded by National Institute of Mental Health (R21 MH100919-01A1, 5R01 MH109466-01) to Hongxin Dong, (R36 MH100912-01A1) to Janitza L. Montalvo-Ortiz and 5F30MH109249-02 to Daniel W. Fisher
Footnotes
Financial Disclosures
The authors declare no conflict of interest.
References
- Abdolmaleky HM, Smith CL, Zhou JR, Thiagalingam S. Epigenetic alterations of the dopaminergic system in major psychiatric disorders. Methods Mol Biol. 2008;448:187–212. doi: 10.1007/978-1-59745-205-2_9. [DOI] [PubMed] [Google Scholar]
- Agid O, Remington G, Kapur S, Arenovich T, Zipursky RB. Early use of clozapine for poorly responding first-episode psychosis. Journal of clinical psychopharmacology. 2007;27:369–73. doi: 10.1097/jcp.0b013e3180d0a6d4. [DOI] [PubMed] [Google Scholar]
- Antonini A, Leenders KL, Reist H, Thomann R, Beer HF, Locher J. Effect of age on D2 dopamine receptors in normal human brain measured by positron emission tomography and 11C-raclopride. Arch Neurol. 1993;50:474–80. doi: 10.1001/archneur.1993.00540050026010. [DOI] [PubMed] [Google Scholar]
- Aoyama Y, Mouri A, Toriumi K, Koseki T, Narusawa S, Ikawa N, Mamiya T, Nagai T, Yamada K, Nabeshima T. Clozapine ameliorates epigenetic and behavioral abnormalities induced by phencyclidine through activation of dopamine D1 receptor. Int J Neuropsychopharmacol. 2014;17:723–37. doi: 10.1017/S1461145713001466. [DOI] [PubMed] [Google Scholar]
- Aupperle P. Management of aggression, agitation, and psychosis in dementia: focus on atypical antipsychotics. American journal of Alzheimer’s disease and other dementias. 2006;21:101–8. doi: 10.1177/153331750602100209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes DE, Robinson B, Csernansky JG, Bellows EP. Sensitization versus tolerance to haloperidol-induced catalepsy: multiple determinants. Pharmacology, biochemistry, and behavior. 1990;36:883–7. doi: 10.1016/0091-3057(90)90094-x. [DOI] [PubMed] [Google Scholar]
- Bressan RA, Erlandsson K, Jones HM, Mulligan RS, Ell PJ, Pilowsky LS. Optimizing limbic selective D2/D3 receptor occupancy by risperidone: a [123I]-epidepride SPET study. J Clin Psychopharmacol. 2003;23:5–14. doi: 10.1097/00004714-200302000-00002. [DOI] [PubMed] [Google Scholar]
- Campbell A, Baldessarini RJ. Effects of maturation and aging on behavioral response to haloperidol in the rat. Psychopharmacology. 1981;73:219–222. doi: 10.1007/BF00422406. [DOI] [PubMed] [Google Scholar]
- Campbell A, Baldessarini RJ, Cremens MC. Dose-catalepsy response to haloperidol in rat: effects of strain and sex. Neuropharmacology. 1988;27:1197–9. doi: 10.1016/0028-3908(88)90018-4. [DOI] [PubMed] [Google Scholar]
- Castellano JF, Fletcher BR, Kelley-Bell B, Kim DH, Gallagher M, Rapp PR. Age-related memory impairment is associated with disrupted multivariate epigenetic coordination in the hippocampus. PLoS One. 2012;7:e33249. doi: 10.1371/journal.pone.0033249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazorla M, de Carvalho FD, Chohan MO, Shegda M, Chuhma N, Rayport S, Ahmari SE, Moore H, Kellendonk C. Dopamine D2 receptors regulate the anatomical and functional balance of basal ganglia circuitry. Neuron. 2014;81:153–64. doi: 10.1016/j.neuron.2013.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen PS, Peng GS, Li G, Yang S, Wu X, Wang CC, Wilson B, Lu RB, Gean PW, Chuang DM, Hong JS. Valproate protects dopaminergic neurons in midbrain neuron/glia cultures by stimulating the release of neurotrophic factors from astrocytes. Mol Psychiatry. 2006;11:1116–25. doi: 10.1038/sj.mp.4001893. [DOI] [PubMed] [Google Scholar]
- Dagnas M, Mons N. Region- and age-specific patterns of histone acetylation related to spatial and cued learning in the water maze. Hippocampus. 2013;23:581–91. doi: 10.1002/hipo.22116. [DOI] [PubMed] [Google Scholar]
- Dong E, Chen Y, Gavin DP, Grayson DR, Guidotti A. Valproate induces DNA demethylation in nuclear extracts from adult mouse brain. Epigenetics. 2010;5:730–5. doi: 10.4161/epi.5.8.13053. [DOI] [PubMed] [Google Scholar]
- Etchepare F, Pambrun E, Begaud B, Verdoux H, Tournier M. Compliance of psychotropic drug prescription with clinical practice guidelines in older inpatients. Fundamental & clinical pharmacology. 2016;30:82–92. doi: 10.1111/fcp.12167. [DOI] [PubMed] [Google Scholar]
- Fink-Jensen A, Schmidt LS, Dencker D, Schulein C, Wess J, Wortwein G, Woldbye DP. Antipsychotic-induced catalepsy is attenuated in mice lacking the M4 muscarinic acetylcholine receptor. Eur J Pharmacol. 2011;656:39–44. doi: 10.1016/j.ejphar.2011.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frieling H, Romer KD, Scholz S, Mittelbach F, Wilhelm J, De Zwaan M, Jacoby GE, Kornhuber J, Hillemacher T, Bleich S. Epigenetic dysregulation of dopaminergic genes in eating disorders. Int J Eat Disord. 2010;43:577–83. doi: 10.1002/eat.20745. [DOI] [PubMed] [Google Scholar]
- Gareri P, De Fazio P, De Fazio S, Marigliano N, Ferreri Ibbadu G, De Sarro G. Adverse effects of atypical antipsychotics in the elderly: a review. Drugs Aging. 2006;23:937–56. doi: 10.2165/00002512-200623120-00002. [DOI] [PubMed] [Google Scholar]
- Gareri P, De Fazio P, Manfredi VG, De Sarro G. Use and safety of antipsychotics in behavioral disorders in elderly people with dementia. Journal of clinical psychopharmacology. 2014a;34:109–23. doi: 10.1097/JCP.0b013e3182a6096e. [DOI] [PubMed] [Google Scholar]
- Gareri P, Segura-Garcia C, Manfredi VG, Bruni A, Ciambrone P, Cerminara G, De Sarro G, De Fazio P. Use of atypical antipsychotics in the elderly: a clinical review. Clin Interv Aging. 2014b;9:1363–73. doi: 10.2147/CIA.S63942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff J, Tsai LH. Histone acetylation: molecular mnemonics on the chromatin. Nat Rev Neurosci. 2013;14:97–111. doi: 10.1038/nrn3427. [DOI] [PubMed] [Google Scholar]
- Graff-Guerrero A, Rajji TK, Mulsant BH, Nakajima S, Caravaggio F, Suzuki T, Uchida H, Gerretsen P, Mar W, Pollock BG, Mamo DC. Evaluation of Antipsychotic Dose Reduction in Late-Life Schizophrenia: A Prospective Dopamine D2/3 Receptor Occupancy Study. JAMA psychiatry. 2015;72:927–34. doi: 10.1001/jamapsychiatry.2015.0891. [DOI] [PubMed] [Google Scholar]
- Greenbaum L, Lerer B. Pharmacogenetics of antipsychotic-induced movement disorders as a resource for better understanding Parkinson’s disease modifier genes. Front Neurol. 2015;6:27. doi: 10.3389/fneur.2015.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekzema E, Herance R, Rojas S, Pareto D, Abad S, Jimenez X, Figueiras FP, Popota F, Ruiz A, Torrent E, Fernandez-Soriano FJ, Rocha M, Rovira M, Victor VM, Gispert JD. The effects of aging on dopaminergic neurotransmission: a microPET study of [11C]-raclopride binding in the aged rodent brain. Neuroscience. 2010;171:1283–6. doi: 10.1016/j.neuroscience.2010.10.012. [DOI] [PubMed] [Google Scholar]
- Ionov ID, Pushinskaya II. Somatostatin antagonist induces catalepsy in the aged rat. Psychopharmacology (Berl) 2013;227:273–6. doi: 10.1007/s00213-012-2961-0. [DOI] [PubMed] [Google Scholar]
- Ishibashi K, Ishii K, Oda K, Kawasaki K, Mizusawa H, Ishiwata K. Regional analysis of age-related decline in dopamine transporters and dopamine D2-like receptors in human striatum. Synapse. 2009;63:282–90. doi: 10.1002/syn.20603. [DOI] [PubMed] [Google Scholar]
- Kaasinen V, Kemppainen N, Nagren K, Helenius H, Kurki T, Rinne JO. Age-related loss of extrastriatal dopamine D(2)-like receptors in women. Journal of neurochemistry. 2002;81:1005–10. doi: 10.1046/j.1471-4159.2002.00895.x. [DOI] [PubMed] [Google Scholar]
- Kaasinen V, Vilkman H, Hietala J, Nagren K, Helenius H, Olsson H, Farde L, Rinne J. Age-related dopamine D2/D3 receptor loss in extrastriatal regions of the human brain. Neurobiol Aging. 2000;21:683–8. doi: 10.1016/s0197-4580(00)00149-4. [DOI] [PubMed] [Google Scholar]
- Kidd SK, Schneider JS. Protection of dopaminergic cells from MPP+-mediated toxicity by histone deacetylase inhibition. Brain Res. 2010;1354:172–8. doi: 10.1016/j.brainres.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum KM, Hiemke C, Schmitt U. Rotarod impairment: catalepsy-like screening test for antipsychotic side effects. The International journal of neuroscience. 2009;119:1509–22. doi: 10.1080/00207450902984002. [DOI] [PubMed] [Google Scholar]
- Kozurkova M, Misurova E, Kropacova K. Effect of aging and gamma radiation on acetylation of rat liver histones. Mech Ageing Dev. 1995;78:1–14. doi: 10.1016/0047-6374(94)01503-e. [DOI] [PubMed] [Google Scholar]
- Lee PE, Gill SS, Freedman M, Bronskill SE, Hillmer MP, Rochon PA. Atypical antipsychotic drugs in the treatment of behavioural and psychological symptoms of dementia: systematic review. Bmj. 2004;329:75. doi: 10.1136/bmj.38125.465579.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Jeong J, Park YU, Kwak Y, Lee SA, Lee H, Son H, Park SK. Valproate alters dopamine signaling in association with induction of Par-4 protein expression. PloS one. 2012;7:e45618. doi: 10.1371/journal.pone.0045618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Xiao H, Isobe K. Histone acetyltransferase activities of cAMP-regulated enhancer-binding protein and p300 in tissues of fetal, young, and old mice. J Gerontol A Biol Sci Med Sci. 2002;57:B93–8. doi: 10.1093/gerona/57.3.b93. [DOI] [PubMed] [Google Scholar]
- Maleszewska M, Mawer J, Tessarz P. Histone modification in aging and lifespan regulation. Curr Mol Bio Rep. 2016;2:26–35. [Google Scholar]
- Markianos M, Hatzimanolis J, Lykouras L. Dopamine receptor responsivity in schizophrenic patients before and after switch from haloperidol to risperidone. Psychiatry Res. 1999;89:115–22. doi: 10.1016/s0165-1781(99)00098-0. [DOI] [PubMed] [Google Scholar]
- Miyamoto J, Tsuji M, Takeda H, Ohzeki M, Nawa H, Matsumiya T. Characterization of the anxiolytic-like effects of fluvoxamine, milnacipran and risperidone in mice using the conditioned fear stress paradigm. European journal of pharmacology. 2004;504:97–103. doi: 10.1016/j.ejphar.2004.09.043. [DOI] [PubMed] [Google Scholar]
- Montalvo-Ortiz JL, Keegan J, Gallardo C, Gerst N, Tetsuka K, Tucker C, Matsumoto M, Fang D, Csernansky JG, Dong H. HDAC inhibitors restore the capacity of aged mice to respond to haloperidol through modulation of histone acetylation. Neuropsychopharmacology. 2014;39:1469–78. doi: 10.1038/npp.2013.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriam S, Sobhani ME. Epigenetic effect of chronic stress on dopamine signaling and depression. Genet Epigenet. 2013;5:11–6. doi: 10.4137/GEG.S11016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neve KA, Seamans JK, Trantham-Davidson H. Dopamine receptor signaling. Journal of receptor and signal transduction research. 2004;24:165–205. doi: 10.1081/rrs-200029981. [DOI] [PubMed] [Google Scholar]
- Nieratschker V, Grosshans M, Frank J, Strohmaier J, von der Goltz C, El-Maarri O, Witt SH, Cichon S, Nothen MM, Kiefer F, Rietschel M. Epigenetic alteration of the dopamine transporter gene in alcohol-dependent patients is associated with age. Addict Biol. 2014;19:305–11. doi: 10.1111/j.1369-1600.2012.00459.x. [DOI] [PubMed] [Google Scholar]
- Peleg S, Sananbenesi F, Zovoilis A, Burkhardt S, Bahari-Javan S, Agis-Balboa RC, Cota P, Wittnam JL, Gogol-Doering A, Opitz L, Salinas-Riester G, Dettenhofer M, Kang H, Farinelli L, Chen W, Fischer A. Altered histone acetylation is associated with age-dependent memory impairment in mice. Science. 2010;328:753–6. doi: 10.1126/science.1186088. [DOI] [PubMed] [Google Scholar]
- Ren M, Leng Y, Jeong M, Leeds PR, Chuang DM. Valproic acid reduces brain damage induced by transient focal cerebral ischemia in rats: potential roles of histone deacetylase inhibition and heat shock protein induction. Journal of neurochemistry. 2004;89:1358–67. doi: 10.1111/j.1471-4159.2004.02406.x. [DOI] [PubMed] [Google Scholar]
- Rinne JO, Hietala J, Ruotsalainen U, Sako E, Laihinen A, Nagren K, Lehikoinen P, Oikonen V, Syvalahti E. Decrease in human striatal dopamine D2 receptor density with age: a PET study with [11C]raclopride. J Cereb Blood Flow Metab. 1993;13:310–4. doi: 10.1038/jcbfm.1993.39. [DOI] [PubMed] [Google Scholar]
- Sen N. Epigenetic Regulation of Memory by Acetylation and Methylation of Chromatin: Implications in Neurological Disorders, Aging, and Addiction. Neuromolecular Med. 2014;29:29. doi: 10.1007/s12017-014-8306-x. [DOI] [PubMed] [Google Scholar]
- Shiroma PR, Geda YE, Mrazek DA. Pharmacogenomic implications of variants of monoaminergic-related genes in geriatric psychiatry. Pharmacogenomics. 2010;11:1305–30. doi: 10.2217/pgs.10.118. [DOI] [PubMed] [Google Scholar]
- Simonini MV, Camargo LM, Dong E, Maloku E, Veldic M, Costa E, Guidotti A. The benzamide MS-275 is a potent, long-lasting brain region-selective inhibitor of histone deacetylases. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:1587–92. doi: 10.1073/pnas.0510341103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh D, Hodgson J. Neuroleptic sensitivity in the elderly: lesson from clinical practice. The Australian and New Zealand journal of psychiatry. 2010;44:1145–6. doi: 10.3109/00048674.2010.515562. [DOI] [PubMed] [Google Scholar]
- Singh P, Thakur MK. Reduced recognition memory is correlated with decrease in DNA methyltransferase1 and increase in histone deacetylase2 protein expression in old male mice. Biogerontology. 2014;15:339–46. doi: 10.1007/s10522-014-9504-5. [DOI] [PubMed] [Google Scholar]
- Siuda D, Wu Z, Chen Y, Guo L, Linke M, Zechner U, Xia N, Reifenberg G, Kleinert H, Forstermann U, Li H. Social isolation-induced epigenetic changes in midbrain of adult mice. Journal of physiology and pharmacology: an official journal of the Polish Physiological Society. 2014;65:247–55. [PubMed] [Google Scholar]
- Suzuki M, Hatano K, Sakiyama Y, Kawasumi Y, Kato T, Ito K. Age-related changes of dopamine D1-like and D2-like receptor binding in the F344/N rat striatum revealed by positron emission tomography and in vitro receptor autoradiography. Synapse. 2001;41:285–93. doi: 10.1002/syn.1085. [DOI] [PubMed] [Google Scholar]
- Sweet RA, Pollock BG. New atypical antipsychotics. Experience and utility in the elderly. Drugs Aging. 1998;12:115–27. doi: 10.2165/00002512-199812020-00004. [DOI] [PubMed] [Google Scholar]
- Tang B, Dean B, Thomas EA. Disease- and age-related changes in histone acetylation at gene promoters in psychiatric disorders. Transl Psychiatry. 2011;1:e64. doi: 10.1038/tp.2011.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toussirot E, Abbas W, Khan KA, Tissot M, Jeudy A, Baud L, Bertolini E, Wendling D, Herbein G. Imbalance between HAT and HDAC activities in the PBMCs of patients with ankylosing spondylitis or rheumatoid arthritis and influence of HDAC inhibitors on TNF alpha production. PloS one. 2013;8:e70939. doi: 10.1371/journal.pone.0070939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremolizzo L, Carboni G, Ruzicka WB, Mitchell CP, Sugaya I, Tueting P, Sharma R, Grayson DR, Costa E, Guidotti A. An epigenetic mouse model for molecular and behavioral neuropathologies related to schizophrenia vulnerability. Proc Natl Acad Sci U S A. 2002;99:17095–100. doi: 10.1073/pnas.262658999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida H, Mamo DC. Dosing of antipsychotics in schizophrenia across the life-spectrum. Progress in neuro-psychopharmacology & biological psychiatry. 2009;33:917–20. doi: 10.1016/j.pnpbp.2009.04.023. [DOI] [PubMed] [Google Scholar]
- Uchida H, Mamo DC, Mulsant BH, Pollock BG, Kapur S. Increased antipsychotic sensitivity in elderly patients: evidence and mechanisms. J Clin Psychiatry. 2009;70:397–405. doi: 10.4088/jcp.08r04171. [DOI] [PubMed] [Google Scholar]
- van Heesbeen HJ, Mesman S, Veenvliet JV, Smidt MP. Epigenetic mechanisms in the development and maintenance of dopaminergic neurons. Development. 2013;140:1159–69. doi: 10.1242/dev.089359. [DOI] [PubMed] [Google Scholar]
- Viana TG, Almeida-Santos AF, Aguiar DC, Moreira FA. Effects of aripiprazole, an atypical antipsychotic, on the motor alterations induced by acute ethanol administration in mice. Basic Clin Pharmacol Toxicol. 2013;112:319–24. doi: 10.1111/bcpt.12036. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Gur RC, Wang GJ, Fowler JS, Moberg PJ, Ding YS, Hitzemann R, Smith G, Logan J. Association between decline in brain dopamine activity with age and cognitive and motor impairment in healthy individuals. Am J Psychiatry. 1998;155:344–9. doi: 10.1176/ajp.155.3.344. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Hitzemann R, Ding YS, Pappas N, Shea C, Piscani K. Decreases in dopamine receptors but not in dopamine transporters in alcoholics. Alcohol Clin Exp Res. 1996;20:1594–8. doi: 10.1111/j.1530-0277.1996.tb05936.x. [DOI] [PubMed] [Google Scholar]
- Vucetic Z, Carlin JL, Totoki K, Reyes TM. Epigenetic dysregulation of the dopamine system in diet-induced obesity. J Neurochem. 2012;120:891–8. doi: 10.1111/j.1471-4159.2012.07649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JL, Evans RL. Evaluation of age and sex differences in locomotion and catalepsy during repeated administration of haloperidol and clozapine in adolescent and adult rats. Pharmacological research: the official journal of the Italian Pharmacological Society. 2008;58:240–6. doi: 10.1016/j.phrs.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis-Martinez D, Richards HW, Timchenko NA, Medrano EE. Role of HDAC1 in senescence, aging, and cancer. Exp Gerontol. 2010;45:279–85. doi: 10.1016/j.exger.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong DF, Young D, Wilson PD, Meltzer CC, Gjedde A. Quantification of neuroreceptors in the living human brain: III. D2-like dopamine receptors: theory, validation, and changes during normal aging. J Cereb Blood Flow Metab. 1997;17:316–30. doi: 10.1097/00004647-199703000-00009. [DOI] [PubMed] [Google Scholar]
- Zeng Y, Tan M, Kohyama J, Sneddon M, Watson JB, Sun YE, Xie CW. Epigenetic enhancement of BDNF signaling rescues synaptic plasticity in aging. J Neurosci. 2011;31:17800–10. doi: 10.1523/JNEUROSCI.3878-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Timeline of the experimental procedures for HDAC inhibitor effect on HAL-induced side effects in aged mice.
Young and aged mice were treated with HAL or HAL+HDAC inhibitors for 14 days. Tissue was collected after the last behavioral test was completed.
Supplementary Figure 2 Divergent effects of HAL and HDAC inhibitors on protein levels of histone acetylation and methylation markers between young and aged mice
Protein levels of histone acetylation H3K9ac, H3K27ac, and H4K12ac markers (Panel A–C), and histone mythylation H3K27me3 marker (Panel D) in young and aged mice. β-actin was used as a control to normalize D2R signal to total protein loaded. Data indicate that HDAC inhibitors VPA and MS-275 increase histone acetylation and decrease histone methylation marker protein levels in aged mice. Data represent mean ± S.E.M. (n = 4 per group). **p<0.01, ***p<0.001, ****p<0.0001
Supplementary Figure 3. The total protein level of H3ac and H4ac
The protein level of H3ac (Panel A) and H4ac (Panel B) in the striatum of young and aged mice. The protein level of total H3ac and H4ac did not show a difference between young and aged mice. Additionally, HAL and HDAC inhibitors did not change H3ac or H4ac expression between groups. (n=4 per group)


