Abstract
Background
Although large series from national joint registries may accurately reflect indications for revision TKAs, they may lack the granularity to detect the true incidence and relative importance of such indications, especially periprosthetic joint infections (PJI).
Questions/purposes
Using a combination of individual chart review supplemented with New Zealand Joint Registry data, we asked: (1) What is the cumulative incidence of revision TKA? (2) What are the common indications for revising a contemporary primary TKA? (3) Do revision TKA indications differ at various followup times after primary TKA?
Methods
We identified 11,134 primary TKAs performed between 2000 and 2015 in three tertiary referral hospitals. The New Zealand Joint Registry and individual patient chart review were used to identify 357 patients undergoing subsequent revision surgery or any reoperation for PJI. All clinical records, radiographs, and laboratory results were reviewed to identify the primary revision reason. The cumulative incidence of each revision reason was calculated using a competing risk estimator.
Results
The cumulative incidence for revision TKA at 15 years followup was 6.1% (95% CI, 5.1%–7.1%). The two most-common revision reasons at 15 years followup were PJI followed by aseptic loosening. The risk of revision or reoperation for PJI was 2.0% (95% CI, 1.7%–2.3%) and aseptic loosening was 1.2% (95% CI, 0.7%–1.6%). Approximately half of the revision TKAs secondary to PJI occurred within 2 years of the index TKA (95% CI, 0.8%–1.2%), whereas half of the revision TKAs secondary to aseptic loosening occurred 8 years after the index TKA (95% CI, 0.4%–0.7%).
Conclusions
In this large cohort of patients with comprehensive followup of revision procedures, PJI was the dominant reason for failure during the first 15 years after primary TKA. Aseptic loosening became more important with longer followup. Efforts to improve outcome after primary TKA should focus on these areas, particularly prevention of PJI.
Level of Evidence
Level III, therapeutic study.
Electronic supplementary material
The online version of this article (doi:10.1007/s11999-017-5396-4) contains supplementary material, which is available to authorized users.
Introduction
Demand for primary TKA continues to increase worldwide [11]. With the aim of improving patient outcomes after surgery, there is an increasing trend toward publication of hospital and surgeon-level data for TKAs [8]. Because of the severe morbidity and costs associated with revision TKA, we first need to understand when and why we revise a primary TKA. This enables strategies to reduce the risk of revision surgery to be developed and prioritized.
Although registry data often have the indication for revision TKA, interpretation is limited by the lack of standardized definitions of revision reasons and objective assessment of radiologic and laboratory parameters [22]. In addition, one study suggested that registry-recorded indications for revision TKA are often inaccurate, particularly regarding periprosthetic joint infections (PJI) [28]. By contrast, large series of revision TKAs from tertiary referral centers are able to provide a more-accurate assessment of revision reasons because standardized definitions can be used. However, such studies lack information regarding the original primary TKA population, and consequently, the true incidence and relative frequency of each indication remain unknown [21, 24]. In addition, the original primary TKA in such studies often was performed in the distant past and may not reflect revision indications for a contemporary primary TKA [6, 20].
Using combined data from the New Zealand Joint Registry and individual patient chart review, we aimed to answer the following questions: (1) What is the cumulative incidence of revision TKA? (2) What are the common indications for revising contemporary primary TKAs? (3) Do revision TKA indications differ at various followups after primary TKA?
Materials and Methods
We performed a multicenter, retrospective study of all 11,134 primary TKAs done at three tertiary hospitals (North Shore Hospital, Auckland City Hospital, and Middlemore Hospital, Auckland, New Zealand) from January 1, 2000, to December 31, 2015. Inclusion criteria were revision TKA during which one or more components was exchanged, removed, manipulated, or added surgically or any reoperation resulting from PJI as defined by the Musculoskeletal Infection Society (MSIS) criteria [9, 17]. Exclusion criteria were unicompartmental knee arthroplasty, constrained TKA including hinged and nonhinged designs, and any tumor prosthesis.
Three hundred fifty-seven patients undergoing subsequent revision surgery or reoperation secondary to PJI were identified using a combination of individual search of patient records at each tertiary hospital, supplemented and verified with New Zealand National Joint Registry data which additionally identified revisions performed at institutions outside the three study hospitals.
The New Zealand Joint Registry’s most recent compliance audit in 2015 showed a capture rate of greater than 95% [16]. All patients were identified using a unique patient identifier (National Health Index) used in the New Zealand health system. Approval from all three district health boards and health and disability ethics committees was obtained. Local hospital data were used to confirm the New Zealand Joint Registry data and to identify patients who underwent revision surgery but were not captured by the registry. If the revision surgery or reoperation was performed at an institution outside the three primary study hospitals, consent was obtained to collect clinical and radiographic data to ensure complete data capture.
All patients received appropriate clinical assessment and medical examination before revision TKA. Clinical findings, laboratory investigation, radiologic data, and operation notes were assessed using a standardized written protocol, and a primary indication for revision TKA was determined independently by two authors (CKK and SR). Disagreements were resolved by consensus in conjunction with a third author (SWY). Where more than one revision reason was thought to contribute, the cause that was considered most important in the decision for the revision procedure was listed as the primary reason.
The revision reason was divided into one of nine categories as defined by Vince [26]: (1) PJI; (2) aseptic loosening; (3) patellofemoral arthrosis; (4) arthrofibrosis or stiffness; (5) tibiofemoral instability; (6) periprosthetic fracture; (7) patella maltracking; (8) polyethylene wear; or (9) extensor mechanism deficiency.
PJI was defined based on the MSIS definition [9, 17]. Aseptic loosening was defined as documented radiographic migration of components larger than 2 mm, progressive radiolucent lines greater than 2 mm, or an intraoperative finding of loose components [27]. If component loosening was present, aseptic loosening was recorded as the primary cause of revision and, if present, polyethylene wear, osteolysis, failure of ingrowth in uncemented implants, and bone collapse causing malalignment were considered secondary or predisposing factors [3]. Technetium bone and CT scans also were reviewed to confirm periprosthetic lucency or increased tracer uptake.
Patients with patellofemoral arthrosis underwent clinical and radiographic examinations (Merchant or patella skyline view) confirming evidence of chondral loss and osteophyte formation before secondary patellar resurfacing.
Arthrofibrosis was defined as a flexion contracture of 15° and/or less than 75° flexion and was considered the primary cause of failure in patients with a stiff but otherwise functional TKA when all other mechanisms were excluded [14, 26].
Primary tibiofemoral instability was considered if investigations excluded aseptic loosening, polyethylene wear, extensor mechanism disruption, PJI, and fracture. Physician office and hospital records were reviewed for clinical documentation of symptomatic instability and examination findings of varus or valgus laxity with the knee assessed at 0° and 30° flexion and/or instability in flexion were considered to assist with diagnosis [13, 25].
Extensor mechanism deficiency included patella and/or quadriceps tendon discontinuity and transverse patella fracture [26]. Patella maltracking or dislocation was defined as symptomatic subluxation and/or dislocation of the patella from the trochlear groove [26].
Polyethylene wear was listed as the primary cause if there was macroscopic evidence of wear and/or delamination on the surface of the polyethylene liner without signs of aseptic loosening [26] or other revision reasons.
Malalignment in the coronal, sagittal, or rotational planes was recorded when present, but was considered a secondary cause of revision rather than one of the nine primary reasons for revision TKA. This follows the rationale of Vince [26], which argues that while malrotation and/or malalignment contribute to revision indications such as patellar instability or arthrofibrosis, they are not classified as indications.
Patient Demographics and Characteristics
A total of 11,134 primary TKAs in 8830 patients met the inclusion criteria. The patients had a mean age of 69 years (range, 18-98 years) (Table 1). The most-common indication for primary TKA was osteoarthritis (96%). Median followup was 5 years (range, 1-16 years). During the study period, 1368 patients died (1653 TKAs). The mortality rate was 9% at 5 years, 28% at 10 years, and 52% at 15 years after the index operation (Table 2).
Table 1.
Demographics for primary and revision TKAs
| Demographic | Primary TKA (n = 11,134) | Revision TKA (n = 357) |
|---|---|---|
| Age at surgery (SD) | 69 (9.7) | 65* (9.7) |
| BMI (kg/m2; SD, total number recorded) | 33 (6.88; 3040) | 33 (7.23; 75) |
| Male (%) | 4792 (43%) | 163 (46%) |
| Indication for primary TKA | ||
| Osteoarthritis | 10,648 | 331 |
| Rheumatoid arthritis | 329 | 20 |
| Other inflammatory arthritis | 48 | 4 |
| Fracture | 50 | 2 |
| Other | 59 | 0 |
| Mean skin to skin time (minutes; range, total number recorded) | 93 (25–402; 10,402) | |
| ASA score | ||
| 1 | 529 (6%) | |
| 2 | 5232 (60%) | |
| 3 | 2862 (33%) | |
| 4 | 53 (0.6%) | |
| Total number of ASA scores recorded (number) | 8676 | |
| Primary surgery details | ||
| Patella resurfaced | 4858 (43.6%) | |
| Cemented TKA | 10,624 (95.4%) | |
| Hybrid TKA | 499 (4.5%) | |
| Uncemented TKA | 11 (0.1%) | |
| Cruciate-retaining knee | 7880 | |
| Posterior-substituting knee | 2278 | |
| Hospital | ||
| A | 4527 (40.7%) | 121 (2.7%†) |
| B | 4897 (44.0%) | 183 (3.7%†) |
| C | 1710 (15.3%) | 53 (3.1%†) |
*Age at the time of revision; †revision rate per hospital; ASA = American Society of Anesthesiologists
Table 2.
Cumulative incidence of revision TKA or reoperation for all reasons and mortality rate
| Variable | Followup (years) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.5 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | |
| Cumulative incidence for revision or reoperation TKA (%) | 0.8 | 1.2 | 1.9 | 2.3 | 2.8 | 3.1 | 3.4 | 3.5 | 3.9 | 4.2 | 4.4 | 4.7 | 5.4 | 5.6 | 5.9 | 6.1 |
| 95% CI | 0.6–0.9 | 1.0–1.4 | 1.6–2.1 | 2.0–2.6 | 2.5–3.1 | 2.7–3.5 | 3.0–3.8 | 3.1–3.9 | 3.4–4.3 | 3.7–4.7 | 3.9–5.0 | 4.1–5.2 | 4.6–6.1 | 4.8–6.4 | 4.9–6.8 | 5.1–7.1 |
| Kaplan-Meier estimate for mortality (%) | 0.6 | 1.1 | 2.4 | 4.2 | 5.9 | 8.6 | 11.7 | 15.0 | 18.6 | 22.7 | 28.0 | 32.4 | 36.0 | 40.3 | 46.5 | 52.1 |
Statistical Analysis
A competing risk estimator that copes with simultaneous risk of different revision indications while accounting for mortality was used to calculate the incidence for each revision TKA reason. The Kaplan-Meier method was used to calculate mortality rate as it was the method being used by the New Zealand Joint Registry for survivorship. The competing risk method was described by Fine and Gray [7] to cope with censoring subjects who experienced failure because of other causes instead of causes of interest. The other causes, which are referred to as competing events, are mutually exclusive to the event of interest. The competing risk method will estimate the event probability adjusted for other causes and was reported to have a higher accuracy in assessing cumulative incidence compared with the traditional Kaplan-Meier method [18].
Outputs from the competing risk method sum to 100% when including all competing events and all event-free probability. Cumulative incidences of different revision TKA reasons at different years of followup along with their 95% CIs were calculated using statistics software R version 3.3.2 (R Foundation for Statistical Computing, Vienna, Austria) [4].
Results
Of the 11,134 primary TKAs, 357 underwent revision or reoperation, with a 15-year incidence of 6%. Cumulative incidence for revision TKA was 1.2% at 1 year, 1.9% at 2 years, 3.1% at 5 years, 4.4% at 10 years, and 6.1% at 15 years after the index primary TKA (Table 2). The annual incidence for revision was highest during the first 3 years, and after 4 years, the annual risk of revision ranged from 0.1% to 0.3% per year.
The five most-common reasons for revision were (1) PJI; (2) aseptic loosening; (3) patellofemoral arthrosis; (4) instability; and (5) stiffness or arthrofibrosis (Fig. 1). There were 169 revision TKAs or reoperations performed owing to PJIs. Eighteen patients had culture-negative PJIs and three who had reoperation TKAs did not have component exchange (two had arthroscopic lavage and one with a monoblock tibial component underwent open débridement and lavage without component exchange). The cumulative incidence for PJI was 0.8% at 1 year, 1% at 2 years, 1.5% at 5 years, and 2% at 15 years after the index operation (Table 3). Fifty-two revision TKAs were performed owing to aseptic loosening. There were 6276 unresurfaced patellae from the original cohort of 11,134 primary TKAs. Forty-nine underwent secondary patella resurfacing owing to patellofemoral arthrosis. Thirty-two revisions were performed for instability and 18 for stiffness or arthrofibrosis, with an incidence of 0.4% and 0.2%, respectively (Fig. 1). The overall prevalence for the remaining four revision TKA categories was (1) nine polyethylene wear; (2) eight periprosthetic fracture; (3) seven patella maltracking; and (4) three extensor mechanism disruption (Table 3).
Fig. 1.
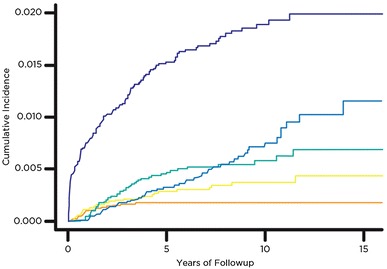
The cumulative incidences for the top five most-common reasons for revision TKAs are presented. The order from most-common to least-common revision reasons are (1) periprosthetic joint infection, (2) aseptic loosening, (3) patellofemoral arthrosis, (4) instability, and (5) stiffness.
Table 3.
Cumulative incidence (%) of reasons for revision TKA at various followups
| Followup (years) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reason for revision | 0.5 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 |
| Infection (95% CI) | 0.56 (0.42–0.7) | 0.76 (0.6–0.92) | 1.03 (0.84–1.22) | 1.18 (0.97–1.38) | 1.41 (1.17–1.64) | 1.52 (1.28–1.77) | 1.65 (1.38–1.91) | 1.68 (1.42–1.95) | 1.80 (1.51–2.08) | 1.86 (1.56–2.15) | 1.89 (1.59–2.19) | 1.93 (1.62–2.24) | 1.99 (1.66–2.32) | 1.99 (1.66–2.32) | 1.99 (1.66–2.32) | 1.99 (1.66–2.32) |
| Aseptic loosening (95% CI) | 0.01 (0–0.03) | 0.02 (0–0.04) | 0.13 (0.06–0.20) | 0.19 (0.1–0.27) | 0.28 (0.17–0.39) | 0.32 (0.2–0.45) | 0.38 (0.24–0.51) | 0.45 (0.30–0.61) | 0.54 (0.36–0.72) | 0.65 (0.44–0.86) | 0.71 (0.5-0.94) | 0.91 (0.51–1.18) | 1.02 (0.69–1.35) | 1.02 (0.69–1.35) | 1.15 (0.73–1.57) | 1.15 (0.73–1.57) |
| Patellofemoral arthrosis (95% CI) | 0 | 0.05 (0.01–0.09) | 0.20 (0.12–0.29) | 0.32 (0.21–0.43) | 0.41 (0.28–0.54) | 0.47 (0.32–0.51) | 0.50 (0.35–0.65) | 0.52 (0.36–0.67) | 0.54 (0.38–0.70) | 0.54 (0.38–0.70) | 0.58 (0.40–0.75) | 0.62 (0.42–0.82) | 0.68 (0.45–0.91) | 0.68 (0.45–0.91) | 0.68 (0.45–0.91) | 0.68 (0.45–0.91) |
| Instability (95% CI) | 0.05 (0.01–0.10) | 0.11 (0.05–0.17) | 0.18 (0.10–0.26) | 0.21 (0.12–0.30) | 0.24 (0.14–0.33) | 0.28 (0.17–0.39) | 0.30 (0.19–0.41) | 0.30 (0.19–0.41) | 0.34 (0.21–0.47) | 0.37 (0.23–0.51) | 0.37 (0.23–0.51) | 0.37 (0.23–0.51) | 0.43 (0.25–0.62) | 0.43 (0.25–0.62 | 0.43 (0.25–0.62 | 0.43 (0.25–0.62 |
| Stiffness (95% CI) | 0.36 (0–0.07) | 0.10 (0.04–0.16) | 0.14 (0.07–0.21) | 0.16 (0.09–0.24) | 0.18 (0.09–0.26) | 0.18 (0.09–0.26) | 0.18 (0.09–0.26) | 0.18 (0.09–0.26) | 0.18 (0.09–0.26) | 0.18 (0.09–0.26) | 0.18 (0.09–0.26) | 0.18 (0.09–0.26) | 0.18 (0.09–0.26) | 0.18 (0.09–0.26) | 0.18 (0.09–0.26) | 0.18 (0.09–0.26) |
| Polyethylene wear (95% CI) | 0 | 0 | 0 | 0 | 0 | 0.01 (0–0.04) | 0.01 (0–0.04) | 0.01 (0–0.04) | 0.01 (0–0.04) | 0.07 (0–0.15) | 0.14 (0.01–0.26) | 0.18 (0.03–0.32) | 0.18 (0.03–0.32) | 0.34 (0.07–0.61) | 0.34 (0.07–0.61) | 0.47 (0.10–0.84) |
| Periprosthetic fracture (95% CI) | 0.03 (0–0.06) | 0.03 (0–0.06) | 0.04 (0–0.07) | 0.04 (0–0.07) | 0.05 (0–0.09) | 0.05 (0–0.09) | 0.07 (0.01–0.12) | 0.07 (0.01–0.12) | 0.09 (0.02–0.16) | 0.09 (0.02–0.16) | 0.09 (0.02–0.16) | 0.13 (0.12–0.25) | 0.13 (0.12–0.25) | 0.13 (0.12–0.25) | 0.13 (0.12–0.25) | 0.13 (0.12–0.25) |
| Patella maltracking (95% CI) | 0.02 (0–0.04) | 0.04 (0–0.07) | 0.06 (0.01–0.1) | 0.06 (0.01–0.1) | 0.06 (0.01–0.1) | 0.07 (0.32–0.51) | 0.07 (0.32–0.51) | 0.07 (0.32–0.51) | 0.07 (0.32–0.51) | 0.07 (0.32–0.51) | 0.07 (0.32–0.51) | 0.07 (0.32–0.51) | 0.07 (0.32–0.51) | 0.07 (0.32–0.51) | 0.07 (0.32–0.51) | 0.07 (0.32–0.51) |
| Extensor mechanism dysfunction (95% CI) | 0.01 (0–0.03) | 0.02 (0–0.43) | 0.03 (0–0.06) | 0.03 (0–0.06) | 0.03 (0–0.06) | 0.03 (0–0.06) | 0.03 (0–0.06) | 0.03 (0–0.06) | 0.03 (0–0.06) | 0.03 (0–0.06) | 0.03 (0–0.06) | 0.03 (0–0.06) | 0.03 (0–0.06) | 0.03 (0–0.06) | 0.03 (0–0.06) | 0.03 (0–0.06) |
| Other (95% CI) | 0.05 (0.01–0.08) | 0.05 (0.01–0.10) | 0.07 (0.02–0.13) | 0.09 (0.03–0.14) | 0.10 (0.04–0.16) | 0.10 (0.04–0.16) | 0.10 (0.04–0.16) | 0.10 (0.04–0.16) | 0.10 (0.04–0.16) | 0.10 (0.04–0.16) | 0.10 (0.04–0.16) | 0.10 (0.04–0.16) | 0.10 (0.04–0.16) | 0.10 (0.04–0.16) | 0.10 (0.04–0.16) | 0.10 (0.04–0.16) |
| TKAs at risk (number) | 10,989 | 10,423 | 8946 | 7766 | 6603 | 5575 | 4775 | 4077 | 3232 | 2526 | 1909 | 1519 | 919 | 756 | 405 | 399 |
The timing of revision or reoperation varied by indication. Aseptic loosening and polyethylene wear combined were the primary causes for revision 8 years after the index surgery (Fig. 2). The annual incidence of PJI was highest during the first 4 years after primary TKA, with aseptic loosening and polyethylene wear becoming the most important indications after 8 years. The cumulative incidence for secondary patella resurfacing was highest during the first 5 years after primary TKA (0.5%) (Table 3). Revision secondary to stiffness tended to present early with all 18 revisions performed within 4 years of the index surgery (Appendix 1. Supplemental material is available with the online version of CORR ®)
Fig. 2.
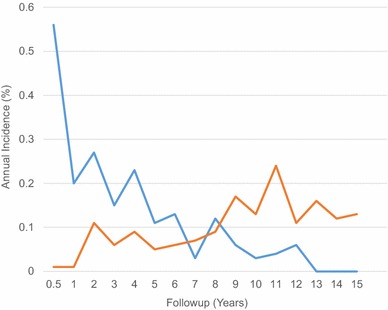
The annual incidence of periprosthetic joint infection versus aseptic loosening and polyethylene wear is shown.
Discussion
Revision surgery after TKA has long been used as the main outcome endpoint by joint registries worldwide [1, 10, 15, 23]. However, such registries lack clinical and radiologic data, making accurate analysis of revision indications difficult. Additionally, some studies show registry capture of certain revision indications is poor [12, 28]. In the current study, we combined local hospital data with national joint registry data to provide more-complete followup of patients who have undergone TKA during a long period and to allow accurate clinical and radiographic analyses of these indications. In contrast to registry findings and previous reports from tertiary revision centers, we found PJI to be the dominant mechanism of revision or reoperation during the first 15 years after primary TKA [15, 19, 21].
There are numerous limitations to our study. First, although the national registry allowed capture of patients who had moved to other cities or had revision surgery at outside institutions, any patient receiving revision surgery overseas would not be captured. Additionally, revision surgery is a rather crude endpoint and any patient who declined revision surgery or who was unable to undergo surgery for medical reasons likewise would not be captured. However, to our knowledge, the combined use of national health patient identifiers and joint registry data to achieve clinical followup of all identified TKA failures is unique, particularly for such a large number of patients across a 15-year period. Second, although we included revision TKAs involving addition or exchange of one or more components, we also recorded any reoperation attributable to confirmed PJI. Although this will tend to increase the relative importance of PJI as a failure mechanism, only three of 169 patients with PJI did not undergo some form of component exchange (ie, revision surgery). Third, there are no universally accepted criteria to define revision indications [2, 16]. We attempted to mitigate this by using the method described by Vince [26], applying standardized criteria to define each mode of failure as objectively as possible. Clinical records, including laboratory investigation, radiographic data, and clinical notes assessed by the reviewers (CKK, SR, and SWY), also were used to identify primary revision reason. However, we acknowledge the subjective nature of identifying the primary revision reason in some cases, as some indications for revision may overlap. One potential criticism of our method is that with our criteria, malalignment was considered a secondary cause of revision, rather than one of the nine primary revision indications. However, we feel the approach used in our study is more robust than that of national joint registries, which vary widely, with the number of recorded indications ranging from eight to 33 across national registries [16]. In addition, registries typically have no defined criteria for PJI [15]. Using the consensus criteria established by the MSIS, we identified 18 patients with culture-negative PJIs and three who had PJIs but were treated operatively without component exchange. Revision indication in such patients would almost certainly be incorrectly identified by registry data capture mechanisms.
The cumulative incidence of revision was 6.1% at 15 years followup and half of these revisions occurred within 3 years of the index primary TKA. This is consistent with the findings from New Zealand Joint Registry data with an overall revision rate of 6.3% at 15 years followup [15]. PJI was the dominant mechanism of failure, accounting for 47% of revision or reoperation TKAs, with a PJI incidence of 1.0% at 2 years and 2.0% at 15 years. The second most-common mechanism was aseptic loosening with a 15-year incidence of 1.2%, accounting overall for 15% of all revision TKAs. This contrasts with previous studies from tertiary referral centers, which reported aseptic loosening to be the most-common cause. Thiele et al. [24] and Sharkey et al. [21] reported aseptic loosening contributed to 39.9% and 21.8% of their revisions, respectively. Similarly, Fehring et al. [6] and Dalury et al. [5] reported 27% and 23.1% of their revisions were secondary to aseptic loosening, respectively. Such reports likely underestimate the importance of PJI as a failure mechanism owing to their status as referral centers. Treatment of patients with PJI often is performed acutely without time for transfer from a primary institution. Patients with more-complex revisions such as massive osteolysis or extensor mechanism disruption also are more likely to be referred, potentially increasing the relative prevalence of these mechanisms at referral centers. Additionally, such reports include revisions of implants placed more than 15 years ago. Although this will emphasize failure mechanisms, such as aseptic loosening, which become more common at long-term followup, it may not reflect failure mechanisms of more-modern prostheses. For example, Sharkey et al. [21] reported 41% of patients were referred from outside institutions, and time to revision ranged from 1 day to 30 years. Thiele et al. [24] reported a substantial number of revision TKAs in their study were performed in patients who were referred from outer regions and with incomplete baseline information, and 16% of their patients had index surgery performed before 2000. Similarly, Fehring et al. [6] reported revision TKAs performed between 1986 and 1999, and most of the patients were from referral areas. In our study, the availability of a known denominator of patients undergoing primary TKA enabled us to calculate the incidence for each revision reason, allowing more-accurate analysis of the relative importance of each mechanism.
National joint registries from Australia, United Kingdom, Sweden, and New Zealand all show aseptic loosening to be the most-common failure mechanism after primary TKA but capture of revision surgery resulting from infection often is poor [10, 15, 23, 28]. Lindgren et al. [12] reported a capture rate of 67% by the Swedish Hip Arthroplasty Register for reoperation resulting from PJI after THA. Zhu et al. [28] reported the New Zealand Joint Registry was 63% accurate in detecting reoperations for PJI (in hip and knee arthroplasties) when compared with data from the International Classification of Diseases, 9th and 10th editions, revision codes. This is consistent with findings of our study, which show a 76% capture rate for revision TKA resulting from PJI. There are some potential reasons for this. First, in our study, many revisions for PJI were performed in an acute setting, where different staffing may compromise reporting protocols to national registries [12]. Second, registry data sheets typically are collected at the time of revision surgery, before culture results are available, and such data sheets do not apply standardized definitions of PJI such as the MSIS criteria used in our study. The importance given to aseptic loosening as a failure mechanism in national registries and revision TKA series is reflected in technologic efforts to improve the outcome of primary TKA; our study suggests future efforts also should place similar emphasis on reducing PJI.
Secondary patella resurfacing for patellofemoral arthrosis was the third most-common reason for revision, accounting for 14% of all revision TKAs, with a 15-year incidence of 0.7%. The New Zealand Joint Registry reported secondary patella resurfacing constituted 24% of all revisions, a higher percentage compared with other registries [1, 15, 23]. This reflects our primary cohort, which had a high number of unsurfaced patella, because this failure mechanism can only occur in this setting.
As expected, the indications for revision or reoperation change with time after surgery. We found the highest incidence of PJI occurred during the first 2 years after primary TKA (1.0%), and after 5 years, the annual incidence was less than 0.2% per year. Some studies report infection as the primary cause for early failure, contributing to 18% to 27% of early revisions [6, 19, 21, 24]. After 8 years, we found aseptic loosening and polyethylene wear became the primary modes of failure and their incidence continued to increase. Aseptic loosening as a mode of failure therefore remains particularly relevant for younger patients, who can be expected to need 15 years of longevity or longer after their TKA.
We found PJI to be the dominant failure mechanism during the first 15 years after modern TKA. Aseptic loosening incidence increases markedly after 8 years and remains an important cause of failure, particularly in younger patients. Efforts to improve outcome after primary TKA should focus on these areas, particularly prevention of PJI.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We thank Toni Hobbs BSc (Department of Orthopaedic Surgery University of Otago, Christchurch) and Chris Frampton PhD (Cant), (Department of Orthopaedic Surgery University of Otago, Christchurch) for providing data from the New Zealand Joint Registry.
Footnotes
Each author certifies that he or she, or a member of his or her immediate family, has no funding or commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved or waived approval for the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
Procedures and data collection were performed at Middlemore Hospital, North Shore Hospital, and Auckland Hospital, Auckland, New Zealand. Data analysis was performed at North Shore Hospital, Auckland, New Zealand.
References
- 1.Australian Orthopaedic Association. National Joint Replacement Registry. Annual Report 2015 - Hip and Knee Arthroplasty. Available at: https://aoanjrr.sahmri.com/en/annual-reports-2015. Accessed May 25, 2016.
- 2.Barr CJ, Barbalace RJ, Wessinger SJ, Bragdon CR, Kwon YM, Malchau H. Validation of a hospital-based joint registry: quantification of errors and maximizing utility. J Arthroplasty. 2012;27:1766–1771. doi: 10.1016/j.arth.2012.04.028. [DOI] [PubMed] [Google Scholar]
- 3.Berend ME, Ritter MA, Meding JB, Faris PM, Keating EM, Redelman R, Faris GW, Davis KE. Tibial component failure mechanisms in total knee arthroplasty. Clin Orthop Relat Res. 2004;428:26–434. doi: 10.1097/01.blo.0000148578.22729.0e. [DOI] [PubMed] [Google Scholar]
- 4.Braun TM, Yuan Z. Comparing the small sample performance of several variance estimators under competing risks. Stat Med. 2007;26:1170–1180. doi: 10.1002/sim.2661. [DOI] [PubMed] [Google Scholar]
- 5.Dalury DF, Pomeroy DL, Gorab RS, Adams MJ. Why are total knee arthroplasties being revised? J Arthroplasty. 2013;28(8 suppl):120–121. doi: 10.1016/j.arth.2013.04.051. [DOI] [PubMed] [Google Scholar]
- 6.Fehring TK, Odum S, Griffin WL, Mason JB, Nadaud M. Early failures in total knee arthroplasty. Clin Orthop Relat Res. 2001;392:315–318. doi: 10.1097/00003086-200111000-00041. [DOI] [PubMed] [Google Scholar]
- 7.Fine JP, Gray RJ. A Proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. doi: 10.1080/01621459.1999.10474144. [DOI] [Google Scholar]
- 8.Haddad FS, Manktelow AR, Skinner JA. Publication of surgeon level data from registers: who benefits? Bone Joint J. 2016;98:1–2. doi: 10.1302/0301-620X.98B1.37709. [DOI] [PubMed] [Google Scholar]
- 9.Huang R, Hu CC, Adeli B, Mortazavi J, Parvizi J. Culture-negative periprosthetic joint infection does not preclude infection control. Clin Orthop Relat Res. 2012;470:2717–2723. doi: 10.1007/s11999-012-2434-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knutson K, Lewold S, Robertsson O, Lidgren L. The Swedish knee arthroplasty register: a nation-wide study of 30,003 knees 1976-1992. Acta Orthop Scand. 1994;65:375–386. doi: 10.3109/17453679408995475. [DOI] [PubMed] [Google Scholar]
- 11.Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780–785. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 12.Lindgren JV, Gordon M, Wretenberg P, Kärrholm J, Garellick G. Validation of reoperations due to infection in the Swedish Hip Arthroplasty Register. BMC Musculoskelet Disord. 2014;15:384. doi: 10.1186/1471-2474-15-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mandalia V, Eyres K, Schranz P, Toms AD. Evaluation of patients with a painful total knee replacement. J Bone Joint Surg Br. 2008;90:265–271. doi: 10.1302/0301-620X.90B3.20140. [DOI] [PubMed] [Google Scholar]
- 15.Nelson CL, Kim J, Lotke PA. Stiffness after total knee arthroplasty. J Bone Joint Surg Am. 2005;87(suppl 1):264–270. doi: 10.2106/00004623-200509001-00009. [DOI] [PubMed] [Google Scholar]
- 16.New Zealand Orthopaedic Association. The New Zealand Joint Registry: Sixteen year report January 1999 to December 2014. Available at: http://nzoa.org.nz/system/files/Web_DH7657_NZJR2014Report_v4_12Nov15.pdf. Accessed October 3, 2016.
- 17.Niinimäki TT. The reasons for knee arthroplasty revisions are incomparable in the different arthroplasty registries. Knee. 2015;22:142–144. doi: 10.1016/j.knee.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 18.Parvizi J, Zmistowski B, Berbari EF, Bauer TW, Springer BD, Valle Della CJ, Garvin KL, Mont MA, Wongworawat MD, Zalavras CG. New definition for periprosthetic joint infection: from the Workgroup of the Musculoskeletal Infection Society. Clin Orthop Relat Res. 2011;469:2992–2994. doi: 10.1007/s11999-011-2102-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Porcher R. CORR Insights®: Kaplan-Meier survival analysis overestimates the risk of revision arthroplasty: a meta-analysis. Clin Orthop Relat Res. 2015;473:3443–3445. doi: 10.1007/s11999-015-4291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schroer WC, Berend KR, Lombardi AV, Barnes CL, Bolognesi MP, Berend ME, Ritter MA, Nunley RM. Why are total knees failing today? Etiology of total knee revision in 2010 and 2011. J Arthroplasty. 2013;28(8 suppl):116–119. doi: 10.1016/j.arth.2013.04.056. [DOI] [PubMed] [Google Scholar]
- 21.Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM. Insall Award paper: Why are total knee arthroplasties failing today? Clin Orthop Relat Res. 2002;404:7–13. doi: 10.1097/00003086-200211000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Sharkey PF, Lichstein PM, Shen C, Tokarski AT, Parvizi J. Why are total knee arthroplasties failing today: has anything changed after 10 years? J Arthroplasty. 2014;29:1774–1778. doi: 10.1016/j.arth.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 23.Siqueira MB, Klika AK, Higuera CA, Barsoum WK. Modes of failure of total knee arthroplasty: registries and realities. J Knee Surg. 2014;28:127–138. doi: 10.1055/s-0034-1396014. [DOI] [PubMed] [Google Scholar]
- 14.The NJR Editorial Board. NJR, National Joint Registry. 12th Annual Report 2015. National Joint Registry for England, Wales, Northern Ireland and the Isle of Man. Surgical data to 31 December 2014. Available at: http://www.njrcentre.org.uk/njrcentre/Portals/0/Documents/England/Reports/12th%20annual%20report/NJR%20Online%20Annual%20Report%202015.pdf. Accessed October 3, 2016.
- 24.Thiele K, Perka C, Matziolis G, Mayr HO, Sostheim M, Hube R. Current failure mechanisms after knee arthroplasty have changed: polyethylene wear is less common in revision surgery. J Bone Joint Surg Am. 2015;97:715–720. doi: 10.2106/JBJS.M.01534. [DOI] [PubMed] [Google Scholar]
- 25.Vince KG. Diagnosis and management of patients with instability of the knee. Instr Course Lect. 2012;61:515–524. [PubMed] [Google Scholar]
- 26.Vince KG. The problem total knee replacement: systematic, comprehensive and efficient evaluation. Bone Joint J. 2014;96(11 suppl A):105–111. [DOI] [PubMed]
- 27.Vyskocil P, Gerber C, Bamert P. Radiolucent lines and component stability in knee arthroplasty: standard versus fluoroscopically-assisted radiographs. J Bone Joint Surg Br. 1999;81:24–26. doi: 10.1302/0301-620X.81B1.9213. [DOI] [PubMed] [Google Scholar]
- 28.Zhu M, Ravi S, Frampton C, Luey C, Young S. New Zealand Joint Registry data underestimates the rate of prosthetic joint infection. Acta Orthop. 2016;87:346–350. doi: 10.3109/17453674.2016.1171639. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


