Abstract
We describe changes in sexual behaviors among men who have sex with men (MSM) following initiation of pre-exposure prophylaxis (PrEP) in a clinic-based sample of MSM initiating PrEP in Providence, Rhode Island. Data were collected at baseline, 3, and 6 months following PrEP initiation including total number of anal sex partners and condom use. A longitudinal mixed effects model assessed changes in number of partners and condom use over time, adjusting for age, race, and education. There was no statistically significant difference in total number of partners over time. There was a significant increase in number of condomless anal sex partners at the 6-month visit compared to baseline (mean change +1.31 partners, 95% confidence interval 0.09–2.53, P = 0.035). As condomless anal sex may increase following PrEP uptake, adherence counseling and efforts to retain patients in PrEP care, especially during periods of non-condom use, are important as PrEP is more widely implemented.
Keywords: Pre-exposure prophylaxis, Men who have sex with men, HIV, Implementation, Behavioral compensation
Introduction
Pre-exposure prophylaxis (PrEP) is highly efficacious for prevention of HIV acquisition among HIV-uninfected men who have sex with men (MSM) [1–3]. PrEP has the potential to substantially alter the trajectory of the HIV epidemic among MSM in the United States as it becomes more widely available [3]. However, a commonly cited concern with PrEP use is that it may undermine other HIV prevention modalities such as condom use and reducing number of sexual partners [4]. Among individuals who have initiated PrEP, a reduction in condom use or increased number of sex partners because of perceived protection from PrEP (behavioral compensation) may lead to increased risk of sexually transmitted infections (STIs) or HIV acquisition, especially if adherence is suboptimal [5, 6]. However, there has been little evidence of changes in risk taking with other public health interventions, such as morning after pills or the human papillomavirus vaccine [4]. Whether PrEP will lead to changes in sexual behaviors remains largely unknown.
There has been no evidence of behavioral compensation among participants in the major PrEP efficacy trials, including iPrEx among MSM [7, 8]. However, participation in a trial does not reflect real-life situations. During the original placebo-controlled trials, the efficacy of PrEP had not yet been established, and participants in the blinded trials were unaware of which arm they were randomized to, and were aware that there was a 50% chance of being on a placebo pill. While participants may not have changed their sexual behaviors during the trial for these reasons, now that efficacy has been established, it is plausible that individuals initiating PrEP may decrease condom use or initiate PrEP with the intention of decreasing condom use. Considerably less data on changes in sexual behaviors following PrEP initiation exists from open label or demonstration studies. Limited qualitative experiences from the San Francisco Demonstration Project suggested that PrEP was used in conjunction with other risk reduction strategies. These data also indicated that some PrEP users felt more comfortable engaging in condomless anal sex while using PrEP, as well as reported reduced anxiety related to acquiring HIV [9]. Additional data from San Francisco indicate increasing rates of STIs following PrEP uptake, suggesting the potential for behavioral changes [3].
In this study, we review sexual behaviors among a cohort of MSM initiating PrEP in a clinical setting in Providence, Rhode Island, using data collected during routine PrEP care [10]. The purpose of this analysis was to explore sexual behavior changes following PrEP initiation by describing immediate changes in sexual behaviors over a 6-month period following PrEP initiation.
Methods
Data was reviewed from a clinical PrEP program in Providence, Rhode Island that was established in 2013 [10]. The PrEP program was established at an STI and HIV prevention clinic, and received referrals from the HIV care and treatment clinic (e.g., individuals in serodiscordant relationships), the STI clinic, and other providers in the state. All MSM presenting to the STI clinic are educated about PrEP by the clinic staff as previously described [11]. Individuals who are interested in PrEP are referred to the on-site PrEP program. On presentation for PrEP, all individuals are provided with education and counseling on safer sex methods including condom use. Individuals are counseled that PrEP is not 100% effective and does not protect against other STIs. Free condoms are available for all individuals. PrEP was prescribed in accordance with Centers for Disease Control and Prevention (CDC) guidelines [12]. Current PrEP guidelines recommend follow-up counseling every 3 months, and repeat STI testing every 6 months [12]. Patients were followed longitudinally every 3 months in accordance with current guidelines. The evaluation and study protocol were approved by the local Institutional Review Board.
Demographic information was collected at the baseline visit and included age, educational status, and race and ethnicity. Sexual behaviors were collected at baseline and each follow-up visit via clinician interview. Patients were asked the number of male partners with whom they had receptive and/or insertive anal sex in the previous 3 months. The total number of partners was calculated as the sum of each individual sexual role. Patients were also asked how many oral sex partners they had had in the previous 3 months. Patients were then asked of how many men they had anal sex with, how many had they not used a condom in the previous 3 months.
The analysis was restricted to individuals who reported having sex with another man and who had two clinical follow-up visits for PrEP (e.g., those who had been on PrEP for at least 6 months and who had been retained in care) as of May 2016. Demographic characteristics are presented as medians and interquartile ranges for continuous variables and proportions for categorical variables. The total number of partners, number of condomless anal sex partners, and number of oral sex partners was calculated at each time point with 95% confidence intervals (CI). A longitudinal mixed effects model with an indicator variable for each time point was built adjusting for age, race (coded as white versus nonwhite due to the small sample), and educational status (coded as college or above versus below). The indicator variable yields an assessment of the statistical significance in the change in number of partners over time. All analyses were run in Stata 14.1 (StataCorp, College Station, TX).
Results
A total of 61 patients met the inclusion criteria for the analysis, were retained in care for at least 6 months, and had clinical follow-up visits at approximately 3 and 6 months following PrEP initiation. The median age of the sample was 31 years (IQR 26 to 46). Nearly three-quarters (73.8%) of participants had a college education or above, nearly one-quarter identified as Hispanic/Latino (24.6%), and 4.9% identified as African American.
At the baseline visit prior to PrEP initiation, the mean number of anal sex partners (receptive and/or insertive) was 4.9 (95% CI 3.9–6), which decreased slightly to 4.7 (95% CI 3.5–5.9) at 3 months and increased to 5.7 (95% CI 3.6–7.8; Fig. 1a) at 6 months. The mean number of condomless anal sex partners at baseline was 2.0 (95% CI 1.2–2.8), increasing to 2.8 (95% CI 1.3–4.3) at 3 months and 3.3 (95% CI 1.7–4.9; Fig. 1b) at 6 months. The mean number of oral sex partners was 6.4 at baseline (95% CI 4.3–8.5), 5.7 at 3 months (95% CI 3.4–8.0), and 6.6 at 6 months (95% CI 4.2–9.1).
Fig. 1.
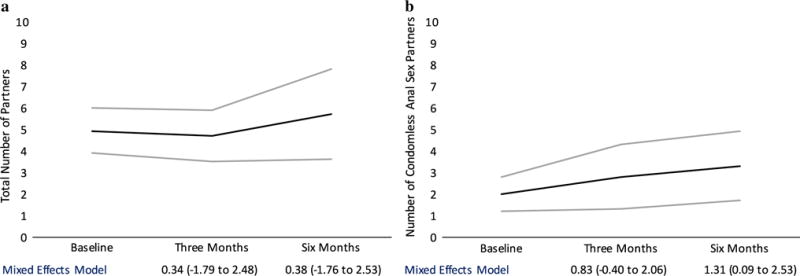
A longitudinal mixed effects model demonstrating changes in a total number of sex partners and b number of condomless anal sex partners at baseline, three and six months after PrEP initiation
There was no statistically significant change over time in total number of anal sex partners or oral sex partners in a longitudinal model (Fig. 1a). In the longitudinal mixed effects model adjusted for age and race/ethnicity, there was a statistically significant increase in mean number of condomless anal sex partners at 6 months compared to baseline (mean increase 1.31 partners, 95% CI 0.09–2.53, P = 0.035; Fig. 1b). This association was stronger in an analysis restricted only to individuals who reported multiple partners in the previous 3 months at a given time point (N = 55). There was an increase in mean number of condomless anal sex partners of 2.01 partners at 3 months (95% CI 0.01–4.02, P = 0.049) and 1.63 at 6 months (95% CI −0.19 to 3.45, P = 0.078). There was no increase in total mean number of partners among those reporting multiple partners at three (1.97, 95% CI −1.50 to 5.45, P = 0.27) or 6 months (−0.09, 95% CI −3.30 to 3.13, P = 0.96).
Discussion
In this sample of MSM prescribed PrEP, the total number of anal and oral sex partners did not change over the first 6 months following PrEP initiation. However, there was a statistically significant increase in number of condomless anal sex partners reported 6 months following PrEP initiation. These results could indicate that although the total number of partners may not change following initiation of PrEP, individuals may feel more protected from HIV and therefore may decrease their use of condoms over time. These results were stronger among individuals who reported more than one partner. Among this subgroup, who similarly had no increase in overall mean number of partners, there was an increase at both three and 6 months in mean number of condomless partners. Although we cannot definitively link these changes to use of PrEP, these results suggest the potential for behavioral compensation with regards to condom use following PrEP initiation in a non-trial setting. Clinicians should be aware of this potential among PrEP patients, although the increase in absolute number of partners was relatively small, with a mean increase of slightly over one partner. Discussion with patients related to the importance of medication adherence to PrEP to achieve maximum prevention benefits, particularly during episodes of non-condom use, may be important to avoid HIV acquisition and mitigate risk. These messages may be particularly effective if supportive advice about risk mitigation, rather than directive messages to use condoms, is offered. Future research related to patient-provider communication and rapport in the context of PrEP is warranted, given the potential for behavioral compensation as noted in this study. While medical providers should generally support and promote condom use, it should not be in such a manner to antagonize a patient and discourage future engagement in care. Patients who do not use condoms are the ones who benefit most from PrEP.
This study adds to a growing body of evidence related to PrEP uptake in ‘real-world’ clinical settings [3, 9, 13]. Evidence from other clinical demonstration projects showed a decrease in the mean number of partners and no change in condomless receptive anal sex [14]. However, there were differences across sites, with increases in condomless receptive anal sex in San Francisco [14]. Another study reporting a clinical setting in San Francisco generally reported low levels of self-reported sexual behavior change [3]. In the context of these studies, our results suggest that some MSM in some settings may participate in more condomless sex after initiating PrEP. Adherence to PrEP may be particularly important during periods of non-condom use. We have previously reported data demonstrating very high rates of adherence in real-world settings [6]. We also found that self-reported adherence correlated with blood concentrations of tenofovir and that patients who are retained in PrEP care generally adhere to PrEP. [6] Patients who are lost to PrEP care may be sub-optimally adherent or may not take medications at all; those patients may need additional outreach or adherence counseling about their HIV transmission risks. Future studies should consider the context of this condomless sex, including whether partnership characteristics (e.g., casual versus primary partners and HIV status of the partners) influence changes in sexual behaviors, and whether adherence and retention in PrEP care play a role in HIV risk taking behaviors. Future work could also consider differences in changes in sexual behaviors in different subgroups of MSM.
The results of this study must be interpreted in the context of several limitations. The data reported here were collected via self-report. Although participants visited the same clinicians for PrEP care and generally there was a high level of rapport, their reports may be subject to social desirability bias or recall bias. In particular, it is possible that rapport built over time, and that participants were more likely to report an accurate assessment of sexual behaviors at follow-up visits. However, the clinic does provide care to a large number of MSM and has a good standing in the community, which may decrease reporting bias. In addition, we did not have data on the sexual behaviors of individuals who were not retained in care. If sexual behaviors change for individuals who initiate PrEP but are not retained in care, addressing retention and care and adherence will be critical. These results may not be generalizable to all MSM taking PrEP, as the results represent the experiences of a relatively small sample of MSM. The duration of follow-up was only 6 months; these data therefore represent short-term sexual behavior outcomes following PrEP. We did not have data on HIV status, viral suppression, or PrEP status of partners, or if they were casual or primary partners. It is possible that some participants changed their behaviors (e.g., condom use) with certain partners whom they knew well or knew to be HIV-uninfected, on PrEP, or virally suppressed. We did not have STI data to assess whether there was an objective change in risk following PrEP uptake.
Despite these limitations, we report early evidence of increasing condomless anal sex in MSM PrEP users in a real world clinic setting, which could be evidence of behavioral compensation following the initiation of PrEP. Despite previous conflicting reports, these results indicate that some PrEP patients may reduce condom use in the period immediately following initiation of PrEP, which may be due to perceived protection from HIV acquisition. Although further work is needed to characterize long-term sexual behavior outcomes among individuals using PrEP, these results suggest that PrEP programs should address the potential for reductions in condom use and provide appropriate counseling related to PrEP adherence, offer support services for retention in PrEP care, and conduct regular STI screening for PrEP patients.
Acknowledgments
Funding PAC is supported by the National Institute of Allergy and Infectious Diseases (1K23AI096923). CEO is supported by the National Institute on Drug Abuse (T32DA013911) and the National Institute of Mental Health (R25MH083620). Additional support was provided by the Lifespan/Tufts/Brown Center for AIDS Research (P30AI042853). RRP is supported by the National Center for Advancing Translational Sciences of the National Institutes of Health (Washington University Institute of Clinical and Translational Sciences UL1TR000448, sub-award KL2TR000450).
Footnotes
Compliance with Ethical Standards
Conflicts of interest KHM has received unrestricted research grants from Gilead Sciences. LM reports institutional programmatic funding, consulting fees, and participation in on the speaker bureau from Gilead Sciences. CEO, ASN, MM, AA, RRP and PAC have declared no conflicts of interest.
Ethical Approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Written informed consent was obtained from all individual participants included in the study.
References
- 1.Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587–99. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grant RM, Anderson PL, McMahan V, Liu AY, Amico KR, Mehrotra M, et al. Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. Lancet Infect Dis. 2014;14:820–9. doi: 10.1016/S1473-3099(14)70847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Volk J, Marcus JL, Phengrasamy T, Blechinger D, Nguyen DP, Follansbee S, et al. No new HIV infections with increasing use of HIV preexposure prophylaxis in a clinical practice setting. Clin Infect Dis. 2015:1–10. doi: 10.1093/cid/civ778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Venter F, Allais L, Richter M. Exposure ethics: does HIV pre-exposure prophylaxis raise ethical problems for the health care provider and policy maker? Bioethics. 2013;28:269–74. doi: 10.1111/bioe.12021. [DOI] [PubMed] [Google Scholar]
- 5.Anderson PL, Glidden DV, Liu A, Buchbinder S, Lama JR, Guanira JV, et al. Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Sci Transl Med. 2012;4:151ra125–151ra125. doi: 10.1126/scitranslmed.3004006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Montgomery MC, Oldenburg CE, Nunn AS, Mena L, Anderson P, Liegler T, et al. Adherence to pre-exposure prophylaxis for HIV prevention in a clinical setting. PLoS ONE. 2016;11:e0157742–e0157742. doi: 10.1371/journal.pone.0157742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marcus JL, Glidden DV, Mayer KH, Liu AY, Buchbinder SP, Amico KR, et al. No evidence of sexual risk compensation in the iPrEx trial of daily oral HIV preexposure prophylaxis. PLoS ONE. 2013;8:e81997. doi: 10.1371/journal.pone.0081997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mugwanya KK, Donnell D, Celum C, Thomas KK, Ndase P, Mugo N, et al. Sexual behaviour of heterosexual men and women receiving antiretroviral pre-exposure prophylaxis for HIV prevention: a longitudinal analysis. Lancet Infect Dis. 2013;13:1021–8. doi: 10.1016/S1473-3099(13)70226-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hojilla JC, Koester KA, Cohen SE, Buchbinder S, Ladzekpo D, Matheson T, et al. Sexual behavior, risk compensation, and HIV prevention strategies among participants in the San Francisco PrEP demonstration project: a qualitative analysis of counseling notes. AIDS Behav. 2016;20:1461–9. doi: 10.1007/s10461-015-1055-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan PA, Mena L, Patel R, Oldenburg CE, Beauchamps L, Perez-Brumer AG, et al. Retention in care outcomes for HIV pre-exposure prophylaxis implementation programmes among men who have sex with men in three US cities. J Int AIDS Soc. 2016;19:1–8. doi: 10.7448/IAS.19.1.20903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan PA, Glynn TR, Oldenburg CE, Montgomery MC, Robinette AE, Almonte A, et al. Implementation of preexposure prophylaxis for human immunodeficiency virus prevention among men who have sex with men at a New England sexually transmitted diseases clinic. Sex Transm Dis. 2016;43:717–23. doi: 10.1097/OLQ.0000000000000514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.US Public Health Service. Preexposure prophylaxis for the prevention of HIV infection in the United States—2014: A Clinical Practice Guideline. Centers for Disease Control and Prevention; [Google Scholar]
- 13.Parker S, Chan PA, Oldenburg CE, Hoffmann M, Poceta J, Harvey J, et al. Patient experiences of men who have sex with men using pre-exposure prophylaxis to prevent HIV infection. AIDS Patient Care STDs. 2015;29:639–42. doi: 10.1089/apc.2015.0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu AY, Cohen SE, Vittinghoff E, Anderson PL, Doblecki-Lewis S, Bacon O, et al. Preexposure prophylaxis for HIV infection integrated with municipal- and community-based sexual health services. JAMA Intern Med. 2015;176:1–11. doi: 10.1001/jamainternmed.2015.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]


