Abstract
The objectives of this review were to assess both the short- and long-term clinical outcomes in patients managed with definitive chemoradiotherapy, and salvage esophagectomy subsequently in comparison to those neoadjuvant chemoradiotherapy followed by planned esophagectomy (NCRS) for esophageal cancer from published literature. Eleven studies comprising 1,906 patients were included, 563 in the salvage group and 1,343 in the NCRS group. Pooled analysis showed no significant difference between salvage and NCRS groups in overall survival [hazard ratio (HR) =1.17; 95% confidence interval (95% CI), 0.94–1.46, P=0.148], postoperative mortality [pooled odds ratios (POR) =1.12; 95% CI, 0.52–2.41, P=0.775], pulmonary complications (POR =1.24; 95% CI, 0.83–1.86, P=0.292) and positive resection margin incidence (POR =1.29; 95% CI, 0.94–1.76, P=0.114). However, within the salvage group there were increases in postoperative morbidity (POR =1.30; 95% CI, 1.00–1.67, P=0.046) and anastomotic leak (POR =1.88; 95% CI, 1.41–2.51, P<0.001). Herein we found that salvage esophagectomy has similar short- and long-term mortality in comparison to planned esophagectomy following neoadjuvant chemoradiotherapy. However, anastomotic leak is increased following salvage esophagectomy suggesting the need for this practice to be reserved for high volume surgeons within high volume centers.
Keywords: Esophageal cancer, salvage esophagectomy, chemotherapy, definitive chemotherapy, neoadjuvant chemotherapy
Introduction
Esophageal cancer is estimated to affect more than 450,000 individuals worldwide with an annually increasing incidence. Despite the advancement in the management of those patients, the prognosis has not changed; the overall 5-year survival of patients with resectable esophageal cancer remains disappointing, ranging from 15% to 34% (1-3).
The oncological management of esophageal cancer with the introduction of neo-adjuvant chemoradiation first started in the early 1980s. Several studies including randomized controlled trials have shown an improved survival with use of neoadjuvant chemoradiotherapy (NCRS) in esophageal cancer suitable for resection especially for squamous cell histological subtype with reported 3-year survival of 14% and a 5-year survival of 13% (4-6).
Definitive combined chemoradiotherapy in the absence of intended surgical resection has been suggested as a treatment option in patients with poor performance status, and also those who opt not to have surgery. Previous randomized controlled trials have demonstrated that surgical resection in treatment of esophageal squamous cell carcinoma does not improve overall survival when compared with definitive chemoradiotherapy (7,8). However, there is an associated mortality of 18% patients in this frail patient cohort, with a 40–75% local recurrence rates following definitive chemoradiotherapy. The subset of patients with disease recurrence that will be considered for salvage esophagectomy (9,10).
Radiotherapy to the thoracic and abdominal cavities has an effect on surgical planes along with systemic effects of cardiac and pulmonary toxicity. These factors and the underlying physiology of these patients ensure salvage esophagectomy is a high-risk procedure. This has previously been reflected by the increased mortality and morbidity rates associated with salvage esophagectomy in small case series.
The objectives of this review are to compare both the short- and long-term clinical outcomes from salvage esophagectomy following definitive chemoradiotherapy In comparison to those receiving planned esophagectomy with NCRS for esophageal cancer from published literature.
Methods
A systematic literature search of MEDLINE (January 1950–October 2016), EMBASE (January 1974–October 2016), Web of Science (January 1990–June 2016) and the Coch-rane Library databases was performed. The search terms ‘(o)esophagectomy’, ‘salvage’, ‘definitive’, ‘neoadjuvant’, ‘(o)esophageal cancer’ and ‘chemoradiotherapy’ and the medical subject headings (MeSH) ‘(o)esophagectomy’, ‘(o)esophageal neoplasm’, ‘chemoradiotherapy’, ‘salvage therapy’, ‘evidence-based medicine’ and ‘evidence-based practice’ were used in combination with the Boolean operators AND or OR.
Abstracts of citations identified by the search were scrutinized by two independent observers (Sheraz Markar and Sara Jamel) to determine eligibility for inclusion in this pooled analysis. Publications were included if they met all of the following criteria:
Patients with diagnosis of esophageal cancer;
Surgical treatment was utilized with a curative intent;
The study compared clinical outcome from esophagectomy after definitive chemoradiotherapy (salvage) to esophagectomy planned after neoadjuvant chemo-radiotherapy (NCRS);
Only articles published from 1995 onwards were included in this analysis (this was to ensure that the studies included reflected current surgical and peri-operative management of esophageal cancer);
The study used only primary data and was not an editorial or systematic review.
The primary outcome was overall survival. Secondary outcomes were the incidence of post-operative mortality [defined as death during hospital admission (in-hospital) or within 30 days of surgery (30-day)], overall postoperative morbidity, specifically anastomotic leak and pulmonary complications (including pneumonia, pneumothorax and respiratory failure), and positive resection margin.
Statistical analysis
Overall survival
The logarithm of the hazard ratio (HR) with 95% confidence interval (95% CI) was used as the primary summary statistic. To estimate HR and its variance, this was extracted from the study directly or required additional calculation depending on the method of data being presented: annual mortality rates, survival curves, number of deaths or percentage freedom from death (11).
Other outcomes
Data from eligible trials were entered into a computerized spreadsheet for analysis. Statistical analysis was performed using StatsDirect 2.5.7 (StatsDirect, Altrincham, UK). Meta-analysis of data was conducted using a random effects model. POR, with 95% CI, were calculated for the effect of salvage esophagectomy on discrete variables (postoperative mortality, morbidity, anastomotic leak, pulmonary complications and positive resection margin). Pooled outcome measures were determined using random-effects models as described by DerSimonian and Laird (12). Heterogeneity among trials was assessed by means of the I2 inconsistency test. This was graded as low (I2<25%), moderate (I2=25–75%) or high (I2>75%). The Egger test was used to assess the funnel for significant asymmetry indicating possible publication or other biases. The significant level was set at P<0.05.
Results
Study and patient demographics
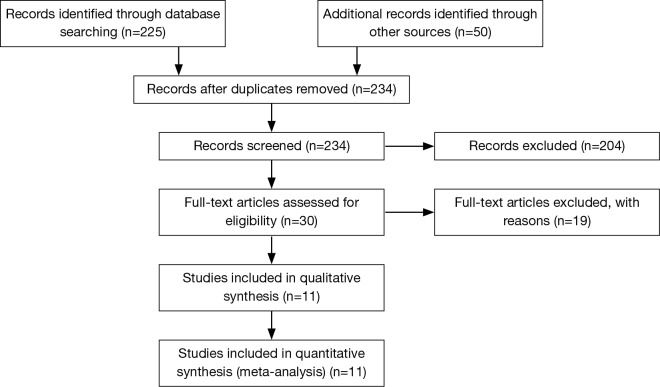
Eleven studies were identified in the initial literature search to meet inclusion criteria (Figure 1) (13-23). After screening, one further publication (23), was excluded as this institution published their outcomes in a follow-up publication, which was included in the analysis (16). Therefore, ten comparative studies were included in this pooled final analysis (13-22). In total 1,906 patients were included, 563 in the salvage group and 1,343 in the NCRS group. As expected the majority of patients were esophageal squamous cell carcinoma, with a large degree of heterogeneity observed in tumor location (Table 1). Similarly, heterogeneity was detected in the analysis of the radiation dosage used in the context of definitive chemoradiotherapy, and the timing of salvage surgery following definitive chemoradiotherapy (Table 2). A summary of clinical outcomes of reported rate of post-operative morbidity and mortality is shown in Table 3.
Figure 1.
PRISMA search strategy.
Table 1. Tumor location and histological subtype for studies included.
| Study | Patient number (salvage) | Patient number (NCRS) | Tumor location (%) | Histological subtype (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Upper (SALV) | Upper (NCRS) | Middle (SALV) | Middle (NCRS) | Lower (SALV) | Lower (NCRS) | Adeno (SALV) | Adeno (NCRS) | SCC (SALV) | SCC (NCRS) | ||||
| Miyata | 33 | 115 | 30 | 42 | 55 | 43 | 15 | 15 | – | – | – | – | |
| Morita | 27 | 197 | 33.3 | 26.9 | 33.3 | 48.2 | 33.3 | 24.9 | – | – | – | – | |
| Takeuchi | 25 | 40 | 32 | 15 | 60 | 72.5 | 8 | 12.5 | – | – | 100 | 100 | |
| Markar | 308 | 540 | 19.2 | 20.1 | 40.9 | 40.6 | 39.9 | 39.2 | 62.7 | 64.9 | 35.4 | 32.8 | |
| Marks | 65 | 521 | – | – | – | – | 95.4 | 97.7 | 100 | 100 | – | – | |
| Nakamra | 27 | 28 | 3.7 | 17.8 | 62.9 | 60.7 | 22.2 | 21.4 | – | – | – | – | |
| Swisher | 13 | 99 | 30.7 | 1 | 23 | 19 | 46 | 80 | 46 | 75 | 54 | 22 | |
| Smithers | 14 | 53 | 7 | 0 | 29 | 9 | 64 | 91 | 64 | 89 | 36 | 9 | |
| Farinella | 16 | 32 | 50 | 9.4 | 18.8 | 21.8 | 31.2 | 68.8 | 12.5 | 43.8 | 87.5 | 53.1 | |
| Tomimaru | 24 | 26 | 20.8 | 34.6 | 54.2 | 42.3 | 25 | 23.1 | 0 | 0 | 100 | 100 | |
| Chao | 27 | 191 | 29.6 | 20.9 | 51.8 | 54.5 | 18.5 | 24.6 | – | – | – | – | |
Salv, salvage esophagectomy; NCRS, neoadjuvant chemoradiotherapy.
Table 2. Total dose of radiation and average time to surgery for all studies.
| Study | Salv group No. | NCRS group No. | Total radiation dose (Gy) | Average time to surgery (days) | |||
|---|---|---|---|---|---|---|---|
| Salv | NCRS | Salv | NCRS | ||||
| Miyata | 33 | 115 | 59.8 | 39.9 | 249 | 38.3 | |
| Morita | 27 | 197 | >60 | 38 | – | – | |
| Takeuchi | 25 | 40 | >50 | <50 | – | – | |
| Markar | 308 | 540 | – | – | – | – | |
| Marks | 65 | 521 | 50 | 48 | 216 | 50 | |
| Nakamra | 27 | 28 | 63 | 38 | 111 | 28 | |
| Swisher | 13 | 99 | 56.7 | 41.4 | – | – | |
| Smithers | 14 | 53 | 60 | 35 | 196 | 28 | |
| Farinella | 16 | 32 | 57.7 | 45 | – | – | |
| Tomimaru | 24 | 26 | 62 | 40 | 180 | 40 | |
| Chao | 27 | 191 | – | – | – | – | |
Salv, salvage esophagectomy; NCRS, neoadjuvant chemoradiotherapy.
Table 3. Clinical outcomes for all studies included.
| Study | Postoperative mortality (%) | Postoperative morbidity (%) | Anastomotic leak (%) | Pulmonary complications (%) | R1/2 resection margin (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Salv | NCRS | Salv | NCRS | Salv | NCRS | Salv | NCRS | Salv | NCRS | |||||
| Miyata | 12 | 4 | – | – | 39 | 13 | – | – | 12 | 12 | ||||
| Morita | 7.4 | 2 | 59 | 40 | 37 | 23 | 29.6 | 14.7 | 29.6 | 22.8 | ||||
| Takeuchi | 5 | 8 | – | – | 24 | – | 44 | 25 | 20 | 22.5 | ||||
| Markar | 8.4 | 9.3 | 63.6 | 58.9 | 17.2 | 10.7 | 42.9 | 40.9 | 12.7 | 11 | ||||
| Marks | 4.6 | 5.2 | – | – | 18.5 | 11.3 | 23.1 | 17.9 | 9.2 | 5.4 | ||||
| Nakamra | 3.7 | 0 | – | – | 3 | 1 | 22.2 | 14.3 | 33 | 39 | ||||
| Swisher | – | 6 | – | – | 38 | 7 | – | – | – | – | ||||
| Smithers | 7 | 2 | 79 | 62 | 14 | 8 | 57 | 30 | – | – | ||||
| Farinella | 0 | 0 | 44 | 37.5 | 25 | 3 | 37.5 | 28 | 19 | 3 | ||||
| Tomimaru | 12.5 | 0 | 50 | 23.1 | 11.5 | 11.5 | 20.8 | 11.5 | 33.3 | 11.5 | ||||
| Chao | 22.2 | 7.9 | – | – | 14.8 | 1.1 | 33.3 | 11.5 | 37.1 | 15.7 | ||||
Salv, salvage esophagectomy; NCRS, neoadjuvant chemoradiotherapy.
Outcome measures
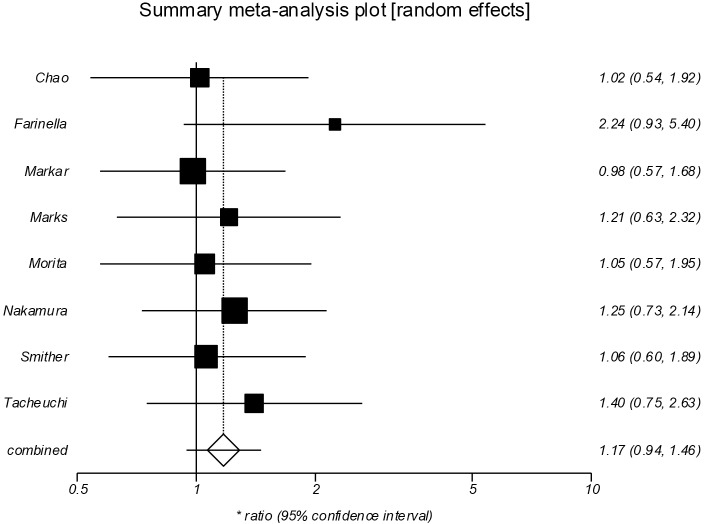
Overall survival (Figure 2)
Figure 2.
Forrest plot showing no significant difference between the groups in overall survival (HR =1.17; 95% CI, 0.94–1.46, P=0.148). HR, hazard ratio.
Pooled analysis of eight studies (13-16,18-21) included 1,711 patients, 506 in the salvage group and 1,205 in the NCRS group. The median follow-up period ranged from 60 to 72 months. The pooled analysis demonstrated no significant difference between salvage and NCRS groups in overall survival (HR =1.17; 95% CI, 0.94–1.46, P=0.148). There was no evidence of significant statistical heterogeneity (I2=0%), however there was significant bias (Egger =3.73, P=0.027).
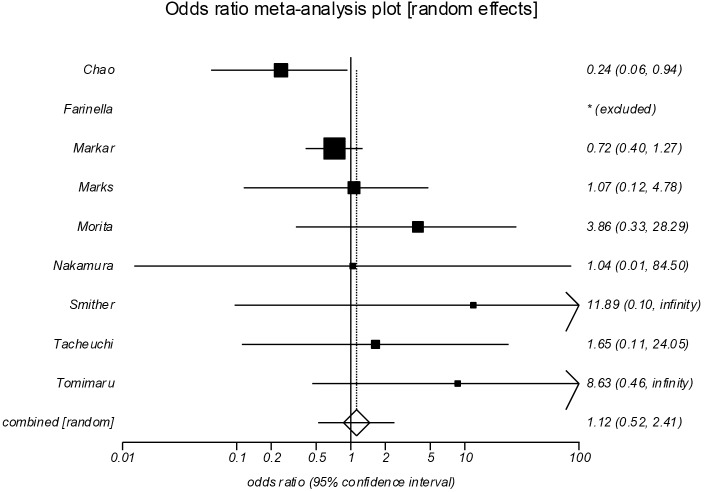
Postoperative mortality (Figure 3)
Figure 3.
Forrest plot showing no significant difference between the groups in postoperative mortality (POR =1.12; 95% CI, 0.52–2.41, P=0.775). POR, pooled odds ratios.
Pooled analysis of nine studies (13-16,18-22) included 1,711 patients, 506 in the salvage group and 1,205 in the NCRS group. There was no significant difference between the groups in postoperative mortality (POR =1.12; 95% CI, 0.52–2.41, P=0.775). There was moderate statistical heterogeneity (I2=44.6%), however no significant evidence of bias (Egger =1.32, P=0.111).
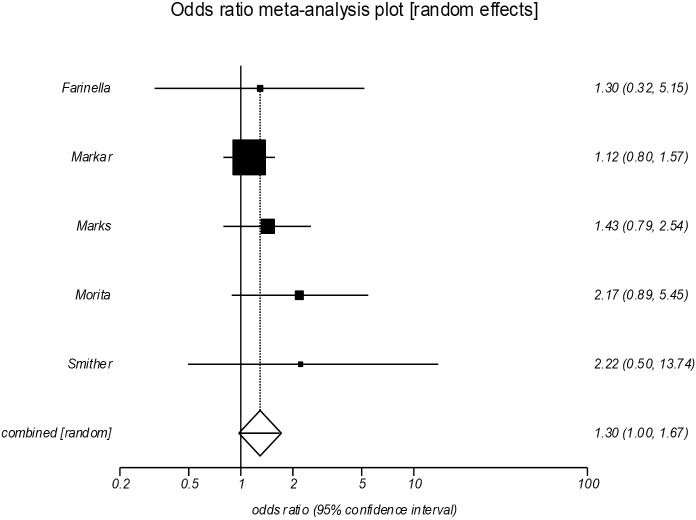
Postoperative morbidity (Figure 4)
Figure 4.
Forrest plot showing an increase in postoperative morbidity following salvage esophagectomy (POR =1.30, 95% CI, 1.00–1.67, P=0.046). POR, pooled odds ratios.
The pooled analysis of five studies (14-16,18,20), revealed a significant increased incidence of overall postoperative morbidity in the salvage group (POR =1.30; 95% CI, 1.00–1.67, P=0.046). There was no evidence of significant statistical heterogeneity (I2=0%), or bias (Egger =1.23, P=0.126).
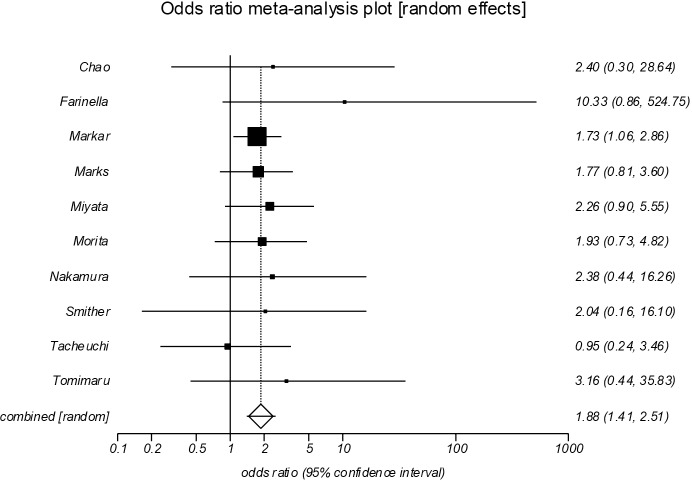
Anastomotic leak (Figure 5)
Figure 5.
Forrest plot showing an increase in anastomotic leak following salvage esophagectomy (POR =1.88; 95% CI, 1.41–2.51, P<0.001). POR, pooled odds ratios.
Anastomotic leak was reported in all included studies (13-22), which showed a significant increase in the salvage group (POR =1.88; 95% CI, 1.41–2.51, P<0.001). There was no evidence of significant statistical heterogeneity (I2=0%), or bias (Egger =0.70; P=0.141).
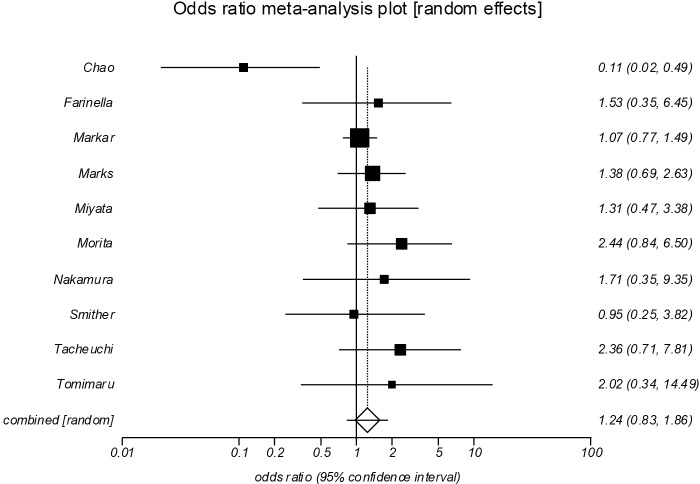
Pulmonary complications (Figure 6)
Figure 6.
Forrest plot showing no significant difference between the groups in pulmonary complications (POR =1.24; 95% CI, 0.83–1.86, P=0.292). POR, pooled odds ratios.
All ten studies reported the incidence of postoperative pulmonary complications (13-22), which showed no significant differences between the groups (POR=1.24, 95% CI, 0.83–1.86, P=0.292). There was evidence of moderate statistical heterogeneity (I2=49.3%), however no significant evidence of statistical bias (Egger =0.19, P=0.834).
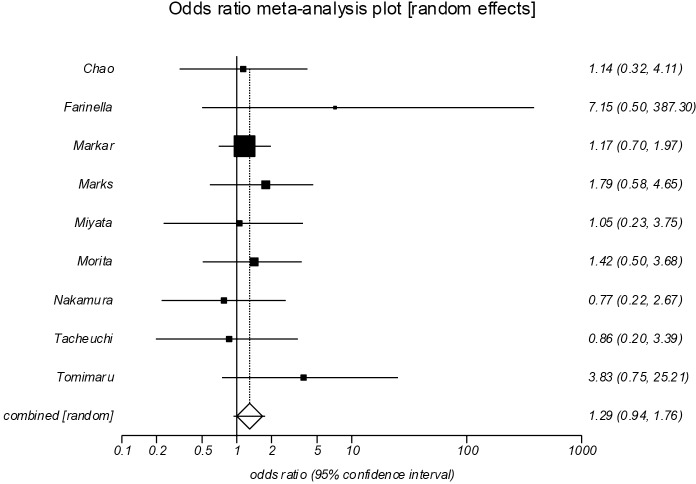
Positive resection margin (Figure 7)
Figure 7.
Forrest plot showing no significant difference between the groups in positive resection margin incidence (POR =1.29; 95% CI, 0.94–1.76, P=0.114). POR, pooled odds ratios.
Nine studies reported the incidence of positive (R1/R2) resection margin (13-19,21,22). Pooled analysis showed no significant difference between the groups in positive resection margin incidence (POR=1.29, 95% CI, 0.94–1.76, P=0.114). There was no evidence of significant statistical heterogeneity (I2=0%), or bias (Egger =0.93, P=0.232).
Discussion
The present study is the largest meta-analysis to date conducted to evaluate the efficacy of undertaking salvage esophagectomy following definitive chemoradiotherapy compared to those undergoing planned esophagectomy with NCRS. The results of this meta-analysis suggest the salvage esophagectomy is comparable to NCRS in terms of postoperative mortality, pulmonary complications, R1/2 resection margin and overall survival. However, salvage was associated with a significant increase in postoperative morbidity and specifically anastomotic leak when compared with NCRS.
It is important to consider the limitations of this meta-analysis in the interpretation of the results gained. Firstly there is a large degree of heterogeneity between the studies included, especially regarding histological subtype of tumor. Further, the indication for salvage esophagectomy as persistent or recurrent disease following definitive chemoradiotherapy was not defined in the majority of studies, which may represent an important confounding variable. The proportion of patients with persistent or recurrent disease following definitive chemoradiotherapy, who were turned down from surgery was not quantified in the majority of studies, suggesting the introduction of selection bias. Finally, the heterogeneity between the studies extends to confounding variables, which may have partially influenced some of the results gained from this meta-analysis.
Analysis of short-term outcomes suggested that salvage esophagectomy was equivalent to NCRS with the exception of anastomotic leak. However it must be emphasized that salvage esophagectomy represents one of the more complex variants of esophagectomy due to the scarring effects of radiotherapy upon the operative field (24). Thus previous surgeon volume-outcome effects observed for all esophagectomy are likely to be amplified in the setting of salvage esophagectomy (25). Suggesting this procedure should only be performed in specialized high volume esophageal surgical units, with the high level of surgical and medical expertise available to manage these complex patients. Further the toxic effects of definitive chemoradiotherapy may show a dose-response relationship, as some studies with total radiation dose up to 60 Gy showing an increase in postoperative mortality within the salvage group (15). However the improvement in tumor response at such high levels of radiotherapy remains unclear, and thus the rationale for using such high radiation doses as part of definitive chemoradiotherapy is questionable.
Salvage esophagectomy was associated with an increase in anastomotic leak when compared with NCRS. This may be in part a reflection of the presumed negative effects that high doses of radiotherapy have upon the micro-circulation of the gastric conduit, resulting in patchy areas of necrosis impairing conduit perfusion and causing anastomotic leak. This further suggests the need for salvage esophagectomy to be performed by high volume experienced esophageal surgeons practicing in high volume centers, with the appropriate infrastructure to rescue patients who develop severe complications following this type of complex surgery (26).
This meta-analysis suggested long-term survival following salvage esophagectomy was equivalent to NCRS. However, it must be acknowledged that the overall treatment pathway of giving patients definitive chemoradiotherapy with follow-up salvage esophagectomy remains unexamined within the current published literature as the denominator of patients remains unquantified. The proportion of patients with persistent or recurrent disease and not considered for salvage esophagectomy due to disease progression or poor patient physiology remains unknown, and therefore the overall efficacy of this treatment approach cannot be fully examined. Further, as described above the majority of included studies grouped both persistent and recurrent disease together. Previous investigations (15), have suggested that persistent disease following definitive chemoradiotherapy may represent a more biological aggressive variant of esophageal cancer. Thus, future studies and treatment recommendations must attempt to distinguish these two distinct patient groups who may both be eligible to receive salvage esophagectomy, but may have a substantially different prognosis.
In conclusion, salvage esophagectomy following definitive chemoradiotherapy appears to have comparable outcomes to NCRS and planned esophagectomy in terms of short- and long-term mortality. However anastomotic leak is increased following salvage esophagectomy indicating the complexity of this procedure, and the need for this to be performed in carefully selected patients, within specialist esophageal cancer centers and by high volume experienced esophageal cancer surgeons.
Acknowledgements
Mr SR Markar is funded by the National Institute of Health Research (NIHR).
Disclaimer: The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Mungo B, Molena D, Stem M, et al. Does neoadjuvant therapy for esophageal cancer increase postoperative morbidity or mortality? Dis Esophagus 2015;28:644-51. 10.1111/dote.12251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.International Agency for Research on Cancer. Oesophageal cancer: estimated incidence, mortality and prevalence worldwide in 2012.
- 3.Allum WH, Stenning SP, Bancewicz J, et al. Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J Clin Oncol 2009;27:5062-7. 10.1200/JCO.2009.22.2083 [DOI] [PubMed] [Google Scholar]
- 4.Tepper J, Krasna MJ, Niedzwiecki D, et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol 2008;26:1086-92. 10.1200/JCO.2007.12.9593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sjoquist KM, Burmeister BH, Smithers BM, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol 2011;12:681-92. 10.1016/S1470-2045(11)70142-5 [DOI] [PubMed] [Google Scholar]
- 6.Van Hagen P, Hulshof M, Van Lanschot J, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. 10.1056/NEJMoa1112088 [DOI] [PubMed] [Google Scholar]
- 7.Teoh AY, Chiu PW, Yeung WK, et al. Long-term survival outcomes after definitive chemoradiation versus surgery in patients with resectable squamous carcinoma of the esophagus: results from a randomized controlled trial. Ann Oncol 2013;24:165-71. 10.1093/annonc/mds206 [DOI] [PubMed] [Google Scholar]
- 8.Bedenne L, Michel P, Bouché O, et al. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol 2007;25:1160-8. 10.1200/JCO.2005.04.7118 [DOI] [PubMed] [Google Scholar]
- 9.Takeuchi S, Ohtsu A, Doi T, et al. A retrospective study of definitive chemoradiotherapy for elderly patients with esophageal cancer. Am J Clin Oncol 2007;30:607-11. 10.1097/COC.0b013e3180ca7c84 [DOI] [PubMed] [Google Scholar]
- 10.Gardner-Thorpe J, Hardwick RH, Dwerryhouse SJ. salvage oesophagectomy after local failure of definitive chemoradiotherapy. Br J Surg 2007;94:1059-66. 10.1002/bjs.5865 [DOI] [PubMed] [Google Scholar]
- 11.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 1998;17:2815-34. [DOI] [PubMed] [Google Scholar]
- 12.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 13.Chao YK, Chan SC, Chang HK, et al. salvage surgery after failed chemoradiotherapy in squamous cell carcinoma of the esophagus. Eur J Surg Oncol 2009;35:289-94. 10.1016/j.ejso.2008.02.014 [DOI] [PubMed] [Google Scholar]
- 14.Farinella E, Safar A, Nasser HA, et al. salvage esophagectomy after failure of definitive radiochemotherapy for esophageal cancer. J Surg Oncol 2016;114:833-7. 10.1002/jso.24429 [DOI] [PubMed] [Google Scholar]
- 15.Markar S, Gronnier C, Duhamel A, et al. salvage surgery after chemoradiotherapy in the management of esophageal cancer: is it a viable therapeutic option? J Clin Oncol 2015;33:3866-73. 10.1200/JCO.2014.59.9092 [DOI] [PubMed] [Google Scholar]
- 16.Marks JL, Hofstetter W, Correa AM, et al. salvage esophagectomy after failed definitive chemoradiation for esophageal adenocarcinoma. Ann Thorac Surg 2012;94:1126-32; discussion 1132-3. 10.1016/j.athoracsur.2012.05.106 [DOI] [PubMed] [Google Scholar]
- 17.Miyata H, Yamasaki M, Takiguchi S, et al. salvage esophagectomy after definitive chemoradiotherapy for thoracic esophageal cancer. J Surg Oncol 2009;100:442-6. 10.1002/jso.21353 [DOI] [PubMed] [Google Scholar]
- 18.Morita M, Kumashiro R, Hisamatsu Y, et al. Clinical significance of salvage esophagectomy for remnant or recurrent cancer following definitive chemoradiotherapy. J Gastroenterol 2011;46:1284-91. 10.1007/s00535-011-0448-0 [DOI] [PubMed] [Google Scholar]
- 19.Nakamura T, Hayashi K, Ota M, et al. salvage esophagectomy after definitive chemotherapy and radiotherapy for advanced esophageal cancer. Am J Surg 2004;188:261-6. 10.1016/j.amjsurg.2004.06.003 [DOI] [PubMed] [Google Scholar]
- 20.Smithers BM, Cullinan M, Thomas JM, et al. Outcomes from salvage esophagectomy post definitive chemoradiotherapy compared with resection following preoperative neoadjuvant chemoradiotherapy. Dis Esophagus 2007;20:471-7. 10.1111/j.1442-2050.2007.00701.x [DOI] [PubMed] [Google Scholar]
- 21.Takeuchi H, Saikawa Y, Oyama T, et al. Factors influencing the long-term survival in patients with esophageal cancer who underwent esophagectomy after chemoradiotherapy. World J Surg 2010;34:277-84. 10.1007/s00268-009-0331-9 [DOI] [PubMed] [Google Scholar]
- 22.Tomimaru Y, Yano M, Takachi K, et al. Factors affecting the prognosis of patients with esophageal cancer undergoing salvage surgery after definitive chemoradiotherapy. J Surg Oncol 2006;93:422-8. 10.1002/jso.20475 [DOI] [PubMed] [Google Scholar]
- 23.Swisher SG, Wynn P, Putnam JB, et al. salvage esophagectomy for recurrent tumors after definitive chemotherapy and radiotherapy. J Thorac Cardiovasc Surg 2002;123:175-83. 10.1067/mtc.2002.119070 [DOI] [PubMed] [Google Scholar]
- 24.Oki E, Morita M, Kakeji Y, et al. salvage esophagectomy after definitive chemoradiotherapy for esophageal cancer. Dis Esophagus 2007;20:301-4. 10.1111/j.1442-2050.2007.00677.x [DOI] [PubMed] [Google Scholar]
- 25.Markar SR, Karthikesalingam A, Thrumurthy S, et al. Volume-outcome relationship in surgery for esophageal malignancy: systematic review and meta-analysis 2000-2011. J Gastrointest Surg 2012;16:1055-63. 10.1007/s11605-011-1731-3 [DOI] [PubMed] [Google Scholar]
- 26.Almoudaris AM, Mamidanna R, Bottle A, et al. Failure to rescue patients after reintervention in gastroesophageal cancer surgery in England. JAMA Surg 2013;148:272-6. 10.1001/jamasurg.2013.791 [DOI] [PubMed] [Google Scholar]