Abstract
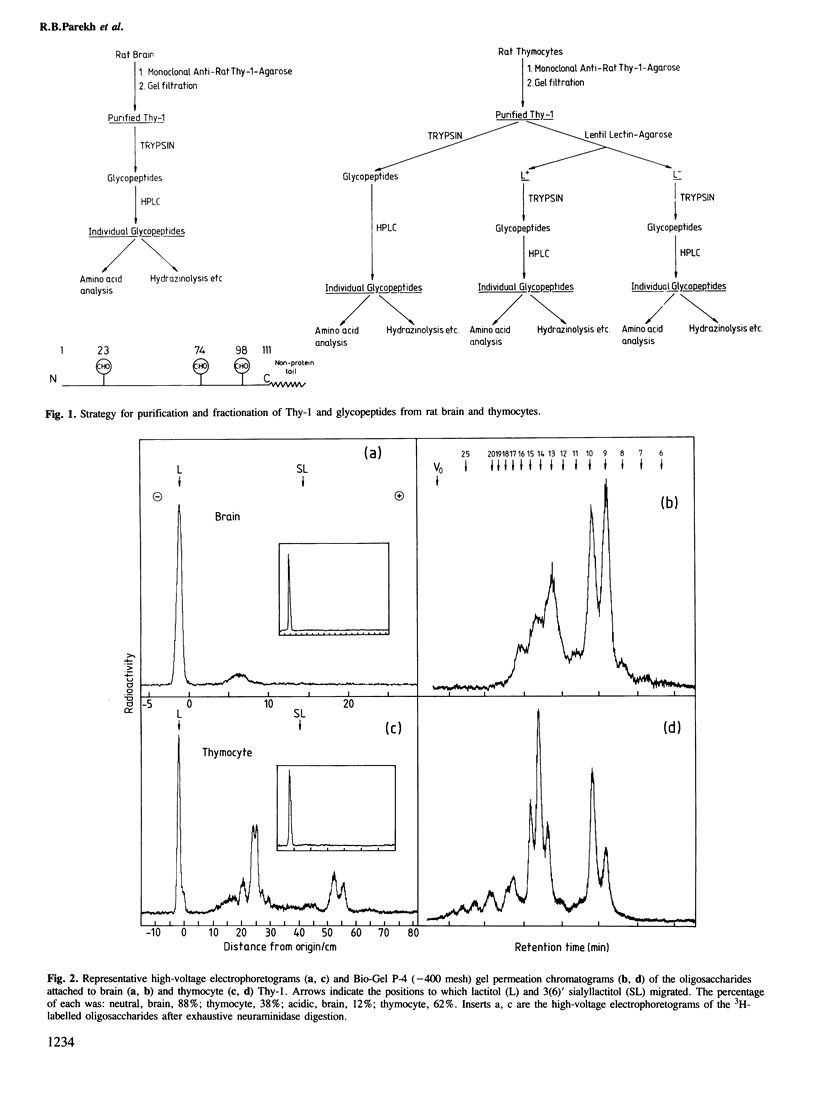
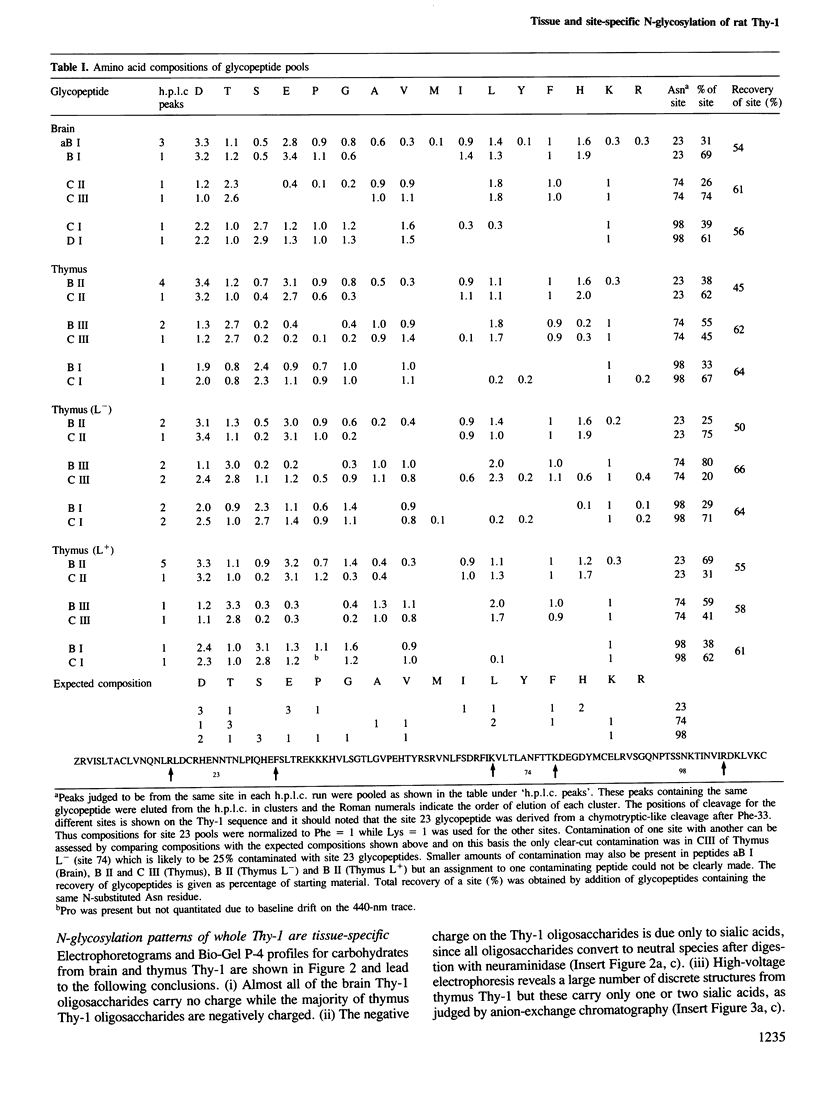
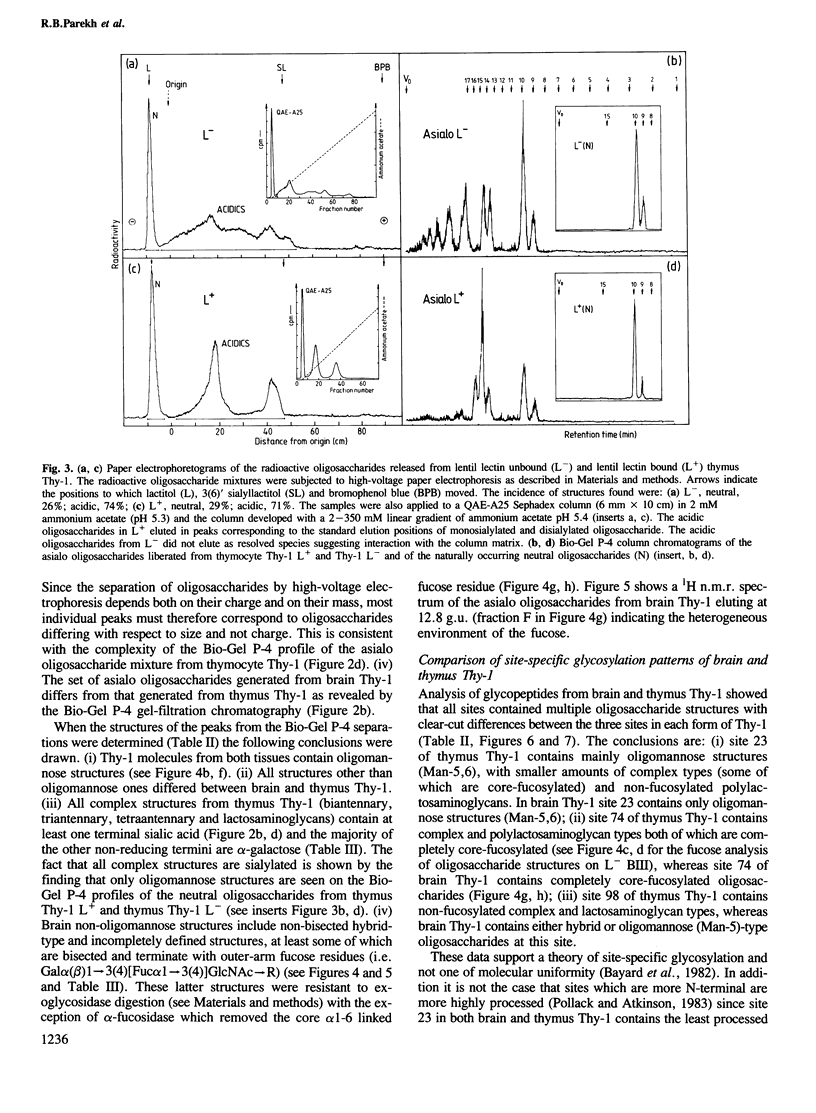
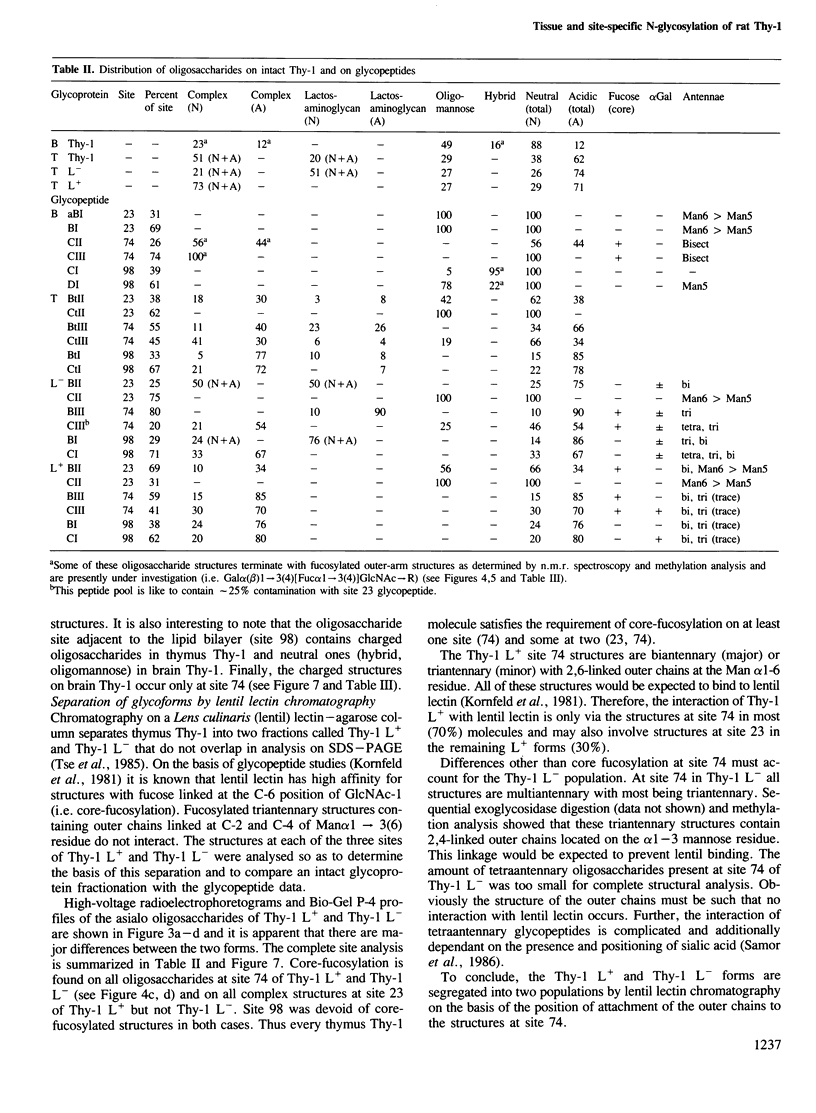
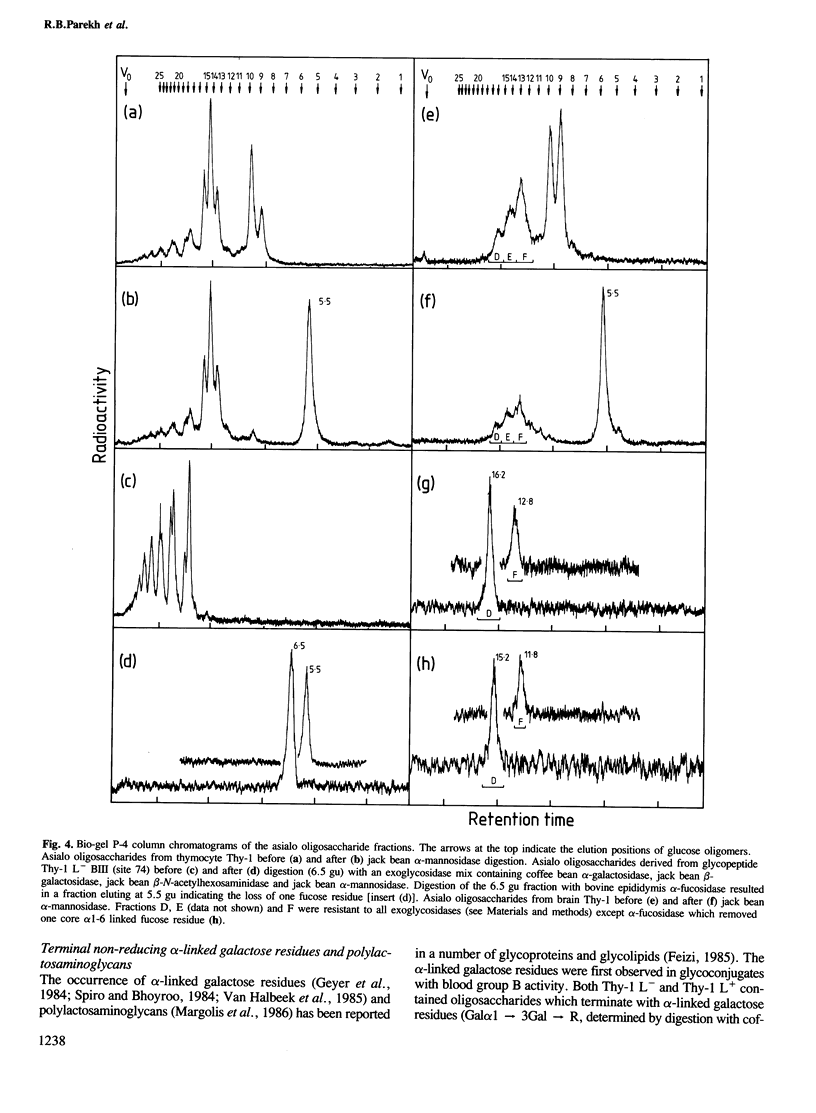
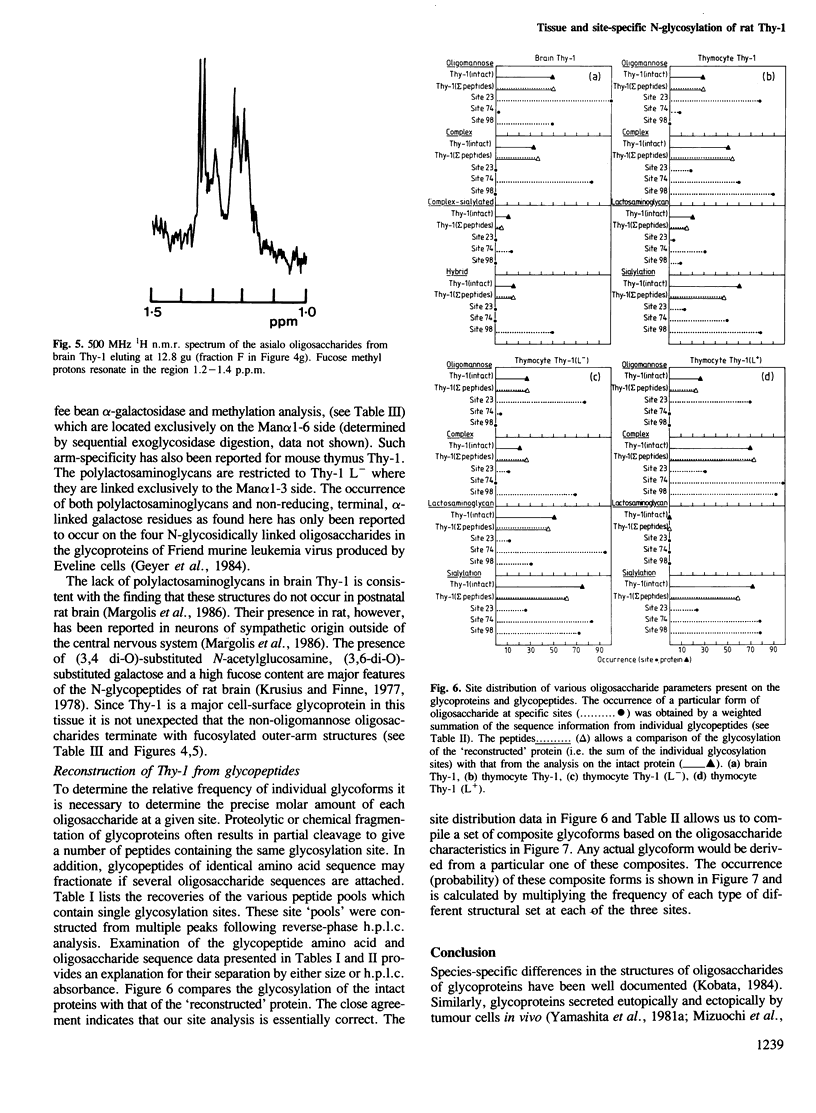
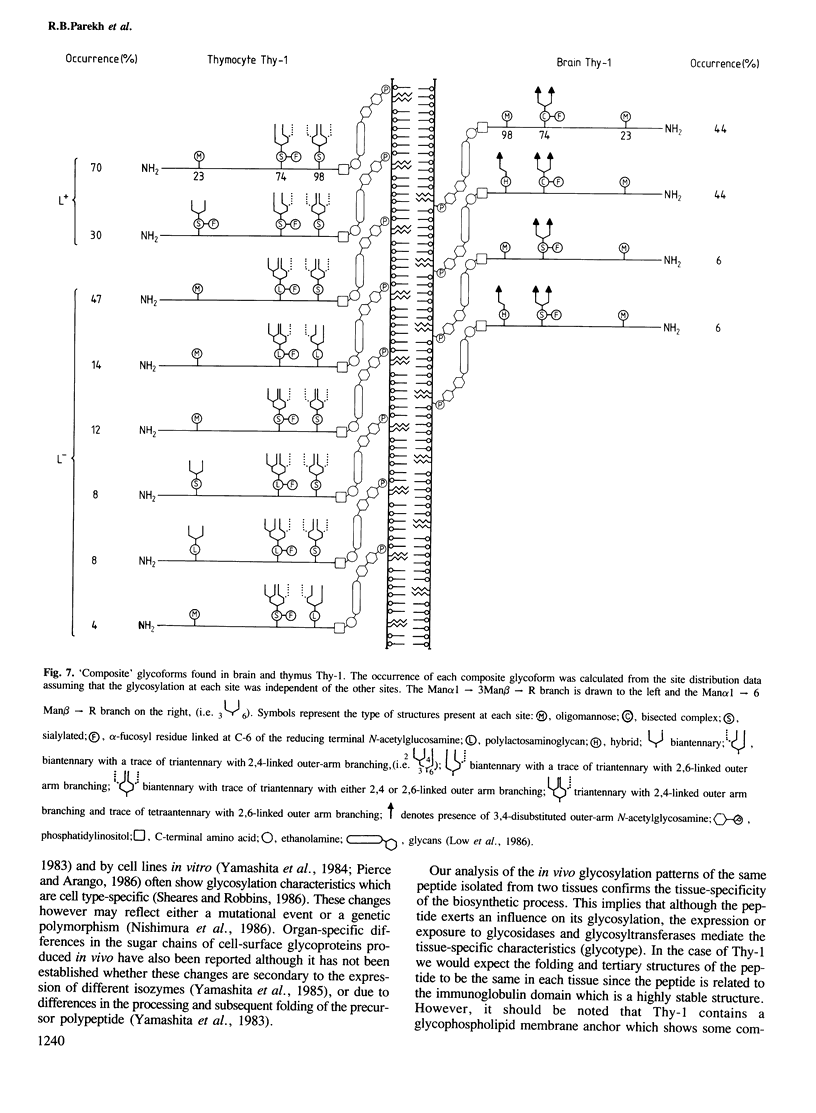
To examine the extent to which protein structure and tissue-type influence glycosylation, we have determined the oligosaccharide structures at each of the three glycosylation sites (Asn-23, 74 and 98) of the cell surface glycoprotein Thy-1 isolated from rat brain and thymus. The results show that there is tissue-specificity of glycosylation and that superimposed on this is a significant degree of site-specificity. On the basis of the site distribution of oligosaccharides, we find that no Thy-1 molecules are in common between the two tissues despite the amino acid sequences being identical. We suggest, therefore, that by controlling N-glycosylation a tissue creates an unique set of glycoforms (same polypeptide but with oligosaccharides that differ either in sequence or disposition). The structures at each of the three sites were also determined for the thymocyte Thy-1 that binds to lentil lectin (Thy-1 L+) and for that which does not (Thy-1 L-). Segregation of intact thymus Thy-1 into two distinct sets of glycoforms by lentil lectin was found to be due to the structures at site 74. Analysis of oligosaccharide structures at the 'passenger' sites (23 and 98) suggests that either Thy-1 L+ and Thy-1 L- molecules are made in different cell-types or that the biosynthesis of oligosaccharides at one site is influenced by the glycosylation at other sites.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barclay A. N., Letarte-Muirhead M., Williams A. F., Faulkes R. A. Chemical characterisation of the Thy-1 glycoproteins from the membranes of rat thymocytes and brain. Nature. 1976 Oct 14;263(5578):563–567. doi: 10.1038/263563a0. [DOI] [PubMed] [Google Scholar]
- Bayard B., Kerckaert J. P., Laine A., Hayem A. Uniformity of glycans within molecular variants of alpha-protease inhibitor with distinct affinity for concanavalin A. Eur J Biochem. 1982 May 17;124(2):371–376. doi: 10.1111/j.1432-1033.1982.tb06602.x. [DOI] [PubMed] [Google Scholar]
- Campbell D. G., Gagnon J., Reid K. B., Williams A. F. Rat brain Thy-1 glycoprotein. The amino acid sequence, disulphide bonds and an unusual hydrophobic region. Biochem J. 1981 Apr 1;195(1):15–30. doi: 10.1042/bj1950015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson S. R. Changes in glycan branching and sialylation of the Thy-1 antigen during normal differentiation of mouse T-lymphocytes. Biochem J. 1985 Mar 1;226(2):519–525. doi: 10.1042/bj2260519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson S. R., Stigbrand T. Partial characterization of the oligosaccharides of mouse thymocyte Thy-1 glycoprotein. Biochem J. 1984 Jul 15;221(2):379–392. doi: 10.1042/bj2210379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson S., Stigbrand T. Carbohydrate complexity of the mouse thymocyte Thy-1 glycoprotein as demonstrated by lectin affinity and isoelectric focusing. Eur J Biochem. 1982 Mar;123(1):1–7. doi: 10.1111/j.1432-1033.1982.tb06490.x. [DOI] [PubMed] [Google Scholar]
- Carlsson S., Stigbrand T., Winblad B. Thy-1 heterogeneity of mouse thymocytes. FEBS Lett. 1979 Dec 1;108(1):116–118. doi: 10.1016/0014-5793(79)81190-4. [DOI] [PubMed] [Google Scholar]
- Dobre M. A., Marx A., Ghetie V. Isolation of a rabbit IgG fraction with cytophilic properties. J Immunol Methods. 1983 May 13;59(3):339–348. doi: 10.1016/0022-1759(83)90194-1. [DOI] [PubMed] [Google Scholar]
- Fatemi S. H., Tartakoff A. M. Hydrophilic anchor-deficient Thy-1 is secreted by a class E mutant T lymphoma. Cell. 1986 Aug 29;46(5):653–657. doi: 10.1016/0092-8674(86)90340-5. [DOI] [PubMed] [Google Scholar]
- Feizi T. Demonstration by monoclonal antibodies that carbohydrate structures of glycoproteins and glycolipids are onco-developmental antigens. Nature. 1985 Mar 7;314(6006):53–57. doi: 10.1038/314053a0. [DOI] [PubMed] [Google Scholar]
- Geyer R., Geyer H., Stirm S., Hunsmann G., Schneider J., Dabrowski U., Dabrowski J. Major oligosaccharides in the glycoprotein of Friend murine leukemia virus: structure elucidation by one- and two-dimensional proton nuclear magnetic resonance and methylation analysis. Biochemistry. 1984 Nov 6;23(23):5628–5637. doi: 10.1021/bi00318a038. [DOI] [PubMed] [Google Scholar]
- Kobata A. Use of endo- and exoglycosidases for structural studies of glycoconjugates. Anal Biochem. 1979 Nov 15;100(1):1–14. doi: 10.1016/0003-2697(79)90102-7. [DOI] [PubMed] [Google Scholar]
- Kornfeld K., Reitman M. L., Kornfeld R. The carbohydrate-binding specificity of pea and lentil lectins. Fucose is an important determinant. J Biol Chem. 1981 Jul 10;256(13):6633–6640. [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Krusius T., Finne J. Characterization of a novel sugar sequence from rat-brain glycoproteins containing fucose and sialic acid. Eur J Biochem. 1978 Mar 15;84(2):395–403. doi: 10.1111/j.1432-1033.1978.tb12180.x. [DOI] [PubMed] [Google Scholar]
- Krusius T., Finne J. Structural features of tissue glycoproteins. Fractionation and methylation analysis of glycopeptides derived from rat brain, kidney and liver. Eur J Biochem. 1977 Sep;78(2):369–379. doi: 10.1111/j.1432-1033.1977.tb11749.x. [DOI] [PubMed] [Google Scholar]
- Letarte M. Isolation and characterization of the Thy-1 glycoproteins of rat brain and thymus. Methods Enzymol. 1984;108:654–665. doi: 10.1016/s0076-6879(84)08125-8. [DOI] [PubMed] [Google Scholar]
- Low M. G., Kincade P. W. Phosphatidylinositol is the membrane-anchoring domain of the Thy-1 glycoprotein. Nature. 1985 Nov 7;318(6041):62–64. doi: 10.1038/318062a0. [DOI] [PubMed] [Google Scholar]
- Margolis R. K., Greene L. A., Margolis R. U. Poly(N-acetyllactosaminyl) oligosaccharides in glycoproteins of PC12 pheochromocytoma cells and sympathetic neurons. Biochemistry. 1986 Jun 3;25(11):3463–3468. doi: 10.1021/bi00359a055. [DOI] [PubMed] [Google Scholar]
- Mizuochi T., Nishimura R., Derappe C., Taniguchi T., Hamamoto T., Mochizuki M., Kobata A. Structures of the asparagine-linked sugar chains of human chorionic gonadotropin produced in choriocarcinoma. Appearance of triantennary sugar chains and unique biantennary sugar chains. J Biol Chem. 1983 Dec 10;258(23):14126–14129. [PubMed] [Google Scholar]
- Morrison M. H., Chaney W. G., Esselman W. J. Molecular weight and charge heterogeneity of Thy-1 glycoprotein in different populations of T-cells. Mol Immunol. 1984 May;21(5):405–413. doi: 10.1016/0161-5890(84)90038-5. [DOI] [PubMed] [Google Scholar]
- Morrison M. H., Lynch R. A., Esselman W. J. Poly-N-acetyllactosamine alterations of Thy-1 glycoprotein in lymphocyte differentiation. Mol Immunol. 1986 Jan;23(1):63–72. doi: 10.1016/0161-5890(86)90172-0. [DOI] [PubMed] [Google Scholar]
- Nishimura R., Shin J., Ji I., Middaugh C. R., Kruggel W., Lewis R. V., Ji T. H. A single amino acid substitution in an ectopic alpha subunit of a human carcinoma choriogonadotropin. J Biol Chem. 1986 Aug 15;261(23):10475–10477. [PubMed] [Google Scholar]
- Parekh R. B., Dwek R. A., Sutton B. J., Fernandes D. L., Leung A., Stanworth D., Rademacher T. W., Mizuochi T., Taniguchi T., Matsuta K. Association of rheumatoid arthritis and primary osteoarthritis with changes in the glycosylation pattern of total serum IgG. Nature. 1985 Aug 1;316(6027):452–457. doi: 10.1038/316452a0. [DOI] [PubMed] [Google Scholar]
- Peterson C. B., Blackburn M. N. Isolation and characterization of an antithrombin III variant with reduced carbohydrate content and enhanced heparin binding. J Biol Chem. 1985 Jan 10;260(1):610–615. [PubMed] [Google Scholar]
- Pierce M., Arango J. Rous sarcoma virus-transformed baby hamster kidney cells express higher levels of asparagine-linked tri- and tetraantennary glycopeptides containing [GlcNAc-beta (1,6)Man-alpha (1,6)Man] and poly-N-acetyllactosamine sequences than baby hamster kidney cells. J Biol Chem. 1986 Aug 15;261(23):10772–10777. [PubMed] [Google Scholar]
- Pollack L., Atkinson P. H. Correlation of glycosylation forms with position in amino acid sequence. J Cell Biol. 1983 Aug;97(2):293–300. doi: 10.1083/jcb.97.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademacher T. W., Homans S. W., Parekh R. B., Dwek R. A. Immunoglobulin G as a glycoprotein. Biochem Soc Symp. 1986;51:131–148. [PubMed] [Google Scholar]
- Regoeczi E., Taylor P., Debanne M. T., März L., Hatton M. W. Three types of human asialo-transferrin and their interactions with the rat liver. Biochem J. 1979 Nov 15;184(2):399–407. doi: 10.1042/bj1840399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samor B., Michalski J. C., Debray H., Mazurier C., Goudemand M., Van Halbeek H., Vliegenthart J. F., Montreuil J. Primary structure of a new tetraantennary glycan of the N-acetyllactosaminic type isolated from human factor VIII/von Willebrand factor. Eur J Biochem. 1986 Jul 15;158(2):295–298. doi: 10.1111/j.1432-1033.1986.tb09750.x. [DOI] [PubMed] [Google Scholar]
- Scudder P., Hanfland P., Uemura K., Feizi T. Endo-beta-D-galactosidases of Bacteroides fragilis and Escherichia freundii hydrolyze linear but not branched oligosaccharide domains of glycolipids of the neolacto series. J Biol Chem. 1984 May 25;259(10):6586–6592. [PubMed] [Google Scholar]
- Seki T., Chang H. C., Moriuchi T., Denome R., Ploegh H., Silver J. A hydrophobic transmembrane segment at the carboxyl terminus of thy-1. Science. 1985 Feb 8;227(4687):649–651. doi: 10.1126/science.2857501. [DOI] [PubMed] [Google Scholar]
- Sheares B. T., Robbins P. W. Glycosylation of ovalbumin in a heterologous cell: analysis of oligosaccharide chains of the cloned glycoprotein in mouse L cells. Proc Natl Acad Sci U S A. 1986 Apr;83(7):1993–1997. doi: 10.1073/pnas.83.7.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiro R. G., Bhoyroo V. D. Occurrence of alpha-D-galactosyl residues in the thyroglobulins from several species. Localization in the saccharide chains of the complex carbohydrate units. J Biol Chem. 1984 Aug 10;259(15):9858–9866. [PubMed] [Google Scholar]
- Swiedler S. J., Freed J. H., Tarentino A. L., Plummer T. H., Jr, Hart G. W. Oligosaccharide microheterogeneity of the murine major histocompatibility antigens. Reproducible site-specific patterns of sialylation and branching in asparagine-linked oligosaccharides. J Biol Chem. 1985 Apr 10;260(7):4046–4054. [PubMed] [Google Scholar]
- Swiedler S. J., Hart G. W., Tarentino A. L., Plummer T. H., Jr, Freed J. H. Stable oligosaccharide microheterogeneity at individual glycosylation sites of a murine major histocompatibility antigen derived from a B-cell lymphoma. J Biol Chem. 1983 Oct 10;258(19):11515–11523. [PubMed] [Google Scholar]
- Tai T., Yamashita K., Ogata-Arakawa M., Koide N., Muramatsu T., Iwashita S., Inoue Y., Kobata A. Structural studies of two ovalbumin glycopeptides in relation to the endo-beta-N-acetylglucosaminidase specificity. J Biol Chem. 1975 Nov 10;250(21):8569–8575. [PubMed] [Google Scholar]
- Takasaki S., Kobata A. Microdetermination of sugar composition by radioisotope labeling. Methods Enzymol. 1978;50:50–54. doi: 10.1016/0076-6879(78)50006-2. [DOI] [PubMed] [Google Scholar]
- Taniguchi T., Mizuochi T., Beale M., Dwek R. A., Rademacher T. W., Kobata A. Structures of the sugar chains of rabbit immunoglobulin G: occurrence of asparagine-linked sugar chains in Fab fragment. Biochemistry. 1985 Sep 24;24(20):5551–5557. doi: 10.1021/bi00341a040. [DOI] [PubMed] [Google Scholar]
- Tse A. G., Barclay A. N., Watts A., Williams A. F. A glycophospholipid tail at the carboxyl terminus of the Thy-1 glycoprotein of neurons and thymocytes. Science. 1985 Nov 29;230(4729):1003–1008. doi: 10.1126/science.2865810. [DOI] [PubMed] [Google Scholar]
- Vaughan L., Lorier M. A., Carrell R. W. alpha 1-Antitrypsin microheterogeneity. Isolation and physiological significance of isoforms. Biochim Biophys Acta. 1982 Mar 4;701(3):339–345. doi: 10.1016/0167-4838(82)90237-0. [DOI] [PubMed] [Google Scholar]
- Williams A. F., Gagnon J. Neuronal cell Thy-1 glycoprotein: homology with immunoglobulin. Science. 1982 May 14;216(4547):696–703. doi: 10.1126/science.6177036. [DOI] [PubMed] [Google Scholar]
- Williams A. F. Immunoglobulin-related domains for cell surface recognition. Nature. 1985 Apr 18;314(6012):579–580. doi: 10.1038/314579a0. [DOI] [PubMed] [Google Scholar]
- Williams D. B., Lennarz W. J. Control of asparagine-linked oligosaccharide chain processing: studies on bovine pancreatic ribonuclease B. An in vitro system for the processing of exogenous glycoproteins. J Biol Chem. 1984 Apr 25;259(8):5105–5114. [PubMed] [Google Scholar]
- Yamashita K., Hitoi A., Taniguchi N., Yokosawa N., Tsukada Y., Kobata A. Comparative study of the sugar chains of gamma-glutamyltranspeptidases purified from rat liver and rat AH-66 hepatoma cells. Cancer Res. 1983 Nov;43(11):5059–5063. [PubMed] [Google Scholar]
- Yamashita K., Hitoi A., Tateishi N., Higashi T., Sakamoto Y., Kobata A. The structures of the carbohydrate moieties of mouse kidney gamma-glutamyltranspeptidase: occurrence of X-antigenic determinants and bisecting N-acetylglucosamine residues. Arch Biochem Biophys. 1985 Aug 1;240(2):573–582. doi: 10.1016/0003-9861(85)90064-5. [DOI] [PubMed] [Google Scholar]
- Yamashita K., Ichishima E., Arai M., Kobata A. An alpha-mannosidase purified from Aspergillus saitoi is specific for alpha 1,2 linkages. Biochem Biophys Res Commun. 1980 Oct 16;96(3):1335–1342. doi: 10.1016/0006-291x(80)90097-2. [DOI] [PubMed] [Google Scholar]
- Yamashita K., Ohkura T., Tachibana Y., Takasaki S., Kobata A. Comparative study of the oligosaccharides released from baby hamster kidney cells and their polyoma transformant by hydrazinolysis. J Biol Chem. 1984 Sep 10;259(17):10834–10840. [PubMed] [Google Scholar]
- Yamashita K., Ohkura T., Yoshima H., Kobata A. Substrate specificity of Diplococcal beta-N-acetylhexosaminidase, a useful enzyme for the structural studies of complex type asparagine-linked sugar chains. Biochem Biophys Res Commun. 1981 May 15;100(1):226–232. doi: 10.1016/s0006-291x(81)80086-1. [DOI] [PubMed] [Google Scholar]
- Yamashita K., Tachibana Y., Nakayama T., Kitamura M., Endo Y., Kobata A. Structural studies of the sugar chains of human parotid alpha-amylase. J Biol Chem. 1980 Jun 25;255(12):5635–5642. [PubMed] [Google Scholar]
- Yamashita K., Tachibana Y., Takeuchi T., Kobata A. Structural study of the sugar chains of alpha-amylases produced ectopically in tumors. J Biochem. 1981 Nov;90(5):1281–1289. doi: 10.1093/oxfordjournals.jbchem.a133593. [DOI] [PubMed] [Google Scholar]


