Abstract
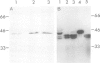
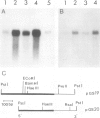
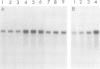
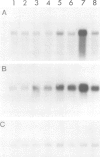
Glutamine synthetase is the key enzyme in the assimilation by plants of reduced nitrogen provided from either the soil or fixed symbiotically in association with Rhizobium. We have isolated a number of cDNA clones for soybean glutamine synthetase (GS) from a nodule-cDNA library, using RNA from polysomes immunoprecipitated by GS antibodies. Transcripts corresponding to two clones differing in their 3' non-translated sequences were present in both root and nodule tissue; however, the concentration in the nodules was several times higher. The relative concentrations of these sequences in both tissues is about 9:1. Availability of ammonium ions [provided as NH4NO3 or (NH4)2SO4] enhanced the expression of both sequences in root tissue within 2 h, reaching a level similar to that in nodules by 8 h, while KNO3 had no effect during this period. When nitrogen fixation was prevented by replacing nitrogen with argon in the root environment or when the nodules were formed by a Fix- mutant of Bradyrhizobium japonicum, the amounts of GS mRNA did not increase over that in roots. These experiments, together with the time course of increase in GS mRNA transcripts, suggest that the genes encoding cytosolic GS are directly induced by the available ammonia.
Keywords: cytosolic glutamine synthetase, gene regulation, nitrogen metabolism, Northern analysis, nodules, Glycine max (soybean)
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergmann H., Preddie E., Verma D. P. Nodulin-35: a subunit of specific uricase (uricase II) induced and localized in the uninfected cells of soybean nodules. EMBO J. 1983;2(12):2333–2339. doi: 10.1002/j.1460-2075.1983.tb01743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullimore J. V., Gebhardt C., Saarelainen R., Miflin B. J., Idler K. B., Barker R. F. Glutamine synthetase of Phaseolus vulgaris L.: organ-specific expression of a multigene family. J Mol Appl Genet. 1984;2(6):589–599. [PubMed] [Google Scholar]
- Fuller F., Künstner P. W., Nguyen T., Verma D. P. Soybean nodulin genes: Analysis of cDNA clones reveals several major tissue-specific sequences in nitrogen-fixing root nodules. Proc Natl Acad Sci U S A. 1983 May;80(9):2594–2598. doi: 10.1073/pnas.80.9.2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhardt C., Oliver J. E., Forde B. G., Saarelainen R., Miflin B. J. Primary structure and differential expression of glutamine synthetase genes in nodules, roots and leaves of Phaseolus vulgaris. EMBO J. 1986 Jul;5(7):1429–1435. doi: 10.1002/j.1460-2075.1986.tb04379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirel B., McNally S. F., Gadal P., Sumar N., Stewart G. R. Cytosolic glutamine synthetase in higher plants. A comparative immunological study. Eur J Biochem. 1984 Jan 2;138(1):63–66. doi: 10.1111/j.1432-1033.1984.tb07881.x. [DOI] [PubMed] [Google Scholar]
- Hirel B., Weatherley C., Cretin C., Bergounioux C., Gadal P. Multiple Subunit Composition of Chloroplastic Glutamine Synthetase of Nicotiana tabacum L. Plant Physiol. 1984 Feb;74(2):448–450. doi: 10.1104/pp.74.2.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lara M., Porta H., Padilla J., Folch J., Sánchez F. Heterogeneity of Glutamine Synthetase Polypeptides in Phaseolus vulgaris L. Plant Physiol. 1984 Dec;76(4):1019–1023. doi: 10.1104/pp.76.4.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layzell D. B., Weagle G. E., Canvin D. T. A highly sensitive, flow through h(2) gas analyzer for use in nitrogen fixation studies. Plant Physiol. 1984 Jul;75(3):582–585. doi: 10.1104/pp.75.3.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T., Zelechowska M., Foster V., Bergmann H., Verma D. P. Primary structure of the soybean nodulin-35 gene encoding uricase II localized in the peroxisomes of uninfected cells of nodules. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5040–5044. doi: 10.1073/pnas.82.15.5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tingey S. V., Walker E. L., Coruzzi G. M. Glutamine synthetase genes of pea encode distinct polypeptides which are differentially expressed in leaves, roots and nodules. EMBO J. 1987 Jan;6(1):1–9. doi: 10.1002/j.1460-2075.1987.tb04710.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma D. P., Haugland R., Brisson N., Legocki R. P., Lacroix L. Regulation of the expression of leghaemoglobin genes in effective and ineffective root nodules of soybean. Biochim Biophys Acta. 1981 Mar 26;653(1):98–107. doi: 10.1016/0005-2787(81)90108-8. [DOI] [PubMed] [Google Scholar]
- Verma D. P., Nash D. T., Schulman H. M. Isolation and in vitro translation of soybean leghaemoglobin mRNA. Nature. 1974 Sep 6;251(5470):74–77. doi: 10.1038/251074a0. [DOI] [PubMed] [Google Scholar]