Abstract
Telomere stability is indispensable for continuous proliferation of cells. Telomere structure is maintained by group of six proteins termed as shelterin. RAP1 and POT1 proteins are significant members of shelterin complex. Expression of RAP1 and POT1 are crucial for telomere maintenance and hence uncontrolled division of cells. Notably, expression of RAP1 and POT1 is unknown in renal cell carcinoma (RCC). In view of these facts, the present study was initiated to investigate the expression of RAP1 and POT1 in RCC and their relationships with clinicopathological features. In total 65 histopathologically confirmed RCC cases and their adjacent normal renal parenchyma were analyzed for gene expression. The mRNA expression of telomere binding proteins RAP1 and POT1 were measured using RT-PCR. Expression of RAP1 was observed to be significantly increased in tumour tissues as compared to corresponding normal renal tissues (P = 0.004). The gene expression of RAP1 was documented to be related to grades of RCC (P = 0.002) and subtypes of RCC (P = 0.01). Although, POT1 expression was up-regulated in RCC tissue, however it was not statistically significant. Also, POT1 expression was not related to grades, stages and subtypes of RCC. This is the first study which shows correlation RAP1 with grades and subtypes of RCC.
Keywords: Kidney, Cancer, Genomics, RAP1, POT 1, Correlation
Introduction
Renal cell carcinoma (RCC) is the most common kidney cancer in adults which constitute ~90 % of all primary adult renal tumors [1]. As, diagnosis of RCC often occurs at advance disease stage hence survival of patient is very poor. The exact cause of RCC carcinogenesis is still not well known. Carcinogenesis is a multistep process that involves chromosome instability which leads to multiple mutations involving activation of oncogenes, loss of tumor suppressor genes. Telomere dysfunction is one of the main probable reasons of chromosome instability [2, 3]. Telomeres are specialized nucleoprotein complexes that are located at the ends of chromosomes [4]. Human telomeres are composed of up to 10–15 kb hexanucleotide TTAGGG repeats and associated telomere binding proteins that generate protective telomere-specific complexes [5, 6]. In higher eukaryotes, a set of six telomere-specific proteins termed “shelterin” has been identified, which are central to telomere maintenance [7]. The telosome/shelterin complex consists of important regulatory telomere binding proteins to include tripeptidyl peptidase1 (TPP1), protection of telomere 1 (POT1), TRF1 interacting nuclear factor 2 (TIN2), repressor activator protein (RAP1), telomere repeat binding factor 1 (TRF1) and TRF2 [8].
Telomere binding proteins TRF1, TRF2 and POT1 bind directly to telomeric repeats and TIN2, TPP1 and RAP1 are interconnected to these proteins thus form a functional complex which caps telomeric ends [6]. POT1 specifically binds to the overhang single-stranded telomeric DNA and shields it from degradation. POT1 recruits telomerase to the single-strand telomeric overhang and thus can act as a telomerase-dependent, positive regulator of telomere length [9, 10]. RAP1 forms a complex with TRF2 and affects the length and heterogeneity of human telomeres and thus play a role in telomere function and end protection [11]. Any changes in telomere binding proteins expressions can lead to telomere dysfunction. Oncogenesis research is now focusing on the shelterin complex. Several studies have shown the association or role of shelterin proteins in various cancers viz. lymphocytic leukemia [12], squamous cell carcinoma [13], non-small cell lung cancer [14] and breast cancer [15]. However, the expressions of telomere binding proteins and their association with clinicopathological parameters in RCC have not been well studied.
In the present study, mRNA expression of the genes encoding the proteins POT1 and RAP1 in RCC and their adjacent normal renal parenchyma were evaluated. The association between expressions of RAP 1 and POT1 and the clinicopathological findings were also assessed.
Materials and Methods
Tissue Samples
The study was approved by the Institute ethics committee and informed consent was taken from all patients. RCC tissue and paired adjacent normal renal parenchyma were obtained from 65 RCC patients who underwent radical nephrectomy. All the tissue samples were immediately snap-frozen and stored at −80 °C till used further. Specimens were confirmed by pathological examination. Tumor staging and grading of clear cell RCC type was performed according to TNM staging [16] and Fuhrman grading [17] respectively.
Total RNA Extraction and Reverse Transcription
Total RNA was extracted from both tumor as well as normal tissue with Trizol reagent (Gibco BRL, Grand Island, NY, USA) according to the manufacturer’s instructions. The concentration was measured by spectrophotometry and formaldehyde denatured agarose gel electrophoresis was used for quality examination. Before reverse transcription, genomic DNA contamination was eliminated by treating 1 μg RNA with 1 U DNase I (Fermentas) for 30 min at 37 °C and then heat-inactivated at 65 °C for 10 min. Total RNA was reverse transcribed into cDNA by using the SuperscriptIII first-strand synthesis system (Invitrogen, USA) as per manufacturer’s protocol. The cDNA samples were stored immediately at −20 °C for succeeding real time PCR analysis.
Quantitative Real-Time PCR
Real-time analysis was performed on 7300 RT-PCR system (Roche, Indianapolis, IN) using the SYBR Green PCR Master Mix (Roche Indianapolis, IN). The oligonucleotide sequences of the primers were as follows:
RAP1 Forward-5′ GCCACCCGGGAGTTTGA 3′
Reverse-5′ GGGTGGATCATCATCACACATAGT-3′
POT1 Forward 5′ CAGAACCTGACGACAGCTTTCC 3′
Reverse 5′ GCACATAGTGGTGTCCTCTCCA 3′
β-actin Forward 5′ CGAGCGCGGCTACAGCTT 3′
Reverse 5′ TCCTTAATGTCACGCACGATTT 3′
The cycles were programmed for 95 °C for a 5 min initial step, then 95 °C for 30 s, 58 °C for 30 s, and 72 °C for 30 s. At the same time no-template controls was also performed in each experiment and no signals were detected in theses. The average threshold cycle for each sample was determined from triplicate reactions and the expression level was normalized to β-actin. The relative amount of mRNA was calculated byΔΔC t method using the following equation [18].
where ΔC t = average C t (RAP1 or POT1) − average C t (β actin).
Statistical Analysis
Statistical analysis was performed using the SPSS 20.0 software package (SPSS, Chicago, IL, USA). The statistical significance of the differences in mRNA expression levels between two groups and more than two groups were analyzed by using Student’s t test and an ANOVA respectively. The correlation between the expression of RAP1 or POT1 and various clinicopathological features was calculated using Spearman’s rank correlation test. A P value of less than 0.05 was considered to indicate statistical significance.
Results
Patient’s Characteristics
A total of 65 cases of histopathologically proven RCC were included in this study. The mean age of the patients in this study was 54 ± 14.6 years. Low stage includes stage 1 and 2 whereas; high stage includes stage 3 and 4. Most of the patients had low stage clear cell RCC (75.0 %), in comparison to the high stages clear cell RCC (25.0 %). Grade 1 and 2 specimens were included in low grade, whereas grade 3 and 4 specimens were included in high grade. Most of the patients were of low grades (68.2 %). Table 1 shows the detail clinical characteristics of all the patients.
Table 1.
Clinical characteristic of the patients
| Patients (n) | 65 |
| Gender, n (%) | |
| Male | 47 (72.3) |
| Female | 18 (27.7) |
| Age (years), mean ± SD | 54 ± 14.6 |
| BMI, mean ± SD | 25.67 ± 0.364 |
| Commonest presenting complaints, n (%) | |
| Hematuria | 36 (55.4) |
| Flank pain | 22 (33.8) |
| Both | 18 (27.7) |
| Incidental radiological examination | 7 (10.8) |
| Histologic subtypes, n (%) | |
| Clear cell RCC | 44 (67.7) |
| Papillary | 12 (18.5) |
| Sarcomatoid | 6 (9.2) |
| Chromophobe | 3 (4.6) |
| Pathologic stages (clear cell), n (%) | |
| T1 | 23 (52.3) |
| T2 | 10 (22.7) |
| T3 | 9 (20.5) |
| T4 | 2 (4.5) |
| Fuhrman grades (Clear Cell), n (%) | |
| I | 9 (20.5) |
| II | 21 (47.7) |
| III | 10 (22.7) |
| IV | 4 (9.1) |
SD standard deviation
RAP1 and POT1 Gene Expressions in RCC
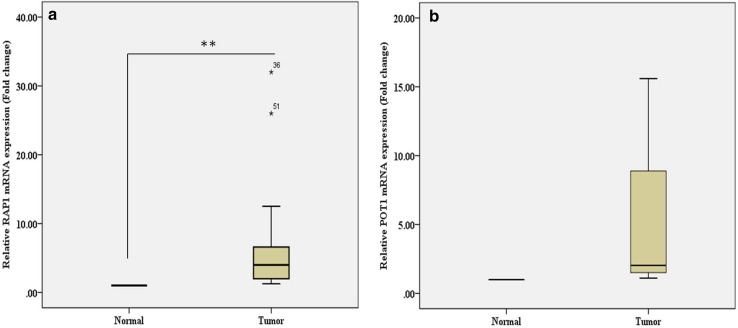
We observed a significantly higher expression of RAP1 mRNA in RCC tissues in comparison to that in adjacent normal renal tissues (fold change median value, 4; P = 0.004; Fig. 1a). Also, a higher expression of POT1 mRNA was observed in RCC tissues as compared to adjacent normal renal parenchyma (fold change median value, 2.02; P = 0.05; Fig. 1b).
Fig. 1.
Box plot diagram shows the gene expression of a RAP 1 and b POT 1 relative to control. The extreme values were noted as open circles and star their sample number mentioned against them. One sample t test was used for statistical analysis. P < 0.05 was considered as significant. ***P < 0.001
Correlation of RAP1 and POT1 Gene Expression with Clinicopathological Parameters
Additionally, an attempt was made to correlate the RAP1 mRNA expressions with various clinicopathological parameters. The RAP1 mRNA expression was significantly declined in clear cell RCC with high grades in comparison to low grade clear cell RCC (low grade vs high grade; 6.60 vs 2.57; P = 0.002; Fig. 2a). Further, a significant difference was noticed in the RAP1 mRNA expression among different subtypes of RCC (Fold change: Median value; ccRCC; 4.75, pRCC; 2.00, ChRCC; 9.00, srCC; 1.8; P = 0.01; Fig. 2b). However, no significant change in RAP1 mRNA expression was observed between low stage and high stage of clear cell RCC (P = 0.673). Conversely, no correlation could be established between POT1 mRNA expression and grades (P = 0.562), stages (P = 0.756) or subtypes of RCC (P = 0.695).
Fig. 2.
The RAP1 mRNA expression level in different a grades and b types of RCC was determined by quantitative real time PCR. The bottom and top edges of the box located at the 25th and 75th percentiles respectively of the sample. The centre horizontal lines are drawn at the median of the sample. The extreme values were noted as stars their sample number mentioned against them. mRNA level of β-actin was used to normalize RAP1 gene expression. Statistical analysis was done by Mann–Whitney and Kruskas–Wallis testrespectively. ***P < 0.001
Discussion
RAP1 and POT1, the telomere associated proteins are part of telomere structure and have an essential role in telomere maintenance. POT1 is involves in directly regulating telomere length. It is homologous in budding and fission yeasts where it is known to regulate the ability of telomerase to elongate telomeres [19]. Studies have shown that human POT1 is the only protein of shelterin complex that binds to telomeric ssDNA [9] which is a substrate of telomerase. Hence, binding of telomerase to its substrate can be modulated by POT1 at least in vitro. Overall, POT1, along with both telomeric ssDNA and TRF2, is critical for regulating telomere length [20]. In this study, POT1 expression was found to be higher in RCC as compared to normal renal parenchyma. But, increased in POT1 expression was not statistically significant. Hence, this increased POT1 expression may be required for maintaining the telomere function in RCC.
Earlier, it has been shown that POT1 expression may be persuaded by tumor stage and telomere length. Previous study in gastric cancer indicated that POT1 is down-regulated in stage I/II whereas it is upregulated in stage III/IV [21]. However, we did not find any correlation between POT1 expressions with stages of RCC. Human RAP1 (hRAP1) is employed to the telomere by TRF2 [22]. It was shown that hRAP1 was shown to have similar functions to its yeast counterpart. Budding yeast RAP1 plays a role in transcription, telomere length regulation, and end capping. Thus, it plays a role in telomere maintenance. In our present study, a significantly higher expression of RAP1 was observed in tumor tissue than in normal renal tissues. This increase in RAP1 expression indicates the protective role of RAP1 in telomere protection in RCC. Previously, it was observed that RAP1 alone suppresses non homologous end joining in human tissue extracts and homologous recombination in mouse cells [23]. Additionally, RAP1 expression was found to be inversely correlated with tumor grades in present study. Its expression was high in low grade tumors and low in high grade tumors.
Tumor grade is a sign of the severity of the malignancy. It refers to the resemblance of the tumor cells to the normal cells of the same tissue type. Low grade cells resemble to normal cells and tend to grow and multiply slowly, whereas high grade tumors grow rapidly. Therefore, inverse relation of RAP1 suggested that its expression might have a protective role in RCC progression. However, there was no difference in RAP1 expression among different stages of clear cell RCC.
Our findings are consistent with the previous study where authors have shown that RAP1 expression is associated with tumor grade and overall survival of the lung cancer patients [14]. Nevertheless, their data strongly support that expression of RAP1 is a promising prognosis marker for lung cancer. In another study by Chen et al. [24] examined the mRNA and protein level expression of RAP1 at different stages of oral cavity squamous cell carcinoma (OCSCC).They have reported strong RAP1 expression as a significant prognostic marker and predictor of aggressive OCSCC. In the present study RAP1 expression was significantly correlated with different subtypes of RCC. This observation supports RAP1 expression as a possible marker of clinical behavior of RCC. However, still little is known about the function, role and mechanism of RAP1 in maintenance of telomeres. It has also shown that depletion of endogenous hRAP1 by small interference RNA leads to longer telomeres and over-expression of a dominant negative form of human RAP1 extends telomeres [25]. The precise role and functions of RAP1 and POT1 are largely unknown in cancer. But, it is evident that the coordinated interaction of these proteins with telomeric DNA is essential to maintain telomere stability. Also, damage in structural components of telomere leads to telomere associated DNA damage response and genome instability [26].
In conclusion, this is the first study exploring expressions of RAP1 and POT1 in different grades, stages and subtypes of RCC. Further, a correlation was established between RAP1 expression with grades and subtypes of RCC. However, an extensive study is required to elucidate the role of RAP1 and POT1 in carcinogenesis of RCC.
Funding
This is a part of a research project funded by Council of Scientific and Industrial Research, New Delhi, India (vide letter no. 27/0218/09/EMR-II). The authors are thankful to Indian council of medical research, New Delhi, India for awarding junior and senior research fellowship (3/1/3/JRF-2010/HRD-67 (11065).
Compliance with Ethical Standards
Conflict of interest
None.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
References
- 1.Chow WH, Dong LM, Devesa SS. Epidemiology and risk factors for kidney cancer. Nat Rev Urol. 2010;7:245–257. doi: 10.1038/nrurol.2010.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murnane JP. Telomere loss as a mechanism for chromosome instability in human cancer. Cancer Res. 2010;70:4255–4259. doi: 10.1158/0008-5472.CAN-09-4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Sullivan RJ, Karseder J. Telomeres: protecting chromosomes against genome instability. Nat Rev Mol Cell Biol. 2010;11:171–181. doi: 10.1038/nrm2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blackburn EH. Structure and function of telomeres. Nature. 1991;350:569–573. doi: 10.1038/350569a0. [DOI] [PubMed] [Google Scholar]
- 5.Griffith JD, Comeau L, Rosenfield S, Stansel RM, Bianchi A, Moss H, et al. Mammalian telomeres end in a large duplex loop. Cell. 1999;97:503–514. doi: 10.1016/S0092-8674(00)80760-6. [DOI] [PubMed] [Google Scholar]
- 6.Liu D, O’Connor MS, Qin J, Songyang Z. Telosome, a mammalian telomere associated complex formed by multiple telomeric proteins. J Biol Chem. 2004;279:51338–51342. doi: 10.1074/jbc.M409293200. [DOI] [PubMed] [Google Scholar]
- 7.Palm W, de Lange T. How shelterin protects mammalian telomeres? Annu Rev Genet. 2008;42:301–334. doi: 10.1146/annurev.genet.41.110306.130350. [DOI] [PubMed] [Google Scholar]
- 8.de Lange T. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev. 2005;19:2100–2110. doi: 10.1101/gad.1346005. [DOI] [PubMed] [Google Scholar]
- 9.Colgin LM, Baran K, Baumann P, Cech TR, Reddel RR. Human POT1 facilitates telomere elongation by telomerase. Curr Biol. 2003;13:942–946. doi: 10.1016/S0960-9822(03)00339-7. [DOI] [PubMed] [Google Scholar]
- 10.Loayza D, de Lange T. POT1 as a terminal transducer of TRF1 telomere length control. Nature. 2003;424:1013–1018. doi: 10.1038/nature01688. [DOI] [PubMed] [Google Scholar]
- 11.Li B, de Lange T. Rap1 affects the length and heterogeneity of human telomeres. Mol Biol Cell. 2003;14:5060–5068. doi: 10.1091/mbc.E03-06-0403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poncet D, Belleville AL, de Roodenbeke CK, de Climens AR, Simon EB, Merle-Beral HL, et al. Changes in the expression of telomere maintenance genes suggest global telomere dysfunction in B-chronic lymphocytic leukemia. Blood. 2008;111:2388–2391. doi: 10.1182/blood-2007-09-111245. [DOI] [PubMed] [Google Scholar]
- 13.Hsu CP, Lee LW, Shai SE, Chen CY. Clinical significance of telomerase and its associate genes expression in the maintenance of telomere length in squamous cell carcinoma of the esophagus. World J Gastroenterol. 2005;11:6941–6947. doi: 10.3748/wjg.v11.i44.6941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin X, Gu J, Lu C, Spitz MR, Wu X. Expression of telomere-associated genes as prognostic markers for overall survival in patients with non-small cell lung cancer. Clin Cancer Res. 2006;12:5720–5725. doi: 10.1158/1078-0432.CCR-05-2809. [DOI] [PubMed] [Google Scholar]
- 15.Salhab M, Jiang WG, Newbold RF, Mokbel K. The expression of gene transcripts of telomere associated genes in human breast cancer: correlation with clinicopathological parameters and clinical outcome. Breast Cancer Res Treat. 2008;109:35–46. doi: 10.1007/s10549-007-9622-8. [DOI] [PubMed] [Google Scholar]
- 16.Sobin LH, Wittekind C. UICC: TNM classification of malignant tumors. 6. New York: Wiley; 2002. [Google Scholar]
- 17.Fuhrman HA, Lasky LC, Lima C. Prognostic significance of morphologic parameters in renal cell carcinoma. Am J Surg Pathol. 1982;6:655–663. doi: 10.1097/00000478-198210000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat Protocol. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 19.Miyoshi T, Kanoh J, Saito M, Ishikawa F. Fission yeast Pot1-Tpp1 protects telomeres and regulates telomere length. Science. 2008;320:1341–1344. doi: 10.1126/science.1154819. [DOI] [PubMed] [Google Scholar]
- 20.Loayza D, Parsons H, Donigian J, Hoke K, de Lange T. DNA binding features of human POT1: a nonamer 5′-TAGGGTTAG-3′ minimal binding site, sequence specificity, and internal binding to multimeric sites. J Biol Chem. 2004;279:13241–13248. doi: 10.1074/jbc.M312309200. [DOI] [PubMed] [Google Scholar]
- 21.Kondo T, Oue N, Yoshida K, Mitani Y, Naka K, Nakayama H, et al. Expression of POT1 is associated with tumor stage and telomere length in gastric carcinoma. Cancer Res. 2004;64:523–529. doi: 10.1158/0008-5472.CAN-03-1196. [DOI] [PubMed] [Google Scholar]
- 22.Chen CH, Chen RJ. Prevalence of telomerase activity in human cancer. Formos Med Assoc. 2011;110:275–289. doi: 10.1016/S0929-6646(11)60043-0. [DOI] [PubMed] [Google Scholar]
- 23.Sfeir A, Kabir S, Van Overbeek M, Celli GB, de Lange T. Loss of Rap1 induces telomere recombination in the absence of NHEJ or a DNA damage signal. Science. 2010;327:1657–1661. doi: 10.1126/science.1185100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen CH, Chuang HC, Huang CC, Fang FM, Huang HY, Tsai HT, et al. Over-expression of Rap-1A indicates a poor prognosis for oral cavity squamous cell carcinoma and promotes tumor cell invasion via Aurora-A modulation. Am J Pathol. 2013;182:516–528. doi: 10.1016/j.ajpath.2012.10.023. [DOI] [PubMed] [Google Scholar]
- 25.O’Connor MS, Safari A, Liu D, Qin J, Songyang Z. The human Rap1 protein complex and modulation of telomere length. J Biol Chem. 2004;279:28585–28591. doi: 10.1074/jbc.M312913200. [DOI] [PubMed] [Google Scholar]
- 26.Sui J, Lin YF, Xu K, Lee KJ, Wang D, Chen BP. DNA PKCs phosphorylates hnRNP-A1 to facilitate the RPA-to POT1 switch and telomere capping after replication. Nucleic Acids Res. 2015;43:5971–5983. doi: 10.1093/nar/gkv539. [DOI] [PMC free article] [PubMed] [Google Scholar]