Abstract
Neurodegenerative molecules play an important role in maintaining a supply for synaptic vesicles; and they are also likely to help regulate the dopamine release which is the primary mechanism of action in pharmacological treatments for attention deficit hyperactivity disorder (ADHD). It is suggested that there could be interactions between α-synuclein and tau in cytoskeletal disorganization and synaptic dystrophy. Therefore, we aim to determine the serum levels of neurodegenerative molecules such as α-synuclein and tau in children with ADHD. The study group consisted of 25 children, aged 6–10, diagnosed with ADHD according to DSM-IV criteria and who appeared at Dicle University, Faculty of Medicine, and Department of Child Psychiatry in Diyarbakır, Turkey. 25 children, having no psychiatric disorders and medical illnesses, were selected as healthy control group. Serum α-synuclein and tau concentrations were determined by Enzyme-Linked Immuno Sorbent Assay. The α-synuclein levels of ADHD were not significantly different than those of controls. The tau levels of ADHD were found to be statistically significantly higher than those of controls. Moreover, α-synuclein levels showed a statistically significantly positive correlation with tau levels in children with ADHD. The results of our preliminary study can suggest that ADHD might possibly share a common disease mechanism with other diseases in terms of tau pathology. Increased serum tau level may be an indication of disturbance of microtubule transportation in the brains of children with ADHD.
Keywords: ADHD, Neurodegeneration, α-Synuclein, Tau protein, Biomarker
Introduction
Attention deficit hyperactivity disorder (ADHD) is a heterogeneous, developmental disorder characterised by lack of attention and hyperactivity. Available theories on pathobiological etiology of ADHD converge around the centrality of dopamine (DA) regulation in the frontosubcortical system, which is the primary mechanism of action in pharmacological treatments for ADHD [1].
Dopamine can affect the accumulation of α-synuclein, a protein mechanistically and genetically involved in the pathogenesis of dopaminergic neuronal death [2]. On the other hand, α-synuclein plays an important role in maintaining a supply for synaptic vesicles in presynaptic terminals; and it may also help to regulate the dopamine release [3]. Other primary function of α-synuclein in dopaminergic neurons may be the regulation of dopamine content and synaptic tone at the synapse. α-synuclein is mainly localized in the presynaptic terminals and regulates the synaptic vesicles in mature neurons [4]. It is suggested there may be interactions between α-synuclein and tau in cytoskeletal disorganization and synaptic dystrophy [5]. Tau is a microtubule-associated protein involved in the normal functioning and viability of neurons by maintaining their proper morphologies and axonal transport. Moreover, it is shown in a study that the function of dopaminergic neurons affected by gene transfer of tau appears to be more sensitive rather than α-synuclein-related damage in this model [6].
It is proposed that the biochemical abnormalities and neurodegeneration process may play significant roles in the etiology of ADHD including dopamine and serotonin neurotransmission pathways [7]. There has been increased attention to ADHD as a heritable neuropsychiatric condition linked to pathogenesis of brain dopamine [8]. Both α-synuclein and tau are important for substantia nigra neurodegeneration models in rats, further indicating their potential as therapeutic targets for human diseases involving loss of dopamine neurons [6].
As far as known, some researchers have suggested that neurobehavioural deficits and pathophysiology of ADHD are associated with microstructural abnormalities of brain white matter, apoptotic neurodegeneration, cytokine-related neurotrophin reflecting glial integrity (S100B), neurotrophic growth factor systems including brain-derived neurotrophic factor, and microglial activation [7, 9–15]. It has also been recommended that markers of glial function and neurodegeneration should be examined in patients with ADHD [7, 12]. However, so far, α-synuclein and tau levels, being associated with neurodegeneration, have not been studied in patients with ADHD.
Considering these aspects, we hypothesized that the α-synuclein and tau levels may have some affects on ADHD, and α-synuclein and tau may be used to comprehend the pathological mechanisms of ADHD. Therefore, we aimed to determine the serum levels of α-synuclein and tau, which have not been investigated previously in children with ADHD although these two markers have been detected in many diseases being associated with neurodegeneration.
Materials and Methods
Subjects and Study Design
This study was carried out in Child Psychiatry Department of Dicle University Training and Research Hospital. The population, taking part in the study, was comprised of 25 children, who were between the ages of 6 and 10 and diagnosed with ADHD prior to treatment. The diagnosis of ADHD was performed in line with the 4th edition criteria of Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) [16].
25 children, having no psychiatric disorders and medical illnesses, were selected as healthy control group. Written consent was obtained from parents of children so that they could participate in voluntary work. ADHD diagnosed children, who had comorbid with oppositional defiant disorders, were also included in the study. Children, who had mental retardation, autism spectrum disorders and tic disorders, history of head trauma, psychotropics, previous or current cortisol therapy, chronic systemic disorders, and clinically active infection, were excluded from the study in order to prevent interference with biochemical parameters. Two experienced psychiatry doctors evaluated the patients. Inter-rater agreement was 0.80. The study protocol was approved by the Non-invasive Clinical Research Ethics Committee of Dicle University (NCREC No: 27.02.2015/167).
Kiddie Schedule for Affective Disorders and Schizophrenia, Present and Lifetime Version (K-SADS-PL)
K-SADS-PL was administered by interviewing the parents of the children and finally summary ratings, which included all sources of information, were achieved. This test was adapted by Gökler et al. [17] for Turkish children and adolescents. K-SADS-PL is a semi-structured diagnostic interview designed to assess current and past episodes of psychopathology in children and adolescents according to DSM-IIIR and DSM-IV criteria [18].
Child and Adolescent Behavior Disorders Screening and Rating Scale
Then, Turgay DSM-IV Based Child and Adolescent Behavior Disorders Screening and Rating Scale (T-DSM-IV-S) was individually administered. This scale, which is an individually administered test including 9 items (questioning lack of inattention), 6 items (questioning hyperactivity), 3 items (questioning impulsivity), 8 items (questioning oppositional defiant disorder), and 15 items (questioning behavior disorders), consisting of 41 items, according to DSM-IV criteria was developed by Turgay [19].
Stroop Test
Then the Stroop test, which is a neuropsychological test reflecting the activity of prefrontal area of the brain, was administered. In the Stroop Test, both processing speed and resistance to interference are measured by requiring the participant to give his/her attention selectively to the task-relevant stimulus and respond to this stimulus and inhibit a competing automatic response [20]. The Stroop test involves the use of four white cards with the dimensions of 14.0 × 21.5 cm. Each card contains an array of six lines comprised of four items. These cards are the “stimuli” of the test; and the reactions, which the participants must give to these stimuli, or the tasks, which the participants must perform, constitute the sections of the test. The scores in each experiment are calculated by rating each section separately. The test evaluates the ability of parallel distributed processing for recognized and unrecognized stimuli, processing speed, and the ability to resist against interference effects of automatic processes [20]. The validity and reliability of Turkish version of this scale were evaluated by Karakaş et al. [21, 22]. Considering the fact that completing the tasks on the card would be affected by variables such as perceptional problems and psychomotor speed, interference effect score was calculated by subtracting the time to recognize colours from the time to complete the interference effect card. The timing, number of mistakes and the corrections were assessed. Children who had pervasive developmental disorder intelligence scores of less than about 70 were excluded from the study [21].
Sample Preparation and Measurement
Laboratory measurements were accomplished in microbiology laboratory of Dicle University Medicine Faculty. Blood samples were drawn by the venipuncture technique into three different tubes without anticoagulant. This sample collection was conducted for both the control and patient group after an overnight (≥12 h) fast. Blood specimens were allowed to clot for 30 min. They were centrifuged at 4000 rpm for 10 min as usual. Thus, blood cells and all large particles in blood samples were precipitated. Yellow and clear serum samples were selected for the study. We removed both hemolyzed and lipemic blood samples. The aliquots of serum samples were kept at −70 °C for measurement of α-synuclein and tau concentrations.
Measurement of Alfa-Synuclein and Tau Protein
Serum α-synuclein and tau concentration were determined by Enzyme-Linked ImmunoSorbent Assay (ELISA) test. These kits (α-synuclein: YEHUA Biological Technology-Catalog Number: YHB0151Hu and Tau: YEHUA Biological Technology-Catalog Number: YHB3036Hu) use ELISA based on biotin double antibody sandwich technology to assay Human α-synuclein and tau levels in serum, blood plasma, saline, urine and other related tissue liquids.
Procedures were performed as follows; 50 μl standards were added to the standard solution wells, 40 μl serum samples and 10 μl α-synuclein or tau proantibodies were added to the sample wells. Then 50 μl streptavidin-HRP was added to each well except empty well, and the plate was covered with seal plate membrane. Plate was shaked gently to mix; and it incubated at 37 °C for 60 min away from light. The plate was washed carefully five times then blotted. 50 μl chromogen reagent A was added to each well, then 50 μl chromogen reagent B was added to each well; and plate was incubated for 10 min at 37 °C away from light for color development. Finally, 50 μl stop solution was added to each well. We measured the optical density (OD) of each well under 450 nm wavelength within 10 min after having added stop solution. According to standard concentrations and corresponding OD values, we calculated the linear regression equation of the standard curve and we determined α-synuclein and tau concentrations of samples.
Statistical Analysis
Statistical analyses were performed using statistics programs with IBM SPSS Statistics 22.0. The normality of the data was assessed by the Shapiro–Wilk normality test and Q–Q graphs. Data were expressed as numbers for categorical variables and mean ± SD or median (25th–75th percentile) for continuous variables. Age comparison between groups was performed using Mann–Whitney U test. Gender comparison was made with the exact method of the Chi-square test. In terms of α-synuclein and tau, comparisons between-groups were performed with independent sample t test. P < 0.05 was considered to be statistically significant.
Results
Our study consisted of 25 children with ADHD (14 female and 11 male) and 25 controls (12 female and 13 male). The mean ages of the controls were 9.5 ± 2.51 and children with ADHD were 8.89 ± 2.54 (p = 0.373). The mean interference effect score of the Stroop Test was 24.7 ± 10.6. The mean attention score of children with ADHD according to parents was 17.9 ± 4.6 points and according to teachers, it was 16.1 ± 5.2 points. The mean hyperactivity–impulsivity score of children with ADHD according to parents was 12.9 ± 8.8 points and according to teachers, it was 13.4 ± 5.8 points. The mean opposition defiance score of children with ADHD according to parents was 9.7 ± 7.6 points and according to teachers, it was 10.2 ± 7.2 points (Table 1).
Table 1.
The basic characteristics of T-DSM-IV-S and the Stroop Test scores of children with ADHD
| Parameters | ADHD group (n = 25) |
|---|---|
| Interference effect | 24.7 ± 10.6 |
| According to parents (mean ± SD) |
According to teachers (mean ± SD) |
|
|---|---|---|
| Attention | 17.9 ± 4.6 | 16.1 ± 5.2 |
| Hyperactivity–impulsivity | 12.9 ± 8.8 | 13.4 ± 5.8 |
| Oppositional defiant | 9.7 ± 7.6 | 10.2 ± 7.2 |
T-DSM-IV-S: Turgay DSM-IV based child and adolescent behaviour disorders screening and rating scale. Data are expressed as mean ± SD for continuous variables
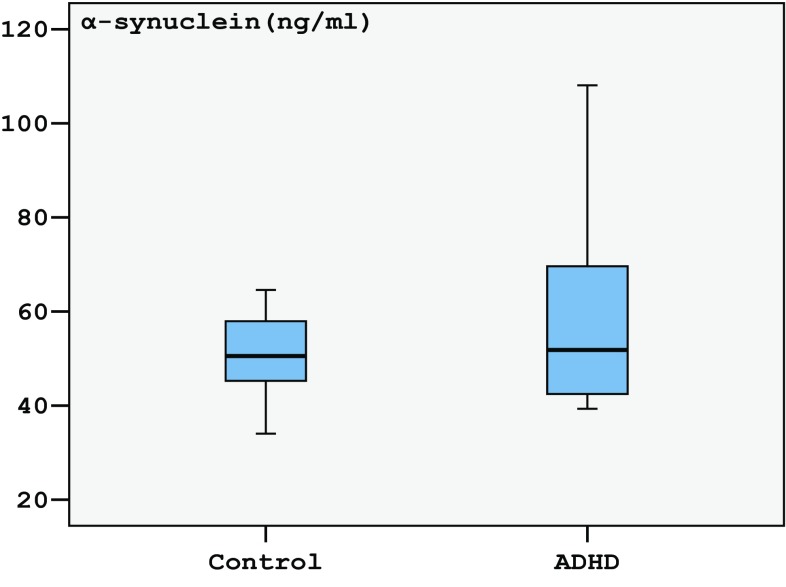
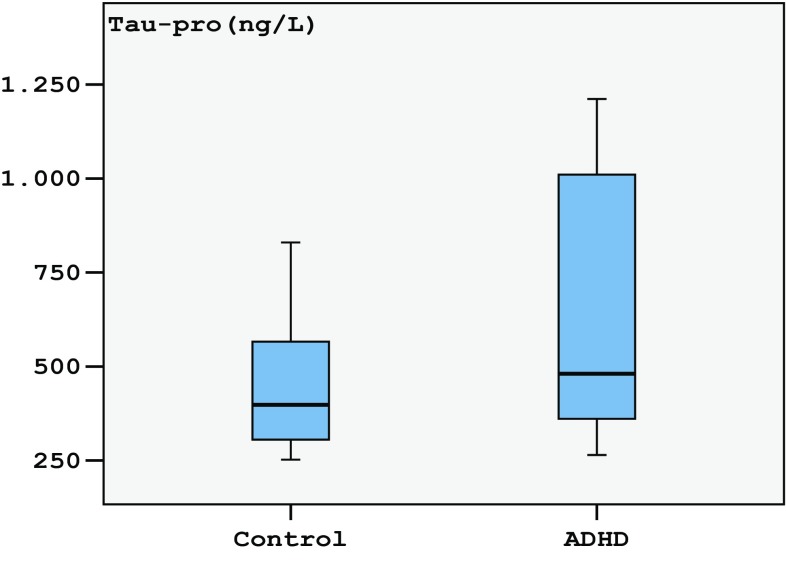
The α-synuclein levels median = 51.41 (25th–75th = 42.45–69.61 ng/ml) of ADHD was not significantly different (p = 0.063) from the median = 50.52 (25th–75th = 44.66–58.67 ng/ml) of controls (Fig. 1; Table 2). The tau levels in children with ADHD median = 470.0 (25th–75th = 360.6–1010.5 ng/l) were found to be statistically significantly higher (p = 0.002) than those of controls’ median = 394.5 (25th–75th = 304.7–566.0 ng/l) (Fig. 2; Table 2).
Fig. 1.
Tukey box plots illustrating the results of the categorical comparison between control and ADHD groups in terms of α-synuclein concentration (p = 0.058)
Table 2.
Main characteristics of α-synuclein and tau protein levels in study groups
| Parameters | Control group (n = 25) |
ADHD group (n = 25) |
p |
|---|---|---|---|
| Gender (M/F) | 13/12 | 11/14 | 0.779 |
| Age (years) | 9.5 ± 2.51 | 8.89 ± 2.54 | 0.373 |
| α-synuclein (ng/ml) | 51.41 (42.45–69.61) | 50.52 (44.66–58.67) | 0.058 |
| Tau protein (ng/l) | 470.0 (360.6–1010.5) | 394.5 (304.7–566.0) | 0.003 |
| α-synuclein (min/max) | (34.0/103.5) | (35.7/108.0) | – |
| Tau protein (min/max) | (251.7/829.9) | (224.7/1211.6) | – |
Data are expressed as numbers for categorical variables and mean ± SD or median (25th–75th percentile) for continuous variables
M male, F female
Fig. 2.
Tukey box plots illustrating the results of the categorical comparison between control and ADHD groups in terms of tau protein concentration (p = 0.003)
On the other hand, the serum distribution of α-synuclein levels in healthy children and children with ADHD was min = 34.0, max = 103.5 and min = 35.7, max = 108.0 ng/ml, respectively. The serum distribution of tau levels in healthy children and children with ADHD was min = 251.7, max = 829.9 and min = 224.7, max = 1211.6 ng/l, respectively (Table 2).
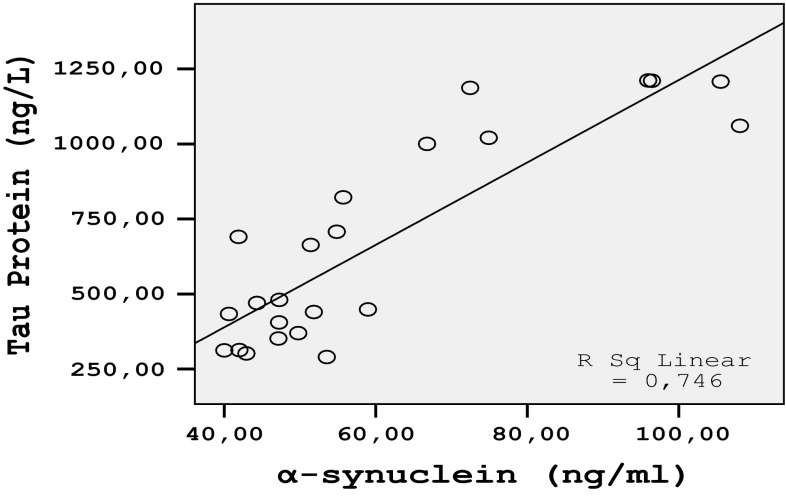
On the other hand, when correlation analysis was administered, α-synuclein levels showed a statistically significantly (r = 0.777; p < 0.01) positive correlation with tau levels (Fig. 3) in children with ADHD, while such a relationship was not found in control group. α-synuclein and tau concentrations did not show any statistically significant correlations in clinical parameters such as stroop test with study groups.
Fig. 3.
Correlation between α-synuclein and tau protein in children with ADHD (r = 0.777; p < 0.01)
Discussion
This is the first report on α-synuclein and tau-protein in serum levels of children with ADHD. In this study, it was found that the level of tau-protein was significantly different in ADHD group when compared to the control group. Moreover, it was specified that tau levels showed a correlation with α-synuclein in children with ADHD.
When the literature was examined, there found a very limited number of studies on diseases caused by tau-protein. However, to the best of our knowledge, till now, tau proteins, which are associated with neurodegeneration, have not been studied in the patients with ADHD. We have not encountered any studies having examined the children with ADHD in terms of α-synuclein and tau-protein when we wished to compare the findings of present study in terms of synucleinopathies and tauopathies. Nevertheless, the toxic effects and mechanisms of Pb on learning and memory was investigated by Zhang et al. [23]. The results of their study showed that Pb exposure can cause excessive accumulation of α-synuclein and hyperphosphorylation of tau. They expressed that pathological hyperphosphorylation and aggregation of tau protein can cause diseases related to nervous system [23]. They also suggested that the increase of α-synuclein is one of the reasons leading to damage on ability of learning and memory as in Pb exposure in rats [23]. Kadak et al. [24] found that serum α-synuclein and tau levels were significantly lower in children with autism spectrum disorder when compared with normal cases. They also showed that the mean serum tau levels were significantly lower in children with autism spectrum disorder as compared with normal cases (mean ± SD:241.23 ± 290.5 and 509.78 ± 269.25 ng/mL, respectively). They suggested that α-synuclein and tau aggregation may lead to synaptic dysfunction; and this may contribute to either neuronal or synaptic dysfunction or neurodegeneration in autism spectrum disorder [24]. Otto et al. [25] found that tau-protein levels were between 233 and 1769 pg/ml (mean ± SD:702 ± 432; median 558 pg/ml) in other dementing disease groups. They also showed that tau-protein levels between 109 and 640 pg/ml (mean ± SD:304 ± 119; median 296 pg/ml) in non-demented controls.
In this study, tau-protein levels were between 251.7 and 829.9 ng/l (mean ± SD:415 ± 128; median 394.5 ng/l) in healthy children. On the other hand, the children with ADHD had a range of tau-protein levels between 224.7 and 1211.6 ng/l (mean ± SD:598 ± 346; median 470.0 ng/l).
Consistent with previous studies, findings obtained in our study have allowed us to determine the serum levels of α-synuclein and tau in ADHD and healthy control groups. We did not find any differences between healthy children and children with ADHD in terms of α-synuclein. This result was not compatible with the results of study conducted by Kadak et al. [24]. However, the mean values of tau serum levels of our study appear to be different from previous studies. Moreover, serum levels of tau in our study seem to be different from previous studies not only in patients but also in the control group. Several factors, such as differences in mean age, measurement techniques and disease groups, testing materials (serum or plasma), exposure to medication or different ethnic origins may be responsible for the discrepancy.
While α-synuclein and tau proteins are typically regarded as intracellular proteins, they have, however, been discovered to be normally present in extracellular biological fluids, including human cerebrospinal fluid (CSF) and blood plasma [26–30]. CSF is in direct contact with the brain interstitial fluid and, thus, probably provides a more accurate evaluation than peripheral blood of α-synuclein and tau metabolism. Nevertheless, the continuous production of CSF necessiates it exit the subarachnoid space surrounding the brain; and probably, as CSF is drained through the subarachnoid granulations into the venous circulation, products released from the brain into the CSF could be transferred to blood when CSF enters the venous circulation [31]. α-synuclein with higher levels than normal has been detected in plasma in Parkinson’s disease (PD) and multiple-system-atrophy patients [5, 26]. Elevated content of oligomeric α-synuclein and tau particles is present in plasma in Alzheimer’s disease or PD patients, compared to controls, showing that changes in the levels and characteristics of extracellular α-synuclein and tau are related with these diseases. Besides, two recent studies have ensured the significance of extracellular α-synuclein in the pathogenic mechanism of α-synucleinopathies [32].
In this study, one of the other most important findings was the demonstration of the statistically significant correlation between serum α-synuclein and tau-protein in children with ADHD.
Recently, Erro Aguirre et al. [33] have shown that there is no apparent clinical correlation between the presence of α-synuclein and tau protein in progressive supranuclear palsy. Nevertheless, they showed that tau protein co-aggregate with α-synuclein in catecholaminergic neurons of progressive supranuclear palsy brains [33]. Therefore, they suggested that their findings were a sign for synergistic interaction between the two proteins [33]. Several lines of evidence based on imaging, genetic and neurochemical studies point toward dysregulation of catecholaminergic systems in ADHD [34]. It was suggested that α-synuclein and tau are important for neurodegeneration models of substantia nigra in rats, further indicated their potential as therapeutic targets for human diseases involving loss of dopamine neurons. It was shown in a study that function of dopaminergic neurons affected by gene transfer of tau appears to be more sensitive rather than α-synuclein-related damage in this model [6].
Although we did not find any significant difference between two groups in terms of serum α-synuclein levels, it was found that there is a statistically significantly correlation between serum α-synuclein and tau-protein in children with ADHD. Considering these aspects, it may be suggested that the results of our study may provide evidence for not only tau pathology but also synergistic interaction between the α-synuclein and tau in children with ADHD. Howover, there are several limitations in present study. Firstly, we collected only serum samples, but cerebrospinal fluid samples were absent and serum dopamine levels could not be measured because of the budget limitation. Lastly, the sample size was small. Up until to this date, there has not been a single study in which an association between clinical ADHD and dopamine-associated α-synuclein and tau has been established. When we consider these aspects, our study could be considered as a preliminary study. Nevertheless, we believe that our findings will provide an important contribution for the literature; and they will generate a different pathophysiologic viewpoint for future researches to be carried out on ADHD.
In conclusion, highly and widely distributed tau serum levels may imply disturbance of microtubule transportation and axonal damage of dopaminergic neurons in ADHD. Within this context, we hypothesized that the serum levels of tau may be affected by ADHD or vice versa; and these molecules may be used to understand the pathological mechanisms of ADHD. We think that it will be of great contribution to determine other potential causes of increased serum tau; and we want to learn whether this increase has any phenotypic effect. However, our knowledge about tau protein levels in cerebrospinal fluid of patients with ADHD remains unclear and deserves further investigation.
Acknowledgments
This research was conducted in centers microbiology laboratory of Dicle University Medical Faculty. We are grateful to all participants and everyone who contributed to this research.
Compliance with Ethical Standards
Conflict of interest
We are relevantly related to the work in this manuscript. We have no relationship, or any other financial source and this manuscript has not been published in any other publication.
Informed consent
We obtaining written informed consent from a parents of children.
Research involving Human Participants and/or Animals
This work is fully relevant to the human ethical. We have the ethical certificate to do this work and we have the ethical number: NCREC No: 27.02.2015/167 for your consideration we have enclosed the scan copy of ethical certificate.
Contributor Information
Ihsan Cetin, Phone: +9 0488 217 35 00, Email: cetiniihsan@gmail.com.
Seref Simsek, Email: drserefsimsek@gmail.com.
References
- 1.Caylak E. Biochemical and genetic analyses of childhood attention deficit/hyperactivity disorder. Am J Med Genet B Neuropsychiatr Genet. 2012;159(6):613–617. doi: 10.1002/ajmg.b.32077. [DOI] [PubMed] [Google Scholar]
- 2.Santa-María I, Hernández F, Smith MA, Perry G, Avila J, Moreno FJ. Neurotoxic dopamine quinone facilitates the assembly of tau into fibrillar polymers. Mol Cell Biochem. 2005;278(1–2):203–212. doi: 10.1007/s11010-005-7499-6. [DOI] [PubMed] [Google Scholar]
- 3.Kokhan VS, Afanasyeva MA, Van’kin GI. α-Synuclein knockout mice have cognitive impairments. Behav Brain Res. 2012;231(1):226–330. doi: 10.1016/j.bbr.2012.03.026. [DOI] [PubMed] [Google Scholar]
- 4.Murphy DD, Rueter SM, Trojanowski JQ, Lee VM. Synucleins are developmentally expressed, and alpha-synuclein regulates the size of the presynaptic vesicular pool in primary hippocampal neurons. J Neurosci. 2000;20(9):3214–3220. doi: 10.1523/JNEUROSCI.20-09-03214.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roy B, Jackson GR. Interactions between tau and asynuclein augment neurotoxicity in a Drosophila model of Parkinson’s disease. Hum Mol Genet. 2014;23(11):3008–3023. doi: 10.1093/hmg/ddu011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klein RL, Dayton RD, Lin WL, Dickson DW. Tau gene transfer, but not alpha-synuclein, induces both progressive dopamine neuron degeneration and rotational behavior in the rat. Neurobiol Dis. 2005;20(1):64–73. doi: 10.1016/j.nbd.2005.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fredriksson A, Archer T. Neurobehavioural deficits associated with apoptotic neurodegeneration and vulnerability for ADHD. Neurotox Res. 2004;6(6):435–456. doi: 10.1007/BF03033280. [DOI] [PubMed] [Google Scholar]
- 8.Shaw P, Gornick M, Lerch J, Addington A, Seal J, Greenstein D, et al. Polymorphisms of the dopamine d4 receptor, clinical outcome, and cortical structure in attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2007;64(8):921–931. doi: 10.1001/archpsyc.64.8.921. [DOI] [PubMed] [Google Scholar]
- 9.Chen L, Huang X, Lei D, He N, Hu X, Chen Y, et al. Microstructural abnormalities of the brain white matter in attention-deficit/hyperactivity disorder. J Psychiatry Neurosci. 2015;40(4):280–287. doi: 10.1503/jpn.140199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolfers T, Onnink AM, Zwiers MP, Arias-Vasquez A, Hoogman M, Mostert JC, et al. Lower white matter microstructure in the superior longitudinal fasciculus is associated with increased response time variability in adults with attention-deficit/hyperactivity disorder. J Psychiatry Neurosci. 2015;40(5):344–351. doi: 10.1503/jpn.140154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y, Horst KK, Kronenberger WG, Hummer TA, Mosier KM, Kalnin AJ, et al. White matter abnormalities associated with disruptive behavior disorder in adolescents with and without attention-deficit/hyperactivity disorder. Psychiatry Res. 2012;202(3):245–251. doi: 10.1016/j.pscychresns.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 12.Oades RD, Dauvermann MR, Schimmelmann BG, Schwarz MJ, Myint AM. Attention-deficit hyperactivity disorder (ADHD) and glial integrity: S100B, cytokines and kynurenine metabolism-effects of medication. Behav Brain Funct. 2010;6:29. doi: 10.1186/1744-9081-6-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shim SH, Hwangbo Y, Yoon HJ, Kwon YJ, Lee HY, Hwang JA, et al. Increased levels of plasma glial-derived neurotrophic factor in children with attention deficit hyperactivity disorder. Nord J Psychiatry. 2015;69(7):546–551. doi: 10.3109/08039488.2015.1014834. [DOI] [PubMed] [Google Scholar]
- 14.Kern JK, Geier DA, King PG, Sykes LK, Mehta JA, Geier MR. Shared brain connectivity issues, symptoms, and comorbidities in autism spectrum disorder, attention deficit/hyperactivity disorder, and tourette syndrome. Brain Connect. 2015;5(6):321–335. doi: 10.1089/brain.2014.0324. [DOI] [PubMed] [Google Scholar]
- 15.Mitchell RH, Goldstein BI. Inflammation in children and adolescents with neuropsychiatric disorders: a systematic review. J Am Acad Child Adolesc Psychiatry. 2014;53(3):274–296. doi: 10.1016/j.jaac.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 16.Owens J, Hoza B. Diagnostic utility of DSM-IV-TR symptoms in the prediction of DSM-IV-TR ADHD subtypes and ODD. J Atten Disord. 2003;7(1):11–27. doi: 10.1177/108705470300700102. [DOI] [PubMed] [Google Scholar]
- 17.Gokler B, Unal F, Pehlivanturk B, Cengel Kultur E, Akdemir D, Taner Y. Reliability and validity of schedule for affective disorders and schizophrenia for school age children-presentand lifetime version-Turkish version (K-SADS-PL-T) Turk J Child Adolesc Ment Health. 2004;11(1):109–116. [Google Scholar]
- 18.Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 19.Turgay A. Disruptive behavior disorders child and adolescent screening and rating scalefor children, adolescents, parents, and teachers. West Blomfield (Michigan): Integrative Therapy Institute Publication. 1994;1(1):1–10.
- 20.Schwartz K, Verhaeghen P. ADHD and Stroop interference from age 9 to age 41 years: a meta-analysis of developmental effects. Psychol Med. 2008;38(11):1607–1616. doi: 10.1017/S003329170700267X. [DOI] [PubMed] [Google Scholar]
- 21.Karakaş S, Erdoğan E, Sak L, Soysal AŞ, Ulusoy T, Ulusoy İY. Stroop Test TBAG Form: standardisation for Turkish culture, reliability and validity. Clin Psychiatry. 1999;2(2):75–88. [Google Scholar]
- 22.Kılıç B, Koçkar A, Irak M, Şener Ş, Karakaş S. The standardization study of the stroop test TBAG Form in children between 6–11 years of age. Turk J Child Adolesc Ment Health. 2002;9(2):86–99. [Google Scholar]
- 23.Zhang J, Cai T, Zhao F, Yao T, Chen Y, Liu X, Luo W, Chen J. The role of α-synuclein and tau hyperphosphorylation-mediated autophagy and apoptosis in lead-induced learning and memory injury. Int J Biol Sci. 2012;8(7):935–944. doi: 10.7150/ijbs.4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kadak MT, Cetin I, Tarakçıoğlu MC, Özer ÖF, Kaçar S, Çimen B. Low serum level α-synuclein and tau protein in autism spectrum disorder compared to controls. Neuropediatrics. 2015;46(6):410–415. doi: 10.1055/s-0035-1565273. [DOI] [PubMed] [Google Scholar]
- 25.Otto M, Wiltfang J, Tumani H, Zerr I, Lantsch M, Kornhuber J, Weber T, Kretzschmar HA, Poser S. Elevated levels of tau-protein in cerebrospinal fluid of patients with Creutzfeldt-Jakob disease. Neurosci Lett. 1997;225(3):210–212. doi: 10.1016/S0304-3940(97)00215-2. [DOI] [PubMed] [Google Scholar]
- 26.El-Agnaf OM, Salem SA, Paleologou KE, Curran MD, Gibson MJ, Court JA, et al. Detection of oligomeric forms of alpha-synuclein protein in human plasma as a potential biomarker for Parkinson’s disease. FASEB J. 2006;20(3):419–425. doi: 10.1096/fj.03-1449com. [DOI] [PubMed] [Google Scholar]
- 27.Zetterberg H, Wilson D, Andreasson U, Minthon L, Blennow K, Randall J, et al. Plasma tau levels in Alzheimer’s disease. Alzheimers Res Ther. 2013;5(2):1–9. doi: 10.1186/alzrt163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Avila J, Lucas JJ, Perez M, Hernandez F. Role of tau protein in both physiological and pathological conditions. Physiol Rev. 2004;84(2):361–384. doi: 10.1152/physrev.00024.2003. [DOI] [PubMed] [Google Scholar]
- 29.Bitsch A, Horn C, Kemmling Y, Seipelt M, Hellenbrand U, Stiefel M, et al. Serum tau protein level as a marker of axonal damage in acute ischemic stroke. Eur Neurol. 2002;47(1):45–55. doi: 10.1159/000047946. [DOI] [PubMed] [Google Scholar]
- 30.Morris M, Maeda S, Vossel K, Mucke L. The many faces of tau. Neuron. 2011;70(3):410–426. doi: 10.1016/j.neuron.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hampel H, Blennow K, Shaw LM, Hoessler YC, Zetterberg H, Trojanowski JQ. Total and phosphorylated tau protein as biological markers of Alzheimer’s disease. Exp Gerontol. 2010;45(1):30–40. doi: 10.1016/j.exger.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Desplats P, Lee HJ, Bae EJ, Patrick C, Rockenstein E, Crews L, et al. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc Natl Acad Sci USA. 2009;106(31):13010–13015. doi: 10.1073/pnas.0903691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Erro Aguirre ME, Zelaya MV, Sánchez Ruiz de Gordoa J, Tuñón MT, Lanciego JL. Midbrain catecholaminergic neurons co-express α-synuclein and tau in progressive supranuclear palsy. Front Neuroanat. 2015;9(1):25. doi: 10.3389/fnana.2015.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maitra S, Sarkar K, Ghosh P, Karmakar A, Bhattacharjee A, Sinha S, et al. Potential contribution of dopaminergic gene variants in ADHD core traits and co-morbidity: a study on eastern Indian probands. Cell Mol Neurobiol. 2014;34(4):549–564. doi: 10.1007/s10571-014-0038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]