Abstract
IL-17 is a pro-inflammatory cytokine implicated a variety of autoimmune diseases. We have recently reported that FGF2 cooperates with IL-17 to protect intestinal epithelium during dextran sodium sulfate (DSS)-induced colitis. Here, we report a pathogenic role of the FGF2-IL-17 cooperation in the pathogenesis of autoimmune arthritis. Combined treatment with FGF2 and IL-17 synergistically induced ERK activation as well as the production of cytokines and chemokines in human synovial intimal resident fibroblast-like synoviocytes (FLS). Furthermore, ectopic expression of FGF2 in mouse joints potentiated IL-17-induced inflammatory cytokine and chemokine production in the tissue. In the collagen-induced arthritis (CIA) model, while ectopic expression of FGF2 in vivo exacerbated tissue inflammation and disease symptom in the wild-type controls, the effect was largely blunted in Il17a −/− mice. Taken together, our study suggests that FGF2 cooperates with IL-17 to promote the pathogenesis of autoimmune arthritis by cooperating with IL-17 to induce inflammatory response.
Introduction
Rheumatoid arthritis (RA) is a common and disabling autoimmune disease with pathology mostly in diarthrodial joints of the feet and hands1, 2. While the etiology remains unclear, the pathogenesis of RA is associated with chronic inflammation in the synovial tissues, which leads to cartilage disruption and bone erosion3, 4.
T cells have long been considered to contribute to the pathogenesis of RA5, 6. Recent studies have further defined the Th17 cells as a major T helper cell subset in promoting chronic inflammation in RA7, 8. IL-17A (also named IL-17), a Th17 signature cytokine, is increased in synovial fluids of RA patients9, 10. Previous studies have demonstrated that impairment of Th17 function or ablation of IL-17 signaling significantly delays the onset and reduces the severity of disease in collagen-induced arthritis (CIA), a mouse model for RA7, 11–13. Importantly, anti-IL-17 and anti-IL-17RA neutralizing antibodies have also shown promising efficacy in treating RA in clinical trials14–17.
IL-17 can induce the production of pro-inflammatory cytokines and chemokines alone or in cooperation with other cytokines such as TNFα13. IL-17 stimulation activates TRAF6-dependent NF-κB and MAPKs activation18 for gene transcription; it also engages a TRAF6-independent, TRAF2/5-dependent signaling for mRNA stabilization19. Act1 is an essential adaptor molecule bridging the IL-17 receptors to the downstream signaling20, 21. Similar to the Il17a-deficient mice, CIA pathogenesis is dramatically reduced in Act1-deficient mice22. IL-17 signaling has been shown to be tightly regulated to prevent excessive inflammation23–26.
In addition to IL-17, the growth factor FGF2 is also increased in RA patients and its level strongly correlates with Larsen’s grade of bone erosion27, 28. FGF2 blocking antibody significantly reduces adjuvant-induced arthritis (AIA) pathogenesis in rat29, suggesting FGF2 may be important for joint inflammation. However, it is not clear how FGF2 functions during the autoimmune pathogenesis. FGF2 signals through its receptor FGFR1. FRS2 (the FGF receptor substrate 2) binds to FGFR1 and recruits the downstream constitutive associated Grb2-Sos1 complex to activate the ERK pathway30–32. FRS2 also binds to SHP2 to form a complex with Grb2 to mediate FGF2-induced ERK signaling32.
We previously reported that IL-17 cooperates with FGF2 to induce ERK activation and target gene expression, a process critical for the repair the damaged intestinal epithelium to protect against dysregulated microbiota-driven colitis33. Here we found that FGF2 also synergized with IL-17 to induce ERK activation to promote autoimmune inflammation during the pathogenesis of arthritis.
Results
Both FGF2 and IL-17 are up-regulated in RA and CIA samples
FGF2 and IL-17 have been separately shown to be elevated in the synovial fluids from RA patients9, 10, 27, 28. To examine the possible cooperation between IL-17 and FGF2 in the pathogenesis of RA, we measured IL-17 and FGF2 levels in the synovium sample from the same RA patients. The result revealed a concurrent up-regulation of FGF2 and IL-17 in the samples from RA patients (Fig. 1A). Both FGF2 and IL-17 are critical for the pathogenesis of inflammatory tissue destruction in rodent models of RA11, 29. Collagen-induced arthritis (CIA) is one of the most widely used mouse models for modeling RA pathology34–37. We found FGF2 and IL-17 were concurrently up-regulated in the joint tissues from mice on the CIA model (Fig. 1B). These data suggest that FGF2 and IL-17 may cooperate to mediate the pathogenesis of RA.
Figure 1.
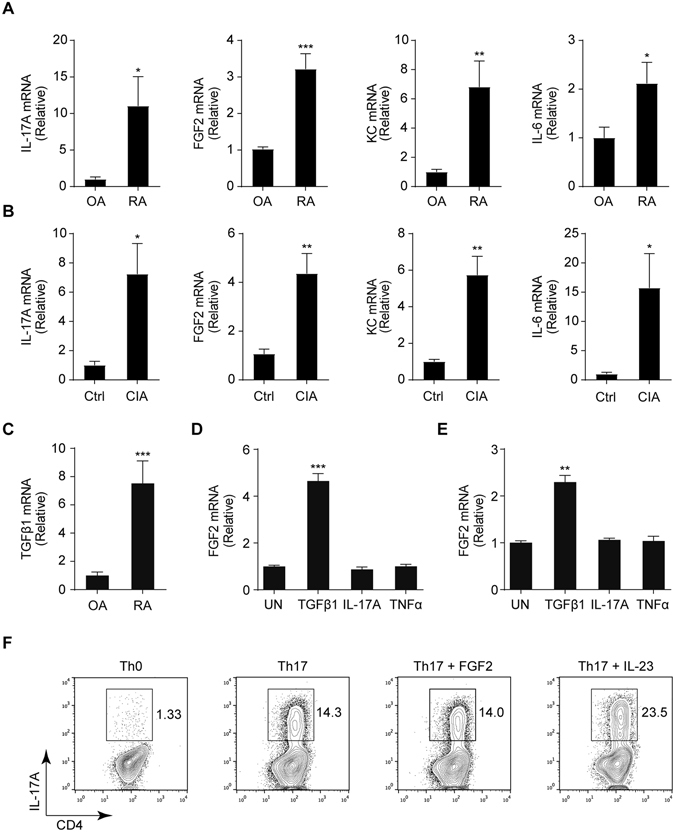
FGF2 and IL-17 are simultaneously elevated in the synovia of RA patients and the joints of CIA model. (A) Quantitative mRNA expression of IL-17, FGF2, IL-6 and KC in the synovia from 13 individuals with rheumatoid arthritis (RA) and 12 individuals with osteoarthritis (OA). (B) Quantitative mRNA expression of IL-17, FGF2, IL-6 and KC in in joints of wild type C57/BL6 left untreated or treated with the condition for CIA (n = 4). (C) Quantitative mRNA expression of TGFβ1 in the synovia from 13 individuals with RA and 12 individuals with OA as in (A). (D and E) Quantitative mRNA expression of FGF2 in human primary FLS cells (D) and MEFs (E) left untreated (UN) or stimulated for 6 hr with TGFβ1 (20 ng/ml), IL-17A (100 ng/ml) or TNFα (20 ng/ml). Cells were serum-starved for 16 hr before treatment. (F) IL-17A levels in in vitro-differentiated Th17 cells by TGFβ1 (2 ng/ml) and IL-6 (40 ng/ml), with or without FGF2 (20 ng/ml) or IL-23 (20 ng/ml). Data are representative of three independent experiments in (B, D–F). Data represent means and s.e.m. in (A–E) *P < 0.05, **P < 0.01, ***P < 0.001 by Student’s t test.
We previously reported that TGFβ induced FGF2 expression in in vitro differentiated CD4+ T cells such as Th17 and Treg cells33. Here we found that TGFβ expression was also up-regulated in the RA samples (Fig. 1C), suggesting that TGFβ also could induce FGF2 expression in infiltrated CD4+ T cells in the autoimmune disease. In addition to T cells, TGFβ induced weak FGF2 expression in resident colonic epithelial cells33. To check potential induction of FGF2 expression in RA resident cells, we purified synovial intimal resident fibroblast-like synoviocytes (FLS) from RA patients, and found that TGFβ but not IL-17 or TNFα induced FGF2 expression in these cells (Fig. 1D and Supplementary Fig. S1) while all the cytokines induced IL-6 expression (Supplementary Fig. S2). Similarly, TGFβ but not IL-17 or TNFα induced FGF2 expression in mouse embryonic fibroblasts (MEFs) (Fig. 1E and Supplementary Fig. S2). These data suggest that TGFβ could induce FGF2 expression in multiple cell types in RA pathogenesis. Furthermore, we found that FGF2 did not promote Th17 development for IL-17 production in vitro (Fig. 1F). Thus, our data suggest that FGF2 and IL-17 do not directly induce each other’s expression.
FGF2 synergizes with IL-17 to induce cytokines and chemokines
Synovial intimal resident fibroblast-like synoviocytes (FLS) are major sources of pro-inflammatory cytokines/chemokines and critically contribute to cartilage destruction38. We tested the FLS responses to FGF2 and IL-17 stimulation. While FGF2 or IL-17 alone exhibited limited effect on the expression of the tested pro-inflammatory cytokines and chemokines in the FLS, combined stimulation with FGF2 and IL-17 synergistically induced production of KC, CXCL2, IL-6, and COX-2 (Fig. 2). To determine whether FGF2 cooperates with IL-17 in vivo, we injected adenoviruses expressing FGF2 and/or IL-17 into the joints of healthy mice. Consistent with in vitro data, FGF2 synergized with IL-17 to induce proinflammatory genes production in mouse joint tissues (Fig. 3A). Histology analysis showed that simultaneous expression of both FGF2 and IL-17 led to severer tissue swelling and immune cell infiltration than that induced by either cytokine alone (Fig. 3B). These results suggest that FGF2 and IL-17 may synergistically promote joint inflammation.
Figure 2.
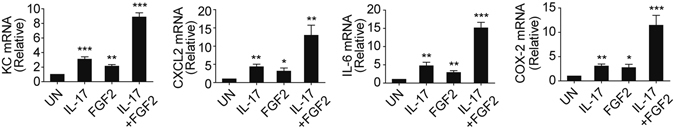
FGF2 synergizes with IL-17 to induce pro-inflammatory gene production. Quantitative mRNA expression of KC, CXCL2, IL-6 and COX-2 in human primary FLS cells left untreated (UN) or stimulated for 6 hr with IL-17 (50 ng/ml), FGF2 (5 ng/ml) alone or with FGF2 plus IL-17. Data are representative of three independent experiments (means and s.e.m.). *P < 0.05, **P < 0.01, ***P < 0.001 by Student’s t test.
Figure 3.

FGF2 synergizes with IL-17 to promote inflammatory pathogenesis. (A) Quantitative mRNA expression of KC, CXCL2, IL-6 and COX-2 in the joints of C57/BL6 mice treated with empty virus (Ad-EV), adenovirus expressing IL-17 (Ad–IL-17), adenovirus expressing FGF2 (Ad–FGF2) or adenovirus expressing IL-17 plus adenovirus expressing FGF2 for multiple times as indicated (n = 4). (B) H&E histology of the representative ankle sections from C57/BL6 mice treated as in (B). Data are representative of three (A and B) independent experiments (mean and s.e.m. in A). *P < 0.05, **P < 0.01, ***P < 0.001 by Student’s t test.
FGF2 cooperates with IL-17 to promote autoinflammatory pathogenesis
To determine the role of the FGF2-IL-17 cooperation during the pathogenesis CIA, we injected FGF2-expressing adenovirus into the joints of Il17a-deficient and wild-type control mice on the CIA model. Consistent with previous report11, IL-17 deficiency reduced CIA clinical score and attenuated the joint tissue damage (Fig. 4A, B). Importantly, we found that ectopic expression of FGF2 severely aggravated the clinical symptoms and exacerbated the joint inflammation in the wild-type mice (Fig. 4A, B). The effect from FGF2 expression was greatly reduced in Il17a-deficient mice (Fig. 4A, B). In addition, IL-17 deficiency also attenuated FGF2-mediated increase of inflammatory cytokine and chemokine production (Fig. 4C). Together, these data suggest FGF2 cooperates with IL-17 to promote CIA pathogenesis.
Figure 4.
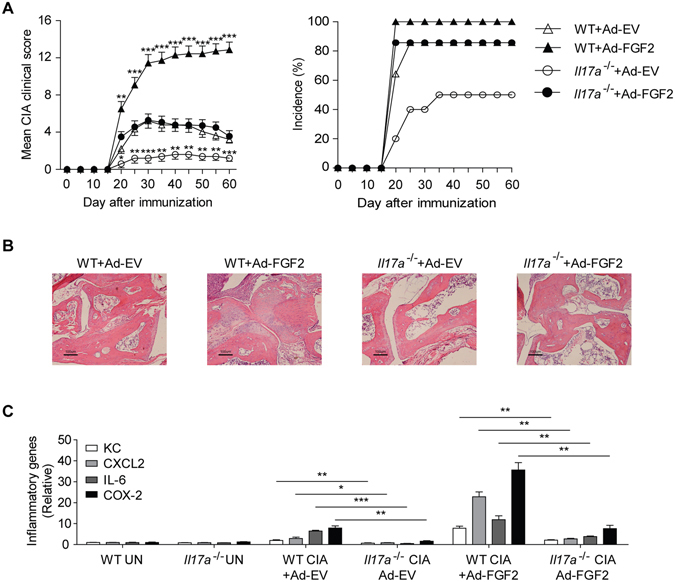
FGF2 cooperates with IL-17 to promote CIA pathogenesis. (A) Mean clinical scores and disease incidence of CIA in C57/BL6 wild type mice or Il17a −/− mice treated with empty virus (Ad-EV) or adenovirus expressing FGF2 (Ad–FGF2) once a week from day 15 after CIA induction (n = 10–14 per group). (B) H&E histology of the representative ankle sections from C57/BL6 wild type or Il17a −/− mice treated as in (A). (C) Quantitative mRNA expression of KC, CXCL2, IL-6 and COX-2 in the joints of C57/BL6 wild type or Il17a −/− mice treated as in (A) (n = 3–4 per group). Data are representative of two (A–C) independent experiments (mean and s.e.m. in A, C). *P < 0.05, **P < 0.01, ***P < 0.001 by Student’s t test.
Act1 negatively regulates FGF2-induced ERK signaling in human primary FLS cells
Act1 is the adaptor molecule for IL-17 signaling. siRNA-mediated knockdown of Act1 expression in the human primary FLS cells abolished IL-17-induced phosphorylation of IκBα, diminished MAPKs activation (Fig. 5A) and blocked IL-17-mediated expression of pro-inflammatory genes (Fig. 5B). In contrast to the positive role of Act1 in IL-17 signaling, we have previously shown that Act1 negatively regulates FGF2-induced ERK signaling in epithelial cells33. Consistently, we found that FGF2-induced ERK activation was enhanced in FLS cells transfected with Act1-targeting siRNA compared to the control siRNA transfected cells (Fig. 6A). Moreover, knocking down of Act1 increased FGF2-induced expression of KC and IL-6 (Fig. 6B). Of note, FGF2-induced activation of JNK and P38 was not affected by the Act1-knockdown (Fig. 6A), suggesting a specific regulation of FGF2-induced ERK signaling by Act1.
Figure 5.
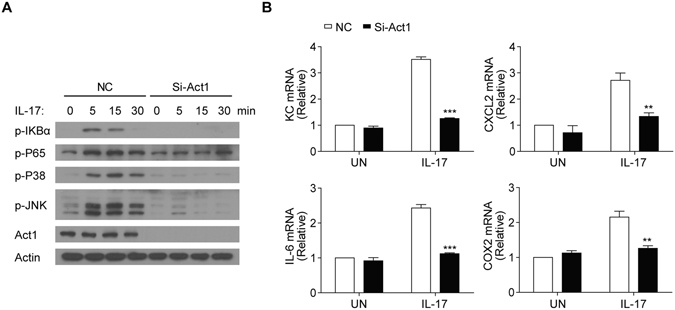
Act1 is required for IL-17 signaling in FLS cells. (A) Western blot analysis of phosphorylated (p-) or total proteins with the indicated antibodies in lysates of human primary FLS cells infected with control virus (NC) or the virus for Act1-specific siRNA (Si-Act1) and then left untreated (0) or treated for the indicated time points with IL-17 (50 ng/ml). (B) Quantitative mRNA expression of KC, CXCL2, IL-6 and COX-2 in the FLS cells infected as in (A) and then left untreated (0) or treated for 1 hr with IL-17 (50 ng/ml). Data are representative of three independent experiments (mean and s.e.m. in B). **P < 0.01, ***P < 0.001 by Student’s t test.
Figure 6.
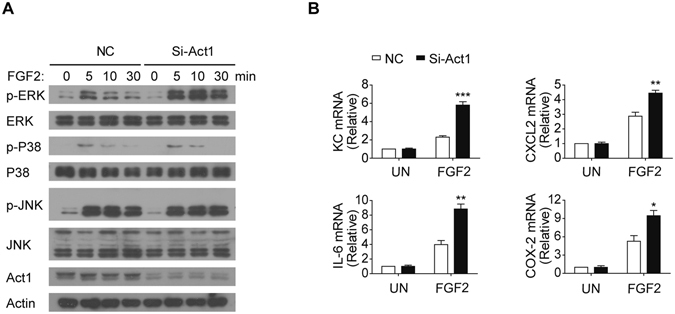
Act1 suppresses FGF2-mediated ERK activation. (A) Western blot analysis with antibodies against the indicated phosphorylated (p-) or total proteins in lysates of human primary FLS cells infected with control lentivirus or the lentivirus for Act1-specific siRNA (Si-Act1), and then left untreated (0) or treated for 5 to 30 min with FGF2 (50 ng/ml). (B) Quantitative mRNA expression of KC, CXCL2, IL-6 and COX-2 in human primary FLS cells infected as in (A) and then left untreated (0) or treated for 1 hr with FGF2 (50 ng/ml). Data are representative of three independent experiments (mean and s.e.m. in B). *P < 0.05, **P < 0.01, ***P < 0.001 by Student’s t test.
We have previously shown that Act1 suppresses FGF2-induced ERK activation by competing with SOS1 for Grb2 binding33. Similarly, we observed that Act1 associated with Grb2 and its signaling partner SHP2 upon FGF2 stimulation in the human primary FLS cells (Fig. 7). These results suggest that Act1 is recruited to FGF2-induced signaling complex to suppress its downstream ERK signaling in human FLS cells.
Figure 7.
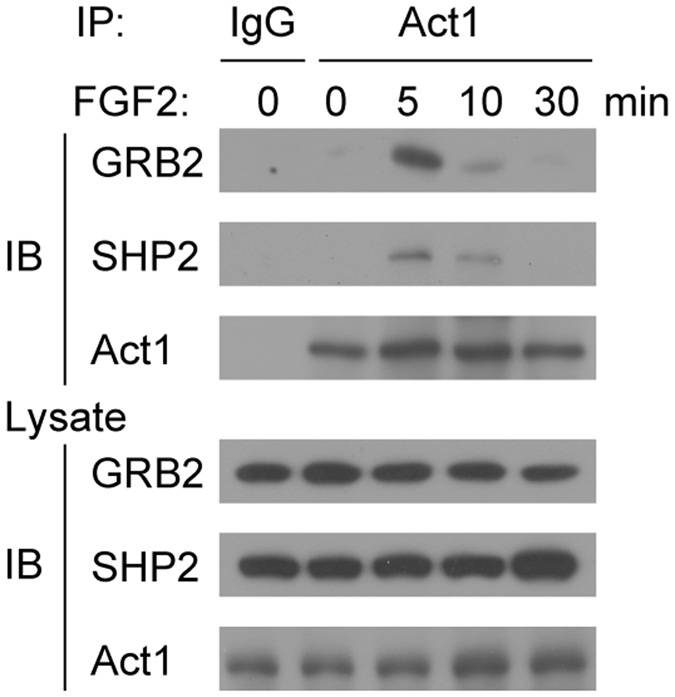
Act1 is recruited to Grb2 complex in FLS cells after FGF2 stimulation. Co-immunoprecipitation of cell lysates from human primary FLS cells. Cells were left untreated (0) or treated for the indicated time points with FGF2 (50 ng/ml). Whole cell lysates were immunoprecipitated with anti-Act1 and immunoblotted with the indicated antibodies. Data are representative of two independent experiments.
FGF2 cooperates with IL-17 to mediate ERK activation for synergistic induction of pro-inflammatory genes
To determine the signaling pathway that mediates the IL-17-FGF2 cooperation in FLS cells, we examined the activation of NF-κB and MAPKs (JNK, P38 and ERK) in response to the stimulation with FGF2, IL-17 or FGF2 plus IL-17. Interestingly, only ERK signaling was synergistically activated by IL-17 and FGF2 in the FLS cells (Fig. 8A), reminiscent of our previous observation in epithelial cells33. Importantly, pharmacological inhibition of ERK activation using the MEK inhibitor PD 98059 drastically reduced the pro-inflammatory cytokine and chemokine induction induced by IL-17 and FGF2 treatment (Fig. 8B). The result was further confirmed by a second inhibitor, PD 0325901, which also suppresses the phosphorylation of ERK1/2 (Fig. 8C). Taken together, these results suggest that FGF2 synergizes with IL-17 to induce pro-inflammatory cytokine and chemokine expression via ERK1/2 activation.
Figure 8.
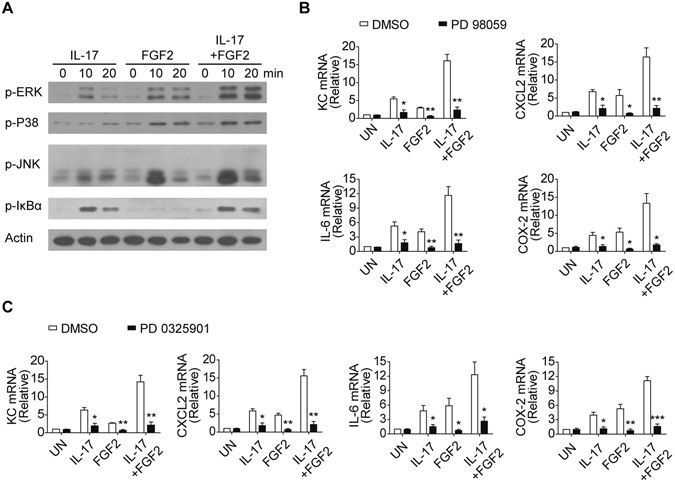
FGF2 synergizes with IL-17 to activate ERK signaling. (A) Western blot analysis of phosphorylated- (p-) Erk, p-P38, p-JNK, p-IκBα and Actin in lysates of human primary FLS cells left untreated (0) or treated for 10 and 20 min with IL-17 (50 ng/ml), FGF2 (5 ng/ml) alone or FGF2 plus IL-17. (B and C) Quantitative mRNA expression of KC, CXCL2, IL-6 and COX-2 in human primary FLS cells left untreated (UN) or stimulated for 6 hr with IL-17 (50 ng/ml), FGF2 (5 ng/ml) alone or FGF2 plus IL-17 in the present of DMSO, or DMSO dissolved Erk inhibitors PD 98059 (20 μM) (B) or PD 0325091 (20 μM) (C). Data are representative of three independent experiments (mean and s.e.m. in B and C). *P < 0.05, **P < 0.01, ***P < 0.001 by Student’s t test.
Discussion
FGF2 is up-regulated in RA patients and critical for the pathogenesis of AIA27–29. However, it remains elusive how the growth factor contributes to the inflammatory autoimmune pathogenesis. Here we demonstrate that FGF2 cooperates with a proinflammatory cytokine IL-17 to synergistically promote autoimmune inflammation. We found that FGF2 and IL-17 were simultaneously up-regulated in the joint tissues of RA as well as CIA. TGFβ was similarly up-regulated in RA samples. We then found that TGFβ induced FGF2 expression in multiple RA relevant cell types including T cells, fibroblasts, and fibroblast-like synoviocytes. We also provided evidence that IL-17 and FGF2 did not induce each other’s expression directly in vitro. However, it is possible that FGF2 may indirectly promote IL-17 expression through inducing IL-6 expression in vivo since IL-6 signaling is critical for Th17 development. We further demonstrated that IL-17 and FGF2 synergistically induced pro-inflammatory genes production in FLS cells and in mice joints in vivo. Importantly, FGF2-mediated effects on the inflammatory pathogenesis of CIA were greatly diminished in Il17a-deficient mice, supporting the cooperation of FGF2 with IL-17 during CIA pathogenesis.
Both the adaptor protein Grb2 and the phosphatase SHP2 are critical for FGF2-induced Ras-ERK signaling39. Grb2 constitutively associates with Sos1, a guanine nucleotide exchange factor (GEF) for Ras. We have previously demonstrated that Act1 directly associates with Grb2 and interfere with FGF2-induced Grb2-Sos1 complex for ERK activation. Act1 is preferentially recruited to IL-17 receptor complex, which releases Act1-mediated suppression on FGF2-induced ERK signaling during co-stimulation of FGF2 and IL-1733. Similarly, here we found that FGF2 induced the association of Act1 with Grb2 and SHP2 in human primary FLS cells and that Act1 suppressed FGF2-induced ERK signaling and production of downstream pro-inflammatory genes in the FLS cells. The data suggest a similar molecular mechanism of Act1-mediated autoimmune inflammation driven by FGF2 and IL-17.
The FLS cells are reported to be the major cell type responsible for autoimmune inflammation38. We utilized the primary FLS cells to investigate the cooperation of FGF2 and IL-17 in promoting inflammation. Because many cell types are responsive to FGF2 and IL-17, our study does not exclude the contribution of IL-17-FGF2 cooperativity in other cell types during the pathogenesis of CIA and RA. Given the wide implication of IL-17 in autoimmune disease, the IL-17-FGF2 cooperation may also contribute to inflammatory pathology of other inflammation-driven autoimmune diseases such as psoriasis.
Methods
Human samples
Tissue specimens from arthritic joints of patients with osteoarthritis or rheumatoid arthritis were obtained as described40. Osteoarthritis and rheumatoid arthritis were diagnosed in accordance with the criteria of the American College of Rheumatology. All individuals provided informed consent. The study was approved by the Research Ethics Board of Shanghai Guanghua Hospital and the associated methods were performed in accordance with the relevant guidelines and regulations.
Mice
Il17a −/− mice on the C57BL/6 background were kindly provided by Dr. Yoichiro Iwakura (University of Tokyo). Il17a −/− and its representative littermate control mice were utilized for experiments. All above mice were maintained in specific pathogen-free conditions. All animal experiments were performed in compliance with the guide for the care and use of laboratory animals and were approved by the institutional biomedical research ethics committee of the Shanghai Institutes for Biological Sciences (Chinese Academy of Sciences).
Induction of CIA
8–10 weeks old Il17a −/− and its representative littermate control mice were injected intradermally with 100 μg emulsified CII (Sigma-Aldrich) plus CFA at several sites of the tail base on day 1 and day 21. Mice were observed every 5 d for scoring of clinical signs, and the observers were blind to evaluate the clinical scores.
Histology
Joint tissues for histological analyses were dissected from CII-immunized mice and immediately fixed with 4% paraformaldehyde. Paraffin-embedded sections of joint were stained with H&E and then examined by light microscopy.
Cell culture
Rheumatoid arthritis derived human fibroblast like synoviocytes (FLS) were kindly provided by Dr. Ningli Li (Shanghai Jiaotong University School of Medicine). The FLS cells, MEF cells, and 293A cells were grown in DMEM medium supplemented with 10% (vol/vol) FBS, penicillin (100 U/ml) and streptomycin (100 μg/ml). 293FT cells were grown in DMEM medium supplemented with 10% (vol/vol) FBS, 1% (vol/vol) NEAA, penicillin (100 U/ml) and streptomycin (100 μg/ml).
In vitro generation of Th17 cells and flow cytometry
Naive CD4+ T cells were purified by magnetic sorting from spleens of C57BL/6 mice. Sorted cells were activated with pre-coated anti-CD3 (5 μg/ml) and soluble anti-CD28 (2 μg/ml) and were then induced to differentiate into Th0 and Th17 cells by adding various cytokines and antibodies as follows: Th0 condition, anti-IFNγ (10 μg/ml) and anti-IL-4 (10 μg/ml); Th17 condition, anti-IFNγ (10 μg/ml), anti-IL-4 (10 μg/ml), TGFβ1 (2 ng/ml) and IL-6 (40 ng/ml), with or without IL-23 (20 ng/ml). Cells were cultured with RPMI-1640 medium containing 10% (vol/vol) FBS, 2-mercaptoethanol (55 μM), penicillin (100 U/ml), and streptomycin (100 μg/ml). Fluorescence-labelled anti-mouse CD4-FITC (GK1.5) and anti-mouse IL-17A-PE (TC11-18H10) were obtained from BD Biosciences. All antibodies were used at 1:100 dilutions. Intracellular staining for IL-17A was performed as follows: differentiated CD4+ T cells were cultured in RPMI-1640 medium containing 10% FBS, 1% L-glutamine, penicillin (100 U/ml), and streptomycin (100 μg/ml) for 4 hours at 37 °C with phorbol 12-myristate 13-acetate (50 ng/ml; Sigma), ionomycin (500 ng/ml; Sigma), and brefeldin A (10 μg/ml; biolegend). After staining with CD4, cells were fixed and permeabilized by using Cytofix/Cytoperm solution (BD Biosciences) to perform the indicated intracellular cytokine staining. FACS Calibur (BD Biosciences) and FlowJo software were used for data acquiring and analysis.
RNA isolation and real-time quantitative PCR
Total RNA was extracted from the indicated cells or mouse joint tissues with TRIzol reagent (Invitrogen) according to the manufacturer’s instructions. RNA samples were reverse-transcribed into cDNA by using a PrimeScript RT Reagent kit (TaKaRa). The cDNA samples were then amplified by quantitative PCR with a SYBR Premix ExTaq kit (TaKaRa) on an ABI PRISM 7900 HT cycler (Applied Biosystems). The expression of indicated genes was normalized to expression of housekeeping gene Rpl13a.
Reagents
Recombinant FGF2 (100-18B) and recombinant TGFβ1 (100-21) were from PeproTech; recombinant IL-17A (7955-IL) and recombinant TNFα (210-TA) were from R&D Systems; Anti-Actin (A4700) were from Sigma. Antibody to Act1 (H300), GRB2 (C-23), phosphorylated Erk (sc-7383) was from Santa Cruz Biotechnology. Antibody to SHP2 (ab31110) was from abcam. Antibodies to phosphorylated IkBa (2859L), p65 (3033S), p38 (9211S) and Jnk (92516) were from Cell Signaling Technology. Antibodies to total Erk (9102), p38 (9212) and Jnk (9252) were also from Cell Signaling Technology. Erk inhibitors PD 98059 (P215) and PD 0325901 (PZ0162) were purchased from Sigma.
Gene knockdown with lentivirus-delivered siRNA
The RNA interfere sequence for knockdown of human Act1 gene was 5′-GCTTCAGAACACTCATGTCTA-3′25; the scrambled sequence for control siRNA was 5′-GGATCCTTGACAATACCAA-3′41. The RNA interfere sequences were constructed into the lentivirus pLSLG vector. Lentivirus pLSLG vectors plus VSVG and delta 8.9 helper vectors were co-transfected into 293FT cells (Invitrogen) for lentiviral packaging. After transfection for 60 hours, virus particles were collected for infection of target cells in the presence of 10 mg/ml polybrene (Sigma). After 4 days of infection, cells were used for indicated treatments.
Adenovirus-mediated gene expression in mice
Mouse IL-17 or FGF2 was constructed into the pAdTrack-CMV vector and then recombined with the pAdEasy-1 vector. Recombinant Ad-IL-17A, Ad-FGF2 or empty vector (Ad-EV) was transfected into QBI-HEK 293A cells (Qbiogene). Viruses were packaged and amplified as described41. After titration, 2 × 108 adenovirus particles were injected into the joints of the indicated mice once a week for different experimental settings.
Co-immunoprecipitation
The FLS cells were treated with or without FGF2, IL-17 or FGF2 plus IL-17 for the indicated time points. Cell extracts were incubated with 20 μl protein A beads (GE Healthcare) plus 0.5 μg of the antibodies against Act1. After overnight incubation at 4 °C, beads were washed with lysis buffer for four times and then analyzed by immunoblotting of indicated antibodies.
Immunoblot analysis
Cells were directly lysed by Triton buffer (0.5% Triton X-100 and 20 mM HEPES, pH 7.6) on ice and the lysates were separated by 10% SDS-PAGE. Separated proteins were transferred onto polyvinylidene fluoride filters by using Mini Trans-Blot (Bio-Rad). The filters were then blocked with Tris-buffered saline with 5% non-fat milk plus 0.1% Tween 20 for 1 hour at room temperature. After blocking, the filters were incubated with primary antibody overnight at 4 °C and subsequently washed for three times with Tris-buffered saline containing 0.1% Tween 20. The filters were then incubated with HRP-conjugated secondary antibodies for 1 hour at room temperature. After washing for three times with Tris-buffered saline containing 0.1% Tween 20, indicated signals were detected by using chemiluminescent HRP substrate (Millipore).
Statistics
Data are presented as mean ± s.e.m. A two-tailed Student’s t test was used for analysis of differences between the groups. P values of <0.05 were considered statistically significant.
Electronic supplementary material
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (81430036, 81230075, 31329002, 91429307, 91329301 and 91542119), the 973 Program (2013CB944904), and the Strategic Priority Research Program of the Chinese Academy of Sciences (Grant No. XDB19000000).
Author Contributions
X.Y.S., S.C., X.R.S., D.Y., M.C., Y.Y. and Z.W. conducted or help with the experiments. N.S. provided clinic samples. N.L. provided the FLS cells. X.Y.S. and Y.Q. designed the experiments and wrote the manuscript. X.L. provided reagents and technical support and edited the manuscript. Y.Q. supervised the study.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Xinrui Shao and Siyuan Chen contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-07597-8
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xinyang Song, Email: xinyang_song@hms.harvard.edu.
Youcun Qian, Email: ycqian@sibs.ac.cn.
References
- 1.Majithia V, Geraci SA. Rheumatoid arthritis: diagnosis and management. The American journal of medicine. 2007;120:936–939. doi: 10.1016/j.amjmed.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 2.Kunkel HG, Williams RC. Rheumatoid Arthritis. Annual review of medicine. 1964;15:37–52. doi: 10.1146/annurev.me.15.020164.000345. [DOI] [PubMed] [Google Scholar]
- 3.Schett G, Gravallese E. Bone erosion in rheumatoid arthritis: mechanisms, diagnosis and treatment. Nat Rev Rheumatol. 2012;8:656–664. doi: 10.1038/nrrheum.2012.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldring SR. Pathogenesis of bone erosions in rheumatoid arthritis. Current opinion in rheumatology. 2002;14:406–410. doi: 10.1097/00002281-200207000-00013. [DOI] [PubMed] [Google Scholar]
- 5.Fox DA. The role of T cells in the immunopathogenesis of rheumatoid arthritis: new perspectives. Arthritis and rheumatism. 1997;40:598–609. doi: 10.1002/art.1780400403. [DOI] [PubMed] [Google Scholar]
- 6.Yocum DE. T cells: pathogenic cells and therapeutic targets in rheumatoid arthritis. Seminars in arthritis and rheumatism. 1999;29:27–35. doi: 10.1016/S0049-0172(99)80035-3. [DOI] [PubMed] [Google Scholar]
- 7.Murphy CA, et al. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. The Journal of experimental medicine. 2003;198:1951–1957. doi: 10.1084/jem.20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sato K, et al. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. The Journal of experimental medicine. 2006;203:2673–2682. doi: 10.1084/jem.20061775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kotake S, et al. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J Clin Invest. 1999;103:1345–1352. doi: 10.1172/JCI5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ziolkowska M, et al. High levels of IL-17 in rheumatoid arthritis patients: IL-15 triggers in vitro IL-17 production via cyclosporin A-sensitive mechanism. Journal of immunology. 2000;164:2832–2838. doi: 10.4049/jimmunol.164.5.2832. [DOI] [PubMed] [Google Scholar]
- 11.Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. Journal of immunology. 2003;171:6173–6177. doi: 10.4049/jimmunol.171.11.6173. [DOI] [PubMed] [Google Scholar]
- 12.Alonzi T, et al. Interleukin 6 is required for the development of collagen-induced arthritis. The Journal of experimental medicine. 1998;187:461–468. doi: 10.1084/jem.187.4.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corneth, O. B. et al. Lack of IL-17 receptor A signaling prevents autoimmune inflammation of the joint and gives rise to a Th2-like phenotype in collagen-induced arthritis. Arthritis and rheumatism (2013). [DOI] [PubMed]
- 14.Genovese MC, et al. Efficacy and safety of secukinumab in patients with rheumatoid arthritis: a phase II, dose-finding, double-blind, randomised, placebo controlled study. Ann Rheum Dis. 2013;72:863–869. doi: 10.1136/annrheumdis-2012-201601. [DOI] [PubMed] [Google Scholar]
- 15.Genovese MC, et al. LY2439821, a humanized anti-interleukin-17 monoclonal antibody, in the treatment of patients with rheumatoid arthritis: A phase I randomized, double-blind, placebo-controlled, proof-of-concept study. Arthritis and rheumatism. 2010;62:929–939. doi: 10.1002/art.27334. [DOI] [PubMed] [Google Scholar]
- 16.Papp KA, et al. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N Engl J Med. 2012;366:1181–1189. doi: 10.1056/NEJMoa1109017. [DOI] [PubMed] [Google Scholar]
- 17.Kellner H. Targeting interleukin-17 in patients with active rheumatoid arthritis: rationale and clinical potential. Therapeutic advances in musculoskeletal disease. 2013;5:141–152. doi: 10.1177/1759720X13485328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwandner R, Yamaguchi K, Cao Z. Requirement of tumor necrosis factor receptor-associated factor (TRAF)6 in interleukin 17 signal transduction. The Journal of experimental medicine. 2000;191:1233–1240. doi: 10.1084/jem.191.7.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun D, et al. Treatment with IL-17 prolongs the half-life of chemokine CXCL1 mRNA via the adaptor TRAF5 and the splicing-regulatory factor SF2 (ASF) Nature immunology. 2011;12:853–860. doi: 10.1038/ni.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qian Y, et al. The adaptor Act1 is required for interleukin 17-dependent signaling associated with autoimmune and inflammatory disease. Nature immunology. 2007;8:247–256. doi: 10.1038/ni1439. [DOI] [PubMed] [Google Scholar]
- 21.Chang SH, Park H, Dong C. Act1 adaptor protein is an immediate and essential signaling component of interleukin-17 receptor. The Journal of biological chemistry. 2006;281:35603–35607. doi: 10.1074/jbc.C600256200. [DOI] [PubMed] [Google Scholar]
- 22.Pisitkun P, Claudio E, Ren N, Wang H, Siebenlist U. The adaptor protein CIKS/ACT1 is necessary for collagen-induced arthritis, and it contributes to the production of collagen-specific antibody. Arthritis and rheumatism. 2010;62:3334–3344. doi: 10.1002/art.27653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qu, F. et al. TRAF6 dependent Act1 phosphorylation by the IKK-related kinases suppresses IL-17-induced NF-kappaB activation. Mol Cell Biol (2012). [DOI] [PMC free article] [PubMed]
- 24.Zhu S, et al. Modulation of experimental autoimmune encephalomyelitis through TRAF3-mediated suppression of interleukin 17 receptor signaling. The Journal of experimental medicine. 2010;207:2647–2662. doi: 10.1084/jem.20100703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi P, et al. Persistent stimulation with interleukin-17 desensitizes cells through SCFbeta-TrCP-mediated degradation of Act1. Science signaling. 2011;4:ra73. doi: 10.1126/scisignal.2001653. [DOI] [PubMed] [Google Scholar]
- 26.Song X, Qian Y. The activation and regulation of IL-17 receptor mediated signaling. Cytokine. 2013;62:175–182. doi: 10.1016/j.cyto.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 27.Manabe N, et al. Involvement of fibroblast growth factor-2 in joint destruction of rheumatoid arthritis patients. Rheumatology. 1999;38:714–720. doi: 10.1093/rheumatology/38.8.714. [DOI] [PubMed] [Google Scholar]
- 28.Qu Z, et al. Expression of basic fibroblast growth factor in synovial tissue from patients with rheumatoid arthritis and degenerative joint disease. Laboratory investigation; a journal of technical methods and pathology. 1995;73:339–346. [PubMed] [Google Scholar]
- 29.Yamashita A, et al. Fibroblast growth factor-2 determines severity of joint disease in adjuvant-induced arthritis in rats. Journal of immunology. 2002;168:450–457. doi: 10.4049/jimmunol.168.1.450. [DOI] [PubMed] [Google Scholar]
- 30.Turner N, Grose R. Fibroblast growth factor signalling: from development to cancer. Nat Rev Cancer. 2010;10:116–129. doi: 10.1038/nrc2780. [DOI] [PubMed] [Google Scholar]
- 31.Tsang M, Dawid IB. Promotion and attenuation of FGF signaling through the Ras-MAPK pathway. Science’s STKE: signal transduction knowledge environment. 2004;2004:pe17. doi: 10.1126/stke.2282004pe17. [DOI] [PubMed] [Google Scholar]
- 32.Eswarakumar VP, Lax I, Schlessinger J. Cellular signaling by fibroblast growth factor receptors. Cytokine & growth factor reviews. 2005;16:139–149. doi: 10.1016/j.cytogfr.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 33.Song X, et al. Growth Factor FGF2 Cooperates with Interleukin-17 to Repair Intestinal Epithelial Damage. Immunity. 2015;43:488–501. doi: 10.1016/j.immuni.2015.06.024. [DOI] [PubMed] [Google Scholar]
- 34.Brand DD. Rodent models of rheumatoid arthritis. Comparative medicine. 2005;55:114–122. [PubMed] [Google Scholar]
- 35.Courtenay JS, Dallman MJ, Dayan AD, Martin A, Mosedale B. Immunisation against heterologous type II collagen induces arthritis in mice. Nature. 1980;283:666–668. doi: 10.1038/283666a0. [DOI] [PubMed] [Google Scholar]
- 36.Brand DD, Latham KA, Rosloniec EF. Collagen-induced arthritis. Nat Protoc. 2007;2:1269–1275. doi: 10.1038/nprot.2007.173. [DOI] [PubMed] [Google Scholar]
- 37.Inglis JJ, Simelyte E, McCann FE, Criado G, Williams RO. Protocol for the induction of arthritis in C57BL/6 mice. Nat Protoc. 2008;3:612–618. doi: 10.1038/nprot.2008.19. [DOI] [PubMed] [Google Scholar]
- 38.Bartok B, Firestein GS. Fibroblast-like synoviocytes: key effector cells in rheumatoid arthritis. Immunological reviews. 2010;233:233–255. doi: 10.1111/j.0105-2896.2009.00859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gotoh N. Regulation of growth factor signaling by FRS2 family docking/scaffold adaptor proteins. Cancer science. 2008;99:1319–1325. doi: 10.1111/j.1349-7006.2008.00840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu S, et al. The microRNA miR-23b suppresses IL-17-associated autoimmune inflammation by targeting TAB2, TAB3 and IKK-alpha. Nature medicine. 2012;18:1077–1086. doi: 10.1038/nm.2815. [DOI] [PubMed] [Google Scholar]
- 41.Song X, et al. IL-17RE is the functional receptor for IL-17C and mediates mucosal immunity to infection with intestinal pathogens. Nature immunology. 2011;12:1151–1158. doi: 10.1038/ni.2155. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


