Abstract
One of the most important complications of diabetes is nephropathy. This study investigates the effects of aqueous garlic extract on inflammation and oxidative stress status in the kidneys of diabetic rats. Male rats were divided into four groups- control rats, diabetic rats, garlic extract-treated diabetic rats, garlic extract-treated normal rats. The glucose, urea, uric acid, and creatinine levels were measured in sera using colorimetric methods. To determine the oxidative stress condition in the kidney tissues, total antioxidant capacity (TAC), malondialdehyde (MDA), and total oxidant status (TOS) were measured using colorimetric methods. Inflammation status was evaluated by the determination of tumor necrosis factor-alpha (TNF-α) gene and protein expression using qRT-PCR and ELISA respectively, while nitric oxide (NO) level in these tissues was measured using the Griess method. Histological examination of Kidneys was carried out by H&E staining. The levels of glucose, urea, and uric acid were found to increase in the serum of diabetic rats and decrease in that of diabetic rats after treatment with garlic. Measurement of MDA, TOS, and TAC revealed oxidative stress in diabetic rats, which improved after receiving the extract. The NO and TNF-α protein levels in diabetic rats were higher than those in control rats. After treatment with garlic, the levels of TNF-α protein and NO became close to the normal levels. Histological results confirmed certain other data as well. Garlic has antioxidant properties; therefore, it can reduce oxidative stress, which plays an important role in the development of diabetic nephropathy. Reduction in oxidative stress has beneficial effects on inflammation because it leads to a decrease in the level of TNF-α.
Keywords: Diabetes, Garlic extract, Oxidative stress, Inflammation
Introduction
Diabetes mellitus (DM) is one of the most significant chronic metabolic disorders characterized by hyperglycemia. The World Health Organization (WHO) predicts that by the year 2030, 366 million people have DM. Characteristic of diabetes is associated with disturbances in the metabolism of carbohydrates, lipids and proteins due to defects in insulin secretion, insulin action or both [1].
Diabetic complications are nephropathy, retinopathy, neuropathy, atherosclerosis and fatty liver. In all these cases continual hyperglycemia plays a significant role in the induction of oxidative stress by increasing glucose autooxidation, nonenzymatic protein glycation and activation of polyol pathway [2]. Also hyperglycemia induced stress sensitive signaling pathways including nuclear factor (NF)-κB. Activation of NF-κB increased cytokine concentrations such as tumor necrosis factor-α (TNF-α). The renal cells are capable to synthesis TNF-α moreover the sensitive to changes of serum’s TNF-α level. This process suggests a causal role for hyperglycemia in the immune activation of diabetes [3].
Since ancient times, consumption of medicinal herbs has considered in treatment of several diseases [4]. In recent years this kind of treatment has received growing attention because it is natural and has a few side effects [5]. Many medicinal plant extracts such as Bougainvillea spectabilis, Moringa oleifera, Curcuma longa, Cynodon dactylon and Trichosanthes dioica were used for treatment of diabetes mellitus due to having hypoglycemic effects [6–10].
One of the most common medicinal plants is Allium sativum (garlic) from Liliaceae family that it is used for the treatment of various diseases such as heart disease, liver dysfunction, cancer, infection and diabetes mellitus [11]. The hypoglycemic and antioxidant property of garlic has been determined in many studies but the effects of this extract on inflammation and its mechanism in diabetes condition has not been clearly elucidated. Therefore in current study, the aqueous extract of garlic was used for treatment of diabetic rats and evaluated the effects of this extract on oxidative stress and inflammation status in the kidneys of type 1 diabetic rats.
Materials and Methods
Preparation of Aqueous Garlic Extract
Fresh garlic bulbs were purchased from local markets in Hamadan, Iran. The plant was taxonomically identified by botanists in the herbarium Department of Biology, Bu-Ali Sina University, Hamadan, Iran. The cloves were peeled, washed and cut into small pieces. About 50 g was blended in 250 mL of distilled water, and homogenized in a mixing machine. The supernatant was filtered through Whatman no. 1 filter paper. Garlic extract was used freshly or quickly frozen until used. Daily 1 mL of this solution/100 g body weight (~2 g/kg) was given to the rats by gavage [12].
Animals and Experimental Design
Adult male Wistar rats weighing around 250–300 g (6–8 week old) were obtained from Hamadan University of Medical Sciences, Hamadan, Iran. The animals housed in standard cages with 12-h light-dark cycles, constant temperature of 25 ± 2 °C and free access to food and water. Investigations were performed conferring to the ethical norms of the Institutional Animal Ethics Committee of Hamadan University of Medical Sciences (Approval No. 9312126433). The animals were acclimatized for at least 5 days under these conditions before the start of the experiments. The rats were divided randomly into four groups each comprising of six animals. For induction of type 1 diabetes streptozotocin (STZ) (Sigma, USA) (Sigma, USA) was used.
Group 1 (C): normal control rats that received single dose of citrate buffer (0.1 M, pH 4.5).
Group 2 (D): Rats that received single dose of STZ (65 mg/kg body weight, intraperitoneal) dissolved in freshly prepared cold citrate buffer (0.1 M, pH 4.5) for induction of Diabetes type 1. To prove the induction of diabetes, 7 days after injection f STZ, blood glucose was measured using a strip operated blood glucose sensor (Accuchek; Roche, Germany). Animals were considered diabetics if their fasting blood glucose concentrations exceed 300 mg/dL. Blood glucose levels were measured once every 10 days during treatment.
Group 3 (D + G): diabetic rats that (similar to group 2) treated with aqueous garlic extract (2 g/kg body weight/day, gavage, 30 days).
Group 4 (G): normal rats that received only aqueous garlic extract (2 g/kg body weight/day, gavage, 30 days).
At the end of treatment period, serum samples were collected from all groups and stored at −20 °C for measuring biochemical parameters. The kidneys were snap-frozen in liquid nitrogen and stored at −80 °C for protein and RNA extraction and a portion of their fixed in 10% formalin for histological evaluation.
Determination of Biochemical Parameters
Biochemical parameters such as glucose, urea, creatinine and uric acid were measured in the serum of rats using commercially available kits (Pars Azmoon diagnostics, Iran).
Preparation of Kidney Tissue Homogenate
Kidney tissue homogenates were prepared on ice using lysis buffer (10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA, 0.1% triton × 100, 10 mM HEPES, 0.5 mM DTT, protease inhibitor cocktail, pH 7.9) and incubated on ice for 20 min. The protein concentrations of kidney homogenate samples were determined by using Bradford method [13].
Measurement of Lipid Peroxidation
Lipid peroxidation in kidney tissue homogenates was determined by malondialdehyde (MDA) assay. The results were expressed as μmol MDA/mg protein content of the samples [14].
Determination of Total Oxidative Status
Total oxidative status (TOS) in kidney tissue homogenates was determined using the oxidation of ferrous ion to ferric ion. The ferric ions form a colored complex with xylenol orange in an acidic medium. Therefore, the color intensity is related to the total number of oxidant molecules present in the sample. The results were expressed as μmol TOS/mg protein content of the samples [15].
Determination of Total Antioxidant Capacity
Total antioxidant capacity (TAC) in kidney tissue homogenates was assessed using ferric reducing antioxidant potential (FRAP) assay according to Benzie and Strain methods. This method is based on the reduction of the ferric tripyridyltriazine (TPTZ) to the blue colored ferrous form at low pH. This reduction is monitored by measuring the absorption change at 593 nm [16].
Determination of Nitric Oxide (NO)
The kidney NO level was determined by measuring the nitrite concentration in the kidney tissue homogenates using Griess method. The results were expressed as μmol nitrite/mg protein content of the samples [17].
Determination of TNF-α Gene Expression by Real Time PCR
Total RNA was extracted from the kidney tissues of control and experimental samples using the RNX-Plus solution (Sinaclon, Iran) according to the manufacturer’s instructions. The purified RNA was quantified by spectrophotometer (A260) and its quality was examined by electrophoresis. 1 μg RNA was reverse transcribed to synthesize cDNA using RevertAid First Strand cDNA Synthesis kit (Thermo Scientific, Lithuania) according to the manufacturer’s instructions. Quantitative Real-time PCR was performed on cDNA samples using the SYBR Premix ExTaq real-time PCR kit (Takara Bio Inc, Japan), according to the manufacturer’s protocols. Primer sequences were as follows: TNF-α (forward), GTCGTAGCAAACCACCAAGC, (reverse), CTCCTGGTATGAAATGGCAAA, 18S rRNA (forward), GTAACCCGTTGAACCCCATT, (reverse), CCATCCAATCGGTAGTAGCG. The relative changes in gene expression were determined using 2−∆∆CT method [18].
Determination of TNF-α Protein
The level of TNF-α in kidney tissue homogenates was evaluated by using rat TNF-α Platinum ELISA kit (eBioscience Bender MedSystems, Austria) according to the manufacturer’s instruction.
Histological Study
In the end of treatment time the kidney tissues of rats were quickly removed and a portion of their fixed in 10% formalin for a week at room temperature. The specimens were dehydrated in ethanol, cleared in xylene, embedded in paraffin, sectioned by microtome and finally stained with hematoxylin and eosin (H&E).
Statistical Analysis
Statistical analysis was performed using the SPSS software version 16 (SPSS, Chicago, IL, USA) and analysis of variance (ANOVA) was used to compare means in different groups. Data were reported as mean ± standard deviation (SD) and significance was taken at P < 0.05.
Results
Effects of Garlic Extract on Body Weights
Table 1 shows the effects of garlic extract on the body weights. In the start of study body weights of rats did not different in any groups. In the end of study in diabetic rats body weights were significantly (P < 0.001) decreased compared with control rats and increased significantly (P < 0.05) in D + G group compared with diabetic rats. Δ body weights were different significantly between control and experimental rats.
Table 1.
Effects of garlic extract on body weight in control and experimental rats
| Groups | Initial body weight (g) | Final body weight (g) | Δ Body weight (g) |
|---|---|---|---|
| C | 266.33 ± 10.08 | 309.17 ± 11.93 | 42.83 ± 6.47 |
| D | 272.4 ± 21.86 | 251 ± 19.24a*** | −15.75 ± 8.49a*** |
| D + G | 251.6 ± 20.07 | 270.2 ± 14.61a**,b* | 18.6 ± 14.66b*** |
| D | 285.33 ± 12.87 | 301.6 ± 13.86 | 20.2 ± 11.34 |
Results are mean ± SD (n = 6)
D + G, diabetic rats that treated with aqueous garlic extract; G, normal rats that treated with aqueous garlic extract
* P < 0.05; ** P < 0.01; *** P < 0.001
aCompare with control (C) rats
bCompare with diabetic rats (D)
Effects of Garlic Extract on Glucose Levels in Duration of Treatment
Effects of garlic on glucose levels in duration of treatment are shown in Table 2. In diabetic rats glucose level was increased significantly (P < 0.001) when compared with normal control rats in 10th, 20th, 30th and 40th day after the injection of STZ. In 10th, 20th and 30th day after oral administration of garlic in D + G group, the glucose level was decreased in comparison with diabetic rats but was not significant. However in 40th day after treatment with garlic, glucose level in treated diabetic rats was decreased significantly (P < 0.05) compared with untreated diabetic rats.
Table 2.
Effects of garlic extract on glucose levels in control and experimental rats
| Groups | 10th day (mg/dL) | 20th day (mg/dL) | 30th day (mg/dL) | 40th day (mg/dL) |
|---|---|---|---|---|
| C | 85.66 ± 8.83 | 86.66 ± 6.97 | 86.5 ± 6.11 | 89.12 ± 10.8 |
| D | 558 ± 32.53a*** | 541.6 ± 76.05a*** | 521.8 ± 101.08a*** | 485.75 ± 113.04a*** |
| D + G | 545.6 ± 57.28a*** | 579.5 ± 37.95a*** | 495 ± 73.89a*** | 321.25 ± 27.09a***,b* |
| G | 83.66 ± 3.44 | 84.5 ± 3.65 | 86.6 ± 3.09 | 90.29 ± 10.1 |
Results are mean ± SD (n = 6)
D + G, diabetic rats that treated with aqueous garlic extract; G, normal rats that treated with aqueous garlic extract
* P < 0.05; *** P < 0.001
aCompare with control (C) rats
bCompare with diabetic rats (D)
Effects of Garlic Extract on Urea, Creatinine and Uric Acid Levels
In Table 3, the levels of urea, creatinine and uric acid in four groups of rats are summarized. The level of creatinine was not different between groups but urea (P < 0.001) and uric acid (P < 0.01) concentrations significantly increased in diabetic rats compared with control rats. Oral administration of garlic decreased significantly the level of urea and uric acid (P < 0.05) in D + G group compared with diabetic rats.
Table 3.
Effects of garlic extract on urea, creatinine and uric acid levels in control and experimental rats
| Groups | Urea (mg/dL) | Creatinine (mg/dL) | Uric acid (mg/dL) |
|---|---|---|---|
| C | 53.6 ± 6.31 | 0.67 ± 0.06 | 1.89 ± 0.39 |
| D | 162.5 ± 30.65a*** | 0.76 ± 0.12 | 3.54 ± 0.32a** |
| D + G | 121.66 ± 15.8a**,b* | 0.77 ± 0.16 | 2.68 ± 0.22b* |
| D | 56 ± 5.61 | 0.75 ± 0.06 | 2.29 ± 0.47 |
Results are mean ± SD (n = 6)
D + G, diabetic rats that treated with aqueous garlic extract; G, normal rats that treated with aqueous garlic extract
* P < 0.05; ** P < 0.01; *** P < 0.001
aCompare with control (C) rats
bCompare with diabetic rats (D)
Effects of Garlic Extract on Oxidative Stress Status in Kidney Tissues
The levels of MDA, TOS and TAC in kidney tissues are shown in Table 4. The level of MDA in kidney tissue homogenates was increased significantly (P < 0.001) in diabetic rats compared with control group and decreased significantly (P < 0.05) in D + G group compared with D group. The level of TOS in kidney tissue homogenates was increased significantly (P < 0.05) in diabetic rats compared with control group and decreased significantly (P < 0.05) in D + G group compared with diabetic rats. The levels of TAC in kidney tissues of diabetic rats were decreased significantly (P < 0.01) compared with control rats and increased significantly (P < 0.05) in D + G rats compared with diabetic rats.
Table 4.
Effects of garlic extract on oxidative status in control and experimental rats
| Groups | MDA (μmol/mg protein) | TOS (μmol/mg protein) | TAC (μmol/mg protein) |
|---|---|---|---|
| C | 0.053 ± 0.006 | 2.983 ± 0.648 | 0.042 ± 0.006 |
| D | 0.114 ± 0.012a*** | 4.307 ± 0.611a* | 0.025 ± 0.006a** |
| D + G | 0.081 ± 0.018a*,b* | 3.191 ± 0.545b* | 0.05 ± 0.016b* |
| D | 0.080 ± 0.013 | 3.121 ± 0.715 | 0.053 ± 0.011 |
Results are mean ± SD (n = 6)
D + G, diabetic rats that treated with aqueous garlic extract; G, normal rats that treated with aqueous garlic extract; MDA, malondialdehyde; TOS, total oxidant status; TAC, total antioxidant capacity
* P < 0.05; ** P < 0.01; *** P < 0.001
aCompare with control (C) rats
bCompare with diabetic rats (D)
Effects of Garlic Extract on NO Level of Kidney Tissues
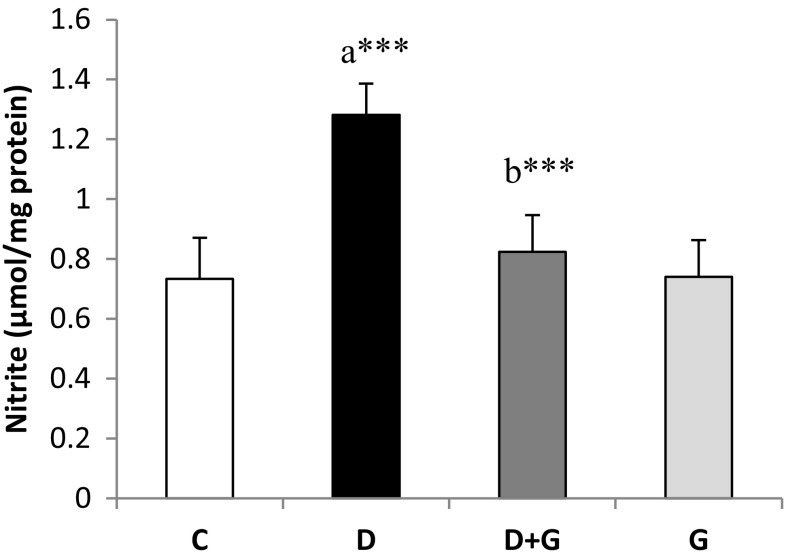
The nitrite level in the kidney tissues of diabetic rats was elevated significantly (P < 0.001) compared to control group and decreased significantly (P < 0.001) in diabetic rats treated with garlic comparison with diabetic rats (Fig. 1).
Fig. 1.
Effects of garlic extract on nitrite level of kidney tissues in control and experimental rats. Results are mean ± SD (n = 6). aCompare with control (C) rats and bcompare with diabetic rats (D). ***P < 0.001; D + G, diabetic rats that treated with aqueous garlic extract; G, normal rats that treated with aqueous garlic extract
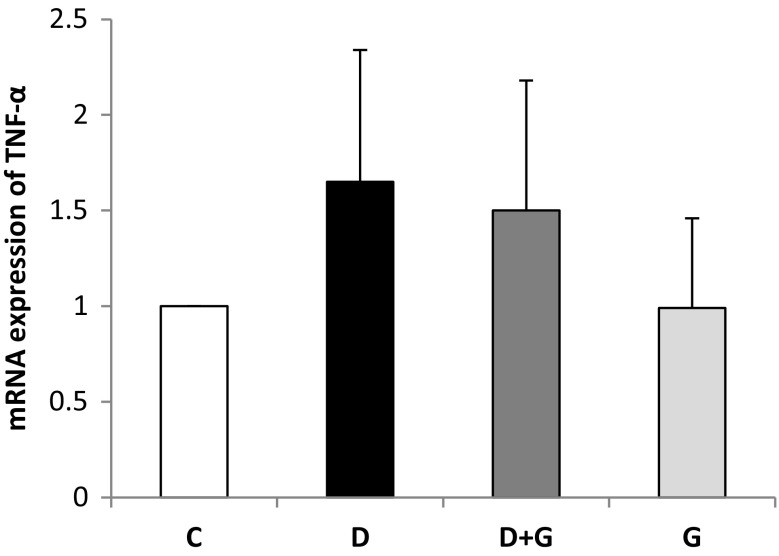
Effects of Garlic Extract on mRNA Folding Changes of TNF-α in Kidney Tissues
The mRNA levels of TNF-α in kidney tissues was shown in Fig. 2. The expression of TNF-α was unaffected in diabetic rats compared to control rats and in D + G group compared to diabetic rats.
Fig. 2.
Effects of garlic extract on mRNA folding changes of tumor necrosis factor-alpha (TNF-α) in kidney tissues of control and experimental rats. C, normal control rats; D, diabetic rats; D + G, diabetic rats that treated with aqueous garlic extract; G, normal rats that treated with aqueous garlic extract
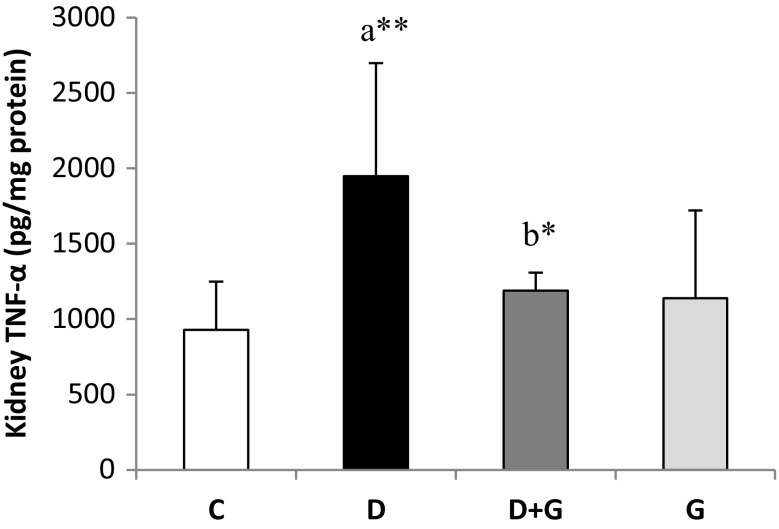
Effects of Garlic Extract on TNF-α Level
The level of TNF-α in kidney tissue homogenates was increased significantly (P < 0.01) in diabetic rats compared with control group and decreased significantly (P < 0.05) in D + G group compared with D group (Fig. 3).
Fig. 3.
Effects of garlic extract on tumor necrosis factor-alpha (TNF-α) level of kidney tissues in control and experimental rats. Results are mean ± SD (n = 6). aCompare with control (C) rats and bcompare with diabetic rats (D). *P < 0.05; **P < 0.01. D + G, diabetic rats that treated with aqueous garlic extract; G, normal rats that treated with aqueous garlic extract
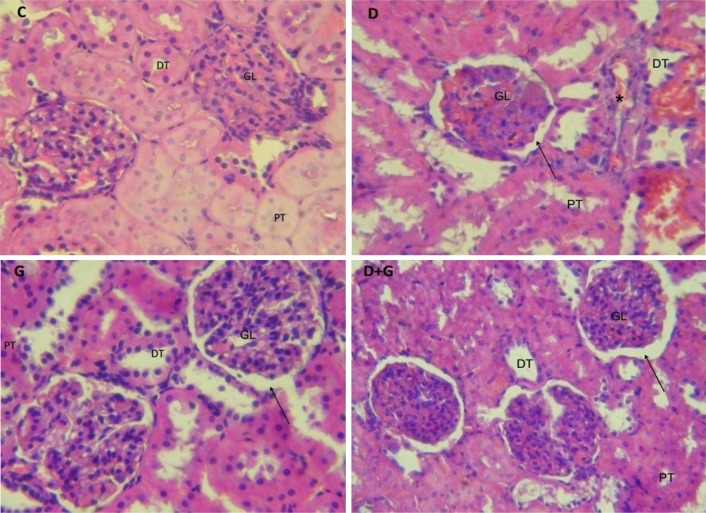
Effects of Garlic Extract on Histopathology of Kidneys
The kidney tissues photomicrographs of control and experimental groups of rats were represented in Fig. 4. In kidney tissue of control rats (C), glomerulus and Bowman capsule space were normal and proximal tube with normal columnar epithelium and distal tube with cubic epithelium were seen. In diabetic animals (D) proximal and distal tubes were expanded. Glomerulus size was normal and similar to control rats but Bowman capsule space was increased. In garlic-treated diabetic rats (D + G) general structure was improved and the expansion of proximal and distal tubes was decreased whereas expansion of Bowman capsule space was not affected. In garlic-treated normal rats (G) a little expansion in proximal and distal tubes were seen but glomerulus size was normal.
Fig. 4.
Light micrographs of H&E staining of kidney tissues of control and experimental rats. C, normal control rats; D, diabetic rats; D + G, diabetic rats that treated with aqueous garlic extract, G: normal rats that treated with aqueous garlic extract; GL, glomerulus; PT, proximal tube; DT, distal tube and asterisk degenerated tubules, arrow Bowman capsule space
Discussion
Diabetes mellitus type 1, induced by streptozotocin in animals, is considered to be a good model for the study of hyperglycemia. STZ causes cytotoxicity in β cells of pancreas and impairs insulin secretion [19]. In recent years, the protective and therapeutic effects of several natural remedies have been investigated in diabetic animal models [20–23]. Allium sativum (garlic) extract has been used as traditional herbal medicine in many cultures for the treatment of several diseases [11]. In this study, the effects of the aqueous extract of garlic on type 1 diabetic rats are evaluated.
The results show that glucose levels in diabetic rats were significantly higher than normal control rats. Daily oral administration of garlic extract for 30 days decreased the serum glucose concentration in diabetic rats. The hypoglycemic effect of garlic has been proved in several studies [12, 24]. It appears that garlic extract can stimulate insulin secretion in the remaining pancreatic β cells. Insulin manages glucose level in the serum of rats by enhancing glucose utilization in peripheral tissues [25].
By the end of the study, the final body weight of control rats increased normally compared to initial body weight (42 g). In diabetic rats, the final body weight decreased in comparison to normal control rats. In other words, Δ body weight of diabetic rats was negative (−15 g). In diabetic condition, due to the absence of insulin, the import of glucose to peripheral tissues decreased while lipolysis in adipose tissues increased. Therefore, the disruption of glucose and lipid metabolism caused a loss of body weight in diabetic animals. Treatment of diabetic rats with garlic extract prevented of loss of body weight (Δ Body weight was 18 g). It appears therefore that garlic can normalize glucose and lipid metabolism and control body weight in diabetic rats that are treated with this extract [25]. We also observed weight gain (Δ Body weight was 20 g) in garlic-treated control rats, but it was lower than normal control rats. It is possible that the gavage caused a stress in garlic-treated control rats and inhibited normal weight gain, which was not the case in normal control rats.
Nephropathy is one of the most important complications of diabetes. It is common to evaluate the serum concentrations of urea, creatinine, and uric acid as standard markers of renal function [1]. Our data show that urea and uric acid concentrations increased in diabetic rats but decreased in those diabetic rats that were treated with garlic extract. These results demonstrate the kidney malfunction in diabetic rats and improvement of kidney function in diabetic rats treated with garlic.
Long term hyperglycemia in diabetic condition causes induction of oxidative stress by increasing the reactive oxygen species (ROS) and decreasing the antioxidant defence. Oxidative stress is hypothesized to play a key role in the pathogenesis of diabetes and its complications. Therefore, one of the most important treatments of diabetes involves the reduction of free radicals in patients and usage of free radical scavengers or antioxidant components such as plant extracts. We demonstrate that MDA and TOS levels elevated in the kidney tissue homogenates of diabetic rats, whereas TAC decreased. Interestingly, these factors return to near-normal levels in diabetic rats after treatment with garlic extract. These results were completely expected because the antioxidant properties of this plant are known. Garlic has organosulfur compounds such as S-allyl cysteine (SAC) that can scavenge free radicals [26, 27].
Many studies consider diabetes mellitus to be an inflammatory disease. The concentrations of different cytokines are found to increase in the serum of patients with diabetes mellitus [3]. TNF-α is one such inflammatory cytokine that increases in the serum of diabetic patients. The kidney cells are capable of biosynthesizing TNF-α moreover the responsing to serum’s TNF-α [28]. The results of current study show that the level of TNF-α protein in the kidney of diabetic rats increased significantly compared to control rats, while the level of TNF-α mRNA did not increase significantly. It seems that the high concentration of TNF-α in serum of diabetic animals causes the accumulation and trapping of cytokine in kidney tissue and hence leads to diabetic nephropathy [29]. On the other hand, it is possible that the posttranscriptional regulation of TNF-α causes the difference between mRNA and protein level of this cytokine [30, 31]. Hyperglycemia and oxidative stress may influence the cis-acting regulatory regions and therefore stabilize mRNA and increase protein level of TNF-α [32]. In diabetic rats, the increase of TNF-α in serum caused the transmission of inflammatory signals through TNF receptor 1 (TNF-R1) to renal cells and thus activated NF-kB. NF-kB induced the up-regulation of inflammatory genes such as iNOS. Therefore, the level of NO increased. NO has the ability to initiate apoptosis and DNA fragmentation [3]. It is possible that NO is an important factor that leads to the destruction of kidney cells and hence to kidney dysfunction.
After oral administration of an aqueous extract of garlic in diabetic rats, the inflammation ameliorated through the reduction of NO and TNF-α levels. As the level of NO decreased in the renal tissues of diabetic rats treated with garlic extract, the function and histology of the kidneys approached normalcy.
It is therefore concluded that the aqueous extract of garlic, due to the antioxidant properties of the plants, must be considered as a good candidate for future studies about the treatment of diabetes mellitus and its abilities to reduce complications in the kidney tissues. In addition, more investigations should be carried out for the evaluation of the detailed mechanism.
Acknowledgments
The present study was funded by Vice-chancellor for Research and Technology, Hamadan University of Medical Sciences (No. 9312126433).
Compliance with Ethical Standards
Conflict of interest
The authors declare that there are no conflicts of interest.
References
- 1.Sivakumar S, Palsamy P, Subramanian SP. Impact of D-pinitol on the attenuation of proinflammatory cytokines, hyperglycemia-mediated oxidative stress and protection of kidney tissue ultrastructure in streptozotocin-induced diabetic rats. Chem Biol Interact. 2010;188(1):237–245. doi: 10.1016/j.cbi.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 2.Saravanan G, Ponmurugan P. S-allylcysteine improves streptozotocin-induced alterations of blood glucose, liver cytochrome P450 2E1, plasma antioxidant system, and adipocytes hormones in diabetic rats. Int J Endocrinol Metab. 2013;11(4):e10927. doi: 10.5812/ijem.10927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ingaramo PI, Ronco MT, Frances DE, Monti JA, Pisani GB, Ceballos MP, et al. Tumor necrosis factor alpha pathways develops liver apoptosis in type 1 diabetes mellitus. Mol Immunol. 2011;48(12–13):1397–1407. doi: 10.1016/j.molimm.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 4.Nasiri A, Ziamajidi N, Behrouj H, Abbasalipourkabir R, Dehghan A. The effects of aqueous extract of chicory root on steatosis, lipid profile and liver damage enzyme markers in tamoxifen-treated rats. Mol Biochem Diagn J. 2014;1(3):185–194. [Google Scholar]
- 5.Behrouj H, Ziamajidi N, Abbasalipourkabir R, Nasiri A, Solemani Asl S. Therapeutic effect of silybum marianum plant extract on tamoxifen-induced fatty liver in rats. Avicenna J Med Biochem. 2015;3(1):e27160. doi: 10.17795/ajmb-27160. [DOI] [Google Scholar]
- 6.Chauhan P, Mahajan S, Kulshrestha A, Shrivastava S, Sharma B, Goswamy HM, Prasad GB. Bougainvillea spectabilis exhibits antihyperglycemic and antioxidant activities in experimental diabetes. J Evid Based Complement Altern Med. 2016;21(3):177–185. doi: 10.1177/2156587215595152. [DOI] [PubMed] [Google Scholar]
- 7.Jaiswal D, Rai PK, Mehta S, Chatterji S, Shukla S, Rai DK, et al. Role of Moringa oleifera in regulation of diabetes-induced oxidative stress. Asian Pac J Trop Med. 2013;6(6):426–432. doi: 10.1016/S1995-7645(13)60068-1. [DOI] [PubMed] [Google Scholar]
- 8.Rai PK, Jaiswal D, Mehta S, Rai DK, Sharma B, Watal G. Effect of Curcuma longa freeze dried rhizome powder with milk in STZ induced diabetic rats. Indian. J Clin Biochem. 2010;25(2):175–181. doi: 10.1007/s12291-010-0032-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rai PK, Jaiswal D, Rai DK, Sharma B, Watal G. Antioxidant potential of oral feeding of Cynodon dactylon extract on diabetes induced oxidative stress. J Food Biochem. 2010;34(1):78–92. doi: 10.1111/j.1745-4514.2009.00265.x. [DOI] [Google Scholar]
- 10.Rai PK, Jaiswal D, Rai DK, Sharma B, Watal G. Effect of water extract of Trichosanthes dioica fruits in streptozotocin induced diabetic rats. Indian J Clin Biochem. 2008;23(4):387–390. doi: 10.1007/s12291-008-0085-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh VK, Singh DK. Pharmacological effects of garlic (Allium sativum L.) Annu Rev Biomed Sci. 2008;10:6–26. doi: 10.5016/1806-8774.2008.v10p6. [DOI] [Google Scholar]
- 12.El-Demerdash FM, Yousef MI, El-Naga NI. Biochemical study on the hypoglycemic effects of onion and garlic in alloxan-induced diabetic rats. Food Chem Toxicol. 2005;43(1):57–63. doi: 10.1016/j.fct.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 13.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 14.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95(2):351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 15.Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem. 2005;38(12):1103–1111. doi: 10.1016/j.clinbiochem.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 16.Benzie IF, Strain J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239(1):70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 17.Miranda KM, Espey MG, Wink DA. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide. 2001;5(1):62–71. doi: 10.1006/niox.2000.0319. [DOI] [PubMed] [Google Scholar]
- 18.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 19.Rezagholizadeh L, Pourfarjam Y, Nowrouzi A, Nakhjavani M, Meysamie A, Ziamajidi N, et al. Effect of Cichorium intybus L. on the expression of hepatic NF-κB and IKKβ and serum TNF-α in STZ− and STZ+ niacinamide-induced diabetes in rats. Diabetol Metab Syndr. 2016;8(1):1. doi: 10.1186/s13098-016-0128-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ziamajidi N, Khaghani S, Hassanzadeh G, Vardasbi S, Ahmadian S, Nowrouzi A, et al. Amelioration by chicory seed extract of diabetes- and oleic acid-induced non-alcoholic fatty liver disease (NAFLD)/non-alcoholic steatohepatitis (NASH) via modulation of PPARalpha and SREBP-1. Food Chem Toxicol. 2013;58:198–209. doi: 10.1016/j.fct.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 21.Shirwaikar A, Rajendran K, Barik R. Effect of aqueous bark extract of Garuga pinnata Roxb. in streptozotocin-nicotinamide induced type-II diabetes mellitus. J Ethnopharmacol. 2006;107(2):285–290. doi: 10.1016/j.jep.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 22.Watal G, Dhar P, Srivastava SK, Sharma B. Herbal medicine as an alternative medicine for treating diabetes. Evid Based Complement Altern Med. 2014;2014:1–2. doi: 10.1155/2014/596071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh RK, Sharma B. Certain traditional indian plants and their therapeutic applications: a review. VRI Phytomed. 2013;1(1):1–11. [Google Scholar]
- 24.Eidi A, Eidi M, Esmaeili E. Antidiabetic effect of garlic (Allium sativum L.) in normal and streptozotocin-induced diabetic rats. Phytomedicine. 2006;13(9):624–629. doi: 10.1016/j.phymed.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 25.Liu CT, Sheen LY, Lii CK. Does garlic have a role as an antidiabetic agent? Mol Nutr Food Res. 2007;51(11):1353–1364. doi: 10.1002/mnfr.200700082. [DOI] [PubMed] [Google Scholar]
- 26.Saravanan G, Ponmurugan P. Ameliorative potential of S-allyl cysteine on oxidative stress in STZ induced diabetic rats. Chem Biol Interact. 2011;189(1–2):100–106. doi: 10.1016/j.cbi.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 27.Hsu CC, Yen HF, Yin MC, Tsai CM, Hsieh CH. Five cysteine-containing compounds delay diabetic deterioration in Balb/cA mice. J Nutr. 2004;134(12):3245–3249. doi: 10.1093/jn/134.12.3245. [DOI] [PubMed] [Google Scholar]
- 28.Navarro JF, Milena FJ, Mora C, Leon C, Claverie F, Flores C, et al. Tumor necrosis factor-alpha gene expression in diabetic nephropathy: relationship with urinary albumin excretion and effect of angiotensin-converting enzyme inhibition. Kidney Int Suppl. 2005;99:S98–S102. doi: 10.1111/j.1523-1755.2005.09918.x. [DOI] [PubMed] [Google Scholar]
- 29.Moriwaki Y, Yamamoto T, Shibutani Y, Aoki E, Tsutsumi Z, Takahashi S, et al. Elevated levels of interleukin-18 and tumor necrosis factor-α in serum of patients with type 2 diabetes mellitus: relationship with diabetic nephropathy. Metabolism. 2003;52(5):605–608. doi: 10.1053/meta.2003.50096. [DOI] [PubMed] [Google Scholar]
- 30.Leverkus M, Yaar M, Eller MS, Tang EH, Gilchrest BA. Post-transcriptional regulation of UV induced TNF-α expression. J Investig Dermatol. 1998;110(4):353–357. doi: 10.1046/j.1523-1747.1998.00154.x. [DOI] [PubMed] [Google Scholar]
- 31.MacKenzie S, Fernàndez-Troy N, Espel E. Post-transcriptional regulation of TNF-α during in vitro differentiation of human monocytes/macrophages in primary culture. J Leukoc Biol. 2002;71(6):1026–1032. [PubMed] [Google Scholar]
- 32.Boado RJ, Pardridge WM. Glucose deprivation and hypoxia increase the expression of the GLUT1 glucose transporter via a specific mRNA cis-acting regulatory element. J Neurochem. 2002;80(3):552–554. doi: 10.1046/j.0022-3042.2001.00756.x. [DOI] [PubMed] [Google Scholar]