Abstract
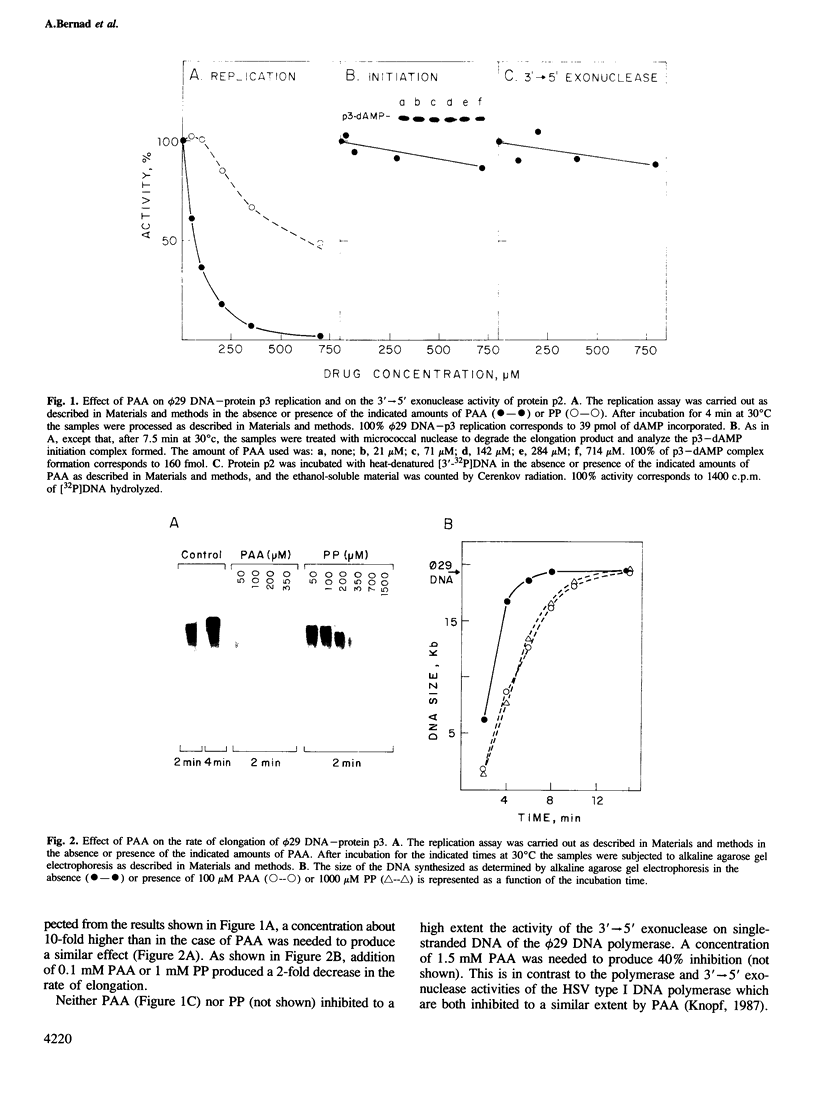
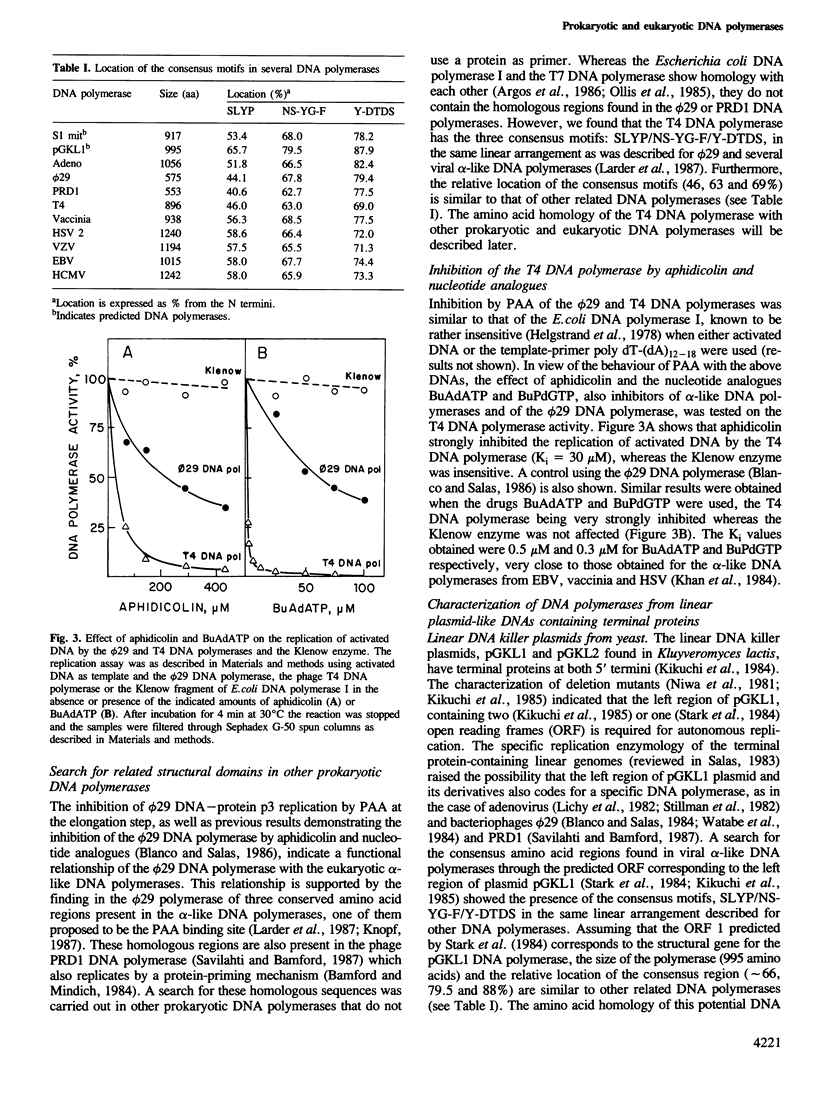
The Bacillus subtilis phage luminal diameter 29 DNA polymerase, involved in protein-primed viral DNA replication, was inhibited by phosphonoacetic acid (PAA), a known inhibitor of alpha-like DNA polymerases, by decreasing the rate of elongation. Three highly conserved regions of amino acid homology, found in several viral alpha-like DNA polymerases and in the luminal diameter 29 DNA polymerase, one of them proposed to be the PAA binding site, were also found in the T4 DNA polymerase. This prokaryotic enzyme was highly sensitive to the drugs aphidicolin and the nucleotide analogues butylanilino dATP (BuAdATP) and butylphenyl dGTP (BuPdGTP), known to be specific inhibitors of eukaryotic alpha-like DNA polymerases. Two potential DNA polymerases from the linear plasmid pGKL1 from yeast and the S1 mitochondrial DNA from maize have been identified, based on the fact that they contain the three conserved regions of amino acid homology. Comparison of DNA polymerases from prokaryotic and eukaryotic origin showed extensive amino acid homology in addition to highly conserved domains. These findings reflect evolutionary relationships between hypothetically unrelated DNA polymerases.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahlquist P., Strauss E. G., Rice C. M., Strauss J. H., Haseloff J., Zimmern D. Sindbis virus proteins nsP1 and nsP2 contain homology to nonstructural proteins from several RNA plant viruses. J Virol. 1985 Feb;53(2):536–542. doi: 10.1128/jvi.53.2.536-542.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleström P., Akusjärvi G., Pettersson M., Pettersson U. DNA sequence analysis of the region encoding the terminal protein and the hypothetical N-gene product of adenovirus type 2. J Biol Chem. 1982 Nov 25;257(22):13492–13498. [PubMed] [Google Scholar]
- Argos P., Tucker A. D., Philipson L. Primary structural relationships may reflect similar DNA replication strategies. Virology. 1986 Mar;149(2):208–216. doi: 10.1016/0042-6822(86)90122-4. [DOI] [PubMed] [Google Scholar]
- Baer R., Bankier A. T., Biggin M. D., Deininger P. L., Farrell P. J., Gibson T. J., Hatfull G., Hudson G. S., Satchwell S. C., Séguin C. DNA sequence and expression of the B95-8 Epstein-Barr virus genome. Nature. 1984 Jul 19;310(5974):207–211. doi: 10.1038/310207a0. [DOI] [PubMed] [Google Scholar]
- Bamford D. H., Mindich L. Characterization of the DNA-protein complex at the termini of the bacteriophage PRD1 genome. J Virol. 1984 May;50(2):309–315. doi: 10.1128/jvi.50.2.309-315.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco L., Gutiérrez J., Lázaro J. M., Bernad A., Salas M. Replication of phage phi 29 DNA in vitro: role of the viral protein p6 in initiation and elongation. Nucleic Acids Res. 1986 Jun 25;14(12):4923–4937. doi: 10.1093/nar/14.12.4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco L., Salas M. Characterization and purification of a phage phi 29-encoded DNA polymerase required for the initiation of replication. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5325–5329. doi: 10.1073/pnas.81.17.5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco L., Salas M. Characterization of a 3'----5' exonuclease activity in the phage phi 29-encoded DNA polymerase. Nucleic Acids Res. 1985 Feb 25;13(4):1239–1249. doi: 10.1093/nar/13.4.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco L., Salas M. Effect of aphidicolin and nucleotide analogs on the phage phi 29 DNA polymerase. Virology. 1986 Sep;153(2):179–187. doi: 10.1016/0042-6822(86)90021-8. [DOI] [PubMed] [Google Scholar]
- Blanco L., Salas M. Replication of phage phi 29 DNA with purified terminal protein and DNA polymerase: synthesis of full-length phi 29 DNA. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6404–6408. doi: 10.1073/pnas.82.19.6404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carthew R. W., Chodosh L. A., Sharp P. A. An RNA polymerase II transcription factor binds to an upstream element in the adenovirus major late promoter. Cell. 1985 Dec;43(2 Pt 1):439–448. doi: 10.1016/0092-8674(85)90174-6. [DOI] [PubMed] [Google Scholar]
- Davison A. J., Scott J. E. The complete DNA sequence of varicella-zoster virus. J Gen Virol. 1986 Sep;67(Pt 9):1759–1816. doi: 10.1099/0022-1317-67-9-1759. [DOI] [PubMed] [Google Scholar]
- Earl P. L., Jones E. V., Moss B. Homology between DNA polymerases of poxviruses, herpesviruses, and adenoviruses: nucleotide sequence of the vaccinia virus DNA polymerase gene. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3659–3663. doi: 10.1073/pnas.83.11.3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenman K., Pedersen-Lane J., West D., Herman R., Maley F., Belfort M. Processing of phage T4 td-encoded RNA is analogous to the eukaryotic group I splicing pathway. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5875–5879. doi: 10.1073/pnas.83.16.5875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escarmis C., García P., Méndez E., López R., Salas M., García E. Inverted terminal repeats and terminal proteins of the genomes of pneumococcal phages. Gene. 1985;36(3):341–348. doi: 10.1016/0378-1119(85)90189-1. [DOI] [PubMed] [Google Scholar]
- García E., Gómez A., Ronda C., Escarmis C., López R. Pneumococcal bacteriophage Cp-1 contains a protein bound to the 5' termini of its DNA. Virology. 1983 Jul 15;128(1):92–104. doi: 10.1016/0042-6822(83)90321-5. [DOI] [PubMed] [Google Scholar]
- Gibbs J. S., Chiou H. C., Hall J. D., Mount D. W., Retondo M. J., Weller S. K., Coen D. M. Sequence and mapping analyses of the herpes simplex virus DNA polymerase gene predict a C-terminal substrate binding domain. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7969–7973. doi: 10.1073/pnas.82.23.7969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunge N., Tamaru A., Ozawa F., Sakaguchi K. Isolation and characterization of linear deoxyribonucleic acid plasmids from Kluyveromyces lactis and the plasmid-associated killer character. J Bacteriol. 1981 Jan;145(1):382–390. doi: 10.1128/jb.145.1.382-390.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helgstrand E., Eriksson B., Johansson N. G., Lannerö B., Larsson A., Misiorny A., Norén J. O., Sjöberg B., Stenberg K., Stening G. Trisodium phosphonoformate, a new antiviral compound. Science. 1978 Sep 1;201(4358):819–821. doi: 10.1126/science.210500. [DOI] [PubMed] [Google Scholar]
- Hirochika H., Sakaguchi K. Analysis of linear plasmids isolated from Streptomyces: association of protein with the ends of the plasmid DNA. Plasmid. 1982 Jan;7(1):59–65. doi: 10.1016/0147-619x(82)90027-0. [DOI] [PubMed] [Google Scholar]
- Huberman J. A. New views of the biochemistry of eucaryotic DNA replication revealed by aphidicolin, an unusual inhibitor of DNA polymerase alpha. Cell. 1981 Mar;23(3):647–648. doi: 10.1016/0092-8674(81)90426-8. [DOI] [PubMed] [Google Scholar]
- Kallin B., Sternås L., Saemundssen A. K., Luka J., Jörnvall H., Eriksson B., Tao P. Z., Nilsson M. T., Klein G. Purification of Epstein-Barr virus DNA polymerase from P3HR-1 cells. J Virol. 1985 May;54(2):561–568. doi: 10.1128/jvi.54.2.561-568.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemble R. J., Thompson R. D. S1 and S2, the linear mitochondrial DNAs present in a male sterile line of maize, possess terminally attached proteins. Nucleic Acids Res. 1982 Dec 20;10(24):8181–8190. doi: 10.1093/nar/10.24.8181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan N. N., Wright G. E., Dudycz L. W., Brown N. C. Butylphenyl dGTP: a selective and potent inhibitor of mammalian DNA polymerase alpha. Nucleic Acids Res. 1984 Apr 25;12(8):3695–3706. doi: 10.1093/nar/12.8.3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan N. N., Wright G. E., Dudycz L. W., Brown N. C. Elucidation of the mechanism of selective inhibition of mammalian DNA polymerase alpha by 2-butylanilinopurines: development and characterization of 2-(p-n-butylanilino)adenine and its deoxyribonucleotides. Nucleic Acids Res. 1985 Sep 11;13(17):6331–6342. doi: 10.1093/nar/13.17.6331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi Y., Hirai K., Gunge N., Hishinuma F. Hairpin plasmid--a novel linear DNA of perfect hairpin structure. EMBO J. 1985 Jul;4(7):1881–1886. doi: 10.1002/j.1460-2075.1985.tb03864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi Y., Hirai K., Hishinuma F. The yeast linear DNA killer plasmids, pGKL1 and pGKL2, possess terminally attached proteins. Nucleic Acids Res. 1984 Jul 25;12(14):5685–5692. doi: 10.1093/nar/12.14.5685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopf C. W. The herpes simplex virus type 1 DNA polymerase gene: site of phosphonoacetic acid resistance mutation in strain Angelotti is highly conserved. J Gen Virol. 1987 May;68(Pt 5):1429–1433. doi: 10.1099/0022-1317-68-5-1429. [DOI] [PubMed] [Google Scholar]
- Knopf K. W. Properties of herpes simplex virus DNA polymerase and characterization of its associated exonuclease activity. Eur J Biochem. 1979 Jul;98(1):231–244. doi: 10.1111/j.1432-1033.1979.tb13181.x. [DOI] [PubMed] [Google Scholar]
- Kouzarides T., Bankier A. T., Satchwell S. C., Weston K., Tomlinson P., Barrell B. G. Sequence and transcription analysis of the human cytomegalovirus DNA polymerase gene. J Virol. 1987 Jan;61(1):125–133. doi: 10.1128/jvi.61.1.125-133.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larder B. A., Kemp S. D., Darby G. Related functional domains in virus DNA polymerases. EMBO J. 1987 Jan;6(1):169–175. doi: 10.1002/j.1460-2075.1987.tb04735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichy J. H., Nagata K., Friefeld B. R., Enomoto T., Field J., Guggenheimer R. A., Ikeda J. E., Horwitz M. S., Hurwitz J. Isolation of proteins involved in the replication of adenoviral DNA in vitro. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 2):731–740. doi: 10.1101/sqb.1983.047.01.084. [DOI] [PubMed] [Google Scholar]
- McDonell M. W., Simon M. N., Studier F. W. Analysis of restriction fragments of T7 DNA and determination of molecular weights by electrophoresis in neutral and alkaline gels. J Mol Biol. 1977 Feb 15;110(1):119–146. doi: 10.1016/s0022-2836(77)80102-2. [DOI] [PubMed] [Google Scholar]
- Moreno M. A., Carrascosa A. L., Ortín J., Viñuela E. Inhibition of African swine fever (ASF virus replication by phosphonoacetic acid. J Gen Virol. 1978 May;39(2):253–258. doi: 10.1099/0022-1317-39-2-253. [DOI] [PubMed] [Google Scholar]
- Moss B., Cooper N. Genetic evidence for vaccinia virus-encoded DNA polymerase: isolation of phosphonoacetate-resistant enzyme from the cytoplasm of cells infected with mutant virus. J Virol. 1982 Aug;43(2):673–678. doi: 10.1128/jvi.43.2.673-678.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata K., Guggenheimer R. A., Hurwitz J. Specific binding of a cellular DNA replication protein to the origin of replication of adenovirus DNA. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6177–6181. doi: 10.1073/pnas.80.20.6177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama Y., Maeno K., Yoshida S. Characterization of human cytomegalovirus-induced DNA polymerase and the associated 3'-to-5', exonuclease. Virology. 1983 Jan 30;124(2):221–231. doi: 10.1016/0042-6822(83)90339-2. [DOI] [PubMed] [Google Scholar]
- Niwa O., Sakaguchi K., Gunge N. Curing of the killer deoxyribonucleic acid plasmids of Kluyveromyces lactis. J Bacteriol. 1981 Dec;148(3):988–990. doi: 10.1128/jb.148.3.988-990.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollis D. L., Brick P., Hamlin R., Xuong N. G., Steitz T. A. Structure of large fragment of Escherichia coli DNA polymerase I complexed with dTMP. 1985 Feb 28-Mar 6Nature. 313(6005):762–766. doi: 10.1038/313762a0. [DOI] [PubMed] [Google Scholar]
- Paillard M., Sederoff R. R., Levings C. S. Nucleotide sequence of the S-1 mitochondrial DNA from the S cytoplasm of maize. EMBO J. 1985 May;4(5):1125–1128. doi: 10.1002/j.1460-2075.1985.tb03749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peñalva M. A., Salas M. Initiation of phage phi 29 DNA replication in vitro: formation of a covalent complex between the terminal protein, p3, and 5'-dAMP. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5522–5526. doi: 10.1073/pnas.79.18.5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto I., Lázaro J. M., García J. A., Hermoso J. M., Salas M. Purification in a functional form of the terminal protein of Bacillus subtilis phage phi 29. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1639–1643. doi: 10.1073/pnas.81.6.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pring D. R., Levings C. S., Hu W. W., Timothy D. H. Unique DNA associated with mitochondria in the "S"-type cytoplasm of male-sterile maize. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2904–2908. doi: 10.1073/pnas.74.7.2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas M. A new mechanism for the initiation of replication of phi 29 and adenovirus DNA: priming by the terminal protein. Curr Top Microbiol Immunol. 1984;109:89–106. doi: 10.1007/978-3-642-69460-8_4. [DOI] [PubMed] [Google Scholar]
- Sridhar P., Condit R. C. Selection for temperature-sensitive mutations in specific vaccinia virus genes: isolation and characterization of a virus mutant which encodes a phosphonoacetic acid-resistant, temperature-sensitive DNA polymerase. Virology. 1983 Jul 30;128(2):444–457. doi: 10.1016/0042-6822(83)90269-6. [DOI] [PubMed] [Google Scholar]
- Stark M. J., Mileham A. J., Romanos M. A., Boyd A. Nucleotide sequence and transcription analysis of a linear DNA plasmid associated with the killer character of the yeast Kluyveromyces lactis. Nucleic Acids Res. 1984 Aug 10;12(15):6011–6030. doi: 10.1093/nar/12.15.6011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stillman B. W., Tamanoi F., Mathews M. B. Purification of an adenovirus-coded DNA polymerase that is required for initiation of DNA replication. Cell. 1982 Dec;31(3 Pt 2):613–623. doi: 10.1016/0092-8674(82)90317-8. [DOI] [PubMed] [Google Scholar]
- Tsurumi T., Maeno K., Nishiyama Y. Nucleotide sequence of the DNA polymerase gene of herpes simplex virus type 2 and comparison with the type 1 counterpart. Gene. 1987;52(2-3):129–137. doi: 10.1016/0378-1119(87)90039-4. [DOI] [PubMed] [Google Scholar]
- Watabe K., Leusch M., Ito J. Replication of bacteriophage phi 29 DNA in vitro: the roles of terminal protein and DNA polymerase. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5374–5378. doi: 10.1073/pnas.81.17.5374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa H., Ito J. Nucleotide sequence of the major early region of bacteriophage phi 29. Gene. 1982 Mar;17(3):323–335. doi: 10.1016/0378-1119(82)90149-4. [DOI] [PubMed] [Google Scholar]






