Abstract
Purpose
To compare the visualization of epiretinal membrane (ERM) using multicolor imaging (MCI) (Heidelberg Engineering, Carlsbad, CA) and conventional white light flood color fundus photography (FP) (Topcon, Tokyo, Japan).
Methods
The paired images of ERM patients who underwent same-day MCI and FP examinations were reviewed. Visibility of the ERM was graded using a scale (0: not visible, 1: barely visible, 2: clearly visible) by masked readers and surface folds were quantitated to quantify the membrane visibility for each method. Images from individual color channels in MCI (green, blue, and infrared) were also graded using same method to further investigate MCI images.
Results
Forty-eight eyes of 42 patients were studied. The average ERM visibility score was 1.8±0.37 for MCI and 1.01±0.63 for FP, respectively (p<0.001). The number of the surface folds detected per quadrant was significantly higher in MCI than FP (6.79±3.32 vs 2.85±2.81, p<0.001). Epiretinal membrane was graded with similar scores on the two modalities in only 43.8% of the eyes; in 56.2%, the ERM was better visualized on MCI than on FP. Conventional FP failed to detect ERM in 11.4% of eyes when the mean central retina thickness was <413 microns. Analysis of laser color reflectance revealed that scanning laser green-reflectance provided better detection of surface folds (5.54±2.12) compared to blue reflectance (4.2±2.34) and infrared reflectance (1.2±0.9).
Conclusion
Multicolor scanning laser imaging provides superior ERM detection and delineation of surface folds than conventional FP, primarily due to the green channel present in the combination-pseudocolor image in MCI.
Keywords: color fundus photography, epiretinal membrane, multicolor imaging, scanning laser imaging, multimodal imaging, multicolor
INTRODUCTION
Conventional fundus photography gives a useful representation of the clinician’s view of the retina and has been used for many years to document and follow and in some cases to diagnose retinal disease.1,2 In recent years, new modalities have added to the information from the fundus exam and standard color imaging; this includes fluorescein angiography (FA),3 which was introduced in the 1960’s, and more recently optical coherence tomography (OCT) imaging.4–7 Simultaneously, scanning laser imaging techniques of the human fundus have been developed,8–12 which have certain advantages over conventional color fundus imaging. A recent development is the incorporation of multicolor SLO images into an OCT fluorescein angiography system, the Heidelberg Spectralis (Heidelberg Engineering, Carlsbad, CA).13,14 The Spectralis scans the retina in 3 different laser wavelengths including blue (486 nm), green (518 nm), and near-infrared (815 nm) and offers a combination-pseudocolor image by combining the three wavelength reflectance images into a single image with each wavelength in one of the three color channels (red, green, and blue). The three monochromatic images allow visualization of distinct information from specific layers of the retina and choroid. Blue laser can capture details of superficial retinal structures, while green laser is highly absorbed by hemoglobin and provides vascular details of the retina in addition to giving a good reflectance image of surface retinal disease. Due to the longer wavelength, near-infrared laser penetrates deeper into the retina, allowing better imaging of the retinal pigment epithelium (RPE) and the choroid.15
Incorporation of conventional color imaging into the Spectralis was not done because the optical pathways are optimized for three laser wavelengths, not white light photography. The overlay of the three laser channels gives a pseudocolor composite image which in some cases looks similar to the clinical findings and color fundus images but not in all cases.
At our institution, we began to incorporate multicolor imaging into our retinal imaging protocol with the Heidelberg Spectralis because the images can be overlaid onto FA or OCT as they are simultaneously or near simultaneously taken, have the same magnification and aspect ratio and are imaged and stored in the same instrument. Since this imaging had the potential to replace color fundus photography, we wished to evaluate it in surface retinal disease (ERM). In this study, we are interested to determine the relationship of multicolor imaging (MCI) images to the clinical image as represented by the fundus color photograph and to determine its fidelity to color fundus photography and see how accurately it represents retinal surface diseases as seen by OCT. We wanted to study this because we noticed that retinal surface changes were well seen on the MCI and did correlate well with retinal surface wrinkling seen clinically and documented by fundus photography. We also noticed that the color rendition is somewhat different when comparing MCI to conventional retinal examination or to color photography and the purpose of this study was to determine the value of MCI in imaging of retinal surface disease such as ERM. We are aware that color fundus photography is not the same as the clinical examination but is a standard used widely to document clinical findings.
METHODS
In our practice, when available, patients with macular disease such as ERM routinely undergo color photography for documentation purposes. Multicolor imaging is performed at the time of OCT scanning in our patients as part of our routine imaging protocol; it takes no appreciable additional time or risk to the patients, we use simultaneous MCI as well as SD-OCT as a part of routine imaging. In this study, we retrospectively reviewed the retinal images of those patients who had ERM involving the macular area and who were referred to the Jacobs Retina Center at the Shiley Eye Institute, University of California, San Diego (UCSD) between July 2013 and December 2015. Only patients who were imaged by both color fundus photography and MCI on the same day and had good quality images were included. Based on our anecdotal experience, MCI works better than color fundus photography through cataract, but we did not study this as a part of study and we excluded patients who had poor quality images due to cataract or severe dry eye. Images with prominent lens reflex obscuring the details of the surface of retina on MCI were also excluded. Color fundus photography was performed with the Topcon TRC50X digital fundus camera (Topcon, Tokyo, Japan) and MCI was performed using the Spectralis SD-OCT (Heidelberg Engineering, Carlsbad, CA). Institutional Review Board approval from UCSD was obtained to review patients’ charts and images.
Evaluation of Epiretinal Membrane Structure
The presence of ERM was confirmed on clinical examination by one physician (W.R.F.) The original MCI and color fundus images for each eye of the patients with clinically confirmed ERM were retrieved and then saved as TIFF files. Epiretinal membrane on FP was defined as an irregular increased reflection on the inner surface of the retina with striae and/or an opaque and gray membrane on MCI, it was seen as a green-yellow superficial structure with attendant retinal folds. Two independent physicians (I.K.M., R.G.) used the same computer screen (1,280 × 1,024 pixels, 17-inch liquid crystal display monitors, NEC Accusync LCD 92V) in the same room to review the images. The ability to visualize the ERM on the MCI and color fundus images was scored from 0 to 2. “Grade 0” indicated that the membrane was not visible, “Grade 1” referred that it was barely visible, “Grade 2” referred that membrane was clearly visible. When there was a disagreement in the grading score, a third imaging specialist was consulted to achieve an acceptable consensus (D.U.B.).
Quantification of Retinal Folds
After completing grading of the visibility of ERM, in order to quantify the visibility of ERM, we have counted the number of surface folds. First, the area with 2000-micron × 2000-micron dimensions on either MCI or CF (on whichever the surface folds were most prominent) was marked by an observer (G.B.). Then, two masked retina specialists (IKM, RG) counted the number of surface folds in the paired image to quantify the ERM features for the statistical analysis in the marked area. Thus both reviewers used the same area to count folds.
The area with 2000-micron × 2000-micron dimensions on either MCI or CF (on whichever the surface folds were most prominent) was marked by another observer (G.B.). Then, two masked retina specialists (IKM, RG) counted the number of surface folds in the paired image to quantify the ERM features for the statistical analysis in the marked area. Masked single-wavelength images that were initially combined to obtain MCI images (i.e., near-infrared reflectance, blue reflectance, and green reflectance) were also graded using the above mentioned grading scale to determine which reflectance image allowed better visualization of the ERM.
Optical Coherence Tomography Confirmation of the Epiretinal Membrane
All SD-OCT images of the patients were reviewed and the presence of ERM was checked on SD-OCT images. The retinal thickness at the point where surface folds were most prominent was obtained on the raster SD-OCT scans taken using the same device (Spectralis, Heidelberg Engineering, Heidelberg, Germany).
Statistical Analysis
The grading score on MCI and color fundus images, the grading score among the three single-wavelength images, and the number of surface folds were determined as outcomes of the study. Kappa coefficient was used to show the inter-graders observation. Paired t-test analysis was used for normally distributed data and the Wilcoxon signed-rank test for non-normally distributed data to compare the grading scores between 2 groups. All statistics analysis was performed with SPSS statistical software version 23 (SPSS Inc, Chicago, IL, US). A P value less than 0.05 was considered to be statistically significant.
RESULTS
Forty-eight eyes of 42 patients with a clinical diagnosis of ERM who underwent same-day conventional color fundus photography and MCI were included the study.
The agreement among the graders in grading the visualization of the ERM was high; K=0.850, p<0.001 for the MCI and K=0.863, p<0.001 for the color fundus photography. The agreement among the graders for visualization of the ERM using the different wavelengths in MCI was also high; K=0.773, p<0.001 for the IR-reflectance, K=0.672, p<0.001 for the blue-reflectance and K=0.729, p<0.001 for the green-reflectance. When there was an inconstancy among the graders, a third observer’s review was obtained and a consensus grade was given.
Out of 48 ERMs, 4 were graded as not visible, 27 as barely visible, and 17 as clearly visible on conventional fundus photography. Epiretinal membrane was visible in all eyes on MCI, where 6 ERMs were graded as barely visible, and 42 as clearly visible. The results of the visibility of the membrane using the 2 imaging modalities are shown in table 1. ERM grading was consistent between the 2 imaging modalities (figure 1) in 21 eyes (43.8%). On the contrary, in 56.2% of eyes (27 eyes) ERM visibility was better on MCI than on conventional color pictures (figure 2, 3). The average grading score for ERM visualization was found to be higher on MCI (1.8±0.37) than on conventional color pictures (1.01±0.63) (p<0.001). The number of the surface folds was also found to be significantly higher in MCI than color fundus images (6.79±3.32 versus 2.85±2.81, p<0.001) (figure 3).
Table 1.
The Visualization of Epiretinal Membrane Using Multicolor Imaging and Color Fundus Photography
| Color Fundus Photography | Total | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| no visible | barely visible | clearly visible | ||||
|
| ||||||
| multicolor | barely visible | Number of eyes | 2 | 4 | 0 | 6 |
| % within multicolor | 33.3% | 66.7% | 0.0% | 100.0% | ||
| % within color | 50.0% | 14.8% | 0.0% | 12.5% | ||
| % of Total | 4.2% | 8.3% | 0.0% | 12.5% | ||
|
|
||||||
| clearly visible | Number of eyes | 2 | 23 | 17 | 42 | |
| % within multicolor | 4.8% | 54.8% | 40.5% | 100.0% | ||
| % within color | 50.0% | 85.2% | 100.0% | 87.5% | ||
| % of Total | 4.2% | 47.9% | 35.4% | 87.5% | ||
|
| ||||||
| Total | Number of eyes | 4 | 27 | 17 | 48 | |
| % within multicolor | 8.3% | 56.3% | 35.4% | 100.0% | ||
| % within color | 100.0% | 100.0% | 100.0% | 100.0% | ||
| % of Total | 8.3% | 56.3% | 35.4% | 100.0% | ||
Figure 1.

shows the paired image of a patient with ERM (figure A, B) that is visible on both imaging techniques. Although retinal surface folds (figure A, B, bottom arrows) are prominent in both imaging modalities, multicolor imaging shows more surface folds (top arrow, figure B). Also, note the difference in color rendition between 2 images (A versus B). The optical coherence tomography image cutting through the area where the surface folds are most prominent (figure C) is shown in the bottom centered image.
Figure 2.
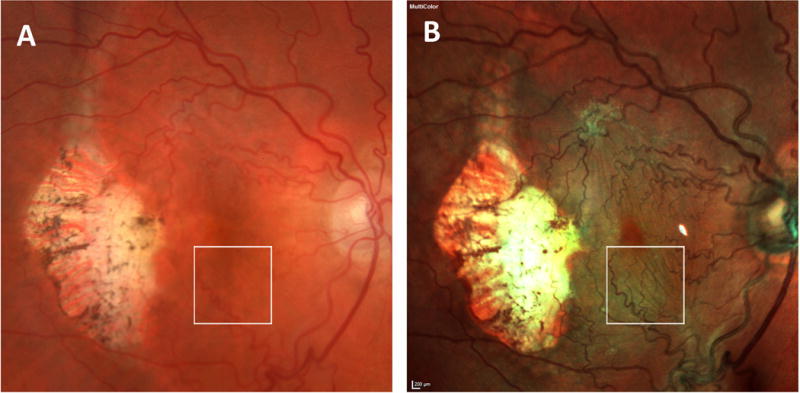
The paired images of a patient with an inconsistent appearance of ERM between color fundus photography (A) and multicolor imaging (B). The surface folds are not visible in image A, however, the surface folds are clearly visible (arrows) in image B. Arrows (figure B) indicate the folds extending towards to superior-temporal quadrant. Note the difference in color rendition between 2 images (A versus B)
Figure 3.
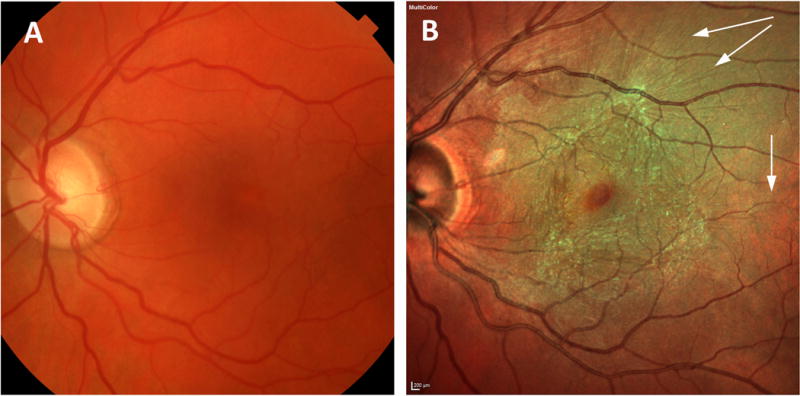
shows the paired images of a patient who had inconsistency in visualization of epiretinal membrane. 2mm×2mm box indicates the area where the surface folds are most prominent. The number of counted surface fold is 0 in color photography and 9 in multicolor imaging.
All patients underwent at least 2 horizontal and vertical OCT scans cutting through the fovea, however, seventy-three percent of eyes (35 eyes) had raster SD-OCT scans of the entire macula. Epiretinal membranes were visible on SD-OCT in all cases (100%) where they were present clinically. The median central retinal thickness in the zone with the most prominent surface retinal folds was 413 microns (range, 370 to 453 microns). The eyes with a non-visible (score=0) ERM grading score on color photography had a mean retinal thickness of 380 microns (range, 370 to 410 microns). When we used the median retinal thickness (413 microns) as a cut-off value, the visibility of the ERM on color photography increased from 31% (when the average retinal thickness was <413 microns) to 52% (when the average retinal thickness was ≥413 microns). Conventional fundus photography failed to detect ERM in 11.4% of the cases that had a mean of central retinal thickness of <413. In MCI, the visibility of the ERM did not differ when using a mean of 413 microns as a cut-off value (48.2% versus 51.7%, table 2).
Table 2.
Categorization of the visibility of ERM using color photography and multicolor imaging based on the central retinal thickness on spectral domain optical coherence tomography on the zone with most prominent surface folds
| Central Retina Thickness <413 microns | Central Retina Thickness ≥413 microns | |||
|---|---|---|---|---|
|
| ||||
| MCI | COLOR | MCI | COLOR | |
| Not Visible, eyes, n (%) | 0 | 4 (11.4%) | 0 | 0 |
| Barely Visible, eyes, n (%) | 2 (5.7%) | 9 (25.7%) | 4 (11.4%) | 11 (31.4%) |
| Clearly Visible, eyes, n (%) | 15 (42.8%) | 1 (2.8%) | 14 (40%) | 10 (28.5%) |
MCI=multicolor imaging, COLOR=color fundus photography, n=number
Among the 3 images with different wavelengths, green-reflectance (1.73±0.44) had statistically higher average grading score than on near-infrared reflectance (0.85± 0.46) (p<0.001) or on blue reflectance (1.51±0.52) (p<0.001) images. The number of surface folds was also found to be higher than that of blue-reflectance (4.2±2.34), and near-infrared reflectance (1.2±0.9) (figure 4).
Figure 4.
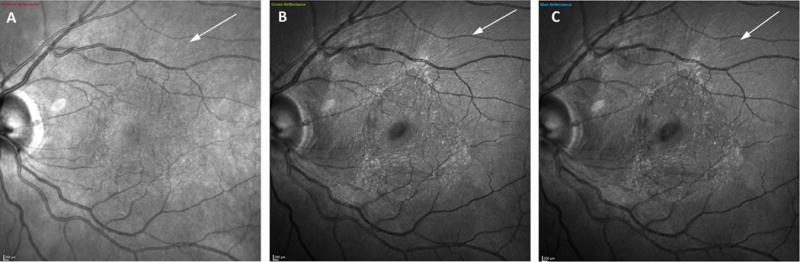
The individual color channels of a patient with epiretinal membrane. Green-reflectance image (Figure B) and blue-reflectance (Figure C) show the hyper-reflective folds (arrow), however, the hyper-reflectivity corresponding to surface folds is not clear in temporal to the fovea in infrared image (figure A).
DISCUSSION
Multicolor imaging is a new method of imaging the retina which can be performed nearly simultaneously with OCT and/or retinal angiography using the Spectralis device. We noted that the color rendering of MCI is not the same as what is seen clinically or by color fundus photography (which use broad spectrum white light), but we noticed that surface retinal structures are seen more prominently on multicolor imaging than on conventional fundus photography. Multicolor imaging is an extremely useful color retinal imaging modality as it is performed simultaneously with scanning laser OCT on the Spectralis instrument. Because the MCI incorporates a combination of single-wavelength images (including green, blue, and infrared),14,15 we did demonstrate that MCI provided superior visualization of the ERM with a higher number of surface folds compared to color fundus photography particularly in the green channel. Although the number of folds in ERM may not be clinically relevant endpoints, we have used this method to quantify the features of the epiretinal membrane. Indeed, short wavelength imaging has been used for many years to visualize surface retinal structures such as the retinal nerve fiber layer.
Fundus photography remains certainly the most common imaging modality to document what can be seen by the ophthalmologist clinically.1 However, it is uncomfortable because of the bright flash and is time consuming when the patient needs to undergo multiple retinal imaging tests because color fundus cameras typically are instruments separate from OCT and other imaging instruments and requires greater optical media clarity and pupil dilation than OCT. In the current study, we demonstrate that MCI offers superior detection of ERM than fundus photography (100% vs 91.7% detection rate). In addition to grading the visibility of the ERM between 2 imaging modality; this was found both with image grading and by quantitating retinal stria. Not surprisingly, we found that retinal thickness was a major determinant of ERM visualization using fundus imaging and found that color fundus photography is less able to visualize ERMs if the retinal thickness is marginally impacted by the ERM. Interestingly, MCI is more sensitive to detect ERM than color photography despite the fact that the color fundus camera has a higher pixel density when compared to the multicolor images (3000 × 2672 pixel for all colors combined on Topcon Color camera vs. 768 × 768-pixel × 3 colors on the Spectralis). The reason for the better ERM visualization in multicolor imaging may also be explained by the higher contrast of the SLO imaging system and the more prominent reflection of surface retinal structures with shorter wavelength imaging such as green or blue. We conclude that ERMs can be better and more conveniently characterized using MCI than color photography. We also found that green reflectance imaging with a 518 nm wavelength was better to detect ERM than blue or infrared reflectance. It is known that retinal surface features are better visualized in short wavelengths, particularly green. Green may be superior to blue in this context because there is more information seen in the green than blue wavelength in general. A retinal image contains very little information in blue light. The retina contains information mainly in the yellow and red region of the color spectrum.
Prior investigations into MCI have focused on other chorioretinal diseases.13,14,16,17 Epiretinal membrane visualization was studied by Reznicek et al,17 comparing the HRA2 (Heidelberg Engineering, Heidelberg, Germany) to the wide-field SLO (Optos PLC, Dunfermline, UK). The authors found that ERMs were best visualized in HRA based green-blue enhanced multicolor enface SLO images, followed by other en-face HRA based multicolor images and SLO en face visualizations obtained with Optomap. Indeed, the Optos SLO uses only red (633 nm) and green (532 nm) wavelength lights when capturing the color composite view. They also showed that the SD-OCT based thickness map provided a good visualization of ERM, but showed in the 54.7% of patients, a larger or smaller extension of the ERM border compared to SD-OCT cross sectional images. In our study, all ERMs were visible on SD-OCT. The difference in used device and the lack of data on the SD-OCT characteristics of the epiretinal membrane make this older study different than ours. We quantitated the lesion findings and, different from other studies and compared the agreement of ERM detection between MCI and color fundus photography. We used the latter as a surrogate for clinical examination and note that MCI imaging may be performed simultaneously with OCT using the Spectralis instrument so may be clinically useful and easy to obtain during OCT.
Our study is the first to evaluate the difference in ERM visualization between conventional color images and multicolor scanning laser scanning ophthalmoscopy. Despite superiority of detecting ERM, interpretation of the pseudo-color images is quite different in multicolor imaging where the membrane is seen as green or yellow in contrast to usual grey-white appearance obtained by real color pictures. A major advantage of MCI over OCT alone is the ability to visualize the entire posterior pole and visualize the wrinkling of the retina by evaluating the retina en face.
In conclusion, we wished to correlate ERM detection with ERM as compared to the clinical impression (color fundus images, confirmed by clinicians). We used retinal fold numbers as a way to objectively quantify ERM; these can be seen clinically, on color photography, by OCT and by the MCI. We thus directly compared two methods of ERM detection and quantification; the clinical one as documented by fundus photographs as well as OCT to determine the ability for MCI, a test done very quickly at the time of OCT as a method to detect and follow ERM in an en-face imaging technique which can be easily compared to clinical exam or photography. We found that MCI clearly improved the ability to detect ERMs compared to conventional fundus camera. When looking at the separate cSLO images obtained using different wavelengths, green-reflectance provided more information than blue or infrared reflectance. Our results suggest that MCI on Heidelberg Spectralis may be used as an additional tool for the evaluation of ERMs, and combined imaging may give more detailed and accurate information without masquerading the disease.
Acknowledgments
Financial Support: Supported in part by UCSD Vision Research Center Core Grant P30EY022589 (WRF), NIH grant EY016323 (DUB) and an unrestricted grant from Research to Prevent Blindness, NY (WRF), The Scientific and Technological Research Center of Turkey (TUBITAK) (IKM). The funding organizations had no role in the design or conduct of this research.
Footnotes
Financial Disclosures: Giulio Barteselli is a full-time employee at Genentech Inc. The other authors have nothing to disclose.
Multicolor imaging provides a higher delineation of retinal folds related with epiretinal membrane than conventional fundus photography.
References
- 1.Early Treatment Diabetic Retinopathy Study Research Group. Grading diabetic retinopathy from stereoscopic color fundus photographs–an extension of the modified Airlie House classification. ETDRS report number 10. Ophthalmology. 1991;98:786–806. [PubMed] [Google Scholar]
- 2.Age-Related Eye Disease Study Research Group. The Age-Related Eye Disease Study system for classifying age-related macular degeneration from stereoscopic color fundus photographs: The Age-Related Eye Disease Study Report Number 6. Am J Ophthalmol. 2001;132:668–681. doi: 10.1016/s0002-9394(01)01218-1. [DOI] [PubMed] [Google Scholar]
- 3.Early Treatment Diabetic Retinopathy from fluorescein angiograms. ETDRS report number 11. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991;98:807–22. [PubMed] [Google Scholar]
- 4.Wang H, Chhablani J, Freeman WR, et al. Characterization of diabetic microaneurysms by simultaneous fluorescein angiography and spectral-domain optical coherence tomography. Am J Ophthalmol. 2012;153:861–867. doi: 10.1016/j.ajo.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freeman SR, Kozak I, Cheng L, et al. Optical coherence tomography-raster scanning and manual segmentation in determining drusen volume in age-related macular degeneration. Retina. 2010;30:431–435. doi: 10.1097/IAE.0b013e3181bd2f94. [DOI] [PubMed] [Google Scholar]
- 6.Brar M, Kozak I, Cheng L, et al. Correlation Between Spectral OCT and Fundus Autofluorescence at the Margins of Geographic Atrophy. Am J Ophthalmol. 2009;148:439–444. doi: 10.1016/j.ajo.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nigam N, Bartsch D-U, Mojana F, et al. Spectral OCT for Imaging ERM, Retinal Edema and Vitreomacular Interface. Retina. 2010;30:246–253. doi: 10.1097/IAE.0b013e3181baf6dc. [DOI] [PubMed] [Google Scholar]
- 8.Freeman WR, Bartsch D-U, Mueller AJ, et al. Simultaneous Indocyanine Green and Fluorescein Angiography Using a Confocal Scanning Laser Ophthalmoscope. Archives of Ophthalmology. 1998;116:455–463. doi: 10.1001/archopht.116.4.455. [DOI] [PubMed] [Google Scholar]
- 9.Bartsch D-U, Freeman WR. Laser-tissue interaction and artifacts in confocal scanning laser ophthalmoscopy and tomography. Neuroscience and Biobehavioral Reviews. 1993;17:459–4567. doi: 10.1016/s0149-7634(05)80123-x. [DOI] [PubMed] [Google Scholar]
- 10.Bartsch D-U, Weinreb RN, Zinser G, et al. Confocal scanning infrared laser ophthalmoscopy for indocyanine green angiography: Preliminary results. Am J Ophthalmol. 1995;120:642–651. doi: 10.1016/s0002-9394(14)72211-1. [DOI] [PubMed] [Google Scholar]
- 11.Bartsch DU, El-Baradi M, El-Musharaf A, Freeman WR. Improved visualization of choroidal neovascularization by scanning laser ophthalmoscope using image averaging. Br J Ophthal. 2005;89:1026–1030. [Google Scholar]
- 12.Lee BR, Kozak I, Bartsch DU, et al. Comparison of a novel confocal scanning laser algorithm with optical coherence tomography in measurement of macular thickness and volume. Retina. 2009;29:1328–1334. doi: 10.1097/IAE.0b013e3181ac7d30. [DOI] [PubMed] [Google Scholar]
- 13.Ben Moussa N, Georges A, Capuano V, et al. MultiColor imaging in the evaluation of geographic atrophy due to age-related macular degeneration. Br J Ophthalmol. 2015;99:842–847. doi: 10.1136/bjophthalmol-2014-305643. [DOI] [PubMed] [Google Scholar]
- 14.Pang CE, Freund KB. Ghost maculopathy: An artifact on near-infrared reflectance and multicolor™ imaging masquerading as chorioretinal pathology. Am J Ophthalmol. 2014;158:171–178. doi: 10.1016/j.ajo.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 15.Murphy R. Multicolor fundus imaging prepares for debut. Retinal Physician. 2012;9:77. [Google Scholar]
- 16.Yehoshua Z, de Amorim Garcia Filho CA, et al. Comparison of Geographic Atrophy Growth Rates Using Different Imaging Modalities in the COMPLETE Study. Ophthalmic Surg Lasers Imaging Retina. 2015;46:413–422. doi: 10.3928/23258160-20150422-03. [DOI] [PubMed] [Google Scholar]
- 17.Reznicek L, Dabov S, Kayat B, et al. Scanning laser ‘en face’ retinal imaging of epiretinal membranes. Saudi J Ophthalmol. 2014;28:134–138. doi: 10.1016/j.sjopt.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]


