Abstract
Structural variation in the primary structure of human T200 glycoprotein has been detected. Three cDNA variants have been characterized each of which encode T200 molecules that differ in size as a result of sequence differences in their amino-terminal regions. The largest form of the molecule is distinguished from the smallest by an insert of 161 amino acids, after the first eight amino-terminal residues. The other variant has an insert at the same location of 47 amino acids identical to residues 75-121 in the larger insert. Both extra domains are rich in serine and threonine residues and are likely to display multiple O-linked oligosaccharides. These structural variants which probably arise by cell-type-specific alternative splicing provide a molecular basis for the previously observed structural and antigenic heterogeneity of T200 glycoprotein. In addition to the variable amino-terminal region, the external domain of human T200 glycoprotein consists of a second cysteine-rich region of about 400 amino acids, a single transmembrane-spanning region and a large cytoplasmic domain of 707 amino acids shared by all of the structural variants and highly conserved between species. The gene encoding human T200 is located on the long arm of chromosome 1.
Full text
PDF





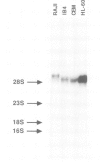
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banga J. P., Guarnotta G., Harte A., Pryce G., Campbell M. A., Quartey-Papafio R., Lydyard P. M., Roitt I. M. A common epitope identified by a monoclonal antibody, MID 2, present on all leucocytes and associated with a group of high molecular weight glycopeptides. Scand J Immunol. 1984 Jan;19(1):11–21. doi: 10.1111/j.1365-3083.1984.tb00895.x. [DOI] [PubMed] [Google Scholar]
- Battifora H. Recent progress in the immunohistochemistry of solid tumors. Semin Diagn Pathol. 1984 Nov;1(4):251–271. [PubMed] [Google Scholar]
- Battifora H., Trowbridge I. S. A monoclonal antibody useful for the differential diagnosis between malignant lymphoma and nonhematopoietic neoplasms. Cancer. 1983 Mar 1;51(5):816–821. doi: 10.1002/1097-0142(19830301)51:5<816::aid-cncr2820510512>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Bourguignon L. Y., Suchard S. J., Nagpal M. L., Glenney J. R., Jr A T-lymphoma transmembrane glycoprotein (gp180) is linked to the cytoskeletal protein, fodrin. J Cell Biol. 1985 Aug;101(2):477–487. doi: 10.1083/jcb.101.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown W. R., Williams A. F. Lymphocyte cell surface glycoproteins which bind to soybean and peanut lectins. Immunology. 1982 Aug;46(4):713–726. [PMC free article] [PubMed] [Google Scholar]
- Childs R. A., Dalchau R., Scudder P., Hounsell E. F., Fabre J. W., Feizi T. Evidence for the occurrence of O-glycosidically linked oligosaccharides of poly-N-acetyllactosamine type on the human leucocyte common antigen. Biochem Biophys Res Commun. 1983 Jan 27;110(2):424–431. doi: 10.1016/0006-291x(83)91166-x. [DOI] [PubMed] [Google Scholar]
- Childs R. A., Feizi T. Differences in carbohydrate moieties of high molecular weight glycoproteins of human lymphocytes of T and B origins revealed by monoclonal autoantibodies with anti-I and anti-i specificities. Biochem Biophys Res Commun. 1981 Oct 30;102(4):1158–1164. doi: 10.1016/s0006-291x(81)80133-7. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Coffman R. L. Surface antigen expression and immunoglobulin gene rearrangement during mouse pre-B cell development. Immunol Rev. 1982;69:5–23. doi: 10.1111/j.1600-065x.1983.tb00446.x. [DOI] [PubMed] [Google Scholar]
- Coffman R. L., Weissman I. L. B220: a B cell-specific member of th T200 glycoprotein family. Nature. 1981 Feb 19;289(5799):681–683. doi: 10.1038/289681a0. [DOI] [PubMed] [Google Scholar]
- Dalchau R., Fabre J. W. Identification with a monoclonal antibody of a predominantly B lymphocyte-specific determinant of the human leukocyte common antigen. Evidence for structural and possible functional diversity of the human leukocyte common molecule. J Exp Med. 1981 Apr 1;153(4):753–765. doi: 10.1084/jem.153.4.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalchau R., Kirkley J., Fabre J. W. Monoclonal antibody to a human leukocyte-specific membrane glycoprotein probably homologous to the leukocyte-common (L-C) antigen of the rat. Eur J Immunol. 1980 Oct;10(10):737–744. doi: 10.1002/eji.1830101003. [DOI] [PubMed] [Google Scholar]
- Dale R. M., McClure B. A., Houchins J. P. A rapid single-stranded cloning strategy for producing a sequential series of overlapping clones for use in DNA sequencing: application to sequencing the corn mitochondrial 18 S rDNA. Plasmid. 1985 Jan;13(1):31–40. doi: 10.1016/0147-619x(85)90053-8. [DOI] [PubMed] [Google Scholar]
- Deane D. L., Cohen B. B., Morton J. E., Steel C. M. Correlation between tumorigenicity and altered glycosylation of a "leukocyte common" antigen in human lymphoid cell lines. Int J Cancer. 1984 Oct 15;34(4):459–462. doi: 10.1002/ijc.2910340405. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebina Y., Ellis L., Jarnagin K., Edery M., Graf L., Clauser E., Ou J. H., Masiarz F., Kan Y. W., Goldfine I. D. The human insulin receptor cDNA: the structural basis for hormone-activated transmembrane signalling. Cell. 1985 Apr;40(4):747–758. doi: 10.1016/0092-8674(85)90334-4. [DOI] [PubMed] [Google Scholar]
- Fabre J. W., Williams A. F. Quantitative serological analysis of a rabbit anti-rat lymphocyte serum and preliminary biochemical characterisation of the major antigen recognised. Transplantation. 1977 Apr;23(4):349–359. doi: 10.1097/00007890-197704000-00009. [DOI] [PubMed] [Google Scholar]
- Hemperly J. J., Murray B. A., Edelman G. M., Cunningham B. A. Sequence of a cDNA clone encoding the polysialic acid-rich and cytoplasmic domains of the neural cell adhesion molecule N-CAM. Proc Natl Acad Sci U S A. 1986 May;83(9):3037–3041. doi: 10.1073/pnas.83.9.3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. Molecular biology of fibronectin. Annu Rev Cell Biol. 1985;1:67–90. doi: 10.1146/annurev.cb.01.110185.000435. [DOI] [PubMed] [Google Scholar]
- Imamura T., Huang C. C., Minowada J., Takahashi M., Moore G. E. Cloning of human hematopoietic cell lines. J Natl Cancer Inst. 1970 Apr;44(4):845–854. [PubMed] [Google Scholar]
- Kincade P. W., Lee G., Watanabe T., Sun L., Scheid M. P. Antigens displayed on murine B lymphocyte precursors. J Immunol. 1981 Dec;127(6):2262–2268. [PubMed] [Google Scholar]
- King W., Thomas-Powell A. L., Raab-Traub N., Hawke M., Kieff E. Epstein-Barr virus RNA. V. Viral RNA in a restringently infected, growth-transformed cell line. J Virol. 1980 Nov;36(2):506–518. doi: 10.1128/jvi.36.2.506-518.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalley P. A., McKusick V. A. Report of the Committee on Comparative Mapping. Cytogenet Cell Genet. 1985;40(1-4):536–566. doi: 10.1159/000132187. [DOI] [PubMed] [Google Scholar]
- Landreth K. S., Kincade P. W., Lee G., Medlock E. S. Phenotypic and functional characterization of murine B lymphocyte precursors isolated from fetal and adult tissues. J Immunol. 1983 Aug;131(2):572–580. [PubMed] [Google Scholar]
- Ledbetter J. A., Rose L. M., Spooner C. E., Beatty P. G., Martin P. J., Clark E. A. Antibodies to common leukocyte antigen p220 influence human T cell proliferation by modifying IL 2 receptor expression. J Immunol. 1985 Sep;135(3):1819–1825. [PubMed] [Google Scholar]
- Lefrancois L., Puddington L., Machamer C. E., Bevan M. J. Acquisition of cytotoxic T lymphocyte-specific carbohydrate differentiation antigens. J Exp Med. 1985 Oct 1;162(4):1275–1293. doi: 10.1084/jem.162.4.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefrancois L., Thomas M. L., Bevan M. J., Trowbridge I. S. Different classes of T lymphocytes have different mRNAs for the leukocyte-common antigen, T200. J Exp Med. 1986 May 1;163(5):1337–1342. doi: 10.1084/jem.163.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Morishima Y., Ogata S., Collins N. H., Dupont B., Lloyd K. O. Carbohydrate differences in human high molecular weight antigens of B- and T-cell lines. Immunogenetics. 1982;15(5):529–535. doi: 10.1007/BF00345912. [DOI] [PubMed] [Google Scholar]
- Morton C. C., Kirsch I. R., Taub R., Orkin S. H., Brown J. A. Localization of the beta-globin gene by chromosomal in situ hybridization. Am J Hum Genet. 1984 May;36(3):576–585. [PMC free article] [PubMed] [Google Scholar]
- Newman W., Targan S. R., Fast L. D. Immunobiological and immunochemical aspects of the T-200 family of glycoproteins. Mol Immunol. 1984 Nov;21(11):1113–1121. doi: 10.1016/0161-5890(84)90122-6. [DOI] [PubMed] [Google Scholar]
- Omary M. B., Trowbridge I. S., Battifora H. A. Human homologue of murine T200 glycoprotein. J Exp Med. 1980 Oct 1;152(4):842–852. doi: 10.1084/jem.152.4.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PULVERTAFT J. V. A STUDY OF MALIGNANT TUMOURS IN NIGERIA BY SHORT-TERM TISSUE CULTURE. J Clin Pathol. 1965 May;18:261–273. doi: 10.1136/jcp.18.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul J. I., Schwarzbauer J. E., Tamkun J. W., Hynes R. O. Cell-type-specific fibronectin subunits generated by alternative splicing. J Biol Chem. 1986 Sep 15;261(26):12258–12265. [PubMed] [Google Scholar]
- Rogers J. H. The origin and evolution of retroposons. Int Rev Cytol. 1985;93:187–279. doi: 10.1016/s0074-7696(08)61375-3. [DOI] [PubMed] [Google Scholar]
- Saga Y., Tung J. S., Shen F. W., Boyse E. A. Sequences of Ly-5 cDNA: isoform-related diversity of Ly-5 mRNA. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6940–6944. doi: 10.1073/pnas.83.18.6940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer J. R., Hozier J. C. High resolution of mouse chromosomes: banding conservation between man and mouse. Science. 1986 Jun 27;232(4758):1632–1635. doi: 10.1126/science.3715469. [DOI] [PubMed] [Google Scholar]
- Scheid M. P., Landreth K. S., Tung J. S., Kincade P. W. Preferential but nonexclusive expression of macromolecular antigens on B-lineage cells. Immunol Rev. 1982;69:141–159. doi: 10.1111/j.1600-065x.1983.tb00453.x. [DOI] [PubMed] [Google Scholar]
- Schneider U., Schwenk H. U., Bornkamm G. Characterization of EBV-genome negative "null" and "T" cell lines derived from children with acute lymphoblastic leukemia and leukemic transformed non-Hodgkin lymphoma. Int J Cancer. 1977 May 15;19(5):621–626. doi: 10.1002/ijc.2910190505. [DOI] [PubMed] [Google Scholar]
- Shen F. W., Saga Y., Litman G., Freeman G., Tung J. S., Cantor H., Boyse E. A. Cloning of Ly-5 cDNA. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7360–7363. doi: 10.1073/pnas.82.21.7360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spickett G. P., Brandon M. R., Mason D. W., Williams A. F., Woollett G. R. MRC OX-22, a monoclonal antibody that labels a new subset of T lymphocytes and reacts with the high molecular weight form of the leukocyte-common antigen. J Exp Med. 1983 Sep 1;158(3):795–810. doi: 10.1084/jem.158.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staden R. Automation of the computer handling of gel reading data produced by the shotgun method of DNA sequencing. Nucleic Acids Res. 1982 Aug 11;10(15):4731–4751. doi: 10.1093/nar/10.15.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staden R. Graphic methods to determine the function of nucleic acid sequences. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):521–538. doi: 10.1093/nar/12.1part2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunderland C. A., McMaster W. R., Williams A. F. Purification with monoclonal antibody of a predominant leukocyte-common antigen and glycoprotein from rat thymocytes. Eur J Immunol. 1979 Feb;9(2):155–159. doi: 10.1002/eji.1830090212. [DOI] [PubMed] [Google Scholar]
- Thomas M. L., Barclay A. N., Gagnon J., Williams A. F. Evidence from cDNA clones that the rat leukocyte-common antigen (T200) spans the lipid bilayer and contains a cytoplasmic domain of 80,000 Mr. Cell. 1985 May;41(1):83–93. doi: 10.1016/0092-8674(85)90063-7. [DOI] [PubMed] [Google Scholar]
- Trowbridge I. S. Interspecies spleen-myeloma hybrid producing monoclonal antibodies against mouse lymphocyte surface glycoprotein, T200. J Exp Med. 1978 Jul 1;148(1):313–323. doi: 10.1084/jem.148.1.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trowbridge I. S., Ralph P., Bevan M. J. Differences in the surface proteins of mouse B and T cells. Proc Natl Acad Sci U S A. 1975 Jan;72(1):157–161. doi: 10.1073/pnas.72.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung J. S., Deere M. C., Boyse E. A. Evidence that Ly-5 product of T and B cells differ in protein structure. Immunogenetics. 1984;19(2):149–154. doi: 10.1007/BF00387858. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Bell J. R., Chen E. Y., Herrera R., Petruzzelli L. M., Dull T. J., Gray A., Coussens L., Liao Y. C., Tsubokawa M. Human insulin receptor and its relationship to the tyrosine kinase family of oncogenes. 1985 Feb 28-Mar 6Nature. 313(6005):756–761. doi: 10.1038/313756a0. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Coussens L., Hayflick J. S., Dull T. J., Gray A., Tam A. W., Lee J., Yarden Y., Libermann T. A., Schlessinger J. Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. 1984 May 31-Jun 6Nature. 309(5967):418–425. doi: 10.1038/309418a0. [DOI] [PubMed] [Google Scholar]
- Watson M. E. Compilation of published signal sequences. Nucleic Acids Res. 1984 Jul 11;12(13):5145–5164. doi: 10.1093/nar/12.13.5145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis J. H., Morton C. C., Bruns G. A., Weis J. J., Klickstein L. B., Wong W. W., Fearon D. T. A complement receptor locus: genes encoding C3b/C4b receptor and C3d/Epstein-Barr virus receptor map to 1q32. J Immunol. 1987 Jan 1;138(1):312–315. [PubMed] [Google Scholar]
- Woollett G. R., Barclay A. N., Puklavec M., Williams A. F. Molecular and antigenic heterogeneity of the rat leukocyte-common antigen from thymocytes and T and B lymphocytes. Eur J Immunol. 1985 Feb;15(2):168–173. doi: 10.1002/eji.1830150211. [DOI] [PubMed] [Google Scholar]