Abstract
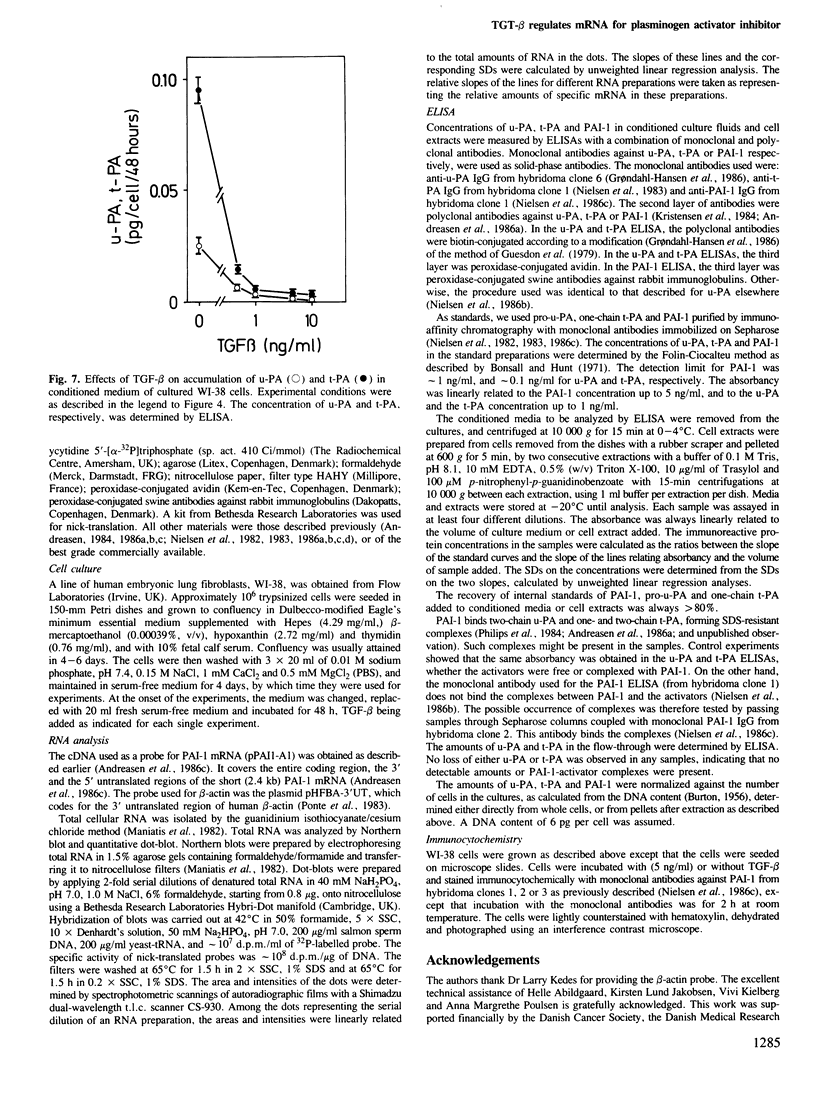
We have studied the mechanism of a transforming growth factor-beta (TGF-beta)-stimulated production of type-1 plasminogen activator inhibitor (PAI-1) in WI-38 human lung fibroblasts. TGF-beta causes an early increase in the PAI-1 mRNA level which reaches a maximal 50-fold enhancement after 8 h. Blocking of protein synthesis with cycloheximide causes an equally strong increase in the level of PAI-1 mRNA. Quantitative studies of the effect of TGF-beta on PAI-1 protein levels in cell extracts and culture media by using monoclonal antibodies are consistent with the effect on PAI-1 mRNA. The results suggest a primary effect of TGF-beta on PAI-1 gene transcription, and also suggest the possibility that the transcription of this gene in non-induced cells may be suppressed by a short-lived negatively regulating protein. Urokinase-type (u-PA) and tissue-type (t-PA) plasminogen activators are decreased in the culture media of TGF-beta-treated cells concomitantly with the increase in PAI-1 accumulation. These findings show that a primary and important biological effect of TGF-beta may be an overall decreased extracellular proteolytic activity, and give an insight into the molecular mechanisms underlying TGF-beta action at the genetic level.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreasen P. A., Christensen T. H., Huang J. Y., Nielsen L. S., Wilson E. L., Danø K. Hormonal regulation of extracellular plasminogen activators and Mr approximately 54,000 plasminogen activator inhibitor in human neoplastic cell lines, studied with monoclonal antibodies. Mol Cell Endocrinol. 1986 May;45(2-3):137–147. doi: 10.1016/0303-7207(86)90141-3. [DOI] [PubMed] [Google Scholar]
- Andreasen P. A., Nielsen L. S., Grøndahl-Hansen J., Skriver L., Zeuthen J., Stephens R. W., Danø K. Inactive proenzyme to tissue-type plasminogen activator from human melanoma cells, identified after affinity purification with a monoclonal antibody. EMBO J. 1984 Jan;3(1):51–56. doi: 10.1002/j.1460-2075.1984.tb01760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen P. A., Nielsen L. S., Kristensen P., Grøndahl-Hansen J., Skriver L., Danø K. Plasminogen activator inhibitor from human fibrosarcoma cells binds urokinase-type plasminogen activator, but not its proenzyme. J Biol Chem. 1986 Jun 15;261(17):7644–7651. [PubMed] [Google Scholar]
- Andreasen P. A., Riccio A., Welinder K. G., Douglas R., Sartorio R., Nielsen L. S., Oppenheimer C., Blasi F., Danø K. Plasminogen activator inhibitor type-1: reactive center and amino-terminal heterogeneity determined by protein and cDNA sequencing. FEBS Lett. 1986 Dec 15;209(2):213–218. doi: 10.1016/0014-5793(86)81113-9. [DOI] [PubMed] [Google Scholar]
- Astedt B., Lecander I., Brodin T., Lundblad A., Löw K. Purification of a specific placental plasminogen activator inhibitor by monoclonal antibody and its complex formation with plasminogen activator. Thromb Haemost. 1985 Feb 18;53(1):122–125. [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonsall R. W., Hunt S. Characteristics of interactions between surfactants and the human erythrocyte membrane. Biochim Biophys Acta. 1971 Oct 12;249(1):266–280. doi: 10.1016/0005-2736(71)90104-0. [DOI] [PubMed] [Google Scholar]
- Chapman H. A., Jr, Stone O. L. Characterization of a macrophage-derived plasminogen-activator inhibitor. Similarities with placental urokinase inhibitor. Biochem J. 1985 Aug 15;230(1):109–116. doi: 10.1042/bj2300109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman P. L., Patel P. D., Cwikel B. J., Rafferty U. M., Sznycer-Laszuk R., Gelehrter T. D. Characterization of the dexamethasone-induced inhibitor of plasminogen activator in HTC hepatoma cells. J Biol Chem. 1986 Mar 25;261(9):4352–4357. [PubMed] [Google Scholar]
- Collen D. On the regulation and control of fibrinolysis. Edward Kowalski Memorial Lecture. Thromb Haemost. 1980 Jun 18;43(2):77–89. [PubMed] [Google Scholar]
- Crutchley D. J., Conanan L. B. Endotoxin induction of an inhibitor of plasminogen activator in bovine pulmonary artery endothelial cells. J Biol Chem. 1986 Jan 5;261(1):154–159. [PubMed] [Google Scholar]
- Danø K., Andreasen P. A., Grøndahl-Hansen J., Kristensen P., Nielsen L. S., Skriver L. Plasminogen activators, tissue degradation, and cancer. Adv Cancer Res. 1985;44:139–266. doi: 10.1016/s0065-230x(08)60028-7. [DOI] [PubMed] [Google Scholar]
- Danø K., Reich E. Serine enzymes released by cultured neoplastic cells. J Exp Med. 1978 Mar 1;147(3):745–757. doi: 10.1084/jem.147.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derynck R., Jarrett J. A., Chen E. Y., Eaton D. H., Bell J. R., Assoian R. K., Roberts A. B., Sporn M. B., Goeddel D. V. Human transforming growth factor-beta complementary DNA sequence and expression in normal and transformed cells. Nature. 1985 Aug 22;316(6030):701–705. doi: 10.1038/316701a0. [DOI] [PubMed] [Google Scholar]
- Erickson L. A., Hekman C. M., Loskutoff D. J. The primary plasminogen-activator inhibitors in endothelial cells, platelets, serum, and plasma are immunologically related. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8710–8714. doi: 10.1073/pnas.82.24.8710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelehrter T. D., Barouski-Miller P. A., Coleman P. L., Cwikel B. J. Hormonal regulation of plasminogen activator in rat hepatoma cells. Mol Cell Biochem. 1983;53-54(1-2):11–21. doi: 10.1007/BF00225243. [DOI] [PubMed] [Google Scholar]
- Ginsburg D., Zeheb R., Yang A. Y., Rafferty U. M., Andreasen P. A., Nielsen L., Dano K., Lebo R. V., Gelehrter T. D. cDNA cloning of human plasminogen activator-inhibitor from endothelial cells. J Clin Invest. 1986 Dec;78(6):1673–1680. doi: 10.1172/JCI112761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golder J. P., Stephens R. W. Minactivin: a human monocyte product which specifically inactivates urokinase-type plasminogen activators. Eur J Biochem. 1983 Nov 15;136(3):517–522. doi: 10.1111/j.1432-1033.1983.tb07771.x. [DOI] [PubMed] [Google Scholar]
- Goustin A. S., Leof E. B., Shipley G. D., Moses H. L. Growth factors and cancer. Cancer Res. 1986 Mar;46(3):1015–1029. [PubMed] [Google Scholar]
- Grimaldi G., Di Fiore P., Locatelli E. K., Falco J., Blasi F. Modulation of urokinase plasminogen activator gene expression during the transition from quiescent to proliferative state in normal mouse cells. EMBO J. 1986 May;5(5):855–861. doi: 10.1002/j.1460-2075.1986.tb04295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grøndahl-Hansen J., Ralfkiaer E., Nielsen L. S., Kristensen P., Frentz G., Danø K. Immunohistochemical localization of urokinase- and tissue-type plasminogen activators in psoriatic skin. J Invest Dermatol. 1987 Jan;88(1):28–32. doi: 10.1111/1523-1747.ep12464827. [DOI] [PubMed] [Google Scholar]
- Guesdon J. L., Ternynck T., Avrameas S. The use of avidin-biotin interaction in immunoenzymatic techniques. J Histochem Cytochem. 1979 Aug;27(8):1131–1139. doi: 10.1177/27.8.90074. [DOI] [PubMed] [Google Scholar]
- Ignotz R. A., Massagué J. Transforming growth factor-beta stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J Biol Chem. 1986 Mar 25;261(9):4337–4345. [PubMed] [Google Scholar]
- Kopitar M., Rozman B., Babnik J., Turk V., Mullins D. E., Wun T. C. Human leucocyte urokinase inhibitor--purification, characterization and comparative studies against different plasminogen activators. Thromb Haemost. 1985 Dec 17;54(4):750–755. [PubMed] [Google Scholar]
- Kristensen P., Larsson L. I., Nielsen L. S., Grøndahl-Hansen J., Andreasen P. A., Danø K. Human endothelial cells contain one type of plasminogen activator. FEBS Lett. 1984 Mar 12;168(1):33–37. doi: 10.1016/0014-5793(84)80201-x. [DOI] [PubMed] [Google Scholar]
- Kruithof E. K., Vassalli J. D., Schleuning W. D., Mattaliano R. J., Bachmann F. Purification and characterization of a plasminogen activator inhibitor from the histiocytic lymphoma cell line U-937. J Biol Chem. 1986 Aug 25;261(24):11207–11213. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laiho M., Saksela O., Andreasen P. A., Keski-Oja J. Enhanced production and extracellular deposition of the endothelial-type plasminogen activator inhibitor in cultured human lung fibroblasts by transforming growth factor-beta. J Cell Biol. 1986 Dec;103(6 Pt 1):2403–2410. doi: 10.1083/jcb.103.6.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laiho M., Saksela O., Keski-Oja J. Transforming growth factor beta alters plasminogen activator activity in human skin fibroblasts. Exp Cell Res. 1986 Jun;164(2):399–407. doi: 10.1016/0014-4827(86)90038-8. [DOI] [PubMed] [Google Scholar]
- Leof E. B., Proper J. A., Getz M. J., Moses H. L. Transforming growth factor type beta regulation of actin mRNA. J Cell Physiol. 1986 Apr;127(1):83–88. doi: 10.1002/jcp.1041270111. [DOI] [PubMed] [Google Scholar]
- Leof E. B., Proper J. A., Goustin A. S., Shipley G. D., DiCorleto P. E., Moses H. L. Induction of c-sis mRNA and activity similar to platelet-derived growth factor by transforming growth factor beta: a proposed model for indirect mitogenesis involving autocrine activity. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2453–2457. doi: 10.1073/pnas.83.8.2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liotta L. A., Goldfarb R. H., Brundage R., Siegal G. P., Terranova V., Garbisa S. Effect of plasminogen activator (urokinase), plasmin, and thrombin on glycoprotein and collagenous components of basement membrane. Cancer Res. 1981 Nov;41(11 Pt 1):4629–4636. [PubMed] [Google Scholar]
- Loskutoff D. J., Edgington T. S. An inhibitor of plasminogen activator in rabbit endothelial cells. J Biol Chem. 1981 May 10;256(9):4142–4145. [PubMed] [Google Scholar]
- Loskutoff D. J., Roegner K., Erickson L. A., Schleef R. R., Huttenlocher A., Coleman P. L., Gelehrter T. D. The dexamethasone-induced inhibitor of plasminogen activator in hepatoma cells is antigenically-related to an inhibitor produced by bovine aortic endothelial cells. Thromb Haemost. 1986 Feb 28;55(1):8–11. [PubMed] [Google Scholar]
- Loskutoff D. J., van Mourik J. A., Erickson L. A., Lawrence D. Detection of an unusually stable fibrinolytic inhibitor produced by bovine endothelial cells. Proc Natl Acad Sci U S A. 1983 May;80(10):2956–2960. doi: 10.1073/pnas.80.10.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medcalf R. L., Richards R. I., Crawford R. J., Hamilton J. A. Suppression of urokinase-type plasminogen activator mRNA levels in human fibrosarcoma cells and synovial fibroblasts by anti-inflammatory glucocorticoids. EMBO J. 1986 Sep;5(9):2217–2222. doi: 10.1002/j.1460-2075.1986.tb04487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins D. E., Rohrlich S. T. The role of proteinases in cellular invasiveness. Biochim Biophys Acta. 1983 Dec 29;695(3-4):177–214. doi: 10.1016/0304-419x(83)90011-2. [DOI] [PubMed] [Google Scholar]
- Nagamine Y., Pearson D., Altus M. S., Reich E. cDNA and gene nucleotide sequence of porcine plasminogen activator. Nucleic Acids Res. 1984 Dec 21;12(24):9525–9541. doi: 10.1093/nar/12.24.9525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen L. S., Andreasen P. A., Grøndahl-Hansen J., Huang J. Y., Kristensen P., Danø K. Monoclonal antibodies to human 54,000 molecular weight plasminogen activator inhibitor from fibrosarcoma cells--inhibitor neutralization and one-step affinity purification. Thromb Haemost. 1986 Apr 30;55(2):206–212. [PubMed] [Google Scholar]
- Nielsen L. S., Grøndahl-Hansen J., Andreasen P. A., Skriver L., Zeuthen J., Danø K. Enzyme-linked immunosorbent assay for human urokinase-type plasminogen activator and its proenzyme using a combination of monoclonal and polyclonal antibodies. J Immunoassay. 1986;7(3):209–228. doi: 10.1080/01971528608060467. [DOI] [PubMed] [Google Scholar]
- Nielsen L. S., Hansen J. G., Andreasen P. A., Skriver L., Danø K., Zeuthen J. Monoclonal antibody to human 66,000 molecular weight plasminogen activator from melanoma cells. Specific enzyme inhibition and one-step affinity purification. EMBO J. 1983;2(1):115–119. doi: 10.1002/j.1460-2075.1983.tb01391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen L. S., Hansen J. G., Skriver L., Wilson E. L., Kaltoft K., Zeuthen J., Danø K. Purification of zymogen to plasminogen activator from human glioblastoma cells by affinity chromatography with monoclonal antibody. Biochemistry. 1982 Dec 7;21(25):6410–6415. doi: 10.1021/bi00268a014. [DOI] [PubMed] [Google Scholar]
- Ny T., Bjersing L., Hsueh A. J., Loskutoff D. J. Cultured granulosa cells produce two plasminogen activators and an antiactivator, each regulated differently by gonadotropins. Endocrinology. 1985 Apr;116(4):1666–1668. doi: 10.1210/endo-116-4-1666. [DOI] [PubMed] [Google Scholar]
- Ny T., Elgh F., Lund B. The structure of the human tissue-type plasminogen activator gene: correlation of intron and exon structures to functional and structural domains. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5355–5359. doi: 10.1073/pnas.81.17.5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ny T., Sawdey M., Lawrence D., Millan J. L., Loskutoff D. J. Cloning and sequence of a cDNA coding for the human beta-migrating endothelial-cell-type plasminogen activator inhibitor. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6776–6780. doi: 10.1073/pnas.83.18.6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannekoek H., Veerman H., Lambers H., Diergaarde P., Verweij C. L., van Zonneveld A. J., van Mourik J. A. Endothelial plasminogen activator inhibitor (PAI): a new member of the Serpin gene family. EMBO J. 1986 Oct;5(10):2539–2544. doi: 10.1002/j.1460-2075.1986.tb04532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philips M., Juul A. G., Thorsen S. Human endothelial cells produce a plasminogen activator inhibitor and a tissue-type plasminogen activator-inhibitor complex. Biochim Biophys Acta. 1984 Nov 6;802(1):99–110. doi: 10.1016/0304-4165(84)90039-4. [DOI] [PubMed] [Google Scholar]
- Philips M., Juul A. G., Thorsen S., Selmer J., Zeuthen J. Immunological relationship between the fast-acting plasminogen activator inhibitors from plasma, blood platelets and endothelial cells demonstrated with a monoclonal antibody against an inhibitor from placenta. Thromb Haemost. 1986 Apr 30;55(2):213–217. [PubMed] [Google Scholar]
- Ponte P., Gunning P., Blau H., Kedes L. Human actin genes are single copy for alpha-skeletal and alpha-cardiac actin but multicopy for beta- and gamma-cytoskeletal genes: 3' untranslated regions are isotype specific but are conserved in evolution. Mol Cell Biol. 1983 Oct;3(10):1783–1791. doi: 10.1128/mcb.3.10.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccio A., Grimaldi G., Verde P., Sebastio G., Boast S., Blasi F. The human urokinase-plasminogen activator gene and its promoter. Nucleic Acids Res. 1985 Apr 25;13(8):2759–2771. doi: 10.1093/nar/13.8.2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A. B., Anzano M. A., Lamb L. C., Smith J. M., Frolik C. A., Marquardt H., Todaro G. J., Sporn M. B. Isolation from murine sarcoma cells of novel transforming growth factors potentiated by EGF. Nature. 1982 Feb 4;295(5848):417–419. doi: 10.1038/295417a0. [DOI] [PubMed] [Google Scholar]
- Roberts A. B., Anzano M. A., Wakefield L. M., Roche N. S., Stern D. F., Sporn M. B. Type beta transforming growth factor: a bifunctional regulator of cellular growth. Proc Natl Acad Sci U S A. 1985 Jan;82(1):119–123. doi: 10.1073/pnas.82.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A. B., Sporn M. B., Assoian R. K., Smith J. M., Roche N. S., Wakefield L. M., Heine U. I., Liotta L. A., Falanga V., Kehrl J. H. Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4167–4171. doi: 10.1073/pnas.83.12.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saksela O. Plasminogen activation and regulation of pericellular proteolysis. Biochim Biophys Acta. 1985 Nov 12;823(1):35–65. doi: 10.1016/0304-419x(85)90014-9. [DOI] [PubMed] [Google Scholar]
- Scott R. W., Bergman B. L., Bajpai A., Hersh R. T., Rodriguez H., Jones B. N., Barreda C., Watts S., Baker J. B. Protease nexin. Properties and a modified purification procedure. J Biol Chem. 1985 Jun 10;260(11):7029–7034. [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B. Autocrine growth factors and cancer. 1985 Feb 28-Mar 6Nature. 313(6005):745–747. doi: 10.1038/313745a0. [DOI] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B., Wakefield L. M., Assoian R. K. Transforming growth factor-beta: biological function and chemical structure. Science. 1986 Aug 1;233(4763):532–534. doi: 10.1126/science.3487831. [DOI] [PubMed] [Google Scholar]
- Sprengers E. D., Verheijen J. H., Van Hinsbergh V. W., Emeis J. J. Evidence for the presence of two different fibrinolytic inhibitors in human endothelial cell conditioned medium. Biochim Biophys Acta. 1984 Sep 28;801(2):163–170. doi: 10.1016/0304-4165(84)90063-1. [DOI] [PubMed] [Google Scholar]
- Stoppelli M. P., Verde P., Grimaldi G., Locatelli E. K., Blasi F. Increase in urokinase plasminogen activator mRNA synthesis in human carcinoma cells is a primary effect of the potent tumor promoter, phorbol myristate acetate. J Cell Biol. 1986 Apr;102(4):1235–1241. doi: 10.1083/jcb.102.4.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan L. M., Quigley J. P. An anticatalytic monoclonal antibody to avian plasminogen activator: its effect on behavior of RSV-transformed chick fibroblasts. Cell. 1986 Jun 20;45(6):905–915. doi: 10.1016/0092-8674(86)90565-9. [DOI] [PubMed] [Google Scholar]
- Wiman B., Chmielewska J., Rånby M. Inactivation of tissue plasminogen activator in plasma. Demonstration of a complex with a new rapid inhibitor. J Biol Chem. 1984 Mar 25;259(6):3644–3647. [PubMed] [Google Scholar]