Abstract
Background
Esophagectomy remains the gold standard in the curative intent treatment of resectable esophageal cancer. However, this procedure is complex and associated with high risk of complications. In an effort to reduce the postoperative morbidity associated with open esophagectomy various minimally invasive techniques have been introduced and developed during the recent years. The aim of the current study was to present our 4.5-year experience of the gradual implementation of various minimally invasive esophagectomy (MIE) techniques in our tertiary referral center
Methods
From May 2012 a transitional period from conventional open esophagectomy to MIE was initiated. This period was preceded by fellowships and visits to expert centers abroad. Thereafter, a gradual implementation and refinement of the new techniques followed. Technique related data were collected prospectively.
Results
Between January 1st 2011 and December 31st 2016 a total of 249 patients underwent an esophagectomy in our unit. Seventy-six cases were performed through a conventional open esophagectomy and 173 by some type of MIE. An increasing utilization of MIE over this time period was seen and finally reached 100% of treatment intentions, during the last 2 years. Ten cases (5.7%) where converted to open approach. A decrease in leak rate, operating time, peroperative bleeding and hospital stay as well as an increasing number of harvested lymph nodes was observed during the implementation period.
Conclusions
The transition from conventional open esophagectomy to MIE was successful at our center. The implementation was overall safe with good postoperative outcomes, although changes in results required technical modifications over time.
Keywords: Minimal invasive esophagectomy (MIE), implementation, learning curve
Introduction
Esophagectomy remains the gold standard in the curative intent treatment of resectable esophageal cancer, often in combination with neoadjuvant chemotherapy or chemoradiotherapy (1-3). However, esophagectomy is a complex invasive procedure requiring exploration of several body cavities for adequate radical lymphadenectomy and restoration of gastrointestinal continuity. Furthermore, patients suffering from esophageal cancer are often elderly with significant comorbidities, which increases the postoperative risks after such a procedure (4). Although, the postoperative mortality after esophagectomy has significantly decreased in high-volume centers over the years, high morbidity rates are still recorded (5,6). In an effort to reduce the postoperative morbidity associated with open esophagectomy various minimally invasive esophagectomy (MIE) techniques have been introduced and developed during the recent years. MIE is believed to minimize the surgical trauma and subsequently the postoperative pain resulting in a faster mobilization and recovery with reduced postoperative complication rates and shorter hospital stay compared to the open procedure. During the last 20 years several studies have been published within the field demonstrating that MIE is a safe technique with an oncological outcome at least equal to open esophagectomy with regard to complete resection rate, number of lymph nodes harvested and postoperative survival (7-9). Moreover, there is an indication of short-term benefits after MIE especially with regards to reduced pulmonary complications and improved health related quality of life compared to the open procedure (10,11).
The aim of the current study was to present our 4.5-year experience of the gradual implementation of various MIE techniques in our tertiary referral center with special focus on the evolution of adaptive changes and refinements of our practice of the procedure over this period.
Methods
Our unit
Center of digestive diseases (CDD) at Karolinska University Hospital, Stockholm is one of the largest and most highly specialized centers in the Nordic countries. It is a tertiary referral center in Stockholm County with a catchment area of approximately 2,500,000 inhabitants. Furthermore, CDD receives referrals for patients with newly diagnosed esophageal and cardia cancer from neighboring counties with an additional population of 650,000. This results in an annual case volume of 40–60 esophageal and resections in the most recent years.
The Unit engages three consultant surgeons (MN, ML, IR), with long laparoscopic experience within benign upper gastrointestinal (UGI) surgery as well as long previous experience in open esophageal cancer surgery.
Introduction of implementation
Table 1 summarizes the different steps of implementation of MIE at CDD, Karolinska, Stockholm. From 2009–2012, one of the current consultant surgeons, IR, spent 3 years in a fellowship in UGI cancer surgery at Bristol Royal Infirmary, Bristol, UK with special focus in MIE. During these years he became confident in both the hybrid laparoscopic assisted esophagectomy (HLAE) technique including laparoscopic gastric mobilization and abdominal lymph node dissection followed by thoracotomy and intrathoracic anastomosis as well as with the minimal invasive McKeown (MIMK) technique, i.e., thoracolaparoscopy followed by cervical anastomosis by performing more than 70 cases. In the meantime, two other consultant surgeons (MN & ML) had the chance to visit another center with extensive experience within the field (VU University Medical Center, VUMC, Amsterdam, the Netherlands) for observation of their technique before the final decision on setup, materials and procedure of choice at CDD.
Table 1. Steps in introduction of MIE at CDD, Karolinska University Hospital, Stockholm.
| Step | Time | Implementation of MIE |
|---|---|---|
| 1 | 2009–2012 | Consultant surgeon IR, 3 years fellowship in minimally invasive UGI-surgery, Bristol Royal Infirmary, Bristol, UK |
| 2 | March 2012 | Consultant surgeons MN& ML visiting center with experience in the field of MIE, AMC, Amsterdam |
| 3 | May 2012 | First case of hybrid esophagectomy, laparoscopic assisted (laparoscopy + thoracotomy) |
| 4 | June 2012 | First case of minimally invasive Mc Keown (thoracoscopy + laparoscopy + cervical anastomosis) with the assistance of professor Cuesta, VUMC Amsterdam |
| 5 | August 2012 and ongoing | Initiation of 2 years fellowship (Japanese fellow) in UGI-surgery at DCC, Karolinska, Stockholm in collaboration with Cancer Institute Hospital, Tokyo, Japan |
| 6 | 2012–2013 | Consultant surgeon ML, 1 year surgical training in minimally invasive UGI-surgery at Flinders Medical Center, Adelaide, Australia |
| 7 | May 2014 | Consultant surgeons IR, MN &ML together with Japanese fellow visited Helsinki University Central Hospital, Helsinki, dr Räsänen, with long experience within the field of MIE and intrathoracic anastomosis |
| 8 | July 2014 | First case of minimally invasive Ivor-Lewis (laparoscopy + thoracoscopy + intrathoracic anastomosis) |
MIE, minimally invasive esophagectomy.
In our department and until May 2012, all patients presented with a resectable lower esophageal cancer or tumors located in the esophagogastric junction underwent mainly open Ivor-Lewis esophagectomy with laparotomy in the supine position followed by right posterolateral thoracotomy in the left lateral decubitus position and intrathoracic anastomosis. An open McKeown esophagectomy with cervical anastomosis was performed for cases with tumors in middle or upper third of esophagus. A few patients per year underwent transhiatal esophagectomy with cervical anastomosis, the main indication being distal esophageal tumor in frail patients.
In May 2012 and as a first test of transition to MIE, the first HLAE was performed safely with all consultants present. This was followed shortly by an additional procedure with satisfactory results and without any technical problems to be encountered. HLAE was chosen as the most feasible MIE in the beginning of this transitional period due to the simple setup and ease to convert to laparotomy if needed. Furthermore, it acted as an indicator in case the advancing to a more demanding procedure would be possible.
In June 2012 the first MIMK was performed. Professor Miguel Cuesta from VUMC, Amsterdam, the Netherlands, with extensive experience within the field was present to assist us with his expertise. For the thoracoscopic part the prone position with single lumen intubation and double lung ventilation was chosen as the preferred setup. The procedure was performed successfully and the whole setup was found to be highly satisfactory.
In August 2012 and as a part of facilitating a parallel implementation of minimally invasive gastric cancer surgery our department established a 2-year UGI-fellowship program with visiting Japanese fellows in collaboration with Cancer Institute Hospital, Tokyo, Japan. This would result to an increased caseload of advanced laparoscopic UGI cancer surgery by almost twofold.
In the years 2012–2013 another consultant surgeon (ML) went for 1-year surgical training at Flinders Medical Center, Adelaide, Australia for further training in minimally invasive UGI-cancer surgery.
Initial phase of implementation
After the preparatory period of HLAE procedures, the phase of transition to complete MIE, including thoracoscopic dissection was initiated. At this point the procedure of choice was the three-stage MIMK procedure, using prone positioning for the thoracoscopic phase. The main reason for choosing this procedure with a cervical anastomosis, being to avoid performing a minimal invasive intrathoracic anastomosis. All esophageal cancer patients had a standardized preoperative investigation and assessment consisting of contrast-enhanced computed tomography (CT) scan of the chest, abdomen and pelvis as well as positron emission tomography (PET) scan. Histological diagnosis was confirmed by preoperative endoscopic biopsies. All the cases were discussed in a multidisciplinary setting where the decision of surgical approach was also taken. At this stage and in order to avoid possible technical difficulties, patients with bulky tumors as well as patients who had an esophageal stent inserted prior to operation where excluded from minimal invasive approach and were offered either a HLAE or a conventional open esophagectomy. The majority of the patients had received neoadjuvant chemotherapy or chemoradiotherapy unless the patient had serious comorbidities or other contraindications. In the first 50 consecutive cases of MIE there were at least two of the senior surgeons (in some cases all three) present during the operation to achieve maximum possible caseload and enhance the learning curve within a shorter period of time.
Refinements of technique
Although the implementation of the MIMK approach was successful and the rate of postoperative morbidity remained similar to the conventional esophagectomy era, by the end of 2013 we started experiencing an increasing severity of cervical anastomotic leakages. This made us question and reconsider our surgical strategy and therefore a team meeting was called upon. There was a concern and a postulation that exposure of the future anastomotic site, especially the gastric fundus in distal esophageal and junctional tumors, to doses of radiation that may impair the healing of the subsequent cervical anastomosis. This hypothesis was studied and the results published by our group in 2016 (12), showing that the future anastomotic site was indeed exposed to biologically relevant doses of radiation and that this was associated to more frequent and severe anastomotic morbidity. The consequence of this was to abandon the MIMK for patients with distal esophageal or esophagogastric junction cancer who had undergone neoadjuvant chemoradiation. These patients were instead offered HLAE, avoiding the cervical anastomosis and thus permitting resection of the uppermost part of the gastric fundus most likely to be exposed to irradiation. Patients without radiation exposure to the gastric fundus, i.e., those treated with surgery alone or neoadjuvant chemotherapy, or with more proximal tumors located in the middle or upper third of the esophagus where the radiation field did not include the stomach, were still offered MIMK. After these adjustments the number of severe anastomotic leaks were promptly reduced.
In the beginning of 2014 and at this point well comfortable with both the laparoscopic and thoracoscopic part of MIMK type MIE we chose to take on a new challenge; the transition from the MIMK and HLAE to the minimally invasive Ivor-Lewis (MIIL) technique, with its challenges of a minimally invasive intrathoracic anastomosis. For this reason our surgical team visited Helsinki University Central Hospital, Helsinki, Finland and Dr Jari Räsänen with extensive experience of intrathoracic esophagogastric anastomoses using circular stapler, in May 2014. After we evaluated the setup in Helsinki we decided to proceed with the transition to MIIL in our unit with some modifications.
In July 2014 the first MIIL was performed successfully in our department. Even for this procedure we chose the prone position since we preferred and mastered this approach after the 2 years of experience of MIMK in our unit. Furthermore, we chose to perform a side-to-side anastomosis instead of a circular one since we found this technique easier to perform in the limited space of the chest cavity. Our MIIL technique has been described in detail together with the initial experience of 46 patients in a recent publication from our group where we could demonstrate that this method is safe with good postoperative results (13). Even during the initial phase of implementation of MIIL (first 20 consecutive cases) always two of the senior surgeons were performing the procedure altering between the resection and reconstruction part of the operation. The aim was a safe and short learning curve of the demanding performance of the minimally invasive intrathoracic anastomosis, as well as the shortening of the operation time. Over the course of the last 2.5 years and due to increased acquired experience in MIE we have been able to perform more and more complicated cases safely resulting in an intention to treat all patients by minimally invasive means regardless of tumor size and preoperative treatment.
Results
Between January 1st 2011 and December 31st 2016 a total of 249 patients underwent an esophagectomy at CDD. Seventy-six cases were performed through a conventional open esophagectomy (Ivor-Lewis, McKeown or transhiatal) and 173 through means of some type of MIE (HLAE, MIMK, MIIL). Until May 2012, all cases were performed by traditional open procedure. Thereafter, there was an increasing tendency in utilization of MIE that reaches 100% over the last 2 years reflecting the acquisition of experience and a sense of security with the implementation of these minimally invasive techniques (Figure 1).
Figure 1.
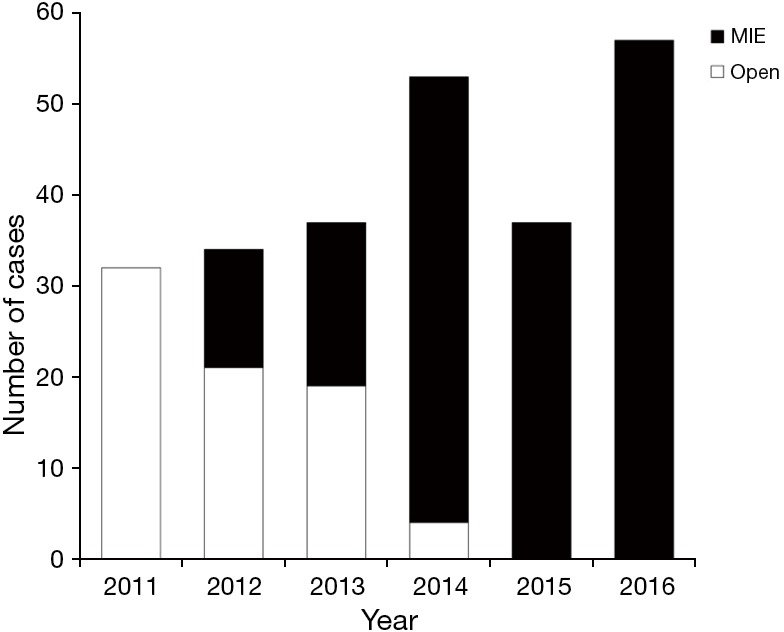
Transition from open to minimally invasive esophagectomy from 2011 to 2016. MIE, minimally invasive esophagectomy.
The evolution of the MIE techniques used is demonstrated in Figure 2 where we can see an increased utilization of MIMK over the first period of the transitional era followed by a decrease between 2013 and 2014 where the surgical strategy was reconsidered due to an increasing severity in cervical anastomotic complications and altered in favor of HLAE in order to avoid cervical anastomoses using irradiated gastric fundus. Since 2015 MIIL has been the procedure of choice in our unit in all distal and junctional tumors.
Figure 2.
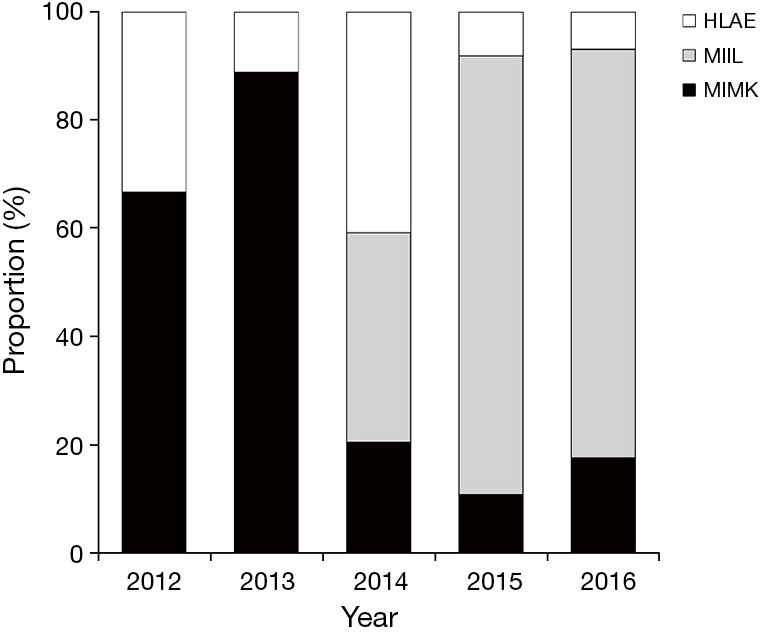
Refinement of minimally invasive technique over time. HLAE, hybrid laparoscopic assisted esophagectomy; MIIL, minimally invasive Ivor-Lewis; MIMK, minimally invasive McKeown.
In the beginning of the implementation of MIE some patients were excluded from this type of surgery due to bulky tumors or preoperatively inserted esophageal stents because of the risk of peroperative challenges perceived as difficult to manage laparoscopically or thoracoscopically. However, over the time, and with increasing volume and experience, we were able to perform advanced cases safely and successfully. Thus, over the last 2 years, all patients presented in our unit with an esophageal or esophagogastric junction cancer are subjects for treatment through some type of minimally invasive technique regardless the tumor location, tumor stage or preoperative treatment (Table 2).
Table 2. Characteristics of patients who were treated by minimally invasive means 2012–2016.
| Variables | Overall (N=173) |
|---|---|
| Age, median (range) (years) | 68.0 (35.0–83.0) |
| Sex (%) | |
| Male | 139 (80.3) |
| Female | 34 (19.7) |
| ASA* (%) | |
| 0 | 2 (1.2) |
| 1 | 61 (36.3) |
| 2 | 93 (55.4) |
| 3 | 11 (6.5) |
| 4 | 1 (0.6) |
| Histological type (%) | |
| Adenocarcinoma | 133 (76.9) |
| Squamous cell carcinoma | 34 (19.7) |
| Other | 6 (3.5) |
| Neoadjuvant treatment (%) | |
| None | 48 (27.7) |
| Chemotherapy | 17 (9.8) |
| Chemoradiotherapy | 108 (62.4) |
| Tumor location (%) | |
| Upper third | 4 (2.3) |
| Middle third | 28 (16.2) |
| Lower third | 6 (3.5) |
| Junction | 134 (77.5) |
| Missing data | 1 (0.6) |
| Stage (%) | |
| 0 | 30 (17.3) |
| I | 33 (19.1) |
| II | 44 (25.4) |
| III | 57 (32.9) |
| Other | 3 (1.7) |
| Not arrived | 6 (3.5) |
*, Missing data: 5.
Nine cases (5.2%) where converted to open approach (laparotomy or thoracotomy) of which 6 occurred during the first 2 years of implementation of MIE. Three cases where converted due to bleeding, four cases due to adhesions, one case due to bulky lymph nodes close to the aortic arch irresectable thoracoscopically and one case due to injury of the right gastroepiploic artery.
In Figure 3, the anastomotic leak rate/year is demonstrated, which is comparable with that in the previous open esophagectomy era (data not shown), apart from 2013 where a peak is noted leading to the modification of our technique and a subsequent significant decrease of anastomotic failure.
Figure 3.
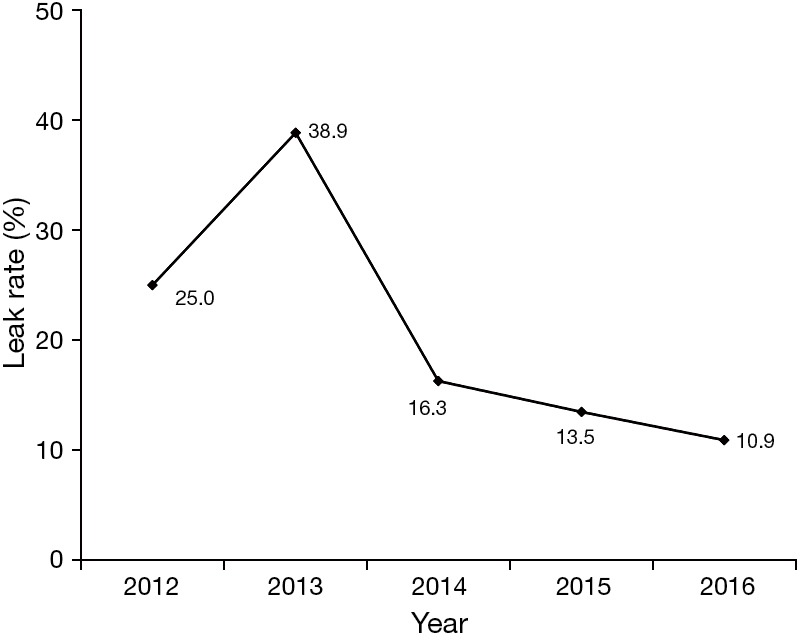
Anastomotic leakage rate over time.
Fifty-nine patients (34.1%) had severe postoperative complications classified as Clavien-Dindo 3 or more and of those 32 (18.5%) with severe pulmonary complications. The postoperative 30-days and 90-days mortality were 2.3% and 4.0% respectively with the majority of deaths occurring in the early years of the transitional period (Table 3).
Table 3. Postoperative complications by type of MIE.
| Variables | MIE | Total (n=173) | ||
|---|---|---|---|---|
| HLAE (n=33) | MIMK (n=48) | MIIL (n=92) | ||
| Clavien-Dindo grade (%) | ||||
| IIIa | 3 (9.1) | 3 (6.2) | 13 (14.1) | 19 (11.0) |
| IIIb | 5 (15.2) | 4 (8.3) | 11 (12.0) | 20 (11.6) |
| IVa | 4 (12.1) | 6 (12.5) | 3 (3.3) | 13 (7.5) |
| V | 3 (9.1) | 1 (2.1) | 3 (3.3) | 7 (4.0) |
| Anastomotic leakage (%) | 6 (18.2) | 14 (29.2) | 14 (15.2) | 34 (19.7) |
| Pulmonary complication (%) | 8 (24.2) | 11 (22.9) | 13 (14.1) | 32 (18.5) |
MIE, minimally invasive esophagectomy; HLAE, hybrid laparoscopic assisted esophagectomy; MIMK, minimally invasive McKeown; MIIL, minimally invasive Ivor-Lewis.
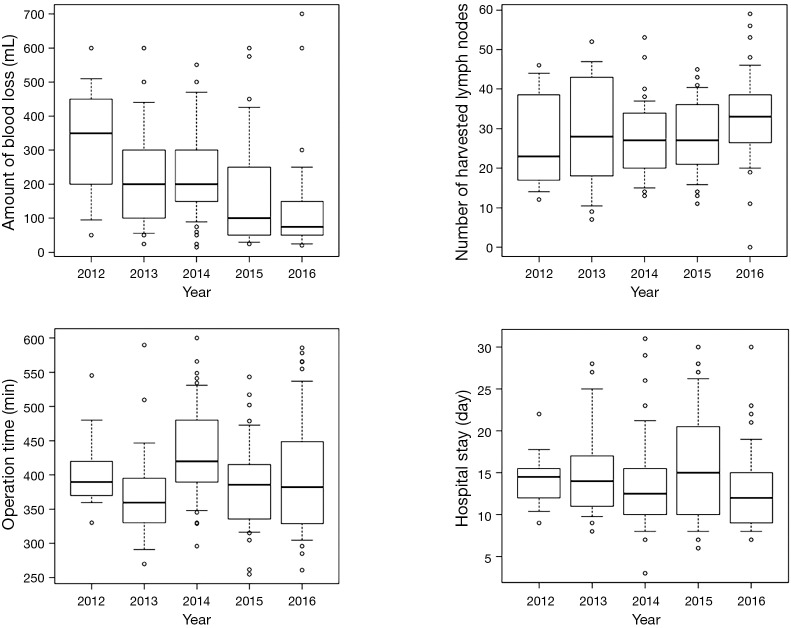
The gaining of experience is depicted by a decrease in both operating time and peroperative bleeding over the implementation period. With regard to operating time, a decrease is demonstrated over the first 2 years during the performance of MIMK followed by a new peak in 2014 where the technique was altered to MIIL. Thereafter, a constant improvement has been noted reaching the lowest level during the last year of our period of MIE practice. Accordingly, a decreasing length of hospital stay can be demonstrated as a proxy for less severe complications and an increasing number of harvested lymph nodes a proxy of the increasing oncological surgical quality (Figure 4).
Figure 4.
Trends of blood loss, operation time, number of harvested lymph nodes and hospital stay over time.
Discussion
In this study, we are presenting our 4.5-year experience of transition from 100% conventional open esophagectomy for cancer to 100% MIE at our center. We are focusing on the gradual implementation and the evolution and refinement of our MIE practice over time.
The utilization of various minimally invasive techniques has increased exponentially over time and several centers have incorporated MIE as the treatment of choice for patients who undergo esophagectomy (14). Over the last years several meta-analyses have been published comparing conventional open esophagectomy and MIE (7,8,15-20). These studies confirm that MIE is feasible and safe and seems to be oncologically equivalent to open technique with regard to radical resection and number of lymph nodes harvested (8,16,18), while long-term survival data of high quality is still lacking. Furthermore, some of these meta-analyses demonstrate a trend to reduced mortality (7) and morbidity (15,17-19) for patients who underwent an MIE compared to those who when operated with conventional open esophagectomy. Moreover, after MIE implementation we have seen a reduction in blood loss and shorter hospital stay (15,17,19).
These favorable findings associated with the implementation of MIE are also supported by the only to date published randomized controlled trial comparing open esophagectomy to MIE. This study showed a significantly reduced risk of pulmonary complications after MIE compared to open esophagectomy (34% vs. 12% respectively) (10). One year follow-up of patients who participated in this randomized study revealed that MIE was associated with a significantly improved quality of life compared to the conventional group (11).
Despite all these encouraging findings, the optimal MIE technique remains debatable and the surgical possibilities range from various hybrid approaches (laparoscopic assisted, thoracoscopic assisted, laparoscopic transhiatal) to a total minimally invasive procedure (15,21), either with intrathoracic or cervical anastomosis.
Triggered by these positive indications we decided to gradually incorporate these techniques into our esophagectomy practice at our center. Before proceeding to implementation of MIE several requirements were fulfilled: (I) the team consisted of surgeons with long experience in both laparoscopic benign UGI-surgery and open esophageal cancer surgery; (II) a member of the team had a previous long fellowship with focus in MIE in a center with long experience within this field; (III) the team visited expert centers abroad for the final evaluation and decision of setup, material, procedure of choice; (IV) the unit could guarantee a steady and high annual case volume required for developing the necessary skills and expertise for these demanding and technically challenging procedures; (V) establishment of 2-year fellowship with rotation of experienced minimal invasive surgeons from a renowned Japanese center of excellence; (VI) collaboration with other expert centers abroad.
Hybrid laparoscopically assisted esophagectomy was initially preferred partly due to its easy setup and our familiarity with laparoscopy and partly due to the safety of conversion to laparotomy in case any difficulty would arise. This, in our opinion technically easier MIE, would act as an indicator of whether we would be able to advance to a more demanding complete MIE procedure.
Furthermore, when it was time to advance to the next stage of implementation of MIE an expert with long experience within the field was invited to perform the first operation together with us. The MIMK was chosen as the next refinement of the technique since a member of the team was familiar with the procedure after a long fellowship abroad and additionally due to the familiarity, within the team, in performance of cervical anastomosis, thus avoiding the technically more complex minimal invasive intrathoracic anastomosis. The prone position was selected as the positioning of choice because of enhanced visualization, improved surgeon’s ergonomics as well as the advantage of double lung ventilation allowing better oxygenation and reducing the risk of postoperative pulmonary complications (22,23).
Another key point to successful transition was the excellent collaboration and support among the members of the team. The first 50 consecutive MIMK were performed by at least 2 of the senior surgeons together, ‘four-handed’. In that way, an increase of the case volume/surgeon and an enhanced learning curve within shorter time could be achieved. Furthermore, we assumed the operation time could be reduced. This excellent collaboration was also decisive when an increasing frequency and severity in cervical anastomotic complications was noted leading to reconsideration of our surgical approach and modification of our operative strategy with subsequently successful results.
The last refinement to our technique was the implementation of MIIL. Despite the challenges of managing a thoracoscopic intrathoracic anastomosis the transition went smoothly due to the experience and confidence gained over time. To date this is the technique of our choice, mainly because it allows us to avoid using the most proximal part of the gastric fundus for the anastomosis, probably reducing the risk of ischemia at the tip of the gastric conduit and also allowing us to avoid using irradiated stomach tissue for the anastomosis, resulting in a lower incidence of anastomotic leaks (24,25).
Our conversion rate is equivalent to that reported by Decker and colleagues (16). Accordingly, our mortality and morbidity rates are also comparable to those demonstrated in other expert centers.
In conclusion, the transition from conventional open esophagectomy to MIE was successful at our center. The implementation was overall safe with good postoperative results, although increased anastomotic complications at one stage required modification of the technique. Currently all patients presenting with esophageal or esophagogastric junction cancer are offered a minimal invasive operation.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Wu PC, Posner MC. The role of surgery in the management of oesophageal cancer. Lancet Oncol 2003;4:481-8. 10.1016/S1470-2045(03)01167-7 [DOI] [PubMed] [Google Scholar]
- 2.Whooley BP, Law S, Murthy SC, et al. Analysis of reduced death and complication rates after esophageal resection. Ann Surg 2001;233:338-44. 10.1097/00000658-200103000-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gebski V, Burmeister B, Smithers BM, et al. Survival benefits from neoadjuvant chemoradiotherapy or chemotherapy in oesophageal carcinoma: a meta-analysis. Lancet Oncol 2007;8:226-34. 10.1016/S1470-2045(07)70039-6 [DOI] [PubMed] [Google Scholar]
- 4.Berger AC, Bloomenthal A, Weksler B, et al. Oncologic efficacy is not compromised, and may be improved with minimally invasive esophagectomy. J Am Coll Surg 2011;212:560-6. 10.1016/j.jamcollsurg.2010.12.042 [DOI] [PubMed] [Google Scholar]
- 5.Ben-David K, Sarosi GA, Cendan JC, et al. Decreasing morbidity and mortality in 100 consecutive minimally invasive esophagectomies. Surg Endosc 2012;26:162-7. 10.1007/s00464-011-1846-3 [DOI] [PubMed] [Google Scholar]
- 6.Braghetto I, Csendes A, Cardemil G, et al. Open transthoracic or transhiatal esophagectomy versus minimally invasive esophagectomy in terms of morbidity, mortality and survival. Surg Endosc 2006;20:1681-6. 10.1007/s00464-006-0009-4 [DOI] [PubMed] [Google Scholar]
- 7.Biere SS, Cuesta MA, van der Peet DL. Minimally invasive versus open esophagectomy for cancer: A systematic review and meta-analysis. Minerva Chir 2009;64:121-33. [PubMed] [Google Scholar]
- 8.Dantoc MM, Cox MR, Eslick GD. Does Minimally Invasive Esophagectomy (MIE) Provide for Comparable Oncologic Outcomes to Open Techniques? A Systematic Review. J Gastrointest Surg 2012;16:486-94. 10.1007/s11605-011-1792-3 [DOI] [PubMed] [Google Scholar]
- 9.Kinjo Y, Kurita N, Nakamura F, et al. Effectiveness of combined thoracoscopic-laparoscopic esophagectomy: Comparison of postoperative complications and midterm oncological outcomes in patients with esophageal cancer. Surg Endosc 2012;26:381-90. 10.1007/s00464-011-1883-y [DOI] [PubMed] [Google Scholar]
- 10.Biere SS, van Berge Henegouwen MI, Maas KW, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet 2012;379:1887-92. 10.1016/S0140-6736(12)60516-9 [DOI] [PubMed] [Google Scholar]
- 11.Maas KW, Cuesta MA, van Berge Henegouwen MI, et al. Quality of Life and Late Complications After Minimally Invasive Compared to Open Esophagectomy: Results of a Randomized Trial. World J Surg 2015;39:1986-93. 10.1007/s00268-015-3100-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klevebro F, Friesland S, Hedman M, et al. Neoadjuvant chemoradiotherapy may increase the risk of severe anastomotic complications after esophagectomy with cervical anastomosis. Langenbecks Arch Surg 2016;401:323-31. 10.1007/s00423-016-1409-0 [DOI] [PubMed] [Google Scholar]
- 13.Irino T, Tsai JA, Ericson J, et al. Thoracoscopic side-to-side esophagogastrostomy by use of linear stapler - a simplified technique facilitating a minimally invasive Ivor-Lewis operation. Langenbecks Arch Surg 2016;401:315-22. 10.1007/s00423-016-1396-1 [DOI] [PubMed] [Google Scholar]
- 14.Lazzarino AI, Nagpal K, Bottle A, et al. Open versus minimally invasive esophagectomy: trends of utilization and associated outcomes in England. Ann Surg 2010;252:292-8. 10.1097/SLA.0b013e3181dd4e8c [DOI] [PubMed] [Google Scholar]
- 15.Verhage RJ, Hazebroek EJ, Boone J, et al. Minimally invasive surgery compared to open procedures in esophagectomy for cancer: A systematic review of the literature. Minerva Chir 2009;64:135-46. [PubMed] [Google Scholar]
- 16.Decker G, Coosemans W, De Leyn P, et al. Minimally invasive esophagectomy for cancer. Eur J Cardiothorac Surg 2009;35:13-20; discussion 20-1. 10.1016/j.ejcts.2008.09.024 [DOI] [PubMed] [Google Scholar]
- 17.Nagpal K, Ahmed K, Vats A, et al. Is minimally invasive surgery beneficial in the management of esophageal cancer? A meta-analysis. Surg Endosc 2010;24:1621-9. 10.1007/s00464-009-0822-7 [DOI] [PubMed] [Google Scholar]
- 18.Sgourakis G, Gockel I, Radtke A, et al. Minimally invasive versus open esophagectomy: Meta-analysis of outcomes. Dig Dis Sci 2010;55:3031-40. 10.1007/s10620-010-1153-1 [DOI] [PubMed] [Google Scholar]
- 19.Guo W, Ma X, Yang S, et al. Combined thoracoscopic-laparoscopic esophagectomy versus open esophagectomy: a meta-analysis of outcomes. Surg Endosc 2016;30:3873-81. 10.1007/s00464-015-4692-x [DOI] [PubMed] [Google Scholar]
- 20.Zhou C, Ma G, Li X, et al. Is minimally invasive esophagectomy effective for preventing anastomotic leakages after esophagectomy for cancer? A systematic review and meta-analysis. World J Surg Oncol 2015;13:269. 10.1186/s12957-015-0661-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shah R, Jobe BA. Open versus minimally invasive esophagectomy: what is the best approach? Minimally invasive esophagectomy. J Gastrointest Surg 2011;15:1503-5. 10.1007/s11605-011-1558-y [DOI] [PubMed] [Google Scholar]
- 22.Noshiro H, Miyake S. Thoracoscopic esophagectomy using prone positioning. Ann Thorac Cardiovasc Surg 2013;19:399-408. 10.5761/atcs.ra.13-00262 [DOI] [PubMed] [Google Scholar]
- 23.Palanivelu C, Prakash A, Senthilkumar R, et al. Minimally Invasive Esophagectomy: Thoracoscopic Mobilization of the Esophagus and Mediastinal Lymphadenectomy in Prone Position-Experience of 130 Patients. J Am Coll Surg 2006;203:7-16. 10.1016/j.jamcollsurg.2006.03.016 [DOI] [PubMed] [Google Scholar]
- 24.Zhang J, Wang R, Liu S, et al. Refinement of Minimally Invasive Esophagectomy Techniques After 15 Years of Experience. J Gastrointest Surg 2012;16:1768-74. 10.1007/s11605-012-1950-2 [DOI] [PubMed] [Google Scholar]
- 25.Zhai C, Liu Y, Li W, et al. A comparison of short-term outcomes between Ivor-Lewis and McKeown minimally invasive esophagectomy. J Thorac Dis 2015;7:2352-8. [DOI] [PMC free article] [PubMed] [Google Scholar]