Abstract
We investigated the association between dietary habits/food group consumption patterns and early risk of metabolic syndrome (MetS), a main cause for metabolic disease. Study participants were recruited from the health promotion center in Dong-A University Hospital and public advertisement. Study subjects (n = 243, 21–80 years) were categorized into three groups: Super-healthy (MetS risk factor [MetS RF] = 0, n = 111), MetS-risk carriers (MetS RF = 1–2, n = 96), and MetS (MetS RF ≥ 3, n = 27). Higher regularity in dietary habits (breakfast-everyday, regular eating time, non-frequent overeating, and non-frequent eating-out) was observed in the Super-healthy group than in the MetS-risk carriers, and particularly in the MetS subjects. The relationship between food group consumption patterns and MetS-risk related parameters were investigated with adjustment for confounding factors. Fruit consumption was positively associated with HDL-cholesterol, and tended to be negatively associated with waist circumference, triglyceride, LDL-cholesterol, and insulin resistance (IR). The consumption of low-fat meats and fish, and vegetables was negatively associated with hs-CRP. Specifically, the consumption of sea-foods belonging to the low-fat fish was negatively associated with fasting glucose, hs-CRP, and interleukin (IL)-6. Anchovy/dried white baits consumption was negatively associated with fasting insulin and IR. Green-yellow vegetables consumption was negatively associated with fasting insulin, IR, and hs-CRP. On the other hand, sugars and fast-foods were positively associated with LDL-cholesterol. Additionally, fast-foods consumption was positively associated with hs-CRP and IL-6 levels. In conclusion, dietary habits/food group consumption patterns are closely associated with MetS-risk related parameters in Koreans. It may suggest useful information to educate people to properly select healthy foods for early prevention of MetS.
Keywords: Dietary habit, Food group consumption pattern, Metabolic syndrome, Inflammation
INTRODUCTION
Metabolic syndrome (MetS) is the name for a group of metabolic disorders including hyperglycemia, hypertension, dyslipidemia, and obesity, particularly abdominal obesity [1]. Uncared metabolic disorders are associated with the increased risk of various chronic diseases related to insulin resistance (IR) such as diabetes, cardiovascular diseases (CVDs), cancer, dementia, and non-alcoholic fatty liver disease [2,3,4,5,6]. The prevalence of MetS has been globally increasing [7,8,9], and the medical expenses related to the treatment of MetS have annually increased from 3.7 trillion won in 2010 to 4.7 trillion won in 2014 (the Health Insurance Review and Assessment Service data). Therefore, it is needed to prevent and control metabolic disorders as an important health issue.
Many causes including age, heredity, and life style, potentially affect the risk of MetS [10,11]. Among them, diet is one of the important causes. Many studies have reported the associations between dietary patterns and MetS [12,13,14,15,16,17]. Westernized diet patterns (i.e., high amounts of saturated fats and simple sugars) have been associated with a higher risk of MetS [12]. In British cohort study, irregular energy intake, particularly from breakfast and between meals was associated with increased cardio-metabolic risk [13]. In addition, rapid eating habit increased the incidence of MetS risk [14]. On the other hand, Mediterranean dietary patterns (i.e., a high consumption of vegetables, fruits, whole cereals, and fish) decreased the risk of MetS [12]. Similarly, the higher meat-eating dietary pattern was associated with a higher prevalence of MetS in Korean male adults in National Cancer Center study [15], higher breakfast skipping was related to an increased risk of obesity and MetS [16], and fast eating behavior was associated with an increased presence risk of non-alcoholic fatty liver disease [17]. However, many studies examining the relationship between dietary patterns and MetS performed in Koreans were implemented with simple survey.
Therefore, this study aimed to investigate if dietary habit and food group consumption patterns are associated with early risk of MetS in non-diabetic healthy Korean adults.
MATERIALS AND METHODS
Study subjects
Study participants (aged over 20 years old) were recruited from the Health Promotion Center in Dong-A University Hospital, and through the public advertisement. Those who have any diagnosis of chronic diseases such as diabetes, CVD, coronary heart disease, stroke, cancer, and any other metabolic diseases were excluded. None of the subjects were taking antihypertensive, lipid-lowering, antidiabetic, or antithrombotic medications. After the screening, a total of 243 Korean adults were finally enrolled in this study. The study was approved by the Institutional Review Board of Dong-A University (2-1040709-AB-N-01-201310-BR-02-04). The informed written consent of all subjects was obtained.
Definition of MetS
MetS was defined by a combination of modified National Cholesterol Education Program-Adult Treatment Panel (NCEP-ATP) III criteria [18], the American Diabetes Association and the Korean society for the study of obesity. MetS risk factors (MetS RFs) include waist circumference (WC) ≥ 90 cm (male) or ≥ 85 cm (female); systolic blood pressure (BP) ≥ 130 mmHg or diastolic BP ≥ 85 mmHg; fasting blood glucose ≥ 100 mg/dL; high-density lipoprotein cholesterol (HDL-C) < 40 mg/dL (male), < 50 mg/dL (female); and triglyceride (TG) ≥ 150 mg/dL. The subjects were divided into 3 groups according to the numbers of MetS RFs: those who have no MetS RFs as ‘Super-healthy’; those who have 1 or 2 MetS RFs as ‘MetS risk carrier’; those who have 3 or more MetS RFs, as ‘MetS.’
Basic information and diet survey
Basic information including demographics, medical history, family history, and physical activity of subjects was collected through a questionnaire interviewed by a registered dietitian. Diet survey consists of usual dietary habits, a semi-quantitative food frequency questionnaire (FFQ), and a 3-day diet record with 24-hour recall method (2 weekdays and 1 weekend) by face to face interview. FFQ consists of 32 questions based on Korean National Health and Nutrition Survey form, and results were expressed as a sum of weekly food consumption based on 1 serving size. Briefly, 32 questions were composed of grains (cooked rice, noodle, bread/sponge cake/cake/crackers/biscuits, rice cake/potato/corn), meat and fishes (red meat/pork/chicken, low fat fishes, squid/shrimp, crab/shellfish/oyster, anchovy/dried white bait, eggs, medium fat fishes, tofu/black bean, hams, high fat fishes, rib/sausage/eel), vegetables (green-yellow, white, young radish/radish leaves, kimchi/diced radish kimchi, seaweeds), fats (nuts, plant oils, mayonnaise/butter/creams/bacon), milk products (milk, yoghurt, ice cream), fruits (fresh fruit/fresh juice), sugars (sugar/syrup/sweetened drink/candy/chocolate), and others (fast-foods, instant noodle, Korean stews, coffee/tea). Energy intake and nutrient content from 3-day diet records were estimated using the Computer Aided Nutritional analysis program (CAN-pro 4.0; the Korean Nutrition Society, Seoul, Korea).
Anthropometric measurements, BP and blood collection
Anthropometric measurements were taken with subjects wearing light clothes except shoes. Height, body weight, and fat and muscle mass were measured by automatic body composition analyzer (N20; AIIA Communication Inc., Seongnam, Korea) without metallic materials. Body mass index (BMI) was calculated as body weight divided by height in square meters (kg/m2). WC was measured by measuring tape. BP was measured at the seated subjects' arm after a rest, using an automatic BP monitor (HEM-7220; OMRON, Matsusaka, Japan). Blood samples were collected in plain and ethylenediaminetetraacetic acid (EDTA)-treated tubes in the morning after an 8-hour fast. Then, samples were separated into serum and plasma, respectively using a centrifuge and stored at −80°C before analysis.
Lipid profiles and glycemic parameters
Serum total cholesterol (TC) and TG levels were measured with kits on a Hitachi 7150 auto-analyzer (Hitachi Ltd., Tokyo, Japan). After precipitation of serum chylomicrons with dextran sulfate magnesium, the concentrations of low-density lipoprotein cholesterol (LDL-C) and HDL-C in the supernatants were enzymatically measured. Fasting serum glucose levels were measured using a glucose oxidase method with a Beckman Glucose analyzer (Beckman Instruments, Irvine, CA, USA). Insulin and C-peptide levels were measured by radioimmuno-assays with commercial kits (ImmunoNucleo Corporation, Stillwater, MN, USA). IR was calculated with the homeostasis model assessment (HOMA) using the following equation:
| HOMA-IR = (fasting insulin [µIU/mL] × fasting glucose [mmol/L])/22.5 |
It developed by Matthews et al. [19] and Choi et al. [20]. Hemoglobin A1c (HbA1c) was measured by a glycated hemoglobin analyzer (SD A1cCare™; SD Biosensor Inc., Suwon, Korea).
Inflammatory markers
High-sensitivity C-reactive protein (hs-CRP) was measured by hs-CRP-latex (II) X2 kit (Seiken Laboratories, Tokyo, Japan). Plasma tumor necrosis factor (TNF)-α and interleukin (IL)-6 were analyzed by Quantikine HS ELISA Kits (R & D Systems, Minneapolis, MN, USA). The color reaction results were measured by iMark™ microplate absorbance reader (Bio-Rad Laboratories, Hercules, CA, USA) at 490 nm.
Statistical analysis
The statistical analysis was carried out with SPSS version 23.0 (SPSS Inc., Chicago, IL, USA). To evaluate differences among the groups, 1-way analysis of variance (ANOVA) with post hoc Bonferroni test and general linear model were used. Pearson and partial correlations were used to describe the association among dietary consumption patterns and MetS RFs. Results were expressed as mean ± standard error, percentages, or correlation co-efficient. A 2-tailed p value under 0.05 was considered significant.
RESULTS
Basic characteristics and biochemical parameters and dietary habits according to MetS risk status
Table 1 presents basic and biochemical characteristics and dietary habits of study subjects according to MetS risk status. As the number of MetS RF increased, people were older, and more obese, particularly had higher WC, and showed dyslipidemia for example, higher levels of triglyceride, LDL-C, TC, and lower levels of HDL-C. Regarding glycemic parameters, fasting levels of glucose, insulin, C-peptide, HOMA-IR, and HbA1C were increased when the number of MetS RF increased. As the number of MetS RF increased, the proportions of males and current alcohol drinkers were increased, but no significant differences were observed in the proportions of cigarette smoking and regular exercising among the 3 groups. Regarding dietary habit, super-healthy people kept higher regularity in everyday breakfast and regular eating time, but non-frequent overeating and non-frequent eating-out than the MetS risk carriers, and particularly than the MetS people.
Table 1. Basic and biochemical characteristics and dietary habits according to MetS risk status.
| Parameters | Super-healthy (n = 111) | MetS risk carriers (n = 96) | MetS (n = 27) | p value* | |
|---|---|---|---|---|---|
| Age, yr | 43.20 ± 1.15 | 46.80 ± 1.49 | 51.60 ± 2.84 | 0.010† | |
| BMI, kg/m2 | 21.90 ± 0.20 | 25.40 ± 0.31 | 27.60 ± 0.55 | < 0.001 | |
| WC, cm | 74.20 ± 0.62 | 86.70 ± 0.92 | 93.10 ± 1.74 | < 0.001 | |
| Systolic BP, mmHg | 110.20 ± 0.84 | 121.10 ± 1.50 | 133.00 ± 2.55 | < 0.001 | |
| Diastolic BP, mmHg | 70.00 ± 0.56 | 77.40 ± 1.01 | 82.20 ± 1.97 | < 0.001 | |
| Triglyceride, mg/dL‡ | 65.50 ± 2.48 | 107.50 ± 6.06 | 195.20 ± 20.60 | < 0.001 | |
| HDL-C, mg/dL | 68.00 ± 1.17 | 58.60 ± 1.37 | 47.70 ± 2.68 | < 0.001 | |
| LDL-C, mg/dL‡ | 114.20 ± 2.45 | 125.70 ± 3.30 | 130.30 ± 7.50 | 0.020 | |
| TC, mg/dL | 186.80 ± 2.42 | 196.90 ± 3.67 | 204.90 ± 8.41 | 0.022 | |
| Glucose, mg/dL‡ | 84.70 ± 0.68 | 95.70 ± 2.03 | 105.10 ± 3.60 | < 0.001 | |
| HbA1C, %‡ | 5.20 ± 0.03 | 5.51 ± 0.05 | 5.74 ± 0.12 | < 0.001 | |
| Insulin, μIU/mL‡§ | 7.10 ± 1.21 | 11.90 ± 1.86 | 23.60 ± 5.15 | < 0.001 | |
| HOMA-IR‡§ | 1.51 ± 0.29 | 3.28 ± 0.73 | 6.33 ± 1.48 | < 0.001 | |
| C-peptide, ng/mL‡¶ | 1.74 ± 0.22 | 2.36 ± 0.22 | 4.09 ± 0.68 | < 0.001 | |
| hs-CRP, mg/dL‡ | 0.59 ± 0.13 | 1.52 ± 0.50 | 1.79 ± 0.42 | < 0.001 | |
| IL-6, pg/mL‡ | 1.11 ± 0.16 | 1.31 ± 0.19 | 1.70 ± 0.44 | 0.015 | |
| TNF-α, pg/mL‡ | 0.96 ± 0.05 | 1.27 ± 0.19 | 1.43 ± 0.22 | 0.043 | |
| Proportion | |||||
| Male | 16 (14.4) | 33 (34.4) | 12 (44.4) | < 0.001 | |
| Current smoker | 7 (6.3) | 11 (11.5) | 1 (3.7) | NS | |
| Current drinker | 29 (26.1) | 40 (41.7) | 13 (48.1) | 0.027 | |
| Dietary habit | |||||
| Breakfast everyday | 59 (53.2) | 51 (53.1) | 13 (48.1) | < 0.050 | |
| Regular eating time | 74 (66.0) | 54 (56.2) | 11 (40.7) | < 0.001 | |
| Frequent overeating | 7 (6.3) | 8 (8.3) | 4 (14.8) | < 0.050 | |
| Frequent eating-out | 14 (12.6) | 16 (16.7) | 5 (18.5) | < 0.050 | |
| Regular exercise | 36 (32.4) | 33 (34.4) | 9 (33.3) | NS | |
Data are means ± SE or number (%).
MetS, metabolic syndrome; BMI, body mass index; WC, waist circumference; BP, blood pressure; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TC, total cholesterol; HbA1C, hemoglobin A1C; HOMA-IR, homeostasis model assessment of insulin resistance index; hs-CRP, high-sensitivity C-reactive protein; IL-6, interleukin-6; TNF-α, tumor necrosis factor-alpha; NS, no statistical significance; SE, standard error; ANOVA, analysis of variance.
*Age adjusted p value; †Unadjusted p value; tested by 1-way ANOVA (unadjusted) or general linear model methods (age adjusted) with Bonferroni method; ‡Tested after log transformed due to the skewed distribution; §n = 147; ¶n = 107.
Food group consumption per week according to MetS risk status
Table 2 shows the sum total of weekly frequency of food group consumption according to MetS risk status. No significant differences were found in the frequency of food group consumption among the 3 groups. In addition, daily intakes of total calorie and other nutrients were not significant different among the 3 groups (data not shown).
Table 2. Patterns of food group consumption per week according to MetS risk status.
| Food group* | Super-healthy (n = 111) | MetS risk carriers (n = 96) | MetS (n = 27) | p value | |
|---|---|---|---|---|---|
| Grains | 18.30 ± 0.64 | 18.30 ± 0.62 | 19.10 ± 1.81 | 0.855 | |
| Meats and fish | 24.40 ± 1.66 | 21.00 ± 1.31 | 21.00 ± 2.00 | 0.230 | |
| Low-fat | 11.10 ± 0.98 | 9.81 ± 0.84 | 8.62 ± 1.11 | 0.342 | |
| Medium-fat | 9.70 ± 0.88 | 8.69 ± 0.76 | 9.30 ± 1.23 | 0.685 | |
| High-fat | 3.53 ± 0.39 | 2.46 ± 0.25 | 3.10 ± 0.94 | 0.112 | |
| Vegetables | 26.60 ± 1.53 | 27.00 ± 1.83 | 23.80 ± 2.54 | 0.670 | |
| Milk products | 9.54 ± 0.86 | 7.88 ± 0.66 | 9.58 ± 1.89 | 0.309 | |
| Fruits | 6.42 ± 0.50 | 5.68 ± 0.48 | 6.31 ± 0.84 | 0.544 | |
| Fast-foods | 1.17 ± 0.12 | 0.99 ± 0.14 | 1.07 ± 0.27 | 0.628 | |
Data are means ± SE; tested by 1-way ANOVA with Bonferroni method.
MetS, metabolic syndrome; SE, standard error; ANOVA, analysis of variance.
*Calculated as sum total of 1-serving sized food consumption in each food group per week.
Relationship between food group consumption patterns and MetS risk related parameters
As stated above, food group consumption patterns were not significantly different according to MetS risk status. Therefore, we sub-analyzed the association between food group consumption pattern and each of MetS risk related parameters. Table 3 shows the relationship between the food group consumption and MetS risk related parameters after adjustment for age, sex, total calorie intake, cigarette smoking, and alcohol consumption which were basic parameters and significantly different among the 3 MetS risk group. The consumptions of low-fat meats and fish (r = −0.144, p = 0.032), and vegetables total (r = −0.188, p = 0.055) were negatively associated with hs-CRP levels. Specifically, the consumption of seafoods which belong to the low-fat fish group was negatively associated with the levels of serum glucose (r = −0.158, p = 0.017), hs-CRP (r = −0.211, p = 0.002), and IL-6 (r = −0.155, p = 0.021). The consumption of anchovy and dried white baits was negatively associated with the levels of insulin (r = −0.186, p = 0.027), HOMA-IR (r = −0.170, p = 0.044), and tended toward negative association with C-peptide (r = −0.170, p = 0.087). In the vegetables group, the consumption of green-yellow vegetables was negatively associated the levels of insulin (r = −0.170, p = 0.043), HOMA-IR (r = −0.180, p = 0.032), hs-CRP (r = −0.162, p = 0.015), and tended toward negative association with glucose (r = −0.114, p = 0.086). In addition, fruits consumption was positively associated with HDL-C (r = 0.156, p = 0.018), tended to be negatively associated with WC (r = −0.126, p = 0.057), triglyceride (r = −0.122, p = 0.065), LDL-C (r = −0.127, p = 0.055), and HOMA-IR (r = −0.157, p = 0.062). On the other hand, the consumption of the sugars (r = 0.157, p = 0.018) and fast-foods (r = 0.141, p = 0.033) was positively associated with LDL-C levels. The fast-foods consumption was also positively associated inflammation markers such as hs-CRP (r = 0.156, p = 0.020) and IL-6 levels (r = 0.132, p = 0.050). However, no significant relationships were observed between milk product consumption and MetS risk related parameters.
Table 3. Relationship between food group consumption patterns and MetS related risk parameters.
| Food group* | Waist | SBP | DBP | TG† | HDL-C | LDL-C† | Glucose† | Insulin† | C-peptide† | IR† | hs-CRP† | IL-6† | TNF-α† | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grains | r | −0.066 | 0.038 | 0.004 | 0.046 | 0.078 | 0.083 | 0.074 | 0.115 | 0.071 | 0.123 | −0.016 | 0.100 | 0.051 | |
| p | 0.321 | 0.569 | 0.957 | 0.489 | 0.242 | 0.213 | 0.269 | 0.173 | 0.481 | 0.144 | 0.814 | 0.136 | 0.451 | ||
| Meats and fish | r | −0.043 | 0.011 | 0.045 | −0.014 | −0.018 | 0.119 | −0.054 | −0.120 | −0.133 | −0.118 | −0.047 | −0.008 | −0.071 | |
| p | 0.519 | 0.863 | 0.498 | 0.831 | 0.792 | 0.072 | 0.413 | 0.154 | 0.182 | 0.161 | 0.483 | 0.901 | 0.294 | ||
| High-fat | r | −0.060 | −0.027 | 0.072 | −0.042 | 0.015 | 0.058 | −0.075 | −0.087 | −0.087 | −0.100 | 0.026 | −0.043 | 0.027 | |
| p | 0.365 | 0.686 | 0.280 | 0.529 | 0.825 | 0.384 | 0.259 | 0.304 | 0.382 | 0.236 | 0.699 | 0.518 | 0.693 | ||
| Medium-fat | r | 0.044 | 0.076 | 0.134 | −0.043 | 0.048 | 0.060 | −0.009 | −0.033 | −0.026 | −0.033 | 0.064 | 0.051 | −0.101 | |
| p | 0.506 | 0.253 | 0.044 | 0.523 | 0.475 | 0.365 | 0.897 | 0.698 | 0.793 | 0.699 | 0.343 | 0.451 | 0.131 | ||
| Low-fat | r | −0.086 | −0.037 | −0.072 | 0.031 | −0.077 | 0.121 | −0.052 | −0.144 | −0.174 | −0.134 | −0.144 | −0.041 | −0.038 | |
| p | 0.196 | 0.573 | 0.276 | 0.643 | 0.248 | 0.069 | 0.431 | 0.087 | 0.081 | 0.112 | 0.032 | 0.545 | 0.576 | ||
| Seafoods among the low-fat fish | |||||||||||||||
| Crab, shellfish, oyster, etc. | r | −0.115 | −0.048 | −0.030 | −0.041 | 0.008 | 0.180 | −0.158 | −0.084 | −0.100 | −0.100 | −0.211 | −0.155 | 0.001 | |
| p | 0.084 | 0.473 | 0.649 | 0.539 | 0.907 | 0.006 | 0.017 | 0.320 | 0.317 | 0.237 | 0.002 | 0.021 | 0.992 | ||
| Anchovy, dried white bait | r | −0.100 | −0.073 | −0.104 | −0.038 | 0.010 | 0.090 | −0.038 | −0.186 | −0.170 | −0.170 | −0.029 | −0.061 | −0.011 | |
| p | 0.133 | 0.272 | 0.117 | 0.568 | 0.881 | 0.177 | 0.569 | 0.027 | 0.087 | 0.044 | 0.665 | 0.368 | 0.865 | ||
| Vegetables total | r | −0.049 | 0.064 | 0.027 | −0.056 | 0.032 | 0.037 | −0.012 | −0.076 | 0.050 | −0.073 | −0.188 | −0.085 | −0.086 | |
| p | 0.461 | 0.337 | 0.684 | 0.396 | 0.628 | 0.574 | 0.854 | 0.369 | 0.620 | 0.391 | 0.005 | 0.207 | 0.203 | ||
| Vegetable subgroup | |||||||||||||||
| Green-yellow | r | −0.075 | 0.051 | 0.020 | −0.057 | 0.012 | 0.029 | −0.114 | −0.170 | −0.117 | −0.180 | −0.162 | −0.081 | −0.084 | |
| p | 0.258 | 0.443 | 0.759 | 0.303 | 0.853 | 0.510 | 0.086 | 0.043 | 0.242 | 0.032 | 0.015 | 0.229 | 0.211 | ||
| Young radish, radish leaves | r | −0.090 | 0.093 | 0.052 | −0.129 | 0.060 | −0.041 | −0.048 | −0.219 | −0.163 | −0.213 | −0.074 | −0.046 | 0.075 | |
| p | 0.175 | 0.164 | 0.434 | 0.052 | 0.370 | 0.541 | 0.475 | 0.009 | 0.101 | 0.011 | 0.271 | 0.494 | 0.264 | ||
| Kimchi, diced radish kimchi | r | −0.043 | −0.014 | 0.004 | 0.040 | 0.042 | 0.040 | 0.032 | 0.044 | 0.195 | 0.046 | −0.194 | −0.081 | −0.163 | |
| p | 0.515 | 0.831 | 0.957 | 0.550 | 0.526 | 0.553 | 0.634 | 0.600 | 0.049 | 0.589 | 0.004 | 0.228 | 0.015 | ||
| Milk products | r | −0.060 | 0.025 | 0.015 | −0.006 | 0.046 | 0.019 | −0.047 | −0.064 | −0.036 | −0.076 | 0.043 | −0.022 | −0.058 | |
| p | 0.370 | 0.704 | 0.816 | 0.933 | 0.485 | 0.780 | 0.482 | 0.447 | 0.716 | 0.366 | 0.526 | 0.742 | 0.389 | ||
| Fruits | r | −0.126 | −0.062 | −0.082 | −0.122 | 0.156 | −0.127 | −0.054 | −0.160 | −0.098 | −0.157 | −0.063 | −0.082 | −0.055 | |
| p | 0.057 | 0.353 | 0.215 | 0.065 | 0.018 | 0.055 | 0.417 | 0.057 | 0.326 | 0.062 | 0.352 | 0.220 | 0.415 | ||
| Sugars: sugar, syrup, candy, chocolate, etc. | r | 0.108 | 0.065 | 0.004 | −0.002 | −0.011 | 0.157 | −0.034 | 0.016 | 0.027 | 0.013 | 0.017 | 0.029 | −0.013 | |
| p | 0.104 | 0.331 | 0.948 | 0.980 | 0.870 | 0.018 | 0.614 | 0.853 | 0.786 | 0.878 | 0.798 | 0.671 | 0.849 | ||
| Fast-foods | r | 0.023 | 0.013 | −0.003 | −0.004 | −0.025 | 0.141 | 0.005 | −0.002 | 0.001 | −0.007 | 0.156 | 0.132 | 0.043 | |
| p | 0.734 | 0.851 | 0.960 | 0.957 | 0.703 | 0.033 | 0.939 | 0.980 | 0.988 | 0.937 | 0.020 | 0.050 | 0.527 | ||
Tested by partial correlation analysis adjusted age, sex, total calorie intake, cigarette smoking, and alcohol drinking.
MetS, metabolic syndrome; SBP, systolic blood pressure; DBP, diastolic blood pressure; TG, triglyceride; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; IR, insulin resistance; hs-CRP, high-sensitivity C-reactive protein; IL-6, interleukin-6; TNF-α, tumor necrosis factor-alpha; r, correlation coefficient.
*Calculated as sum total of one-serving sized food consumption in each food group per week; †Tested after log-transformed.
Comparison of adiposity and inflammation according to MetS risk status and fast-food consumption
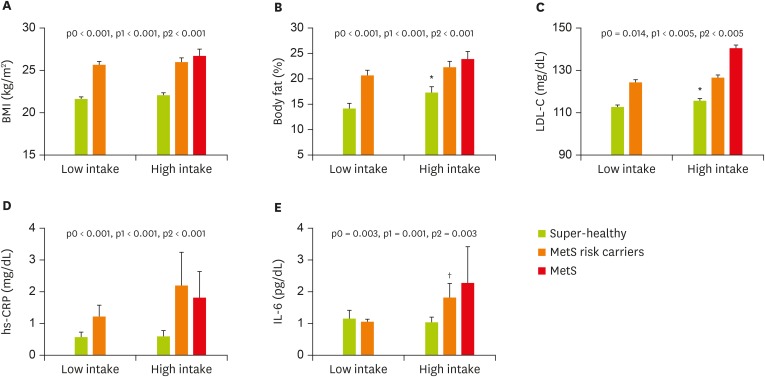
As shown in Table 3, fast-foods consumption which was highly correlated with MetS and cardiovascular risk (LDL-C, IL-6, and TNF-α) was selected for sub-analysis. We sub-grouped study subjects by fast-food consumption level (the lower 50 percentiles in study subjects “lower intake group”: they consumed fast-foods less than once per week, vs. the upper 50 percentiles in study subjects “higher intake group”: they consumed fast-foods once and more per week). In addition, each of fast-foods consumption group was subdivided into super-healthy, MetS risk carriers, and MetS according to MetS RF status. Finally, study subjects were categorized into 6 sub-groups (the low intake group: super healthy [n = 61], MetS risk carrier [n = 75]; the high intake group: super healthy [n = 50], MetS risk carrier [n = 38], and MetS [n = 10]) (Figure 1). Interestingly, subjects with MetS were observed only in the high fast-food intake group. All subjects in the high fast-foods intake group were more obese, and had higher levels of body fat (%) and LDL-C than those in the low fast-foods intake group. Circulating levels of hs-CRP and IL-6 were also significantly higher in the high fast-foods intake group than in the low fast-foods intake group. Particularly, IL-6 levels in the high fast-foods intake group were increased as the number of MetS RF increased.
Figure 1.
Adiposity and inflammation according to MetS risk status and fast-food intake level. Data are means ± SE.
p0, unadjusted p value; p1, p value adjusted for age and sex; p2, p value adjusted for age, sex, total calorie intake, cigarette smoking, and alcohol drinking; BMI, body mass index; LDL-C, low-density lipoprotein cholesterol; hs-CRP, high-sensitivity C-reactive protein; IL-6, interleukin-6; MetS, metabolic syndrome; SE, standard error; ANOVA, analysis of variance.
*Tested after log-transformed; †Tested by 1-way ANOVA with Bonferroni method or general linear model with adjustment.
DISCUSSION
This cross-sectional study was performed to examine the association between food group consumption pattern, and early risk of MetS in non-diabetic healthy Korean adults. We identified that dietary habits and selected food consumption patterns are significantly associated with early alteration of MetS risk related parameters. This is the first study to elucidate the relationship between food consumption patterns and MetS risk status focusing on early alteration of endocrine metabolism in healthy Korean adults.
In the present study, we observed that dietary habit such as meal regularity, and the frequencies of having breakfast, overeating and eating-out were related to MetS risk. Specifically, higher proportions of irregular eating, less breakfast eating, and frequent overeating and eating out were observed in subjects with MetS RFs, particularly in those with MetS than in super-healthy people. Our results are partly in accordance with previous reports [13,21,22,23]. Pot et al. [13] reported that individuals with a more irregular intake of energy, especially during breakfast, had the increased cardio-metabolic risk. Odegaard et al. [21] also demonstrated that having breakfast everyday was strongly associated with the reduced risk of metabolic conditions. Shin et al. [22] showed that subjects with MetS more overeat than those without MetS. Also, people with MetS showed higher rate of eating out than those without MetS in the study by Ohta et al. [23].
Regarding the association between food group consumption patterns and MetS risk related parameters, the low-fat meats and fish consumption was negatively correlated with the inflammatory response in this study. Particularly, higher seafood consumption was associated with lower levels of hs-CRP and IL-6. However, contrary to our results, Nanri et al. [24] reported that seafood consumption pattern was significantly associated with elevated hs-CRP levels in men. Precise mechanisms for the relationship between seafood intake and inflammation need to be elucidated. In addition, higher consumption of anchovy and dried white baits were associated with lower levels of insulin and HOMA-IR. Anchovy and dried white baits which belong to low-fat fish group are rich sources of dietary calcium. The Korean population study based on the Korea National Health and Nutrition Examination Survey 2008–2011 shows that calcium intake may reduce the prevalence of MetS in postmenopausal women [25]. Calcium supplementation also improved insulin sensitivity in adult obese rats [26].
Vegetable consumption was related to inflammatory response and IR in this study. Among vegetables, higher green-yellow vegetables intake was associated with lower levels of hs-CRP and HOMA-IR. Similar result was observed in the report of Nettleton et al. [27]: consumption of green leafy vegetables, whole grains, fruits, and nuts were negatively related to the levels of hs-CRP and IL-6 in a multi-ethnic population. Cook et al. [28] also found in their overweight Latino youth participants that dark green and deep yellow/orange vegetables were positively correlated with insulin sensitivity, but not with IR. In the present study, young radish and their leaves were also negatively associated with insulin levels and IR. However, there were no other reports for the relationship and the mechanisms, thereby further studies being needed. In addition, kimchi consumption was inversely associated with inflammatory responses (i.e., hs-CRP and TNF-α). It is partly in accordance with the report of Oh et al. [29] presenting that consumption of rice combined with kimchi was related to a lower risk of MetS. However, no significant changes were observed in proinflammatory cytokines after 4-week kimchi consumption in Chinese people performed by Lee et al. [30]. To elucidate the relationship between kimchi consumption and inflammation, long-term prospective observation studies are needed.
The fruits consumption was inversely associated with MetS related parameters in the current study. Fruits have plenty of nutrients and bioactive compounds, such as vitamins, minerals, antioxidants, phytochemicals, etc. Daily intake of flavanones was associated with lipid-lowering properties in type 2 diabetic patients with MetS [31]. According to the Tehran lipid glucose study, higher intake of red/purple fruits and vegetables (FVs) was related to lower body weight and abdominal fat gain after 3-year follow-up. Yellow, green, and white FVs were related to favorable lipid parameters [32]. These results are similar with our results. Park et al. [33] also exhibited that higher intake of fruits in Korean women had lower incidences of MetS. Based on our result and previous reports, fruits consumption may be beneficial for controlling lipid profiles, particularly in people exposed at early status of MetS risk.
The current study also presented that LDL-C levels were positively related with consumptions of sugary foods (chocolate, syrup, candy, chocolate, etc.) as well as fast-foods (burgers, pizzas, fries, instant noodles, etc.). Stanhope et al. [34] reported through their 2-week intervention study that consumption of high-fructose corn syrup sweetened beverages for 2 weeks increased concentrations of LDL-C, apoB, and other risk factors for CVD. Aeberli et al. [35] showed that LDL particle size was reduced after high fructose and high sucrose consumption, and more atherogenic LDL subclass distribution was observed when fructose-containing sugar sweetened beverages. Maersk et al. [36] also showed that daily intake of sucrose-sweetened soft drinks for 6-month increased ectopic fat accumulation and blood lipids. In addition, fast food consumption was significantly related to the increased serum levels of triglyceride and LDL-C in Iranian adults, particularly in middle aged adults [37]. Fast food consumption was also associated with higher LDL-C and other MetS related parameters in the adolescents [38].
In the present, we sub-categorized study subjects according to their fast-foods intake levels (less than 1 time per week vs. 1 time and more per week), and observed higher adiposity and inflammation in higher fast-foods intake group particularly with MetS risk. Anderson et al. [39] found the odds of being obese were higher in subjects consuming fast-foods more than once a week than people who consumed less than once a week. El-Seweidy et al. [40] reported that higher expression of genes related to inflammation was induced by higher fast-food consumption.
This study has a limitation that the number of the study subjects was relatively small to be generalized to all population. Further studies with larger population and longitudinal observation are needed. Despite the limitation, these results may suggest useful information to educate people to properly select healthy foods for early prevention of MetS and CVD.
ACKNOWLEDGEMENTS
We thank the research volunteers who participated in the study described in this manuscript.
Footnotes
Funding: This study was supported by the National Research Foundation of Korea Grant funded by the Korean Government (2016R1A2B4013627).
Conflict of Interest: The authors declare that they have no competing interests.
References
- 1.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC., Jr International Diabetes Federation Task Force on Epidemiology and Prevention; Hational Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; International Association for the Study of Obesity. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 2.Klein BE, Klein R, Lee KE. Components of the metabolic syndrome and risk of cardiovascular disease and diabetes in Beaver Dam. Diabetes Care. 2002;25:1790–1794. doi: 10.2337/diacare.25.10.1790. [DOI] [PubMed] [Google Scholar]
- 3.Ford ES. Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome: a summary of the evidence. Diabetes Care. 2005;28:1769–1778. doi: 10.2337/diacare.28.7.1769. [DOI] [PubMed] [Google Scholar]
- 4.Micucci C, Valli D, Matacchione G, Catalano A. Current perspectives between metabolic syndrome and cancer. Oncotarget. 2016;7:38959–38972. doi: 10.18632/oncotarget.8341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ng TP, Feng L, Nyunt MS, Feng L, Gao Q, Lim ML, Collinson SL, Chong MS, Lim WS, Lee TS, Yap P, Yap KB. Metabolic syndrome and the risk of mild cognitive impairment and progression to dementia: follow-up of the Singapore longitudinal ageing study cohort. JAMA Neurol. 2016;73:456–463. doi: 10.1001/jamaneurol.2015.4899. [DOI] [PubMed] [Google Scholar]
- 6.Feng RN, Du SS, Wang C, Li YC, Liu LY, Guo FC, Sun CH. Lean-non-alcoholic fatty liver disease increases risk for metabolic disorders in a normal weight Chinese population. World J Gastroenterol. 2014;20:17932–17940. doi: 10.3748/wjg.v20.i47.17932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O'Neill S, O'Driscoll L. Metabolic syndrome: a closer look at the growing epidemic and its associated pathologies. Obes Rev. 2015;16:1–12. doi: 10.1111/obr.12229. [DOI] [PubMed] [Google Scholar]
- 8.Choi JH, Woo HD, Lee JH, Kim J. Dietary patterns and risk for metabolic syndrome in Korean women: a cross-sectional study. Medicine (Baltimore) 2015;94:e1424. doi: 10.1097/MD.0000000000001424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lim S, Shin H, Song JH, Kwak SH, Kang SM, Won Yoon J, Choi SH, Cho SI, Park KS, Lee HK, Jang HC, Koh KK. Increasing prevalence of metabolic syndrome in Korea: the Korean National Health and Nutrition Examination Survey for 1998–2007. Diabetes Care. 2011;34:1323–1328. doi: 10.2337/dc10-2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eapen D, Kalra GL, Merchant N, Arora A, Khan BV. Metabolic syndrome and cardiovascular disease in South Asians. Vasc Health Risk Manag. 2009;5:731–743. doi: 10.2147/vhrm.s5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al Thani M, Al Thani AA, Al-Chetachi W, Al Malki B, Khalifa SA, Haj Bakri A, Hwalla N, Nasreddine L, Naja FA. ‘High Risk’ lifestyle pattern is associated with metabolic syndrome among Qatari women of reproductive age: a cross-sectional national study. Int J Mol Sci. 2016;17:E698. doi: 10.3390/ijms17060698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martínez-González MA, Martín-Calvo N. The major European dietary patterns and metabolic syndrome. Rev Endocr Metab Disord. 2013;14:265–271. doi: 10.1007/s11154-013-9264-6. [DOI] [PubMed] [Google Scholar]
- 13.Pot GK, Hardy R, Stephen AM. Irregular consumption of energy intake in meals is associated with a higher cardiometabolic risk in adults of a British birth cohort. Int J Obes. 2014;38:1518–1524. doi: 10.1038/ijo.2014.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu B, Haruyama Y, Muto T, Yamazaki T. Association between eating speed and metabolic syndrome in a three-year population-based cohort study. J Epidemiol. 2015;25:332–336. doi: 10.2188/jea.JE20140131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woo HD, Shin A, Kim J. Dietary patterns of Korean adults and the prevalence of metabolic syndrome: a cross-sectional study. PLoS One. 2014;9:e111593. doi: 10.1371/journal.pone.0111593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chung SJ, Lee Y, Lee S, Choi K. Breakfast skipping and breakfast type are associated with daily nutrient intakes and metabolic syndrome in Korean adults. Nutr Res Pract. 2015;9:288–295. doi: 10.4162/nrp.2015.9.3.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee S, Ko BJ, Gong Y, Han K, Lee A, Han BD, Yoon YJ, Park S, Kim JH, Mantzoros CS. Self-reported eating speed in relation to non-alcoholic fatty liver disease in adults. Eur J Nutr. 2016;55:327–333. doi: 10.1007/s00394-015-0851-z. [DOI] [PubMed] [Google Scholar]
- 18.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 19.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 20.Choi ES, Rhee EJ, Kim JH, Won JC, Park CY, Lee WY, Oh KW, Park SW, Kim SW. Insulin sensitivity and insulin secretion determined by homeostasis model assessment and future risk of diabetes mellitus in Korean men. Korean Diabetes J. 2008;32:498–505. [Google Scholar]
- 21.Odegaard AO, Jacobs DR, Jr, Steffen LM, Van Horn L, Ludwig DS, Pereira MA. Breakfast frequency and development of metabolic risk. Diabetes Care. 2013;36:3100–3106. doi: 10.2337/dc13-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shin A, Lim SY, Sung J, Shin HR, Kim J. Dietary intake, eating habits, and metabolic syndrome in Korean men. J Am Diet Assoc. 2009;109:633–640. doi: 10.1016/j.jada.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 23.Ohta Y, Tsuchihashi T, Arakawa K, Onaka U, Ueno M. Prevalence and lifestyle characteristics of hypertensive patients with metabolic syndrome followed at an outpatient clinic in Fukuoka, Japan. Hypertens Res. 2007;30:1077–1082. doi: 10.1291/hypres.30.1077. [DOI] [PubMed] [Google Scholar]
- 24.Nanri H, Nakamura K, Hara M, Higaki Y, Imaizumi T, Taguchi N, Sakamoto T, Horita M, Shinchi K, Tanaka K. Association between dietary pattern and serum C-reactive protein in Japanese men and women. J Epidemiol. 2011;21:122–131. doi: 10.2188/jea.JE20100110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim MK, Chon SJ, Noe EB, Roh YH, Yun BH, Cho S, Choi YS, Lee BS, Seo SK. Associations of dietary calcium intake with metabolic syndrome and bone mineral density among the Korean population: KNHANES 2008–2011. Osteoporos Int. 2017;28:299–308. doi: 10.1007/s00198-016-3717-1. [DOI] [PubMed] [Google Scholar]
- 26.Conceição EP, Moura EG, Soares PN, Ai XX, Figueiredo MS, Oliveira E, Lisboa PC. High calcium diet improves the liver oxidative stress and microsteatosis in adult obese rats that were overfed during lactation. Food Chem Toxicol. 2016;92:245–255. doi: 10.1016/j.fct.2016.04.015. [DOI] [PubMed] [Google Scholar]
- 27.Nettleton JA, Steffen LM, Mayer-Davis EJ, Jenny NS, Jiang R, Herrington DM, Jacobs DR., Jr Dietary patterns are associated with biochemical markers of inflammation and endothelial activation in the Multi-Ethnic Study of Atherosclerosis (MESA) Am J Clin Nutr. 2006;83:1369–1379. doi: 10.1093/ajcn/83.6.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cook LT, O'Reilly GA, Goran MI, Weigensberg MJ, Spruijt-Metz D, Davis JN. Vegetable consumption is linked to decreased visceral and liver fat and improved insulin resistance in overweight Latino youth. J Acad Nutr Diet. 2014;114:1776–1783. doi: 10.1016/j.jand.2014.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oh IM, Joung HJ, Oh SW, Yoon YS, Yoo KH, Park JE, Park JS, Jang EJ, Park SJ, Park SW, Kim SJ, Baik HW. Relationship of combined consumption of rice and kimchi, Korean traditional diet and the risk of metabolic syndrome in healthy Korean volunteers. J Korean Soc Parenter Enter Nutr. 2013;5:110–116. [Google Scholar]
- 30.Lee H, Kim DY, Lee MA, Jang JY, Choue R. Immunomodulatory effects of kimchi in Chinese healthy college students: a randomized controlled trial. Clin Nutr Res. 2014;3:98–105. doi: 10.7762/cnr.2014.3.2.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oh JS, Kim H, Vijayakumar A, Kwon O, Choi YJ, Huh KB, Chang N. Association between dietary flavanones intake and lipid profiles according to the presence of metabolic syndrome in Korean women with type 2 diabetes mellitus. Nutr Res Pract. 2016;10:67–73. doi: 10.4162/nrp.2016.10.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mirmiran P, Bahadoran Z, Moslehi N, Bastan S, Azizi F. Colors of fruits and vegetables and 3-year changes of cardiometabolic risk factors in adults: Tehran lipid and glucose study. Eur J Clin Nutr. 2015;69:1215–1219. doi: 10.1038/ejcn.2015.49. [DOI] [PubMed] [Google Scholar]
- 33.Park S, Ham JO, Lee BK. Effects of total vitamin A, vitamin C, and fruit intake on risk for metabolic syndrome in Korean women and men. Nutrition. 2015;31:111–118. doi: 10.1016/j.nut.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 34.Stanhope KL, Bremer AA, Medici V, Nakajima K, Ito Y, Nakano T, Chen G, Fong TH, Lee V, Menorca RI, Keim NL, Havel PJ. Consumption of fructose and high fructose corn syrup increase postprandial triglycerides, LDL-cholesterol, and apolipoprotein-B in young men and women. J Clin Endocrinol Metab. 2011;96:E1596–E1605. doi: 10.1210/jc.2011-1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aeberli I, Gerber PA, Hochuli M, Kohler S, Haile SR, Gouni-Berthold I, Berthold HK, Spinas GA, Berneis K. Low to moderate sugar-sweetened beverage consumption impairs glucose and lipid metabolism and promotes inflammation in healthy young men: a randomized controlled trial. Am J Clin Nutr. 2011;94:479–485. doi: 10.3945/ajcn.111.013540. [DOI] [PubMed] [Google Scholar]
- 36.Maersk M, Belza A, Stødkilde-Jørgensen H, Ringgaard S, Chabanova E, Thomsen H, Pedersen SB, Astrup A, Richelsen B. Sucrose-sweetened beverages increase fat storage in the liver, muscle, and visceral fat depot: a 6-mo randomized intervention study. Am J Clin Nutr. 2012;95:283–289. doi: 10.3945/ajcn.111.022533. [DOI] [PubMed] [Google Scholar]
- 37.Bahadoran Z, Mirmiran P, Golzarand M, Hosseini-Esfahani F, Azizi F. Fast food consumption in Iranian adults; dietary intake and cardiovascular risk factors: Tehran lipid and glucose study. Arch Iran Med. 2012;15:346–351. [PubMed] [Google Scholar]
- 38.Marlatt KL, Farbakhsh K, Dengel DR, Lytle LA. Breakfast and fast food consumption are associated with selected biomarkers in adolescents. Prev Med Rep. 2015;3:49–52. doi: 10.1016/j.pmedr.2015.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anderson B, Rafferty AP, Lyon-Callo S, Fussman C, Imes G. Fast-food consumption and obesity among Michigan adults. Prev Chronic Dis. 2011;8:A71. [PMC free article] [PubMed] [Google Scholar]
- 40.El-Seweidy MM, Hashem RM, Abo-El-matty DM, Mohamed RH. Frequent inadequate supply of micronutrients in fast food induces oxidative stress and inflammation in testicular tissues of weanling rats. J Pharm Pharmacol. 2008;60:1237–1242. doi: 10.1211/jpp.60.9.0017. [DOI] [PubMed] [Google Scholar]