Abstract
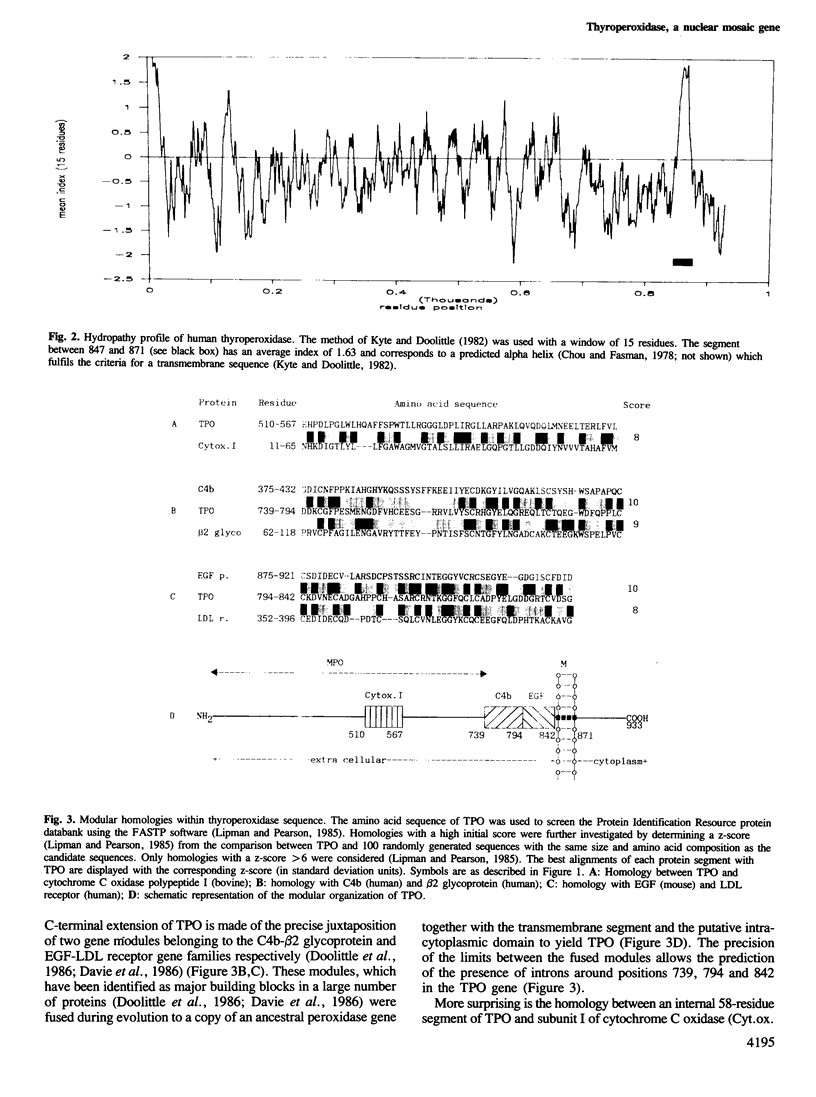
A lambda gt11 cDNA library was constructed from a normal human thyroid and screened with a rabbit anti-porcine thyroperoxidase antibody. A series of thyroperoxidase (TPO) clones were obtained which allowed determination of the complete primary structure of the protein. The library was also screened with serum from a patient with Hashimoto's thyroiditis, an autoimmune disease characterized by the presence in the serum of high titers of autoantibodies directed against the 'microsomal antigen' (McAg). Comparison of the cDNA sequences from TPO clones and McAg clones provides definite proof that the McAg is TPO. A short segment of TPO was characterized as bearing a major epitope involved in autoimmunity. The primary structure of TPO was 42% homologous to myeloperoxidase (MPO). It contains, in addition, a C-terminal extension with a membrane anchor region contiguous to two domains encoded by modules belonging to the EGF and C4b gene families. The existence in TPO of still another domain presenting a significant homology with a putative heme-binding region of cytochrome C oxidase polypeptide I raises the possibility that a mitochondrial gene module has contributed a piece to the evolution of a typical nuclear mosaic gene.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander N. M. Purification of bovine thyroid peroxidase. Endocrinology. 1977 Jun;100(6):1610–1620. doi: 10.1210/endo-100-6-1610. [DOI] [PubMed] [Google Scholar]
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Empirical predictions of protein conformation. Annu Rev Biochem. 1978;47:251–276. doi: 10.1146/annurev.bi.47.070178.001343. [DOI] [PubMed] [Google Scholar]
- Czarnocka B., Ruf J., Ferrand M., Carayon P., Lissitzky S. Purification of the human thyroid peroxidase and its identification as the microsomal antigen involved in autoimmune thyroid diseases. FEBS Lett. 1985 Oct 7;190(1):147–152. doi: 10.1016/0014-5793(85)80446-4. [DOI] [PubMed] [Google Scholar]
- Davie E. W., Ichinose A., Leytus S. P. Structural features of the proteins participating in blood coagulation and fibrinolysis. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 1):509–514. doi: 10.1101/sqb.1986.051.01.062. [DOI] [PubMed] [Google Scholar]
- Doolittle R. F., Feng D. F., Johnson M. S., McClure M. A. Relationships of human protein sequences to those of other organisms. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 1):447–455. doi: 10.1101/sqb.1986.051.01.054. [DOI] [PubMed] [Google Scholar]
- Farrelly F., Butow R. A. Rearranged mitochondrial genes in the yeast nuclear genome. Nature. 1983 Jan 27;301(5898):296–301. doi: 10.1038/301296a0. [DOI] [PubMed] [Google Scholar]
- Hadler H. I., Dimitrijevic B., Mahalingam R. Mitochondrial DNA and nuclear DNA from normal rat liver have a common sequence. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6495–6499. doi: 10.1073/pnas.80.21.6495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs H. T., Grimes B. Complete nucleotide sequences of the nuclear pseudogenes for cytochrome oxidase subunit I and the large mitochondrial ribosomal RNA in the sea urchin Strongylocentrotus purpuratus. J Mol Biol. 1986 Feb 20;187(4):509–527. doi: 10.1016/0022-2836(86)90330-x. [DOI] [PubMed] [Google Scholar]
- Johnson K. R., Nauseef W. M., Care A., Wheelock M. J., Shane S., Hudson S., Koeffler H. P., Selsted M., Miller C., Rovera G. Characterization of cDNA clones for human myeloperoxidase: predicted amino acid sequence and evidence for multiple mRNA species. Nucleic Acids Res. 1987 Mar 11;15(5):2013–2028. doi: 10.1093/nar/15.5.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Libert F., Ruel J., Ludgate M., Swillens S., Alexander N., Vassart G., Dinsart C. Complete nucleotide sequence of the human thyroperoxidase-microsomal antigen cDNA. Nucleic Acids Res. 1987 Aug 25;15(16):6735–6735. doi: 10.1093/nar/15.16.6735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Magnusson R. P., Gestautas J., Seto P., Taurog A., Rapoport B. Isolation and characterization of a cDNA clone for porcine thyroid peroxidase. FEBS Lett. 1986 Nov 24;208(2):391–396. doi: 10.1016/0014-5793(86)81055-9. [DOI] [PubMed] [Google Scholar]
- Morishita K., Kubota N., Asano S., Kaziro Y., Nagata S. Molecular cloning and characterization of cDNA for human myeloperoxidase. J Biol Chem. 1987 Mar 15;262(8):3844–3851. [PubMed] [Google Scholar]
- Ohtaki S., Kotani T., Nakamura Y. Characterization of human thyroid peroxidase purified by monoclonal antibody-assisted chromatography. J Clin Endocrinol Metab. 1986 Sep;63(3):570–576. doi: 10.1210/jcem-63-3-570. [DOI] [PubMed] [Google Scholar]
- Portmann L., Hamada N., Heinrich G., DeGroot L. J. Anti-thyroid peroxidase antibody in patients with autoimmune thyroid disease: possible identity with anti-microsomal antibody. J Clin Endocrinol Metab. 1985 Nov;61(5):1001–1003. doi: 10.1210/jcem-61-5-1001. [DOI] [PubMed] [Google Scholar]
- Powell R., Neilan J., Gannon F. Plaque dot assay. Nucleic Acids Res. 1986 Feb 11;14(3):1541–1541. doi: 10.1093/nar/14.3.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROITT I. M., LING N. R., DONIACH D., COUCHMAN K. G. THE CYTOPLASMIC AUTO-ANTIGEN OF THE HUMAN THYROID. I. IMMUNOLOGICAL AND BIOCHEMICAL CHARACTERISTICS. Immunology. 1964 Jul;7:375–393. [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virion A., Courtin F., Dème D., Michot J. L., Kaniewski J., Pommier J. Spectral characteristics and catalytic properties of thyroid peroxidase-H2O2 compounds in the iodination and coupling reactions. Arch Biochem Biophys. 1985 Oct;242(1):41–47. doi: 10.1016/0003-9861(85)90477-1. [DOI] [PubMed] [Google Scholar]
- Weitzman S. A., Stossel T. P., Harmon D. C., Daniels G., Maloof F., Ridgway E. C. Antineutrophil autoantibodies in Graves' disease. Implications of thyrotropin binding to neutrophils. J Clin Invest. 1985 Jan;75(1):119–123. doi: 10.1172/JCI111663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]