Abstract
Introduction and Hypothesis
To compare hypotheses regarding why obesity is associated with stress urinary incontinence (SUI): 1) obesity increases demands on the continence system (e.g. higher cough pressure) vs 2) obesity compromises urethral function and urethrovaginal support.
Methods
A secondary analysis was performed using data from a case-control study of SUI in women. Measurements of urethrovaginal support (POP-Q point Aa, urethral axis), urethral function (maximal urethral closure pressure (MUCP)), and measures of continence system demands (intravesical pressures at rest and during maximal cough) were analyzed. Cases and controls were divided into three body mass index (BMI) groups: normal (18.5–24.9 kg/m2); overweight (25.0–29.9 kg/m2); and obese (≤30 kg/m2). Logistic regression models where created to investigate variables related to SUI for each BMI group. Structural Equation Modeling was used to test the direct and indirect relationships between BMI, SUI, maximal cough pressure, MUCP, and POP-Q point Aa.
Results
108 continent controls and 103 stress-incontinent women were included. MUCP was the factor most strongly associated with SUI for all BMI groups. Maximal cough pressure was significantly associated with SUI for obese women (OR 3.191 (1.326, 7.683), p<.01), but not for normal or overweight women. Path model analyses showed a significant relationship between BMI and SUI through maximal cough pressure (indirect effect, p=.038), but not through MUCP (indirect effect, p=.243) or POP-Q point Aa (indirect effect, p=.410).
Conclusions
Our results support the first hypothesis: obesity is associated with SUI because of increased intravesical pressure, which therefore increases demand on the continence mechanism.
Keywords: Obesity, pelvic floor disorders, urinary stress incontinence, urodynamics
Introduction
Stress urinary incontinence (SUI) and obesity are both common conditions that impact quality of life. Between 2011 and 2012, approximately 66% of adult women ≥ 20 years of age in the United States were overweight or obese (Body Mass Index (BMI) ≥ 25.0 kg/m2) [1]. Nearly one in five women is estimated to have urinary incontinence [2], with SUI being the most common type [3]. It is well-established that obesity is associated with an increase in the prevalence and severity of SUI [4,5]. For each 5-unit increase in BMI, there is a 10% increased odds of SUI [6], so that among women with a BMI ≥ 40 kg/m2, the prevalence of SUI is nearly 70% [7]. We know that weight loss is associated with improvement in SUI symptoms. There is Level 1 evidence showing that overweight and obese women who lose 5–10% of their weight experience an approximately 70% reduction in SUI episodes [8]. However, while the relationship between obesity and SUI is well-described, the exact mechanism of action is not known.
Stress incontinence may result from any of the following three factors: 1) compromised urethral function; 2) damage to urethrovaginal support; and 3) increased abdominal pressure that places greater demands on the continence system. In the Research on Stress incontinence Etiology (ROSE) study, we compared continent controls to stress incontinent women and determined that urethral function, as measured by maximal urethral closure pressure (MUCP), was the factor most strongly associated with SUI and that urethral support played a less important role [9]. BMI was also found to be an independent predictor of SUI. However, the complex relationship between obesity and the various components of the continence mechanism has not been fully delineated.
Using data from the ROSE study, we performed a secondary analysis to measure the impact of BMI on the continence mechanism. Specifically, we sought to test two competing hypotheses concerning the mechanism by which obesity may cause SUI: 1) obesity increases demands on an otherwise normal continence system (e.g. higher cough pressure); and 2) obesity compromises urethral function and urethrovaginal support.
Materials and Methods
The current study is a secondary analysis of an IRB-approved case-control study of women with stress urinary incontinence (HUM00043944). Detailed methodology has been previously described by DeLancey et al. 2008 and is briefly reviewed here [9]. Women with daily demonstrable stress incontinence were recruited along with a group of asymptomatic controls from the community who were chosen to be of similar age, race, parity, and hysterectomy status. All participants underwent a clinical examination and completed a three-day voiding diary and structured symptom questionnaire. Demographic data, as well as medical and surgical history were also obtained. Stress-incontinent women met all of the following criteria: 1) answered “yes” to the question “Do you currently experience urine leakage with cough, sneeze, laugh, lift, or exercise on a daily basis?”; 2) demonstrated a minimum of one SUI episode on at least two out of the three days of the voiding diary; and 3) had a positive stress test with 300 mL in the bladder. Controls (continent women) met the following criteria: 1) fewer than six episodes of incontinence in the last 12 months; 2) no urinary leakage on a three-day voiding diary; and 3) a negative stress test with 300 mL in the bladder. Women who reported symptoms of urgency incontinence were included as cases only if the stress component was their predominant incontinence symptom and they met the other study criteria.
Clinic examination consisted of a standardized POP-Q examination and urodynamic testing. Assessment of levator ani muscle function using an instrumented speculum that measures force in Newtons generated at rest and during maximal voluntary contraction was also performed and has been previously presented [9]. Urodynamic testing was performed with 300 mL of normal saline in the bladder. Parameters collected included: intravesical pressures at rest and during maximal cough, cough leak point pressures (LPPs), MUCP profile, and uroflow testing. The average of three serial MUCP measurements was used for analysis. Measurements were categorized into the following mechanistic domains: urethral function (MUCP), urethral support (POP-Q point Aa; urethral axis with the cotton-tipped swab at rest, during strain, and during Kegel augmentation), and intravesical pressure (intravesical pressure at rest and during maximal cough).
To analyze the relationship between BMI and SUI, cases and controls were divided into three BMI groups based on the World Health Organization definitions: normal (18.5–24.9 kg/m2); overweight (25.0–29.9 kg/m2); and obese (≥ 30 kg/m2). Descriptive statistics including means, standard deviations, and proportions were obtained for the overall sample and for the BMI and incontinence status groups. Simple linear regression models were used to assess group differences between continuous measures and simple binary logistic regression models were used to assess group differences between binary measures. The outcome of incontinence status was further modeled by multivariable binary logistic regressions stratified by BMI group. Standardized variables were used in these models and were created by converting the original measure to a z-score.
Path models from the Structural Equation Modeling (SEM) framework were then used to test the relationships between BMI and incontinence while accounting for the effects of other plausibly significant variables including maximal cough pressure, MUCP, and POP-Q point Aa. Path analysis is a statistical technique used to analyze a set of simultaneous regression models. Path models investigate whether the effect of one variable on the outcome of interest (e.g. the effect of BMI on SUI) is mediated by one or more variables (e.g. increased BMI causes SUI because it increases maximal cough pressure, weakens urethral function, and/or compromises urethrovaginal support). The latter are termed “mediating variables.” Correlations between a predictor and an outcome can be analyzed in a way that distinguishes between direct effects and indirect effects within the path analysis framework. Direct effects investigate the influence of one variable directly on another. An indirect effect investigates how changes in a predictor variable produce changes in a mediating variable that, in turn, produces changes in the outcome of interest. Unstandardized beta coefficients are presented and all continuous variables were converted to z-scores for the presented path models, which yield coefficient interpretations that mirror those from a regression analysis with standardized variables. BMI was measured continuously rather than categorically in the presented path models to provide more statistical power and a clearer presentation of hypotheses.
Path models with binary outcomes were fit as probit regressions using the weighted least squared (WLSMV) estimator in MPLUS (Version 6.1 [Computer Software]. Los Angeles, CA: Muthen & Muthen.) All other statistical analyses were conducted using Stata (StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP.) and p-values less than .05 were considered statistically significant. When comparing those who are obese and have stress incontinence (n=45) to those who are of normal weight with stress incontinence (n=26), we have 80% statistical power to detect effects that are considered practically important and moderately large when assuming an alpha of .05, a two-tailed independent sample’s t-test between the two groups, and an effect size of .7 (nQuery Advisor + nTerim 4. Boston, MA: Statistical Solutions).
Results
A total of 211 women were included in the analyses, with 108 continent controls and 103 stress-incontinent women (Table 1). When comparing continent to stress-incontinent women, no differences were seen in terms of age, race, vaginal parity, or comorbidities including hypertension, diabetes, chronic lung disease, or heart disease. There were also no differences in these variables across BMI groups. Stress-incontinent women were found to have a slightly higher prevalence of arthritis or rheumatism compared to continent controls.
Table 1.
Demographics and Medical Comorbidities by BMI Category in Women With and Without Stress Urinary Incontinence
| Normala | Overweighta | Obesea | P Valuesb | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Controls (N=46) 1 |
Stress Incontinent (N=26)
2 |
Controls (N=32) 3 |
Stress Incontinent (N=32)
4 |
Controls (N=30) 5 |
Stress Incontinent (N=45)
6 |
Overall Controls vs.
Stress Incontinent (1, 3, 5 vs 2, 4, 6) |
Controls Across BMI
Groups (1 vs 3 vs 5) |
Stress Incontinent
Across BMI Groups (2 vs 4 vs 6) |
|
| Age, years | 48.1 ± 12.2 | 43.6 ± 8.4 | 45.4 ± 12.8 | 48.6 ± 9.6 | 49.7 ± 7.7 | 49.4 ± 8.9 | .977 | .249 | .059 |
| Caucasian | 100 (46) | 88.0 (22/25) | 97.0 (29/30) | 97.0 (31) | 90.0 (27) | 95.0 (42/44) | .491 | .290 | .368 |
| Vaginal Births | 2 (1,3) | 2 (1,3) | 2 (2,2) | 2 (1,3) | 2 (1,3) | 2 (1,3) | .416 | .554 | .143 |
| BMI, kg/m2 | 23.0 ± 1.4 | 23.0 ± 1.4 | 27.1 ± 1.4 | 27.8 ± 1.5 | 35.4 ± 3.7 | 36.5 ± 4.9 | <.001 | <.001 | <.001 |
| Hypertension | 13.0 (6) | 8.0 (2/25) | 16.1 (5/31) | 18.8 (6) | 16.7 (5) | 24.4 (11) | .519 | .888 | .202 |
| Diabetes | 2.2 (1) | 4.0 (1/25) | 6.5 (2/31) | 0 | 0 | 6.7 (3) | .665 | .347 | .636 |
| Chronic Lung Diseasec | 13.0 (6) | 0 | 6.5 (2/31) | 21.9 (7) | 16.7 (5) | 24.4 (11) | .293 | .434 | .792 |
| Cardiac Diseased | 8.7 (4) | 4.0 (1/25) | 0 | 9.4 (3) | 6.7 (2) | 4.4 (2) | .735 | .746 | .617 |
| Arthritis/Rheumatism | 19.6 (9) | 32.0 (8/25) | 32.3 (10/31) | 37.5 (12) | 31.0 (9/29) | 48.9 (22) | .023 | .366 | .339 |
Data presented as mean ± SD, median (IQR), or % (n/N).
BMI categories as follows: Normal (18.5–24.9 kg/m2), Overweight (25.0 –29.9 kg/m2), and Obese (>30 kg/m2)
P values determined using simple linear regression or logistic regressions.
Chronic Lung Disease includes bronchitis, asthma, emphysema, COPD
Cardiac Disease includes coronary artery disease, congestive heart failure, prior myocardial infarction or angina
Table 2 shows the results for clinic examination and urodynamic variables between controls and SUI women and also across BMI groups. As previously published, compared to continent controls, stress-incontinent women had lower MUCP, less urethrovaginal support as measured by POP-Q point Aa and the cotton-tipped swab test at rest and during Kegel, and higher intravesical pressure with maximal cough. Resting bladder pressures were similar between groups.
Table 2.
Comparison of Pelvic Floor Measures in Women With and Without Stress Urinary Incontinence by BMI Category
| Normala | Overweighta | Obesea | P Valuesb | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Controls (N=46) 1 |
Stress Incontinent (N=26)
2 |
Controls (N=32) 3 |
Stress Incontinent (N=32)
4 |
Controls (N=30) 5 |
Stress Incontinent (N=45)
6 |
Overall Controls vs. Stress Incontinent
(1, 3, 5 vs 2, 4, 6) |
Controls Across BMI Groups (1 vs 3 vs 5) |
Stress Incontinent Across BMI Groups
(2 vs 4 vs 6) |
|
| Measures of Urethral Function | |||||||||
| Urethral Pressure, cm H2O | |||||||||
| Maximal Urethral Closure Pressure | 69.8 ± 22.3 | 36.4 ± 11.7 | 70.8 ± 24.9 | 42.9 ± 19.0 | 70.1 ± 20.6 | 41.9 ± 18.3 | <.001 | .977 | .429 |
| Measures of Urethrovaginal Support | |||||||||
| POP-Q Point Aa, cm | −1.2 ± 0.8 | −0.4 ± 1.0 | −0.8 ± 0.9 | −0.6 ± 0.8 | −1.1 ± 0.9 | −0.7 ± 0.7 | <.001 | .220 | .414 |
| Urethral Axis (degrees from horizontal) Using Cotton-Tipped Swab | |||||||||
| At Rest | −11.2 ± 15.0 | −5.0 ± 9.6 | −4.6 ± 15.7 | −3.8 ± 12.7 | −0.2 ± 12.1 | 4.0 ± 10.7 | .001 | .001 | .006 |
| During Strain | 24.8 ± 19.5 | 27.1 ± 22.8 | 27.5 ± 19.9 | 25.8 ± 21.4 | 22.4 ± 18.0 | 33.5 ± 17.5 | .102 | .602 | .190 |
| During Kegel | −25.6 ± 14.1 | −13.1 ± 15.3 | −18.3 ± 16.4 | −15.1 ± 15.7 | −16.9 ± 21.6 | −8.3 ± 13.6 | <.001 | .039 | .169 |
| Measures Relating to Increased Demands on the Continence System | |||||||||
| Intravesical Pressure, cm H2O | |||||||||
| At Rest | 17.6 ± 12.8 | 15.5 ± 4.6 | 19.0 ± 4.5 | 21.0 ± 4.3 | 23.6 ± 4.6 | 24.7 ± 5.0 | .148 | .002 | <.001 |
| With Maximal Cough | 132.4 ± 28.5 | 136.8 ± 38.2 | 142.4 ± 34.2 | 156.5 ± 34.0 | 170.6 ± 35.3 | 185.9 ± 44.4 | .001 | <.001 | <.001 |
Data presented as mean ± SD
BMI categories as follows: Normal (18.5–24.9 kg/m2), Overweight (25.0– 29.9 kg/m2), and Obese (>30 kg/m2)
P values determined using simple linear regression or logistic regressions.
Next, continent controls were compared across BMI groups and were found to have similar urethral function (MUCP) and POP-Q point Aa; however, urethral axis at rest and during Kegel increased with BMI. Finally, increasing BMI was associated with greater demands on the continence system as measured by intravesical pressure at rest and with maximal cough. Among the stress-incontinent women, the same significant differences were observed, with one exception being that urethral axis during Kegel was not significantly different across BMI groups.
Figure 1 shows the results for cough LPPs by BMI group for stress incontinent women. As BMI group increases, so does the average cough LPP. Pairwise comparisons revealed significantly greater cough LPPs for the obese group compared to the normal weight group (167.5 vs. 129.6 cmH2O, p=.001) and also compared to the overweight group (167.5 vs. 138.5 cmH2O, p=.008). Similar results were found with Valsalva leak point pressures (data not shown).
Figure 1.
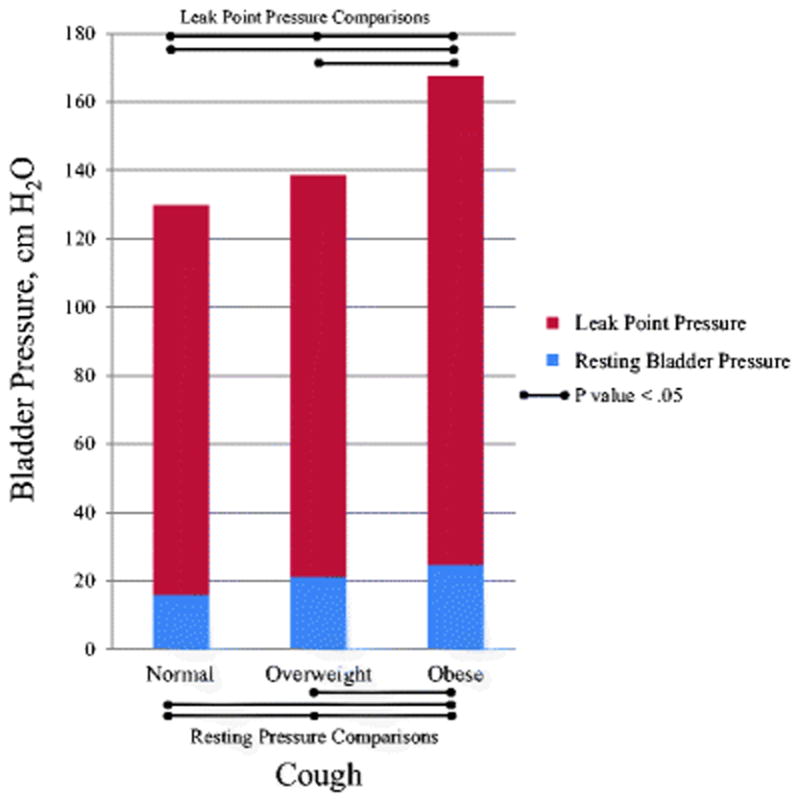
Resting and Leak Point Pressures During Cough in Stress Incontinent Women by BMI Group
Logistic regression models were run to identify factors independently associated with stress incontinence in each of the three BMI groups (Table 3). Increasing MUCP was associated with decreasing odds of stress incontinence and for all groups, MUCP was the factor most highly associated with stress incontinence. Within the normal BMI group, increasing age was associated with decreased odds of stress incontinence, and as the value of point Aa increased (indicating less urethrovaginal support) so did the odds of stress incontinence. Maximal cough pressure was not predictive of stress incontinence in this group. In the overweight group, increasing age also decreased the odds of stress incontinence. Both the location of point Aa and intravesical pressure during maximal cough approached, but did not reach, significance. Finally, among obese women, maximal cough pressure and decreasing urethrovaginal support (point Aa) were predictive of stress incontinence, but age was not significantly associated. To summarize, the factors predictive of stress incontinence differed depending on patient BMI. Maximal cough pressure was significantly associated with stress incontinence in obese women, but not for those who were normal weight or overweight. Furthermore, the association between MUCP and stress incontinence was strongest for women in the normal BMI group.
Table 3.
Logistic Regression Models of Factors Associated With Stress Incontinence Stratified by BMI Groupa
| Standardized Odds Ratios (95% Confidence Intervals) | |||
|---|---|---|---|
| Normal (N=72) | Overweight (N=64) | Obese (N=75) | |
| Constant | 0.166 (0.041, 0.662)b | 0.993 (0.504, 1.956) | 0.679 (0.260, 1.773) |
| Age | 0.197 (0.062, 0.627)c | 0.381 (0.169, 0.859)b | 0.556 (0.202, 1.527) |
| Maximal Urethral Closure Pressure | 0.006 (0.0004, 0.104)d | 0.102 (0.033, 0.320)d | 0.046 (0.011, 0.202)d |
| POP-Q Point Aa | 3.378 (1.188, 9.607)b | 1.871 (0.921, 2.802)e | 2.826 (1.218, 6.560)b |
| Maximal Cough Pressure | 1.09 (0.393, 3.023) | 2.199 (0.944, 5.123)e | 3.191 (1.326, 7.683)c |
| Pseudo R2 | .71d | .377d | .507d |
BMI categories as follows: Normal (18.5–24.9 kg/m2), Overweight (25.0– 29.9 kg/m2), and Obese (>30 kg/m2)
p<.05
p<.01
p<.001
p<.10 (approaches statistical signficance)
Next, path models from the SEM framework were created to test our null hypotheses concerning the mechanisms by which higher BMI may increase the occurrence of stress incontinence (Figure 2 and 3). Figure 2 shows the analysis of Hypothesis 1, which states that increases in BMI are associated with SUI due to the increased demands on the continence system. In the context of the path model where all relationships are tested simultaneously, the direct relationship between BMI and maximal cough pressure (a) and the relationship between maximal cough pressure and stress incontinence (b) are both positive and statistically significant. The direct relationship between BMI and SUI is positive and approaches statistical significance (p=.053). The indirect effect of BMI on SUI through maximal cough (a & b) is positive and statistically significant (p=.038). This indicates that as BMI increases, maximal cough pressure also increases, which, in turn, increases the occurrence of stress incontinence. This model provides support for the hypothesis that increases in BMI influence stress incontinence by increasing demand on the continence system. Note that this model has zero degrees of freedom, which renders fit indices uninformative.
Figure 2.
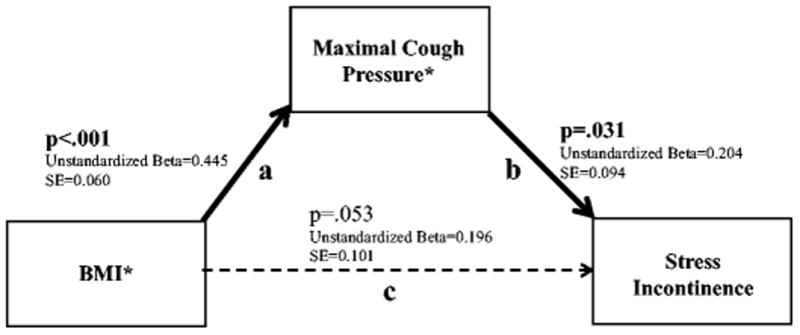
Structural Equation Modeling showing the relationships between BMI, Maximal Cough Pressure, and Stress Urinary Incontinence
*Standardized values used for analyses
Direct, Indirect and Total Effects:
Direct effect of BMI on Stress Incontinence (c)=0.196 (SE=0.101), p=.053
Indirect effect of BMI on Stress Incontinence through Maximal Cough Pressure (a & b)=0.091 (SE=0.044), p=.038 Total effect of BMI on Stress Incontinence (a & b & c)=0.286 (SE=0.091), p=.002
Fit Indices:
Chi(0)=<.001, p<.001; RMSEA <.001 (90% CI: <.001, <.001); CFI 1.000
Note: This model has zero degrees of freedom, which renders fit indices uninformative.
Figure 3.
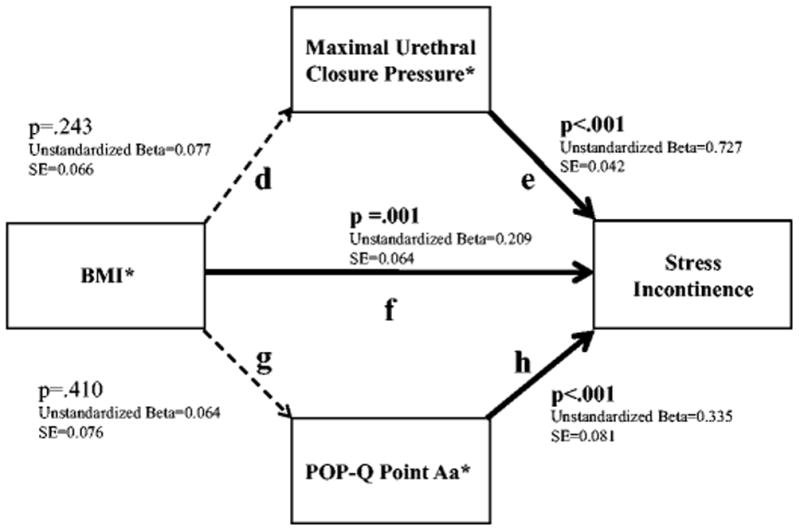
Structural Equation Modeling Showing the Relationships Between BMI and Measures of Urethral Function, Urethrovaginal Support, and Stress Urinary Incontinence. *Standardized values used for analyses
Direct, Indirect and Total Effects:
Direct effect of BMI on Stress Incontinence (f)=0.209 (SE=0.064), p=.001
Indirect effect of BMI on Stress Incontinence through Maximal Urethral Closure Pressure (d & e)=0.056 (SE=0.048), p=.243 Indirect effect of BMI on Stress Incontinence through POP-Q Point Aa (f & h)=0.021 (SE=0.026), p=.410
Total effect of BMI on Stress Incontinence (d & e & g & h & f)=0.286 (SE=0.091), p=.002
Fit Indices:
Chi(1)=0.012, p=0.913; RMSEA <.001 (90% CI: <.001, .073); CFI 1.000
Figure 3 shows results for Hypothesis 2 and depicts the relationships between the continence mechanism factors of urethrovaginal support (point Aa) and urethral function (MUCP) as they relate to the occurrence of SUI. While MUCP (e), point Aa (h), and BMI are each significantly and positively associated with SUI, associations were not found between BMI and either MUCP (d) or point Aa (g) in the context of the path model. Further, the indirect effects of BMI on SUI through either MUCP or point Aa are not statistically significant (p=.243 and p=.410, respectively), indicating that the data do not fully support the hypothesized model even though acceptable model fit statistics were obtained. Similar results were found in the incontinent group when evaluating the degree to which leak point pressure is predicted by the continence mechanisms of MUCP (Unstandardized Beta=0.870 (SE=0.146), p < .001), point Aa (Unstandardized Beta=−0.227 (SE=0.091), p=.013), and BMI (Unstandardized Beta=0.294 (SE=0.077), p < .001), but associations were not found between BMI and either MUCP (Unstandardized Beta=0.001 (SE=.057), p=.98) or point Aa (Unstandardized Beta=−0.005 (SE=0.092), p=.96). Further, the indirect effects of BMI on leak point pressure through either MUCP or point Aa are not statistically significant (Unstandardized Beta=0.001 (SE=0.054), p=.984; Unstandardized Beta=0.001 (0.023), p=.956, respectively). The fit indices further indicate that data did not support the hypothesized model (Chi(1)=1.721, p<.001; RMSEA=.092 (90% CI: <.001, .319); CFI=.98).
Discussion
In this secondary analysis of a case-control study, we quantified the effect of BMI on the three components of the continence mechanism. We identified that the primary association between obesity and SUI is higher cough pressures which place increased demands on the continence system rather than changes in urethral function or urethrovaginal support.
Our findings show that obese women have higher resting intravesical pressures and also generate more pressure during maximal cough compared to women with BMI in the normal or overweight range. Data from our study support the theory that this increased load on the continence mechanism overrides the urethral closure pressure and causes leakage. Therefore, even in the presence of a normally supported and normally functioning urethra, the sheer amount of intraabdominal pressure generated in obese women is what leads to SUI. These results confirm those of Richter et al., who analyzed urodynamic parameters for normal, overweight, and obese women undergoing surgery for SUI [10]. They reported similar results showing that obese women have higher Valsalva LPPs, as well as higher intravesical and intraabdominal pressures at rest. Our study extends the literature by showing that reduced urethral pressure—the dominant cause of SUI—may not be primarily responsible for the increased incontinence observed in obese women. Among women with stress incontinence, those who were obese had similar MUCP measurements compared to those who were normal or overweight, which further supports the fact that obesity may not impair urethral function.
According to our data, excess weight can be seen as a reversible “load” on the continence mechanism. This helps explain why weight loss, either medical or surgical, improves SUI symptoms in overweight and obese women [7,8,11–13]. In a study of overweight and obese incontinent women undergoing a three-month medical weight loss intervention, Subak et al. reported significant correlations between weight loss and intravesical pressure at maximal capacity [14]. Bump et al. also found significant improvements in intravesical pressure and magnitude of intravesical pressure increases with coughing in women who underwent surgical weight loss [15]. In both of these previously mentioned studies, weight loss was also associated with a significant reduction in urinary incontinence symptoms.
The synthetic mid-urethral sling is considered the gold standard for surgical management of SUI. While some studies have shown a statistically lower success rate following surgical management of stress incontinence [16], overall the safety and success of synthetic mid-urethral slings for obese women is well-established [17–19]. In a meta-analysis on surgical treatment of pelvic floor disorders and obesity, Greer et al reported a statistically significant 4% lower cure rate for obese versus non-obese women following tension-free vaginal tape. However, overall cure rate among obese women was still high at 81% [17]. A more recent systematic review also reported a 4% lower objective cure rate among obese versus non-obese women following synthetic mid-urethral sling procedures; however, this difference was not statistically significant (79.2% vs 83.3%, p = .56) [18]. In both studies, bladder perforation rates were lower in obese women. Therefore, while weight loss may be a reasonable first approach to medical management of SUI symptoms in obese women, current evidence shows the synthetic mid-urethral sling is also a safe and effective treatment for this population.
Strengths of this study include having a control group of asymptomatic women who are of similar age, race, and parity, as well as the use of a standardized and comprehensive clinical evaluation that assesses each aspect of the continence mechanism in several ways. Only women with predominant SUI symptoms (and no other urogynecologic complaints) were included, which limits confounding factors. Our study is also strengthened by our statistical methodology and use of path models from the SEM framework, which allowed us to analyze complex relationships between multiple variables simultaneously and test for both direct and indirect effects. Because this is a secondary analysis, our data were limited to that originally collected for the primary study. Therefore, the results of our regression analyses are less stable than they would otherwise be with a larger sample size and we were unable to control for confounding factors, such as socioeconomic status and depression, that could impact the prevalence of SUI [20,21]. Some urodynamic parameters, such as leak point pressure, may demonstrate greater variation based on user technique, and therefore may be subject to a larger margin of error. However, the urodynamics in this study were all performed by experienced nurses, which limits the influence of technical variability. Furthermore, obesity is a complex disease that affects multiple organ systems. It is not possible for any single study to address all potentially associated factors (e.g. metabolic, neurologic, psychologic, etc.). However, this study provides important new information regarding the association between obesity and pelvic floor factors specific to urinary continence.
In conclusion, our results support the hypothesis that obese women have a higher prevalence of SUI because they generate higher intravesical pressures, thus placing more demand on the continence mechanism. Because it does not appear that obesity damages the continence mechanism directly, it seems clinically rational for weight loss to be recommended as an initial treatment of SUI for obese women.
Acknowledgments
This research was supported by the National Institutes of Health Office of Research on Women’s Health grant P50 HD044406. Investigator support for CWS was provided by the National Institute of Child Health and Human Development WRHR Career Development Award K12 HD065257.
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
Author Contributions:
CW Swenson: Data analysis, manuscript writing/editing
GE Kolenic: Data analysis, manuscript writing/editing
ER Trowbridge: Protocol/project development, data collection, manuscript editing
MB Berger: Protocol/project development, data collection, manuscript editing
C Lewicky-Gaupp: Protocol/project development, data collection, manuscript editing
RU Margulies: Protocol/project development, data collection, manuscript editing
DM Morgan: Protocol/project development, data collection, data analysis, manuscript writing/editing
DE Fenner: Protocol/project development, data collection, manuscript editing
JO DeLancey: Protocol/project development, data collection, data analysis, manuscript writing/editing
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311(8):806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu JM, Vaughan CP, Goode PS, Redden DT, Burgio KL, Richter HE, Markland AD. Prevalence and trends of symptomatic pelvic floor disorders in U.S. women. Obstet Gynecol. 2014;123(1):141–148. doi: 10.1097/AOG.0000000000000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hannestad YS, Rortveit G, Sandvik H, Hunskaar S Norwegian EsEoIitCoN-T. A community-based epidemiological survey of female urinary incontinence: the Norwegian EPINCONT study. Epidemiology of Incontinence in the County of Nord-Trondelag. J Clin Epidemiol. 2000;53(11):1150–1157. doi: 10.1016/s0895-4356(00)00232-8. [DOI] [PubMed] [Google Scholar]
- 4.Dwyer PL, Lee ET, Hay DM. Obesity and urinary incontinence in women. Br J Obstet Gynaecol. 1998;95(1):91–96. doi: 10.1111/j.1471-0528.1988.tb06486.x. [DOI] [PubMed] [Google Scholar]
- 5.Hunskaar S. A systematic review of overweight and obesity as risk factors and targets for clinical intervention for urinary incontinence in women. Neurourol Urodyn. 2008;27(8):749–757. doi: 10.1002/nau.20635. [DOI] [PubMed] [Google Scholar]
- 6.Kuh D, Cardozo L, Hardy R. Urinary incontinence in middle aged women: childhood enuresis and other lifetime risk factors in a British prospective cohort. J Epidemiol Community Health. 1999;53(8):453–458. doi: 10.1136/jech.53.8.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burgio KL, Richter HE, Clements RH, Redden DT, Goode PS. Changes in urinary and fecal incontinence symptoms with weight loss surgery in morbidly obese women. Obstet Gynecol. 2007;110(5):1034–1040. doi: 10.1097/01.AOG.0000285483.22898.9c. [DOI] [PubMed] [Google Scholar]
- 8.Wing RR, Creasman JM, West DS, Richter HE, Myers D, Burgio KL, Franklin F, Gorin AA, Vittinghoff E, Macer J, Kusek JW, Subak LL Program to Reduce Incontinence by D, Exercise. Improving urinary incontinence in overweight and obese women through modest weight loss. Obstet Gynecol. 2010;116(2 Pt 1):284–292. doi: 10.1097/AOG.0b013e3181e8fb60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeLancey JO, Trowbridge ER, Miller JM, Morgan DM, Guire K, Fenner DE, Weadock WJ, Ashton-Miller JA. Stress urinary incontinence: relative importance of urethral support and urethral closure pressure. J Urol. 2008;179(6):2286–2290. doi: 10.1016/j.juro.2008.01.098. discussion 2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Richter HE, Kenton K, Huang L, Nygaard I, Kraus S, Whitcomb E, Chai TC, Lemack G, Sirls L, Dandreo KJ, Stoddard A. The impact of obesity on urinary incontinence symptoms, severity, urodynamic characteristics and quality of life. J Urol. 2010;183(2):622–628. doi: 10.1016/j.juro.2009.09.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Richter HE, Creasman JM, Myers DL, Wheeler TL, Burgio KL, Subak LL Program to Reduce Incontinence by D, Exercise Research G. Urodynamic characterization of obese women with urinary incontinence undergoing a weight loss program: the Program to Reduce Incontinence by Diet and Exercise (PRIDE) trial. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(12):1653–1658. doi: 10.1007/s00192-008-0694-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelly CJ, Vichayavilas PE. Weight loss for urinary incontinence in overweight and obese women. N Engl J Med. 2009;360(21):2256. doi: 10.1056/NEJMc090431. author reply 2257. [DOI] [PubMed] [Google Scholar]
- 13.Deitel M, Stone E, Kassam HA, Wilk EJ, Sutherland DJ. Gynecologic-obstetric changes after loss of massive excess weight following bariatric surgery. J Am Coll Nutr. 1988;7(2):147–153. doi: 10.1080/07315724.1988.10720232. [DOI] [PubMed] [Google Scholar]
- 14.Subak LL, Whitcomb E, Shen H, Saxton J, Vittinghoff E, Brown JS. Weight loss: a novel and effective treatment for urinary incontinence. J Urol. 2005;174(1):190–195. doi: 10.1097/01.ju.0000162056.30326.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bump RC, Sugerman HJ, Fantl JA, McClish DK. Obesity and lower urinary tract function in women: effect of surgically induced weight loss. Am J Obstet Gynecol. 1992;167(2):392–397. doi: 10.1016/s0002-9378(11)91418-5. discussion 397–399. [DOI] [PubMed] [Google Scholar]
- 16.Hellberg D, Holmgren C, Lanner L, Nilsson S. The very obese woman and the very old woman: tension-free vaginal tape for the treatment of stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18(4):423–429. doi: 10.1007/s00192-006-0162-2. [DOI] [PubMed] [Google Scholar]
- 17.Greer WJ, Richter HE, Bartolucci AA, Burgio KL. Obesity and pelvic floor disorders: a systematic review. Obstet Gynecol. 2008;112(2 Pt 1):341–349. doi: 10.1097/AOG.0b013e31817cfdde. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weltz V, Guldberg R, Lose G. Efficacy and perioperative safety of synthetic mid-urethral slings in obese women with stress urinary incontinence. Int Urogynecol J. 2015;26(5):641–648. doi: 10.1007/s00192-014-2567-7. [DOI] [PubMed] [Google Scholar]
- 19.Lovatsis D, Gupta C, Dean E, Lee F. Tension-free vaginal tape procedure is an ideal treatment for obese patients. Am J Obstet Gynecol. 2003;189(6):1601–4. doi: 10.1016/j.ajog.2003.09.041. discussion 1604–5. [DOI] [PubMed] [Google Scholar]
- 20.Sung VW, West DS, Hernandez AL, Wheeler TL, 2nd, Myers DL, Subak LL Program to Reduce Incontinence by D, Exercise. Association between urinary incontinence and depressive symptoms in overweight and obese women. Am J Obstet Gynecol. 2009;200(5):557 e551–555. doi: 10.1016/j.ajog.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tincello D, Sculpher M, Tunn R, Quail D, van der Vaart H, Falconer C, Manning M, Timlin L. Patient characteristics impacting health state index scores, measured by the EQ-5D of females with stress urinary incontinence symptoms. Value Health. 2010;13(1):112–118. doi: 10.1111/j.1524-4733.2009.00599.x. [DOI] [PubMed] [Google Scholar]


