Abstract
The aim of the present study was to formulate a fermented whey product using probiotic bacteria and whey protein concentrate (WPC containing 70% of proteins) to obtain a fermented product with low lactose and β-lactoglobulin (BLG) content. Several factors such as starter culture concentration (1, 5, and 10%) and fermentation time (2, 4, 6, 8, and 10 h) were optimized on the basis of growth activity in terms of viable count, pH, and acidity. Starter culture concentration and fermentation time of 5% and 10 h, respectively, show the optimal viable count (9.8 × 1010) and pH (4.42) as per the requirement. Proteolysis of WPC by a mixed culture of Lactobacillus acidophilus and Lactobacillus bulgaricus was found to be 98.8 μg/ml. Hydrolysis of whey proteins by probiotic bacteria was detected by SDS-PAGE. A significant (p ≤ 0.05) decrease in cell viability and pH was observed as the refrigerated storage period goes on increasing (0–15 days).
Keywords: Whey, Prebiotic, Probiotic, β-lactoglobulin, Cell viability
Introduction
Whey, a byproduct of cheese industry typically disposed off as waste into the environment, causes serious environmental hazards due to its high Chemical Oxygen Demand (57–75 g L−1) and Biological Oxygen Demand (35–40 g L−1). Numerous efforts have been made over the past years in order to control the environmental pollution by utilizing the whey (Gonzalez-Martıinez et al. 2002). Different fractions of whey such as whey protein, whey protein concentrate, lactalbumin, and lactoglobulin are separated from the liquid whey using ultrafiltration and spray drying and used in pharma and food industries (Yee et al. 2007).
Whey protein consists of four major bioactive peptides, i.e., β-lactoglobulin, α-lactalbumin, bovine serum albumin, and immunoglobulin (Sousa et al. 2012). Among these four major bioactive peptides, β-lactoglobulin is the major whey protein consisting of around 58% of whey. It has a molecular weight of 18.4 kDa and contains 162 amino acid residues (Ye et al. 2000). This protein is the major cause of milk intolerance and also causes allergy. Allergenicity of this protein can be reduced by hydrolysis. Moreover, chemical hydrolysis can lead to the formation of toxic substrates like lyso-alanine (Sinha et al. 2007). Hydrolysis by using probiotic microorganisms is one of the best alternatives to reduce allergenicity.
Probiotic microorganisms mainly lactic acid bacteria (LAB) are more pronounced in food industry to improve food digestibility, texture, shelf life, and sensory profile of the final product (Wood 1997). Whey fermentation by using LAB improves the protein and lactose digestibility. It also contributes to the flavor and texture of the end product (Pescuma et al. 2010). Recently, probiotic-based foods are considered as the most up-surged area of research due to their health benefits. Regular consumption of probiotic microorganisms improves lactose digestion, regulate the bowel function, stimulate the immune system, and inhibit the pathogens (Ouwehand and Salminen 1998).
Prebiotics improves the growth of probiotic microorganisms. Oats are the major source of beta-glucan which is the functional component of cereal fibers and act as a prebiotic. Release of low-molecular weight fatty acids during beta-glucan fermentation in the colon release anticarcinogenic compound and prevents the risk of cancer. It also prevents the risk of cardiovascular disease by reducing the LDL-cholesterol (Angelov et al. 2006).
To the best of our knowledge, fermentation of whey with the addition of oats to prepare symbiotic fermented product has not yet been reported in literature. Therefore, in the present study attempts were made to develop a symbiotic product with added benefits of improved digestibility. The objectives of the present study were to (1) study growth profiling of probiotic microorganism, (2) investigate the effect of the addition of oats on consumer acceptability, and (3) perform proteolytic and hydrolytic assessment.
Materials and methods
Microorganisms and media
Lyophilized culture of probiotic strains (Lactobacillus acidophilus NCDC 291, Lactobacillus bulgaricus NCDC 304) used in this work was purchased from National Dairy research Institute, Karnal, Haryana, India. Whey protein concentrate was obtained from Mahaan Pvt. Ltd. Delhi, India. Oats used in the present study as a prebiotic was purchased from a local market of Delhi city. Procured oats was ground in a laboratory-scale hammer mill (W-series, Schutte-Buffalo Hammer mill, LLC, NY, USA) and passed through an 18-mesh (British Standard) sieve. Lactobacillus MRS medium procured from HiMedia which constituted 0.3 g l-cysteine (oxygen scavenging component to accelerate the growth of probiotic strains) per liter medium was used for revival of lyophilized culture of probiotics (Homayouni et al. 2008).
Whey protein concentrate (WPC) fermentation medium was prepared by adding 5% whey protein concentrate (WPC70, 70% w/w protein) to the distilled water and heat treating at 80 °C for 30 min in a waterbath (SBB Aqua12 plus, 15 liter capacity, Grant Instruments, Cambridge Ltd, UK). After heat treatment, pre-autoclaved 10% (v/v) glucose was aseptically added to the WPC fermentation medium. Sterility of fermentation medium was checked as suggested by Varga (2006) by spreading a small volume onto potato dextrose agar (PDA), nutrient agar (NA), and MRS agar for the growth of fungi/yeast, bacteria, and any indigenous microorganism (Lactobacillus), respectively. Strain compatibility was tested as suggested by Pescuma et al. (2010).
Growth profile of probiotic strains in MRS-cysteine medium
To examine the growth pattern of probiotic bacteria in MRS-cysteine medium, static fermentation was carried out at 37 °C. Seed culture was prepared by inoculating 1% lyophilized culture (0.5% Lactobacillus acidophilus NCDC 291 + 0.5% Lactobacillus bulgaricus NCDC 304) in MRS-cysteine medium and then incubated statically at 37 °C for 24 h. Primary seed culture (10%, v/v) was then transferred into secondary seed culture (MRS-cysteine broth) and cultivated for 24 h at 37 °C in static condition. Secondary seed culture (10%, v/v) was again transferred to MRS-cysteine broth and incubated at 37 °C for 24 h, which was designated as tertiary seed culture. After each 2 h of incubation, whole cell culture fluid (2 ml) was withdrawn from the tertiary culture to monitor the growth profile of probiotic bacteria. Optical density was measured at 600 nm (OD of 0.6 corresponds to a ~9.00 log CFU/ml according to McFarland scale).
Fermentation condition
Seed culture for WPC medium was prepared from the static fermentation of MRS-cysteine broth till tertiary seed culture for 10 h. After that, whole cell culture fluid was centrifuged (10,000 rpm for 10 min at 4 °C), washed twice with sterile saline solution (0.85% NaCl), and resuspended in WPC fermentation medium to its original volume. Cell count of culture was 5.9 × 1011 CFU/ml. In order to optimize the inoculum size, different concentrations of seed culture (1, 5, 10%) were inoculated in the WPC fermentation medium and fermentation was carried out at 37 °C for 10 h. Effect of the presence of prebiotics, i.e., oats (5%), was also checked on the growth of probiotics with different inoculum sizes (1, 5, and 10%) along with control which did not contain oats. Viable cells of probiotic bacteria were determined by serial dilution followed by pour plate method on MRS-cysteine agar plates.
Optimized inoculum size (5%) was used to observe the growth profile of probiotic bacteria. Seed cultures and inoculum were prepared as described earlier. Probiotic bacterial inoculum (5%, v/v) was used to inoculate WPC fermentation medium containing 5% (w/v) whey protein concentrate, 5% (w/v) oats, and 10% (v/v) glucose. Fermentation was performed statically in sealed bottles containing 70 ml of WPC fermentation medium in 100-ml schott bottle and incubated at 37 °C for 10 h. Samples were aseptically withdrawn after every 2 h during 10 h and monitored for cell viability and changes in pH and TSS. Cell viability was determined by plating appropriate dilutions of the cultures in MRS-cysteine agar plates by pour plate method.
Proteolysis and hydrolytic assessment
Proteolytic activity of probiotic bacteria in the fermented sample was determined using the o-phthaldialdehyde (OPA) test as suggested by Pescuma et al. (2010). Hydrolytic assessment was performed by SDS-PAGE as suggested by Schagger and Jagow (1987) with few modifications. Fermented and non-fermented WPC medium was first treated with SDS (10%) for 10 min at 90 °C and centrifuged at 10,000 rpm for 10 min. Supernatant was collected and 2 µl of each sample was prepared separately in denaturing 4× buffer and heated at 100 °C for 5 min before electrophoresis. Gel was run in a Tris–glycine buffer at a constant current of 25 mA. After electrophoresis, proteins were stained with Coomassie Brilliant Blue R-250.
Product development and sensory analysis
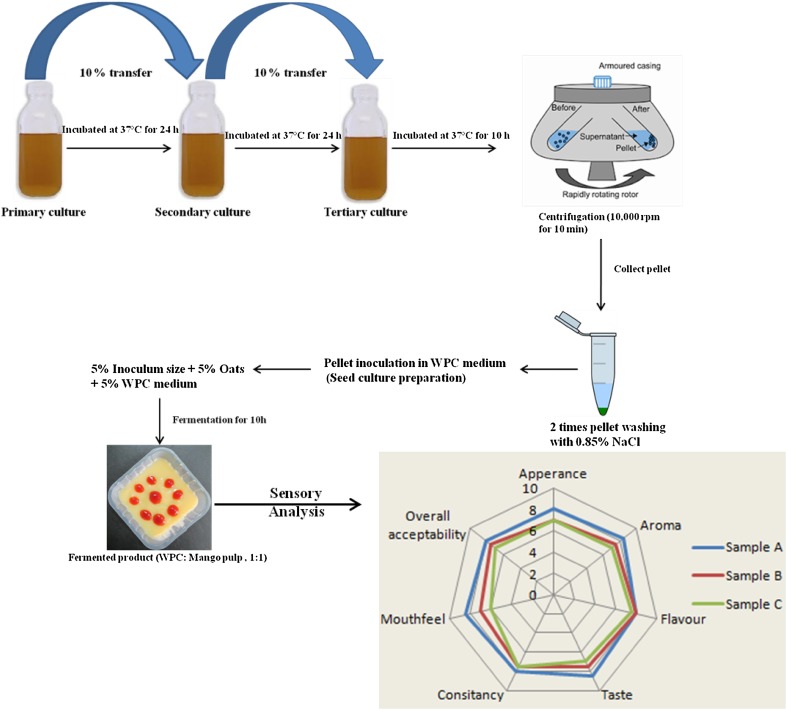
WPC medium was fermented for 10 h at 37 °C and after that fermentation was terminated by cooling down the sample to 4 °C. Fermented WPC was diluted with mango variety Ataulfo (Mangifera indica) pulp (1:1, 2:1, 3:1) previously prepared by grinding fresh mango pieces with the addition of 20% sugar followed by pasteurization at 80 °C for 30 min. Procedure for preparation of fermented whey-based product is shown in Fig. 1. The resulting product was poured into sterile glass bottles and stored at 4 °C.
Fig. 1.
Procedure for preparation of fermented whey-based product and sensory analysis (Samples A, B, and C are fermented whey containing oats and mango pulp in the ratios of 1:1, 2:1, and 3:1, respectively)
All samples of different blends (1:1, 2:1, 3:1) of fermented whey containing oats and mango pulp were prepared for sensory attributes such as appearance, aroma, taste, flavor, consistency, mouth feel, and overall acceptability. The samples were then evaluated by 20 semi-trained panelists (research students and staff members) from the Department of Food Technology and Nutrition. They were asked to score their preferences for all the mentioned sensory attributes of the samples using nine-point hedonic scales, where 9 = extremely like and 1 = extremely dislike. Each panelist evaluated all samples (identified by unique three-digit codes) in a balanced sequential order. Training sessions were held until panel members could identify the same sample that was coded differently in a session.
Chemical analysis
Changes in pH and TSS were determined by a digital pH meter (Thermo scientific, Orion 2 Star pH Benchtop) and a digital refractometer (Kruss optronic, Germany), respectively. Lactic acid production of the fermented sample was determined as suggested by Bulatovic et al. (2012).
Shelf life studies
Optimized fermented product prepared from (1:1) fermented whey containing oats and mango pulp was packed in schott glass bottles. These samples were stored under refrigerated conditions (4 °C) and assessed for microbial stability, pH, and TSS during the 15-day storage.
Statistical analysis
Analysis of variance test was carried out using commercial statistical package, SPSS ver. 11.5 (SPSS Inc., Chicago, IL, USA). All results of chemical analysis were recorded as mean ± SD of three replicates. Mean values were compared and significant differences were given using Duncan’s LSD test (p ≤ 0.05).
Results and discussion
Strain compatibility and growth profile of probiotic bacteria in MRS-cysteine medium
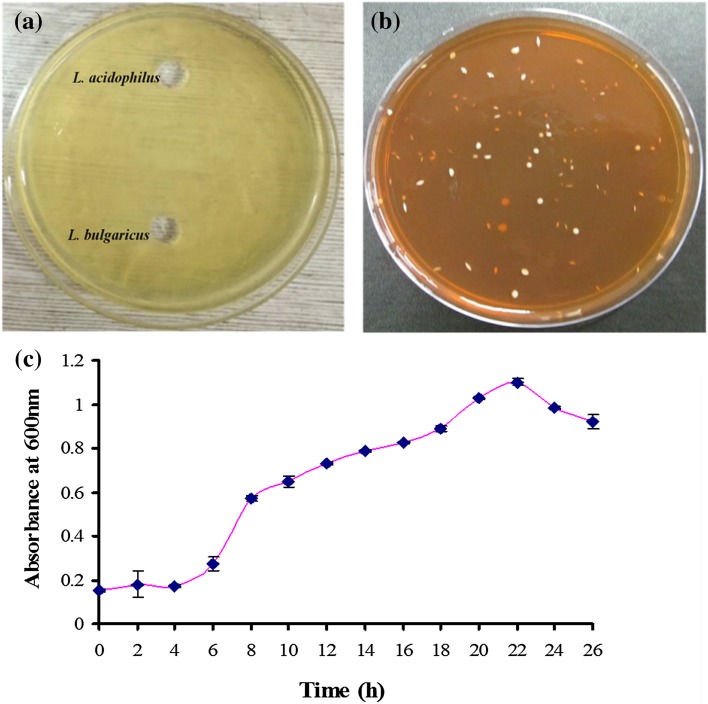
Strain compatibility was checked according to the method suggested by Pescuma et al. (2010). Both the strains were found to be compatible to each other (Fig. 2). Therefore, the mixed culture of Lactobacillus acidophilus NCDC 291 and Lactobacillus bulgaricus NCDC 304 was used for further studies. Growth profile of probiotic bacteria clearly indicates that the exponential phase triggered from the 6th h of incubation and remained till the 22nd h. Afterwards, the growth of probiotic bacteria entered the decline phase (Fig. 2). It was also observed that the absorbance (0.252–0.562) has been doubled from 6 to 8 h. In accordance with the McFarland scale, at 10-h interval absorbance (0.628) was observed to be indicating the cell density of around 9.00 log CFU/ml. Therefore, 10 h fermentation time has been considered as the optimum fermentation time and was continued for further studies.
Fig. 2.
a Strain compatibility, b growth of probiotic bacterial colonies on MRS-cysteine agar plate, and c growth curve of probiotic culture in MRS-cysteine medium
Effect of different inoculum sizes on fermentation medium
WPC medium with and without oats was inoculated with the starter culture suspension of Lactobacillus bulgaricus NCDC 304 and Lactobacillus acidophilus NCDC 291 (1, 5, and 10%) aiming to obtain the required levels of viable cells as per the probiotic requirement (106–107 CFU/ml) (Mattila-Sandholm et al. 1999). In the present study, 10-h fermentation was considered in order to minimize the risk of contamination and to obtain the desired properties of probiotic product. The higher microbial count in the medium containing oats was attributed to the function of oats as prebiotics. Therefore, WPC medium containing oats was considered for further studies. Desirable properties as per the probiotic product such as viable count and pH (4.0–4.5) were obtained with 5% inoculum size (Table 1), whereas in 1 and 10% inoculum sizes the viable count and pH were not as per the desired ratio required for probiotic product (Ying et al. 2013).
Table 1.
Effect of different inoculum sizes and prebiotics (oats) on fermentation medium
| Inoculum size (%) | Sample A | Sample B | ||
|---|---|---|---|---|
| (CFU/ml) | pH | (CFU/ml) | pH | |
| 1 | 5.6 × 104 | 6.42 | 2.4 × 106 | 5.96 |
| 5 | 7.9 × 107 | 5.06 | 9.8 × 1010 | 4.42 |
| 10 | 8.2 × 108 | 4.23 | 2.1 × 1011 | 3.85 |
Sample A: fermented whey-based product without oats; Sample B: fermented whey-based product containing oats
Growth profile of probiotic bacteria in WPC fermentation medium with oats
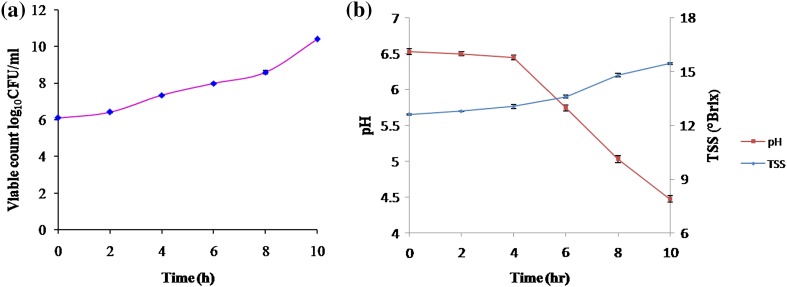
Growth profile of probiotic bacteria was checked for every 2-h interval and it was inferred that probiotic bacterial population progressively increased with up to 10 h of incubation (2.5 × 1010 CFU/ml) (Fig. 3). pH of the fermentation medium decreased from 6.5 to 4.3 which confirms the active growth of probiotic bacteria and lactic acid production (Fig. 3). These results are consistent with those of an earlier study reported by Angelov et al. (2006) for the fermentation of oat-based mash using probiotic microorganism (Lactobacillus plantarum B28). Total soluble solid increased from 12.6 to 15.5 (°Brix) due to gelation of whey protein with the fermentation period (Fig. 3). Fermentation was terminated after 10 h of incubation because probiotic microorganisms are sensitive to pH. At an acidic pH (less than 4), probiotic bacterial viable count decreases due to the acidification of cytoplasm and increase in energy consumption for the maintenance of intracellular pH (Shabala et al. 2006). Loss of probiotic bacterial viability also reduced the functionality of product as a sufficient number of viable bacteria (>7 log10CFU/g of product) are required to confer health benefits (Ying et al. 2013).
Fig. 3.
a Growth profile of probiotic bacteria in WPC fermentation medium containing oats. b Effect of change in pH and TSS during fermentation
Lactic acid production in WPC medium
Fermentation activity of probiotic cultures was evaluated by determining the volumetric productivity of lactic acid production. After 10-h fermentation, the volumetric productivity of Lactobacillus bulgaricus NCDC 304 and Lactobacillus acidophilus NCDC 291 culture was found to be 0.675 (g l−1 h−1).
Chemical analysis of mango-flavored fermented product
WPC medium was fermented for 10 h and then fermentation was terminated by cooling down the sample to 4 °C, mixed with mango pulp in the ratio of 1:1, and analyzed for total soluble solids, pH, and titratable acidity (Table 2). pH of the fermented sample was lesser than the non-fermented sample due to the production of lactic acid.
Table 2.
Chemical analysis of fermented and non-fermented products
| Sample 1 | Sample 2 | Sample 3 | |
|---|---|---|---|
| 0-day storage | |||
| pH | 6.42 ± 0.05a | 4.31 ± 0.02b | 4.13 ± 0.03c |
| Total soluble solids (TSS) | 12.56 ± 0.05c | 15.33 ± 0.15b | 19.33 ± 0.35a |
| Titratable acidity (°SH) | 4.03 ± 0.06c | 30.23 ± 0.02b | 31.76 ± 0.15a |
| Viable count | – | 9.1 × 1010 | 8.5 × 1010 |
| 7-day storage | |||
| pH | 6.40 ± 0.08a | 4.08 ± 0.03b | 4.10 ± 0.03b |
| Total soluble solids (TSS) | 12.60 ± 0.0c | 15.50 ± 0.10b | 19.46 ± 0.15b |
| Titratable acidity (°SH) | 4.02 ± 0.01b | 31.56 ± 0.20a | 31.80 ± 0.26a |
| Viable count | – | 9.5 × 1010 | 8.9 × 1010 |
| 15-day storage | |||
| pH | 6.40 ± 0.08b | 3.99 ± 0.02b | 4.03 ± 0.02a |
| Total soluble solids (TSS) | 12.70 ± 0.08c | 15.73 ± 0.15b | 19.73 ± 0.20a |
| Titratable acidity (°SH) | 4.02 ± 0.0b | 31.86 ± 0.15a | 32.16 ± 0.55a |
| Viable count | – | 8.8 × 109 | 9.5 × 108 |
Mean values with different superscripts in the same row differ significantly (Duncan’s LSD test, P < 0.05). Sample 1: non-fermented whey-based product containing oats; Sample 2: fermented whey-based product containing oats; Sample 3: fermented whey-based product containing oats and mango pulp
Proteolytic and hydrolytic assessment
Proteolytic activity by mixed culture of probiotic strains were started from 67.1 µg/ml leucine and reached at 98.8 µg/ml leucine at 10-h fermentation. Low proteolytic activity during the first 8 h of fermentation can be related with the consumption of free amino acids by microorganisms (Pescuma et al. 2010). Therefore, a small increment was observed in the production of free amino acids as fermentation was carried out only for 10 h. Increase in leucine concentration (determined by OPA test) with the fermentation time shows the application of whey-based fermented product in sports nutrition as leucine is a branched chain essential amino acid (Pasin and Miller 2000).
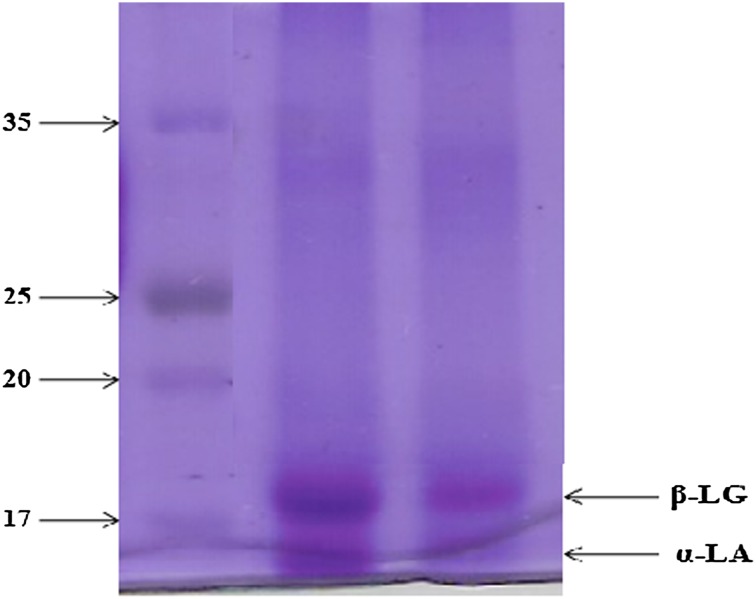
Hydrolysis of whey protein was analyzed by SDS-PAGE. Figure 4 shows the effect of probiotic bacteria on whey protein pattern in fermented media in comparison to non-fermented media. It can be clearly seen from Fig. 4 that the concentration of α-lactalbumin (α-LA) and β-lactoglobulin (β-LG) decreased in WPC fermentation medium as compared with the non-fermented medium. This shows the proteolytic potential of probiotic bacteria which may also play an important role in lowering the antigenicity of α-LA and β-LG.
Fig. 4.
Hydrolysis of whey proteins (lane 1 pre-stained protein molecular weight marker, lane 2 non-fermented whey sample, lane 3 fermented whey sample)
Sensory evaluation
Sensory evaluations of samples of different blends (1:1, 2:1, 3:1) of fermented whey containing oats and mango pulp are depicted in Fig. 1. According to a consensus made with the panelists during sensory evaluation, it was determined that the main descriptors that characterized the product were acidity, sweetness, and pungent flavor due to fermentation of oats. Outcome of sensory evaluation revealed that pungent flavor increased with an increase in concentration of the fermented whey-based product containing oats, resulting in a decrease in acceptability scores. Blending of fermented WPC medium containing oats with mango pulp (1:1) improved the product appearance without affecting cell viability. According to Thamer and Penna (2005), acid production defines the quality characteristics and shelf life of the product; a probiotic fermented food should reach up to pH 4.6, which is close to that obtained in this study, to ensure light flavor and avoid adverse effects of low pH on probiotic bacteria. Increase in acidity with fermentation is due to conversion of lactose into lactic acid.
Storage study
During the 15 days of refrigerated storage, viable count of food product was decreased (8.5 × 1010 to 9.5 × 108 CFU/ml). These results are in accordance with Mendoza et al. (2007) who also reported a decline in the total viable count of Lactobacillus reuteri and Bifidobacterium bifidum of whey-based probiotic beverage stored at 4 ± 1 °C. Significant decrease in pH (Table 2) was also observed for fermented samples as the storage period goes on increasing (0 to 15 days) due to the production of lactic acid by probiotic microorganisms. A similar significant (p ≤ 0.05) increase in TSS was observed for fermented samples as the storage period goes on increasing (0 to 15 days). The results of this study are consistent with Mashayekh et al. (2015) where the number of viable cells of probiotic bacteria in pineapple, apple, and mangifera juice mixture was reduced due to acidic condition of beverage.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest regarding the publication of this paper.
References
- Angelov A, Gotcheva V, Kuncheva R, Hristozova T. Development of a new oat-based probiotic drink. Int J Food Microbiol. 2006;112:75–80. doi: 10.1016/j.ijfoodmicro.2006.05.015. [DOI] [PubMed] [Google Scholar]
- Bulatovic ML, Rakin MB, Mojovic LV, Nikolic SB, Vukasinovic Sekulic MS, Dukic Vukovic AP. Selection of Lactobacillus Strains for functional whey-based beverage production. J Food Sci Eng. 2012;2:705–711. [Google Scholar]
- Gonzalez-Martıinez C, Becerra M, Chafer M, Albors A, Carot JM, Chiralt A. Influence of substituting milk powder for whey powder on yoghurt quality. Trends Food Sci Tech. 2002;13:334–340. doi: 10.1016/S0924-2244(02)00160-7. [DOI] [Google Scholar]
- Homayouni A, Azizi A, Ehsani MR, Yarmand MS, Razavi SH. Effect of microencapsulation and resistant starch on the probiotic survival and sensory properties of symbiotic icecream. Food Chem. 2008;111:50–55. doi: 10.1016/j.foodchem.2008.03.036. [DOI] [Google Scholar]
- Mashayekh S, Hashemiravan M, Dokht MF. Study on production possibility of probiotic fermented beverage based on mixture of pineapple, apple and mango juices. J Chem Pharm Res. 2015;7:1132–1137. [Google Scholar]
- Mattila-Sandholm T, Matto J, Saarela M. Lactic acid bacteria with health claims—interactions and interference with gastrointestinal flora. Int Dairy J. 1999;9:25–35. doi: 10.1016/S0958-6946(99)00041-2. [DOI] [Google Scholar]
- Mendoza AH, Robles VJ, Angulo JO, Cruz JDL, Garcia HS. Preparation of a whey based probiotic product with Lactobacillus reuteri and Bifidobacterium bifidum. Food Technol Biotech. 2007;45:27–31. [Google Scholar]
- Ouwehand AC, Salminen SJ. The health effects of cultured milk products with viable and non-viable bacteria. Int Dairy J. 1998;8:749–758. doi: 10.1016/S0958-6946(98)00114-9. [DOI] [Google Scholar]
- Pasin G, Miller SL (2000) US Whey proteins and sport nutrition. Application monographs of the US dairy export council
- Pescuma M, Hebert EM, Mozzi F, Valdez GFD. Functional fermented whey-based beverage using lactic acid bacteria. Int J Food Microbiol. 2010;141:73–81. doi: 10.1016/j.ijfoodmicro.2010.04.011. [DOI] [PubMed] [Google Scholar]
- Schagger H, Jagow GV. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Shabala L, Mcmeekin T, Budde B, Siegumfeldt H. Listeria innocua and Lactobacillus delbrueckii subsp. bulgaricus employ different strategies to cope with acid stress. Int J Food Microbiol. 2006;110:1–7. doi: 10.1016/j.ijfoodmicro.2006.01.026. [DOI] [PubMed] [Google Scholar]
- Sinha R, Radha C, Prakash J, Kaul P. Whey protein hydrolysate: functional properties, nutritional quality and utilization in beverage formulation. Food Chem. 2007;101:1484–1491. doi: 10.1016/j.foodchem.2006.04.021. [DOI] [Google Scholar]
- Sousa GT, Lira FS, Rosa JC, De Oliveira EP, Oyama LM, Santos RV, Pimentel GD. Dietary whey protein lessens several risk factors for metabolic diseases: a review. Lipids Health Dis. 2012;11:67. doi: 10.1186/1476-511X-11-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thamer KG, Penna ALB. Efeito do teor de soro, açu´ car e de frutooligossacarı´deos sobre a populaça˜o de bacte´ rias la´ticas probio´ticas em bebidas fermentadas. Revista Brasileira de Cieˆncias Farmaceˆuticas. 2005;41:393–400. [Google Scholar]
- Varga L. Effect of acacia (Robinia pseudoacacia L.) honey on the characteristic microflora of yogurt during refrigerated storage. Int J Food Microbiol. 2006;108:272–275. doi: 10.1016/j.ijfoodmicro.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Wood BJB. Microbiology of Fermented Foods. London: Blackie Academic & Professional; 1997. [Google Scholar]
- Ye X, Yoshida S, Ng T. Isolation of lactoperoxidase, lactoferrin, α-lactalbumin, β-lactoglobulin B and β-lactoglobulin A from bovine rennet whey using ion exchange chromatography. Int J Biochem Cell B. 2000;32:1143–1150. doi: 10.1016/S1357-2725(00)00063-7. [DOI] [PubMed] [Google Scholar]
- Yee KWK, Wiley DE, Bao J. Whey protein concentrate production by continuous ultrafiltration: operability under constant operating conditions. J Membrane Sci. 2007;290:125–137. doi: 10.1016/j.memsci.2006.12.026. [DOI] [Google Scholar]
- Ying D, Schwander S, Weerakkody R, Sanguansri L, Gantenbein-Demarchi C, Augustin MA. Microencapsulated Lactobacillus rhamnosus GG in whey protein and resistant starch matrices: probiotic survival in fruit juice. J Funct Foods. 2013;5:98–105. doi: 10.1016/j.jff.2012.08.009. [DOI] [Google Scholar]