Abstract
Background
The first and only randomized trial comparing open esophagectomy (OE) with minimally invasive esophagectomy (MIE) showed a significant lower incidence of post-operative respiratory infections in the patients who underwent MIE. In order to identify which specific factors are related to a better respiratory outcome in this trial an additional analysis was performed.
Methods
This was a prospective, multicenter, randomized controlled trial. Eligible patients, with a resectable intrathoracic esophageal carcinoma, including the gastro-esophageal (GE) junction tumors and Eastern Cooperative Oncology Group ≤2, were randomized to either MIE or OE. Respiratory infection investigated was defined as a clinical manifestation of (broncho-) pneumonia confirmed by thorax X-ray and/ or Computed Tomography scan and a positive sputum culture. A logistic regression model was used.
Results
From 2009 to 2011, 115 patients were randomized in 5 centers. Eight patients developed metastasis during neoadjuvant therapy or had an irresectable tumor and were therefore excluded from the analysis. Fifty-two OE patients were comparable to 55 MIE patients with regard to baseline characteristics. In-hospital mortality was not significantly different [2% (open group) and 4% (MIE group)]. A body mass index (BMI) ≥26 and OE were associated with a roughly threefold risk of developing a respiratory infection.
Conclusions
Overweight patients and OE are independently associated with a significant higher incidence of post-operative respiratory infections, i.e., pneumonia.
Keywords: Minimally invasive, esophagectomy, respiratory infections, pneumonia, open esophagectomy (OE), obesity
Introduction
Transthoracic esophageal resection with gastric tube reconstruction is to date the only curative option for patients with resectable esophageal cancer. However, this open resection carries a significant risk of morbidity and death (50–70% and 5% respectively) (1). The main morbidity encountered are respiratory infections (57–28%) (1,2).
Minimally invasive esophagectomy (MIE) performed by right thoracoscopy in prone position and laparoscopy will reduce significantly the rate of respiratory complications (3-5). Thoracoscopy can be performed in a lateral thoracic position with a right lung block or in prone position without selective lung block. Several studies have reported significantly low post-operative respiratory infection rates and shorter hospital stay after MIE with comparable oncological results than the open procedure (4-9).
The first randomized trial comparing OE with MIE showed a significant lower incidence of respiratory infections in the patients who underwent MIE (5). In order to identify which specific factors are related to a better outcome in this trial an additional analysis was performed.
Methods
Study design
A multicenter, randomized clinical trial was conducted between June 2009 and March 2011., including patients with esophageal cancer from 5 European centers; VU University Medical Center (Amsterdam, the Netherlands), Academic Medical Center (Amsterdam, the Netherlands), Canisius Wilhelmina Hospital (Nijmegen, the Netherlands), Hospital Universitario Josep Trueta (Girona, Spain), and IRCCS Clinico San Donato, University of Milan, (Milan, Italy). Patients with a European Clinical Oncology Group (ECOG) performance status of 0-2 with resectable esophageal cancer of intrathoracic esophagus or type I esophagogastric junction with an indication for neoadjuvant therapy were eligible for inclusion (NTR TC 2452). Patients were randomized (in a 1:1 fashion) for either an OE or MIE in prone position. All patients underwent a pre-conditioning with physiotherapy and adequate nutrition through a duodenal feeding tube if necessary. All patients had pre-operative neoadjuvant therapy according to chemoradiotherapy of CROSS scheme or chemotherapy (10,11). Further information about participating centers, peri-operative management can be found in previous publications (5).
Esophagectomy
The OE and the MIE surgical interventions consisted of a two-stage esophageal resection with gastric tube formation followed by cervical or thoracic anastomosis. For patients undergoing the open procedure a double tube was placed for selective intubation whereas patients undergoing MIE were positioned in prone decubitus and there was no need for selective intubation with the exception of patients in whom an intrathoracic anastomosis was planned (a bronchus blocker was placed in the right bronchus and inflated only during the anastomosis phase).
Complications
Post-operative morbidity was separated into surgical and respiratory complications. Surgical morbidity included anastomotic leakage, chylous leakage, and re-operations. Respiratory infection was defined as post-operative (broncho-) pneumonia confirmed by thorax X-ray and/or computed tomography (CT) scan and a positive sputum culture.
Statistical analysis
Statistical analysis was performed by SPSS version 18 (SPSS, Chicago, IL, USA). Data are presented as mean with standard deviation (SD) or median with range where appropriate. Univariate logistic regression was performed, and statistically significant variables at the P<0.10 level were entered into a multivariate model by backward elimination. The following variables were entered into the logistic regression models: age, sex, body mass index (BMI), comorbidities (including smoking and alcohol consumption), ASA score, type of surgery (minimally invasive vs. OE), level of anastomosis, and type of carcinoma. Statistical significance was set at P<0.05.
Results
Demographic parameters
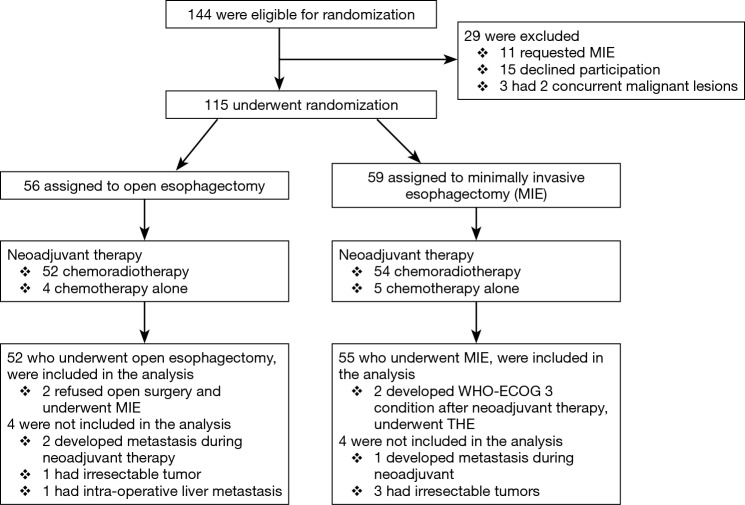
Out of 144 eligible patients, 115 patients were randomly assigned to OE or MIE. Eight patients were not included in the analysis due to different reasons (5). Finally, 52 patients were analyzed in the open group and 55 patients in the minimally invasive group. Two patients in open group refused open surgery and underwent MIE. Two patients in the MIE group developed a WHO-ECOG condition of 3 during neoadjuvant therapy and underwent a transhiatal esophagectomy. The flow chart and demographic, and clinical pathological characteristics of the two study groups, comorbidity and type of neoadjuvant therapy, are depicted in Figure 1 and Tables 1,2).
Figure 1.
Enrollment and outcomes. MIE, minimally invasive esophagectomy; THE, transhiatal esophagectomy.
Table 1. Demographic and clinical characteristics of the patients.
| Demographic parameters | Open esophagectomy (n=52) | MIE (n=55) | P value |
|---|---|---|---|
| Gender | ns | ||
| Male | 43 | 39 | |
| Female | 9 | 16 | |
| Age^ (years) | 62 [42–75] | 62 [34–75] | ns |
| BMI | 24 (±3.7) | 25 (±3.7) | ns |
| Alcohol consumption [(>2 standard drinks/day] | 22 | 21 | ns |
| Smoking | 18 | 16 | ns |
| Respiratory comorbidity | 4 | 6 | ns |
| Diabetes Mellitus | 10 | 8 | ns |
| Cardiac comorbidity | 9 | 14 | ns |
| ASA-classification | ns | ||
| 1 | 14 | 10 | |
| 2 | 31 | 32 | |
| 3 | 6 | 13 | |
| 4 | 1 | 0 | |
| Type carcinoma | ns | ||
| Adenocarcinoma | 35 | 33 | |
| Squamous cell carcinoma | 16 | 22 | |
| Other | 1 | 0 | |
| Location of tumor¶ | ns | ||
| Upper third | 2 | 1 | |
| Middle third | 20 | 23 | |
| Lower third/junction | 30 | 30 | |
| Neoadjuvant therapy | ns | ||
| Chemoradiotherapy | 48 | 50 | |
| Chemotherapy alone | 4 | 5 |
MIE, minimally invasive esophagectomy; BMI, body mass index; ASA, American Association of Anesthesiologist classification system; ns, not significant. ^, skewed distribution, median (range), Mann Whitney U test applied; ¶, American Joint Committee on Cancer (AJCC) site classification of thoracic and abdominal esophagus, one patient in the MIE group had a cardia carcinoma per-operatively and subsequently underwent a Merendino gastric resection.
Table 2. Pathological specimen parameters.
| Pathology | Open esophagectomy (n=52) | MIE (n=55) | P value |
|---|---|---|---|
| Type carcinoma | ns | ||
| Adenocarcinoma | 34 | 26 | |
| Squamous cell carcinoma | 10 | 16 | |
| Other | 0 | 2 | |
| No residual tumor present | 8 | 11 | |
| Total LN resected^ | 21 [7–47] | 20 [3–44] | ns |
| Resection margin | ns | ||
| R0 | 47 | 54 | |
| R1 | 5 | 1 | |
| Stage~ | ns | ||
| 0 | 0 | 1 | |
| I | 4 | 4 | |
| IIa | 16 | 17 | |
| IIb | 6 | 9 | |
| III | 14 | 11 | |
| IV | 5 | 4 | |
| No residual tumor/no LN metastasis | 7 | 9 |
MIE, minimally invasive esophagectomy; ns, not significant. ^, skewed distribution, median (range), Mann Whitney U test applied. LN, Lymph Node; ~, Staging based on the AJCC 6th Edition.
Morbidity and mortality
Conversion rate was 11% (6 patients). Five cases were converted to thoracotomy and one case to laparotomy. Reasons for conversion included persistent hypercapnia (1 patient), pleural adhesions (2 patients), inadequate intrathoracic anastomosis (2 patients), and extensive adhesions around the celiac trunk (1 patient).
There was no difference in ICU stay between the groups (1 vs. 1 day). However, a significant shorter hospital stay was observed in the MIE group [15 (7–120) vs. 11 (7–80) days]. Moreover, MIE patients experienced less pain in the first 10 days post-operatively (mean VAS 3±2 vs. 2±2).
The overall in-hospital incidence of post-operative pneumonia was significant in favor of the MIE group [19 (37%) vs. 7 (13%), P=0.004].
Four patients (8%) in the open and 7 patients (13%) in the MIE group had an anastomotic leakage (not significant). One patient (2%) in the MIE group developed a mediastinitis without an anastomotic leakage; one patient in the open group developed an empyema without anastomotic leakage. There was significant more recurrent nerve palsy in the open group [8 (15%) vs. 1 (2%), P=0.012]. There was no relationship between recurrent nerve palsy and respiratory infections: 4 patients in the OE group with recurrent nerve palsy had a respiratory infection during the in-hospital period; the other 4 patients in the OE group and the 1 in the MIE group experienced no respiratory infections. One patient in the MIE group had a pulmonary embolism. Re-operations were required in 5 patients (10%) in the open group and 8 patients (15%) in the MIE group. There were no significant differences in hospital mortality between the two groups, 1.8% (1 patient open) versus 3.8% (2 patients MIE).
Logistic regression for pulmonary morbidity
The results of the univariate and multivariate regression model for the specific respiratory infections are shown on Tables 3 and 4. Multivariate analysis demonstrated an increased risk for respiratory infection in patients who had a body mass index (BMI) ≥26 and in patients who underwent OE.
Table 3. Univariate logistic regression models for pneumonia.
| Parameters involved in the analysis | Odds ratio | 95% confidence interval | P value |
|---|---|---|---|
| Age (≥51 vs. ≤50) | 0.58 | 0.23–1.5 | 0.263 |
| Sex (male vs. female) | 2.53 | 0.68–9.30 | 0.164 |
| BMI (≥26 vs. <26) | 3.781 | 1.19–11.99 | 0.024 |
| Alcohol consumption (>2 standard drinks vs. ≤2 standard drinks) | 0.77 | 0.32–1.88 | 0.560 |
| Smoking (yes vs. no) | 1.35 | 0.84–2.19 | 0.215 |
| ASA score | 1.36 | 0.41–4.48 | 0.620 |
| Cardiac comorbidity (yes vs. no) | 1.67 | 0.52–5.50 | 0.387 |
| Respiratory comorbidity (yes vs. no) | 0.44 | 0.11–1.70 | 0.234 |
| Diabetic comorbidity (yes vs. no) | 1.15 | 0.34–3.86 | 0.822 |
| Previous surgery (yes vs. no) | 1.72 | 0.70–4.25 | 0.237 |
| Surgical approach (open vs. MIE) | 3.95 | 1.49–10.45 | 0.006 |
| Level of anastomosis (cervical vs. intrathoracic) | 2.10 | 0.71–6.18 | 0.178 |
| Histology (adeno- vs. squamous cell carcinoma) | 0.77 | 0.24–2.55 | 0.674 |
BMI, body mass index; ASA, American Association of Anesthesiologist classification system; MIE, minimally invasive esophagectomy.
Table 4. Multivariate logistic regression model for pneumonia.
| Predictive factors for respiratory infection | Odds ratio | 95% confidence interval | P value |
|---|---|---|---|
| BMI (≥26 vs. <26) | 3.49 | 1.07–11.40 | 0.038 |
| Surgical approach (open vs. MIE) | 3.71 | 1.38–10.03 | 0.010 |
BMI, body mass index. MIE, minimally invasive esophagectomy.
Discussion
This study investigated factors associated with respiratory infections in a randomized trial comparing open transthoracic esophagectomy with MIE. Respiratory infection was associated with a BMI ≥26 and OE in both a univariate and multivariate analysis.
The association of BMI and post-operative complications were investigated in the past. Recent studies have shown conflicting results. Healy et al. identified a BMI >30 as a risk factor for respiratory complications in 150 consecutive patients (12). In contrary, Grotenhuis et al. investigated a larger cohort of 556 patients who underwent esophagectomy and found no association with complications in obese patients (13). In our study overweight (BMI ≥26) was associated in the multivariate analysis with a threefold higher incidence of pneumonia.
Moreover, MIE was associated with a significant lower incidence of respiratory infections. MIE has previously been identified as a factor with a significant better respiratory outcome. Zingg et al. showed that MIE was associated with a decreased risk of respiratory failure (14). In their series the majority of the MIE procedures were performed thoracoscopically assisted, i.e., thoracoscopy and laparotomy. Briez et al., compared two cohorts of patients, one group underwent a hybrid procedure combining laparoscopy and thoracotomy and the other group a full open approach. They found a significant difference of 15.7% versus 42.9% in favor of the hybrid MIE (15). In our study, the MIE procedures included a thoracoscopy and laparoscopy. It is likely that the combination of both approaches has more significant impact on post-operative outcome. The surgical access-related trauma and possibly less impairment of the respiratory function in the post-operative period have been shown to be associated with lower pulmonary morbidity (5,8). In addition, the approach with the patient in prone position could also contribute to a better outcome (16,17). A study comparing MIE in prone position with MIE in lateral decubitus showed that prone positioning resulted in a significant shorter operation time than in lateral decubitus (16). The major cause for this was the better exposure. Also, the prone position allows better ventilation and oxygenation of the ipsilateral lung, which is blocked in the lateral decubitus position. The outcome of this study has been confirmed by other studies (17,18). Atelectasis could be promoted in the collapsed lung, which is a major contribution to post-operative respiratory infection (19). This prone approach, with partial lung collapse during the intrathoracic anastomosis, could therefore result in a lower percentage of respiratory infection.
Currently, preoperative physiotherapy and other preconditioning measures together with perioperative fast track protocols have reduced the numbers of pulmonary infections after esophageal surgery and the numbers will improve further with the adoption of MIE with those perioperative protocols (20).
The main shortcoming of studies that investigate predictive factors is patient selection. This is of course intrinsic in retrospective analyses. This is especially true for identified factors such as surgical approach (MIE or open surgery). This study is an additional analysis of a randomized trial comparing OE with MIE. Patient selection is therefore not present as the trial was powered for respiratory infections.
In conclusion, overweight patients and OE are independently associated with a significant higher incidence of respiratory infections, i.e., pneumonia.
Acknowledgements
Funding by Digestive Surgery Foundation of the Unit of Digestive Surgery of the VU University Medical Centre.
Ethical Statement: This study received ethical approval (Institutional Review Board number HGE 2008/001 and DB 7/12/2011 from the Research Ethics Committee of Vrije University Medical Center in Amsterdam) and the whole TIME protocol was registered in the Netherlands Trial Register: NTR TC 2452), and written informed consent was obtained from all patients.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Hulscher JB, Van Sandick JW, De Boer AG, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Engl J Med 2002;347:1662-9. 10.1056/NEJMoa022343 [DOI] [PubMed] [Google Scholar]
- 2.Burmeister BH, Smithers BM, Gebski V, et al. Surgery alone versus chemoradiotherapy followed by surgery for resectable cancer of the oesophagus: a randomised controlled phase III trial. Lancet Oncol 2005;6:659-68. 10.1016/S1470-2045(05)70288-6 [DOI] [PubMed] [Google Scholar]
- 3.Cuschieri A. Thoracoscopic subtotal oesophagectomy. Endosc Surg Allied Technol 1994;2:21-5. [PubMed] [Google Scholar]
- 4.Palanivelu C, Prakash A, Senthilkumar R, et al. Minimally invasive esophagectomy: Thoracoscopic mobilization of the esophagus and mediastinal lymphadenectomy in prone position - Experience of 130 patients. J Am Coll Surg 2006;203:7-16. 10.1016/j.jamcollsurg.2006.03.016 [DOI] [PubMed] [Google Scholar]
- 5.Biere SS, van Berge Henegouwen MI, Maas KW, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet 2012;379:1887-92. 10.1016/S0140-6736(12)60516-9 [DOI] [PubMed] [Google Scholar]
- 6.Luketich JD, Alvelo-Rivera M, Buenaventura PO, et al. Minimally invasive esophagectomy: outcomes in 222 patients. Ann Surg 2003;238:486-94; discussion 494-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zingg U, Mcquinn A, Divalentino D, et al. Minimally invasive versus open esophagectomy for patients with esophageal cancer. Ann Thorac Surg 2009;87:911-9. 10.1016/j.athoracsur.2008.11.060 [DOI] [PubMed] [Google Scholar]
- 8.Nguyen NT, Hinojosa MW, Smith BR, et al. Minimally invasive esophagectomy: lessons learned from 104 operations. Ann Surg 2008;248:1081-91. 10.1097/SLA.0b013e31818b72b5 [DOI] [PubMed] [Google Scholar]
- 9.Biere SS, Cuesta MA, van der Peet DL. Minimally invasive versus open esophagectomy for cancer: a systematic review and meta-analysis. Minerva Chir 2009;64:121-33. [PubMed] [Google Scholar]
- 10.van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. 10.1056/NEJMoa1112088 [DOI] [PubMed] [Google Scholar]
- 11.Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11-20. 10.1056/NEJMoa055531 [DOI] [PubMed] [Google Scholar]
- 12.Healy LA, Ryan AM, Gopinath B, et al. Impact of obesity on outcomes in the management of localized adenocarcinoma of the esophagus and esophagogastric junction. J Thorac Cardiovasc Surg 2007;134:1284-91. 10.1016/j.jtcvs.2007.06.037 [DOI] [PubMed] [Google Scholar]
- 13.Grotenhuis BA, Wijnhoven BP, Hötte GJ, et al. Prognostic value of body mass index on short-term and long-term outcome after resection of esophageal cancer. World J Surg 2010;34:2621-7. 10.1007/s00268-010-0697-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zingg U, Smithers BM, Gotley DC, et al. Factors associated with postoperative pulmonary morbidity after esophagectomy for cancer. Ann Surg Oncol 2011;18:1460-8. 10.1245/s10434-010-1474-5 [DOI] [PubMed] [Google Scholar]
- 15.Briez N, Piessen G, Torres F, et al. Effects of hybrid minimally invasive oesophagectomy on major postoperative pulmonary complications. Br J Surg 2012;99:1547-53. 10.1002/bjs.8931 [DOI] [PubMed] [Google Scholar]
- 16.Fabian T, Martin J, Katigbak M, et al. Thoracoscopic esophageal mobilization during minimally invasive esophagectomy: a head-to-head comparison of prone versus decubitus positions. Surg Endosc 2008;22:2485-91. 10.1007/s00464-008-9799-x [DOI] [PubMed] [Google Scholar]
- 17.Kubo N, Ohira M, Yamashita Y, et al. Thoracoscopic esophagectomy in the prone position versus in the lateral position for patients with esophageal cancer: a comparison of short-term surgical results. Surg Laparosc Endosc Percutan Tech 2014;24:158-63. 10.1097/SLE.0b013e31828fa6d7 [DOI] [PubMed] [Google Scholar]
- 18.Markar SR, Wiggins T, Antonowicz S, et al. Minimally invasive esophagectomy: Lateral decubitus versus prone positioning; systematic review and pooled analysis. Surg Oncol 2015;24:212-9. 10.1016/j.suronc.2015.06.001 [DOI] [PubMed] [Google Scholar]
- 19.Yatabe T, Kitagawa H, Yamashita K, et al. Better postoperative oxygenation in thoracoscopic esophagectomy in prone positioning. J Anesth 2010;24:803-6. 10.1007/s00540-010-0968-4 [DOI] [PubMed] [Google Scholar]
- 20.Le Roy B, Pereira B, Bouteloup C, et al. Effect of prehabilitation in gastro-oesophageal adenocarcinoma: study protocol of a multicentric, randomised, control trial-the PREHAB study. BMJ Open 2016;6:e012876. 10.1136/bmjopen-2016-012876 [DOI] [PMC free article] [PubMed] [Google Scholar]