Dear Sir
Methylphenidate, an amphetamine-like psychomotor stimulant, alleviates fatigue in many patient populations and is recommended, and often prescribed off-label, to improve fatigue in multiple sclerosis (MS).1 Recent research also supports that methylphenidate can improve balance and walking in various populations2–4 but no published studies have evaluated the effects of repeated doses of methylphenidate on fatigue, balance or walking in MS.
If effective for mobility and fatigue in MS, methylphenidate would provide an inexpensive (~$15–$20/month) option that could be administered at a range of doses to optimize effects. We therefore completed a 6-week, double-blind, randomized controlled trial pilot study to compare the effects of escalating doses of oral methylphenidate to matched placebo, on balance, walking, and fatigue in MS.
We recruited 24 people aged 20 to 65 years with MS, poor balance, and walking difficulties, from the VA Portland Health Care System and the surrounding community. We excluded those taking methylphenidate, modafinil, or armodafinil and those with contraindications to methylphenidate. Participants presented for a baseline visit and three follow-up visits, each two weeks ± two days apart. They were randomly allocated in a 1:1 ratio to receive methylphenidate or placebo, with the dose escalating at each visit, from 10 mg b.i.d. to 20 mg b.i.d. to 30 mg b.i.d. Participants exited the study at 6 weeks, or earlier for drug intolerance. At each visit participants completed the Modified Fatigue Index Scale (MFIS), Activities-specific Balance Confidence (ABC) Scale, Timed 25-Foot Walk (T25FW), and the oral Symbol Digit Modalities Test (SDMT) and we fit a Bayesian hierarchical model to the complete set of outcomes at each time point to estimate differences in outcomes between groups.
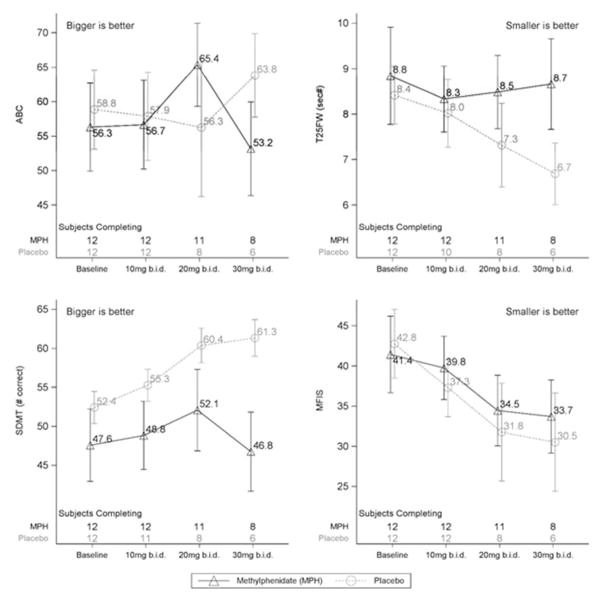
At baseline groups were generally well matched. In general, average performance on all measures improved over time in both groups, with greater improvements in the placebo group than in the methylphenidate group at all doses (Figure). The T25FW, MFIS, and SDMT showed at least 60% probability of a deleterious effect of methylphenidate compared to placebo at all doses. By the 30mg b.i.d. dose there is at least 80% certainty that methylphenidate was less beneficial than placebo for all outcomes. For the ABC there was, at best, no deleterious impact of methylphenidate at 10mg b.i.d. and 20mg b.i.d., but by 30mg b.i.d. there was also a negative effect compared to placebo for this outcome.
Figure.
Mean outcomes at each visit/dose level. Error bars are ± 1 standard error. The number of participants contributing data at each time point are shown above the x-axis in each plot.
There were similar adverse events (AEs) in both groups. The most common in both groups were typical for methylphenidate, including insomnia (6 methylphenidate, 4 placebo), and gastrointestinal symptoms (3 methylphenidate, 4 placebo). In the methylphenidate group two exited after the 20mg b.i.d. dose (1 for escalating blood pressure, 1 for nightmares) and two exited during the 30mg b.i.d. dose (1 for insomnia, 1 for tachycardia). In the placebo group two exited after the 10mg b.i.d. dose (1 for hypertension, 1 for a fall) and four exited during the 20mg b.i.d. dose (2 for hypertension, 1 for a family emergency, and 1 felt lightheaded). Adherence was high (94% for methylphenidate, 95% for placebo) and blinding was good (60% participants correct, 40% examiners correct).
Although limited by small sample size, this study suggests that methylphenidate at up to 60mg daily, in divided doses, is no more effective than placebo for improving balance, walking or fatigue in people with MS. The lack of benefit for balance and walking may be because methylphenidate is most likely to help balance and walking in MS by improving cognition, and cognition in this sample, as reflected by mean SDMT scores of around 50, was within normal range for age, limiting potential for improvement. The lack of benefit for fatigue is more concerning because study participants had severe fatigue (mean baseline MFIS score around 41 to 42) and therefore had potential to improve. If the lack of improvements with methylphenidate compared to placebo in this study accurately represents the effects in most people with MS, why is this medication recommended and used clinically? In our experience, people with MS who take methylphenidate commonly report feeling better. But, does this reflect activation and euphoria similar to that produced by other stimulants such d-amphetamine and cocaine. Methylphenidate may make people feel good without actually improving MS-related fatigue.
Based on the results of this study, a full-scale trial to examine benefits of methylphenidate for fatigue, balance or walking in MS is not recommended and, given the habit-forming potential of this medication, we recommend clinicians carefully assess the effectiveness of methylphenidate for the prescribed indication as distinct from other reasons patients may wish to take this drug.
Acknowledgments
Funding
This research was supported by a pilot grant from the National MS Society (#PP1748), and was conducted with support from the National Center for Rehabilitative Auditory Research (NCRAR). Study data were collected and managed using REDCap electronic data capture tools hosted at OHSU. REDCap (Research Electronic Data Capture), a secure, web-based application designed to support data capture for research studies, is supported at OHSU by (1 UL RR024140 01).
Contributor Information
MH Cameron, Department of Neurology, Oregon Health & Science University and VA Portland Health Care System, Portland, OR, USA.
G McMillan, National Center for Rehabilitative Auditory Research, VA Portland Health Care System, Portland, OR, USA.
References
- 1.Bakshi R. Fatigue associated with multiple sclerosis: Diagnosis, impact and management. Multiple Sclerosis. 2003;9(3):219–227. doi: 10.1191/1352458503ms904oa. [DOI] [PubMed] [Google Scholar]
- 2.Ben-Itzhak R, Giladi N, Gruendlinger L, Hausdorff JM. Can methylphenidate reduce fall risk in community-living older adults? A double-blind, single-dose cross-over study. J Am Geriatr Soc. 2008;56(4):695–700. doi: 10.1111/j.1532-5415.2007.01623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Auriel E, Hausdorff JM, Herman T, Simon ES, Giladi N. Effects of methylphenidate on cognitive function and gait in patients with parkinson’s disease: A pilot study. Clin Neuropharmacol. 2006;29(1):15–17. doi: 10.1097/00002826-200601000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Bart O, Podoly T, Bar-Haim Y. A preliminary study on the effect of methylphenidate on motor performance in children with comorbid DCD and ADHD. Res Dev Disabil. 2010;31(6):1443–1447. doi: 10.1016/j.ridd.2010.06.014. [DOI] [PubMed] [Google Scholar]