Abstract
Implementation of (robot assisted) minimally invasive esophagectomy and increased knowledge of the relation between the autonomic nervous system and the immune response have led to new insights regarding the surgical anatomy of the esophagus. First, two layers of connective tissue were identified; the aorto-esophageal and aorto-pleural ligaments that separate the peri-esophageal compartment, containing vagus nerves, carinal lymph nodes and trachea, from the para-aortic compartment; containing thoracic duct and azygos vein. Second the surgical anatomy of the pulmonary vagus nerve branches has been described in detail. Based on the hypothesis that sparing the vagal nerve branches may be important a method to spare the pulmonary branches of the vagus nerve during thoracoscopic esophagectomy was validated in a cadaver study. Further studies will now investigate the impact of these new insights in the surgical anatomy of the esophagus in clinical practice.
Keywords: Esophagus, anatomy, connective tissue layers, vagus nerve
Introduction
In 1913 the first successful resection of the thoracic esophagus for cancer was performed, the patient surviving for 12 years (1). The esophagus was replaced by an extracorporeal rubber tube that connected the remnant of the cervical esophagus to the stomach. Despite this initial success only 2 of the subsequent 25 patients survived the postoperative period (2). Fortunately, in the following century the desperately needed technical innovation resulted in acceptable postoperative survival and quality of life (3-9). The most recent development was the minimally invasive transthoracic esophagectomy (8). Morbidity was significantly reduced compared to the former open approaches, with similar oncologic outcome (9).
Following introduction of minimally invasive surgery several anatomical issues arose that required further study. First, robot assisted and conventional minimally invasive surgery optimize the view of the surgical field by using up to 10× magnification. This enabled visualization of previously undescribed tissue planes in the mediastinum. These naturally existing tissue planes may be used as dissection plane during minimally invasive surgery. Secondly, a function of the vagus nerve has been identified that may important for patients undergoing an esophagectomy. The inflammatory response is regulated via the vagus nerve and vagus nerve stimulation has been shown to be effective in many inflammatory models (10,11). Since pulmonary (inflammatory) complications occur relatively frequently following esophagectomy, compared to other thoracic procedures, we hypothesized that pulmonary vagotomy, an integral part of esophagectomy, may be an important factor in this regard.
In this review the general anatomy of the esophagus will be summarized briefly followed by new insights in the surgical anatomy of the esophagus including the course of the pulmonary vagus nerve branches.
General anatomy
The esophagus is a slender tube traversing part of the neck, the thorax and abdomen in its course from the pharynx to the stomach. From inside outwards it is constituted of mucosa, submucosa, a circular muscle layer, a longitudinal muscle layer and adventitia (12). Important structures that are intimately related to the esophagus are the trachea and pericardium ventrally; the azygos vein and right pleura on the right laterally, the spine and thoracic duct dorsally, and the aorta and left pleura left laterally.
The esophagus requires sphincters to prevent air and liquid uncontrollably being sucked into the esophagus due to the negative intrathoracic pressure. In the neck the upper esophageal sphincter is found, which is the caudal part of the inferior pharyngeal constrictor, located at the pharynx-esophagus transition. The lower esophageal sphincter is the part of the esophageal musculature at the level of the diaphragm up to the stomach which is able to generate a higher pressure. It serves as a functional sphincter and cannot be distinguished morphologically. Its sphincteric action is reinforced by the right crus of the diaphragm which envelops the esophagus and contracts during inspiration, thereby serving as an external sphincter.
The esophageal mucosa and submucosa contain a dense uninterrupted network of arterioles (13). In the neck these are supplied by multiple small branches from the inferior thyroid artery. In the thorax 4–5 esophageal arteries arise directly from the aorta. Also 1–2 esophageal branches arise from bronchial arteries and occasionally (20%) from an intercostal artery. In the abdomen there are generally multiple branches, from the left inferior phrenic artery, left gastric artery and short gastric arteries (13,14). Due to the uninterrupted network of intramural arterioles it is possible to leave a completely mobilized thoracic esophagus in situ when incurable cancer is discovered preoperatively, without esophageal ischemia or perforation in 72% percent of patients (15). The veins draining the esophagus generally course next to the supplying arteries.
The esophagus is characterized by a dense network of submucosal lymph channels that are mainly longitudinally oriented. Lymph node stations collecting the lymph are located in the neck, mediastinum, and along the left gastric artery up to the coeliac lymph nodes. The lymph node map of the International Association for the Study of Lung Cancer is commonly used to classify the mediastinal lymph nodes (16). Importantly, the variation in the number of mediastinal lymph nodes is very large, ranging from 11 to 54 lymph nodes (17). Regarding the abdominal lymph nodes the lymph node map of the Japanese Society for gastric cancer is used (18). The thoracic duct arises from the cisterna chyli and courses dorsal to the esophagus, receiving esophageal lymph channels on its course to the left venous angle.
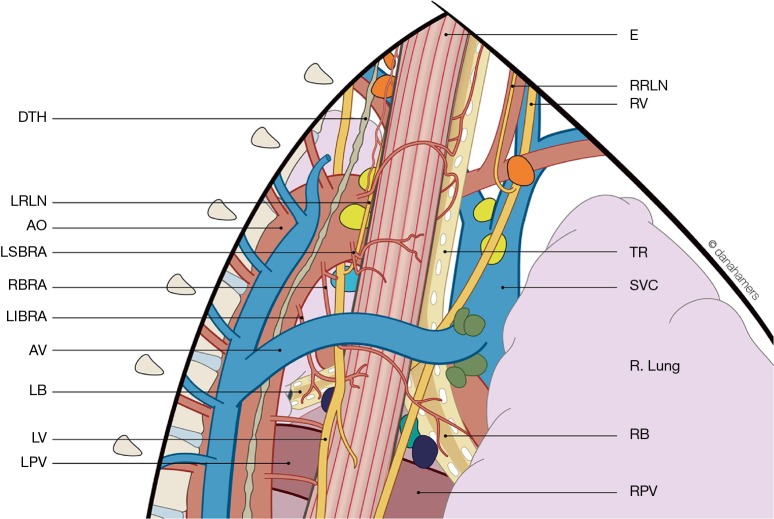
Recently a study of 25 thoracoscopic esophagectomies was performed, aiming to describe the anatomy of the supracarinal esophagus from a thoracoscopic viewpoint (19). The resulting anatomic description is shown in Figure 1, emphasizing the complex anatomy of this confined region.
Figure 1.
Surgical anatomy of the supracarinal esophagus from a thoracoscopic viewpoint (Cuesta et al., Surg Endosc 2016, rights obtained). DTH, ductus thoracicus; LRLN, left recurrent laryngeal nerve; AO, aorta; LSBRA, left superior bronchial artery; RBRA, right bronchial artery; LIBRA; left inferior bronchial artery; AV, azygos vein; LB, left bronchus; LV, left vagus nerve; LPV, left pulmonary vein; E, esophagus; RRLN, right recurrent laryngeal nerve; RV, right vagus nerve; TR, trachea; SVC, superior vena cava; RB, right bronchus; RPV, right pulmonary vein.
Connective tissue layers and compartments
Recently the TIME trial has shown that thoracoscopic esophagectomy reduces pulmonary complications, which was followed by increased application of this approach (9). During thoracoscopic esophageal resections in vivo previously undescribed connective tissue layers were encountered. The knowledge and application of naturally existing tissue planes and compartments has been shown to be crucial in colorectal surgery. Therefore, the connective tissue layers and compartments surrounding the esophagus were recently studied in vivo during thoracoscopic esophagectomies (n=55) and using MRI, and in cadavers (n=2) with the aid of magnetic resonance imaging (MRI) and histology of large tissue sections (20,21). In these studies two regions were distinguished: a region superior to the aortic arch (superior mediastinum and neck) and one inferior to the aortic arch.
The connective tissue planes and compartments superior to the aortic arch are summarized in Figure 2. Above the aortic arch the esophagus and trachea traverse the visceral compartment. The posterior and posterolateral border of this compartment is formed by the alar fascia, which connects the right and left carotid sheets, passing dorsally to the esophagus. The anterior and anterolateral border is formed by the strap muscles. This visceral compartment also contains recurrent laryngeal nerves, the thyroid gland and lymph nodes. During thoracoscopic esophagectomy and dissection in the superior mediastinum the alar fascia can be seen posterior to the esophagus as a connective tissue layer spreading to the left and right lateral sides, containing the esophageal branches of the inferior thyroid artery.
Figure 2.
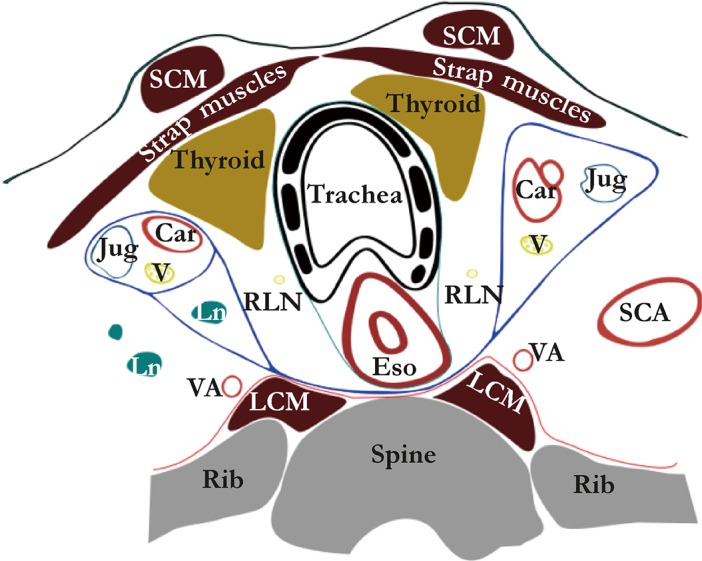
Peri-esophageal connective tissue layers above the aortic arch (Weijs et al. 2016, rights obtained). The blue line represents the alar fascia and the carotid sheaths; the green line represents the visceral fascia and the red line represents the perivertebral fascia. Car, carotid artery; Eso, esophagus; Jug, internal jugular vein; LCM, longus colli muscle; Ln, lymph node; Rln, recurrent laryngeal nerve; SCA, subclavian artery; SCM, sternocleidomastoid muscle; V, vagus nerve; VA, vertebral artery.
Inferior to the aortic arch the mediastinum is traditionally divided into the anterior, middle and posterior mediastinum (12). The boundaries of the posterior mediastinum are the pericardium anteriorly, the pleura laterally and the spine posteriorly. A connective tissue layer coursing from the descending aorta to the esophagus, the aorto-esophageal ligament and a parallel connective tissue layer coursing from descending aorta to the right pleural reflection, the aorta-pleural ligament, divide the posterior mediastinum into two separate compartments (Figure 3). The anterior part, named the peri-esophageal compartment, contains the esophagus, vagus nerves, trachea and carinal lymph nodes. The posterior part, named the para-aortic compartment, contains the azygos vein, thoracic duct and occasionally lymph nodes. Interestingly, the aorto-esophageal ligament can be visualized on magnetic resonance imaging. Further studies should determine if this could aid in preoperative planning and staging of esophageal cancer.
Figure 3.
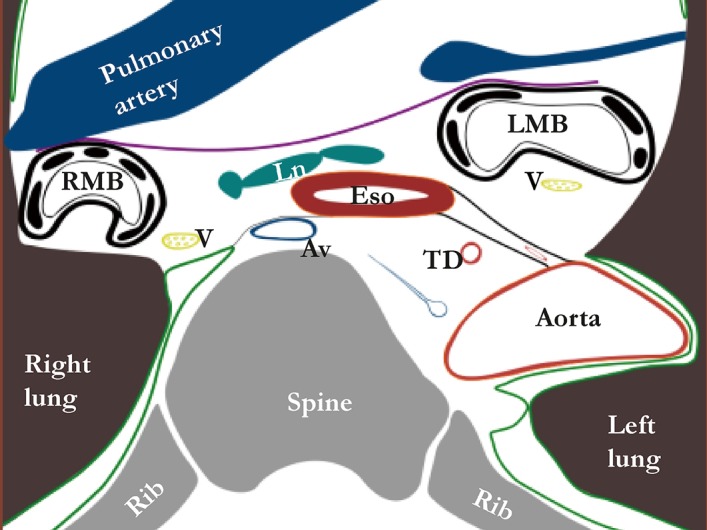
Peri-esophageal connective tissue layers below the level of the aortic arch (Weijs et al. 2016, rights obtained). The green line represents pleura, the purple line represents a connective tissue layer coursing from left to right main bronchus, the black line represents the aorto-esophageal ligament and the gray line represents the aorto-pleural ligament. Av, azygos vein; LMB, left main bronchus; Ln, lymph node; RMB, right main bronchus; TD, thoracic duct.
Pulmonary branches of the vagus nerve
Pulmonary complications remain frequent following esophagectomy, for example pneumonia is reported in 28% to 40% of patients (22). Since this differs from other thoracic surgical procedures, where pneumonia rates are much lower, an explanation may be found in the specific structures that are resected. In this respect the vagus nerve should be mentioned since it regulates many important pulmonary functions, such as the cough reflex, mucous production and bronchus diameter (23). Furthermore the vagus nerve has been shown to be important in the regulation of inflammation. Multiple other factors have been identified that have a causative role in the development of pulmonary complications following esophagectomy, for example one-lung ventilation, aspiration and sputum stasis. However these factors all have in common that inflammation is the final common pathway through which they manifest in a pneumonia or acute respiratory distress syndrome. This is supported by studies showing that a postoperative systemic inflammatory response syndrome following esophagectomy is predictive of subsequent pulmonary complications (24). Therefore pulmonary vagotomy during esophagectomy may be a pivotal factor in development of postoperative pulmonary complications and sparing the pulmonary branches of the vagus nerve may be beneficial.
As the precise anatomy of the pulmonary vagus nerve branches was unclear, an anatomical study was performed in six human cadavers (25). This study provided a map for the development of a method to spare the pulmonary branches of the vagus nerve in 10 human cadavers (26).
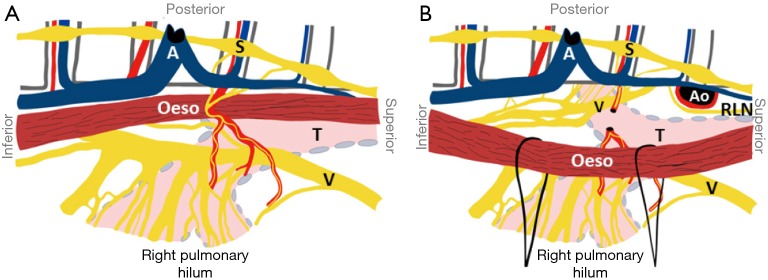
Both lungs are supplied by a small anterior pulmonary plexus and a large posterior pulmonary plexus (containing 74–77% of the total lung supply). The right anterior plexus is located just above the right pulmonary artery and the left anterior plexus just anterior to the left pulmonary artery. The right posterior pulmonary plexus consists of a median of 13 nerve branches that sequentially arise from the vagus nerve from where it crosses the superior edge of the right main bronchus (Figure 4). The left posterior pulmonary plexus consists of a median of 13 nerve branches that sequentially arise from the vagus nerve between where it crosses the superior edge of the pulmonary artery and the lower edge of the pulmonary vein. The posterior pulmonary plexus is segmentally organized: the cranialmost branches innervate the superior lung lobe, the caudalmost innervate the middle and inferior lung lobes. In order to spare the innervation of both superior and middle/inferior lung lobes, all branches up to the caudalmost largest branch of the vagus nerve should be spared. The right caudalmost large pulmonary vagus nerve branch is found a median of 11 mm caudal to inferior edge of the right main bronchus and the left caudalmost large pulmonary vagus nerve branch is located a median of 13 mm caudal to the inferior edge of the left main bronchus.
Figure 4.
Anatomy of the pulmonary branches of the vagus nerve from the viewpoint of right transthoracic esophagectomy (Weijs et al. 2015, rights obtained). A, azygos vein; Ao, aorta; Oeso, esophagus; RLN, left recurrent laryngeal nerve; S, sympathetic trunk; T, trachea; V, vagus nerve.
It was feasible to spare the pulmonary branches of the vagus nerve during thoracoscopic esophagectomy in human cadavers, using these descriptions. On the right side, a median of nine pulmonary vagus nerve branches could be spared, of which four coursed to the middle and inferior lung lobes. On the left side a median of 10 pulmonary vagus nerve branches could be spared, of which four coursed to the inferior lung lobe. The subcarinal (station 7) lymph nodes were always removed completely. Peri-bronchial lymph nodes (station 10L and R) were left behind in eight cases, however, it is questionable if these would have been resected during conventional esophagectomy.
Conclusions
Introduction of minimally invasive surgery and new insights in the function of the vagus nerve required a better understanding of the corresponding anatomy. For the esophagus this resulted in refined descriptions of the surgical anatomy from a thoracoscopic viewpoint, the peri-esophageal fascias and mapping of pulmonary vagus nerve branches.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Torek F. The first successful case of resection of the thoracic portion of the oesophagus for carcinoma. Surg Gynecol Obstet 1913;16:614-7. [Google Scholar]
- 2.Torek F. Carcinoma of the thoracic portion of the esophagus. Arch Surg 1925;10:353-60. 10.1001/archsurg.1925.01120100365019 [DOI] [Google Scholar]
- 3.Ohsawa T. The surgery of the oesophagus. Arch Jpn Chir 1933;10:605-95. [Google Scholar]
- 4.Lewis I. The surgical treatment of carcinoma of the oesophagus; with special reference to a new operation for growths of the middle third. Br J Surg 1946;34:18-31. 10.1002/bjs.18003413304 [DOI] [PubMed] [Google Scholar]
- 5.McKeown KC. Total three-stage oesophagectomy for cancer of the oesophagus. Br J Surg 1976;63:259-62. 10.1002/bjs.1800630403 [DOI] [PubMed] [Google Scholar]
- 6.Orringer MB, Sloan H. Esophagectomy without thoracotomy. J Thorac Cardiovasc Surg 1978;76:643-54. [PubMed] [Google Scholar]
- 7.Omloo JM, Lagarde SM, Hulscher JB, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the mid/distal esophagus: five-year survival of a randomized clinical trial. Ann Surg 2007;246:992-1000; discussion 1000-1. 10.1097/SLA.0b013e31815c4037 [DOI] [PubMed] [Google Scholar]
- 8.Cuschieri A. Endoscopic subtotal oesophagectomy for cancer using the right thoracoscopic approach. Surg Oncol 1993;2 Suppl 1:3-11. 10.1016/0960-7404(93)90052-Z [DOI] [PubMed] [Google Scholar]
- 9.Biere SS, van Berge Henegouwen MI, Maas KW, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet 2012;379:1887-92. 10.1016/S0140-6736(12)60516-9 [DOI] [PubMed] [Google Scholar]
- 10.Tracey KJ. Reflex control of immunity. Nat Rev Immunol 2009;9:418-28. 10.1038/nri2566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luyer MD, Greve JW, Hadfoune M, et al. Nutritional stimulation of cholecystokinin receptors inhibits inflammation via the vagus nerve. J Exp Med 2005;202:1023-9. 10.1084/jem.20042397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gray H. Chapter XI: Splanchnology. In: Lewis WH (editor). Anatomy of the human body, 20th edition. New York: Philadelphia, Lea & Febiger; 1918. [Google Scholar]
- 13.Liebermann-Meffert DM, Luescher U, Neff U, et al. Esophagectomy without thoracotomy: is there a risk of intramediastinal bleeding? A study on blood supply of the esophagus. Ann Surg 1987;206:184-92. 10.1097/00000658-198708000-00011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swigart LL, Siekert RG, et al. The esophageal arteries; an anatomic study of 150 specimens. Surg Gynecol Obstet 1950;90:234-43, illust. [PubMed] [Google Scholar]
- 15.Weijs TJ, Toxopeus EL, Ruurda JP, et al. Leaving a mobilized thoracic esophagus in situ when incurable cancer is discovered intraoperatively. Ann Thorac Surg 2015;99:490-4. 10.1016/j.athoracsur.2014.08.041 [DOI] [PubMed] [Google Scholar]
- 16.Rusch VW, Asamura H, Watanabe H, et al. The IASLC lung cancer staging project: a proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol 2009;4:568-77. [DOI] [PubMed] [Google Scholar]
- 17.Ziyade S, Pinarbasili NB, Ziyade N, et al. Determination of standard number, size and weight of mediastinal lymph nodes in postmortem examinations: reflection on lung cancer surgery. J Cardiothorac Surg 2013;8:94. 10.1186/1749-8090-8-94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kajitani T. The general rules for the gastric cancer study in surgery and pathology. Part I. Clinical classification. Jpn J Surg 1981;11:127-39. 10.1007/BF02468883 [DOI] [PubMed] [Google Scholar]
- 19.Cuesta MA, van der Wielen N, Weijs TJ, et al. Surgical anatomy of the supracarinal esophagus based on a minimally invasive approach: vascular and nervous anatomy and technical steps to resection and lymphadenectomy. Surg Endosc 2017;31:1863-70. 10.1007/s00464-016-5186-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cuesta MA, Weijs TJ, Bleys RL, et al. A new concept of the anatomy of the thoracic oesophagus: the meso-oesophagus. Observational study during thoracoscopic esophagectomy. Surg Endosc 2015;29:2576-82. 10.1007/s00464-014-3972-1 [DOI] [PubMed] [Google Scholar]
- 21.Weijs TJ, Goense L, van Rossum PS, et al. The peri-esophageal connective tissue layers and related compartments: visualization by histology and magnetic resonance imaging. J Anat 2017;230:262-71. 10.1111/joa.12552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weijs TJ, Berkelmans GH, Nieuwenhuijzen GA, et al. Immediate Postoperative Oral Nutrition Following Esophagectomy: A Multicenter Clinical Trial. Ann Thorac Surg 2016;102:1141-8. 10.1016/j.athoracsur.2016.04.067 [DOI] [PubMed] [Google Scholar]
- 23.Mazzone SB, Canning BJ. Autonomic neural control of the airways. Handb Clin Neurol 2013;117:215-28. 10.1016/B978-0-444-53491-0.00018-3 [DOI] [PubMed] [Google Scholar]
- 24.D’Journo XB, Michelet P, Marin V, et al. An early inflammatory response to oesophagectomy predicts the occurrence of pulmonary complications. Eur J Cardiothorac Surg 2010;37:1144-51. 10.1016/j.ejcts.2009.11.033 [DOI] [PubMed] [Google Scholar]
- 25.Weijs TJ, Ruurda JP, Luyer MD, et al. Topography and extent of pulmonary vagus nerve supply with respect to transthoracic oesophagectomy. J Anat 2015;227:431-9. 10.1111/joa.12366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weijs TJ, Ruurda JP, Luyer MD, et al. Preserving the pulmonary vagus nerve branches during thoracoscopic esophagectomy. Surg Endosc 2016;30:3816-22. 10.1007/s00464-015-4683-y [DOI] [PubMed] [Google Scholar]