Abstract
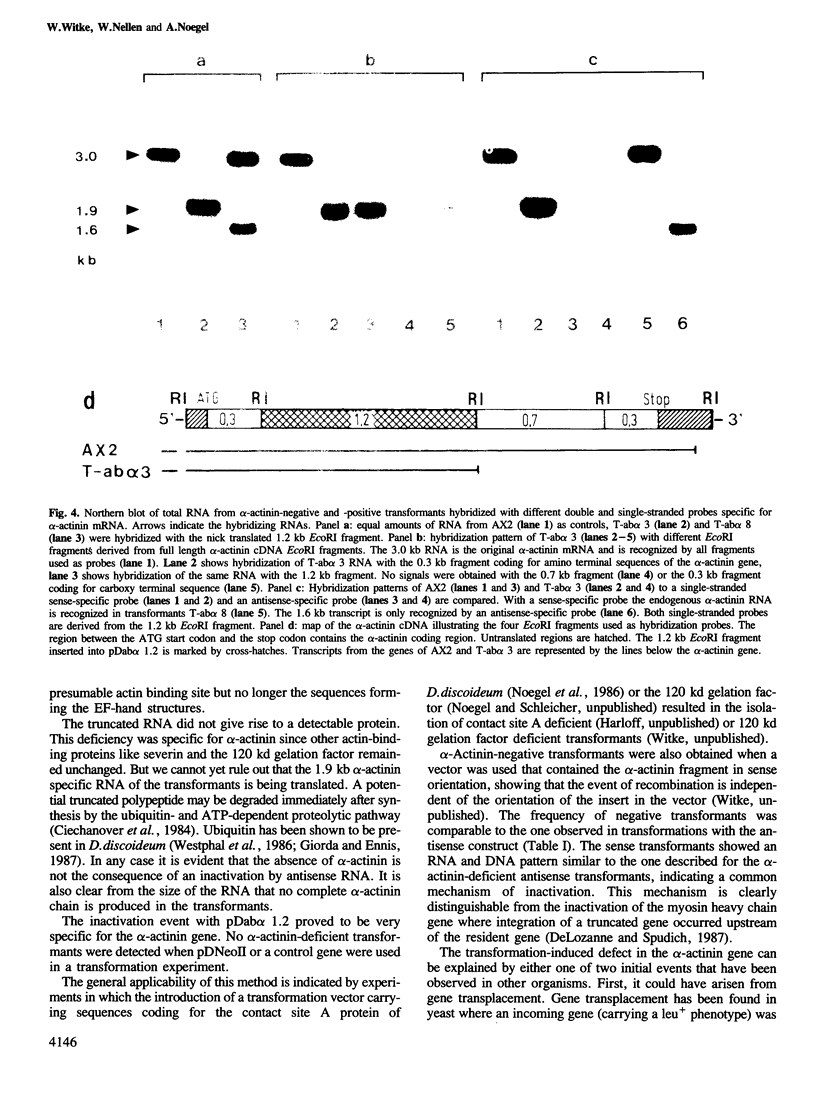
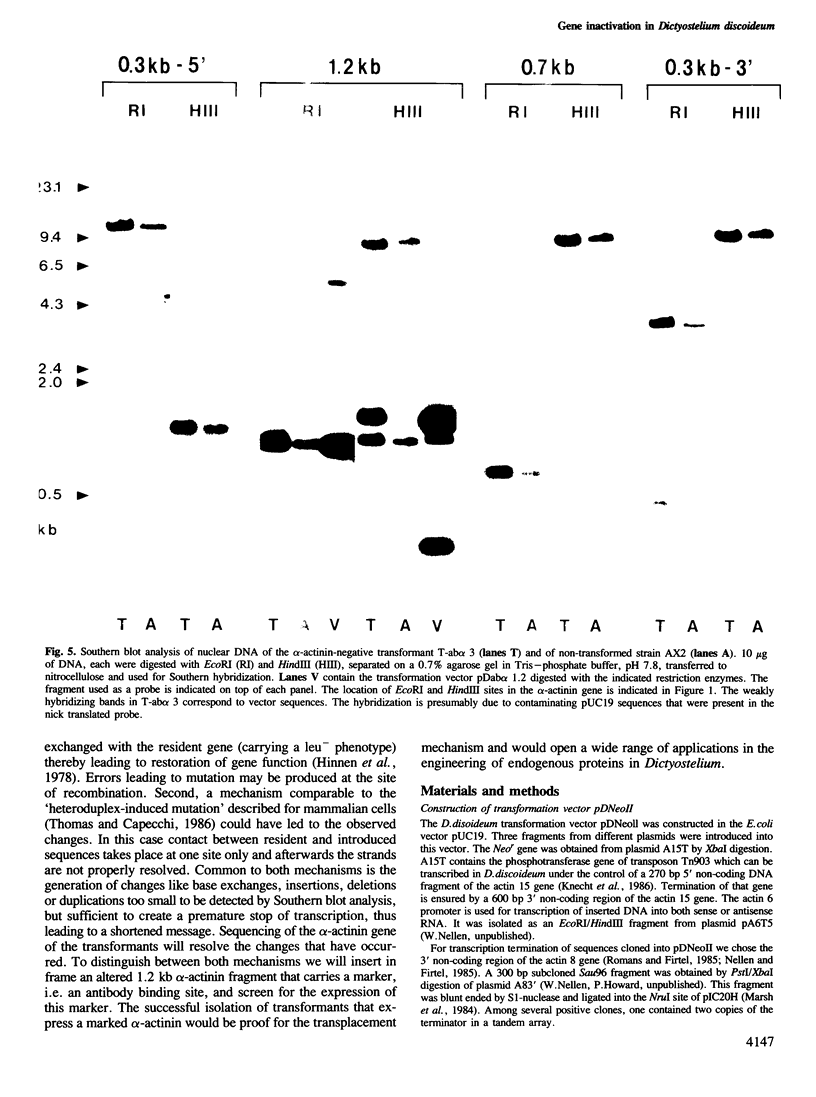
Mutation of the alpha-actinin gene in Dictyostelium has been achieved by transforming cells with the Dictyostelium transformation vector pDNeoII containing a 1.2 kb fragment of the alpha-actinin gene. Transformants deficient in alpha-actinin, an actin-binding protein, produced an altered mRNA that lacked the 3' portion of the coding region. The defect in alpha-actinin production was not due to integration of the vector within the gene, but was apparently caused by errors produced during homologous recombination between the introduced alpha-actinin sequence and its complementary sequence in the coding region of the endogenous gene.
Full text
PDF
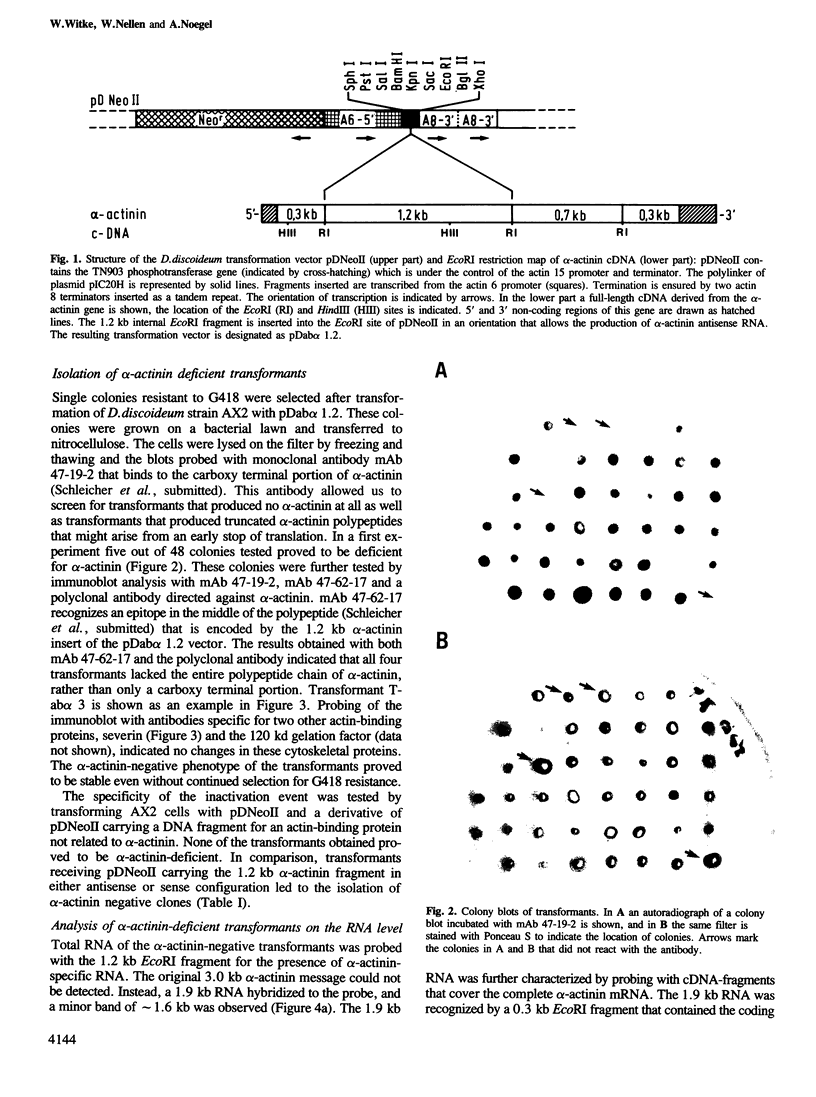


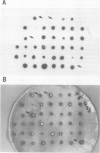
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baron M. D., Davison M. D., Jones P., Patel B., Critchley D. R. Isolation and characterization of a cDNA encoding a chick alpha-actinin. J Biol Chem. 1987 Feb 25;262(6):2558–2561. [PubMed] [Google Scholar]
- Ciechanover A., Finley D., Varshavsky A. The ubiquitin-mediated proteolytic pathway and mechanisms of energy-dependent intracellular protein degradation. J Cell Biochem. 1984;24(1):27–53. doi: 10.1002/jcb.240240104. [DOI] [PubMed] [Google Scholar]
- Crowley T. E., Nellen W., Gomer R. H., Firtel R. A. Phenocopy of discoidin I-minus mutants by antisense transformation in Dictyostelium. Cell. 1985 Dec;43(3 Pt 2):633–641. doi: 10.1016/0092-8674(85)90235-1. [DOI] [PubMed] [Google Scholar]
- De Lozanne A., Spudich J. A. Disruption of the Dictyostelium myosin heavy chain gene by homologous recombination. Science. 1987 May 29;236(4805):1086–1091. doi: 10.1126/science.3576222. [DOI] [PubMed] [Google Scholar]
- Gerisch G., Hagmann J., Hirth P., Rossier C., Weinhart U., Westphal M. Early Dictyostelium development: control mechanisms bypassed by sequential mutagenesis. Cold Spring Harb Symp Quant Biol. 1985;50:813–822. doi: 10.1101/sqb.1985.050.01.099. [DOI] [PubMed] [Google Scholar]
- Giorda R., Ennis H. L. Structure of two developmentally regulated Dictyostelium discoideum ubiquitin genes. Mol Cell Biol. 1987 Jun;7(6):2097–2103. doi: 10.1128/mcb.7.6.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnen A., Hicks J. B., Fink G. R. Transformation of yeast. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1929–1933. doi: 10.1073/pnas.75.4.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht D. A., Cohen S. M., Loomis W. F., Lodish H. F. Developmental regulation of Dictyostelium discoideum actin gene fusions carried on low-copy and high-copy transformation vectors. Mol Cell Biol. 1986 Nov;6(11):3973–3983. doi: 10.1128/mcb.6.11.3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht D. A., Loomis W. F. Antisense RNA inactivation of myosin heavy chain gene expression in Dictyostelium discoideum. Science. 1987 May 29;236(4805):1081–1086. doi: 10.1126/science.3576221. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marsh J. L., Erfle M., Wykes E. J. The pIC plasmid and phage vectors with versatile cloning sites for recombinant selection by insertional inactivation. Gene. 1984 Dec;32(3):481–485. doi: 10.1016/0378-1119(84)90022-2. [DOI] [PubMed] [Google Scholar]
- Nellen W., Firtel R. A. High-copy-number transformants and co-transformation in Dictyostelium. Gene. 1985;39(2-3):155–163. doi: 10.1016/0378-1119(85)90309-9. [DOI] [PubMed] [Google Scholar]
- Nellen W., Silan C., Firtel R. A. DNA-mediated transformation in Dictyostelium discoideum: regulated expression of an actin gene fusion. Mol Cell Biol. 1984 Dec;4(12):2890–2898. doi: 10.1128/mcb.4.12.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noegel A., Gerisch G., Stadler J., Westphal M. Complete sequence and transcript regulation of a cell adhesion protein from aggregating Dictyostelium cells. EMBO J. 1986 Jul;5(7):1473–1476. doi: 10.1002/j.1460-2075.1986.tb04384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noegel A., Witke W., Schleicher M. Calcium-sensitive non-muscle alpha-actinin contains EF-hand structures and highly conserved regions. FEBS Lett. 1987 Sep 14;221(2):391–396. doi: 10.1016/0014-5793(87)80962-6. [DOI] [PubMed] [Google Scholar]
- Orr-Weaver T. L., Szostak J. W., Rothstein R. J. Yeast transformation: a model system for the study of recombination. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6354–6358. doi: 10.1073/pnas.78.10.6354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romans P., Firtel R. A. Organization of the Dictyostelium discoideum actin multigene family. Flanking sequences show subfamily homologies and unusual dyad symmetries. J Mol Biol. 1985 Jun 5;183(3):311–326. doi: 10.1016/0022-2836(85)90003-8. [DOI] [PubMed] [Google Scholar]
- Thomas K. R., Capecchi M. R. Introduction of homologous DNA sequences into mammalian cells induces mutations in the cognate gene. Nature. 1986 Nov 6;324(6092):34–38. doi: 10.1038/324034a0. [DOI] [PubMed] [Google Scholar]
- Thomas K. R., Folger K. R., Capecchi M. R. High frequency targeting of genes to specific sites in the mammalian genome. Cell. 1986 Feb 14;44(3):419–428. doi: 10.1016/0092-8674(86)90463-0. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevor K., Linney E., Oshima R. G. Suppression of endo B cytokeratin by its antisense RNA inhibits the normal coexpression of endo A cytokeratin. Proc Natl Acad Sci U S A. 1987 Feb;84(4):1040–1044. doi: 10.1073/pnas.84.4.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallraff E., Schleicher M., Modersitzki M., Rieger D., Isenberg G., Gerisch G. Selection of Dictyostelium mutants defective in cytoskeletal proteins: use of an antibody that binds to the ends of alpha-actinin rods. EMBO J. 1986 Jan;5(1):61–67. doi: 10.1002/j.1460-2075.1986.tb04178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witke W., Schleicher M., Lottspeich F., Noegel A. Studies on the transcription, translation, and structure of alpha-actinin in Dictyostelium discoideum. J Cell Biol. 1986 Sep;103(3):969–975. doi: 10.1083/jcb.103.3.969. [DOI] [PMC free article] [PubMed] [Google Scholar]