Abstract
Objectives
The aim of this systematic literature review was to assess the clinical level of evidence of commercially available demineralised bone matrix (DBM) products for their use in trauma and orthopaedic related surgery.
Methods
A total of 17 DBM products were used as search terms in two available databases: Embase and PubMed according to the Preferred Reporting Items for Systematic Reviews and Meta Analyses statement. All articles that reported the clinical use of a DBM-product in trauma and orthopaedic related surgery were included.
Results
The literature search resulted in 823 manuscripts of which 64 manuscripts met the final inclusion criteria. The included manuscripts consisted of four randomised controlled trials (level I), eight cohort studies (level III) and 49 case-series (level IV). No clinical studies were found for ten DBM products, and most DBM products were only used in combination with other grafting materials. DBM products were most extensively investigated in spinal surgery, showing limited level I evidence that supports the use Grafton DBM (Osteotech, Eatontown, New Jersey) as a bone graft extender in posterolateral lumbar fusion surgery. DBM products are not thoroughly investigated in trauma surgery, showing mainly level IV evidence that supports the use of Allomatrix (Wright Medical, London, United Kingdom), DBX (DePuy Synthes, Zuchwil, Switzerland), Grafton DBM, or OrthoBlast (Citagenix Laval, Canada) as bone graft extenders.
Conclusions
The clinical level of evidence that supports the use of DBM in trauma and orthopaedic surgery is limited and consists mainly of poor quality and retrospective case-series. More prospective, randomised controlled trials are needed to understand the clinical effect and impact of DBM in trauma and orthopaedic surgery.
Cite this article: J. van der Stok, K. A. Hartholt, D. A. L. Schoenmakers, J. J. C. Arts. The available evidence on demineralised bone matrix in trauma and orthopaedic surgery: A systemati c review. Bone Joint Res 2017;6:423–432. DOI: 10.1302/2046-3758.67.BJR-2017-0027.R1.
Keywords: Bone defects, Bone grafts, Demineralised bone matrix
Article focus
What is the available clinical level of evidence that supports the use of demineralised bone matrix (DBM) in trauma and orthopaedic surgery?
Key messages
The majority of the available clinical level evidence supporting the use of DBM products in trauma and orthopaedic surgery consist of case series (level IV evidence).
The use of DBM products has been most extensively investigated in spinal surgery, with level I evidence that supports the use of Grafton DBM (Osteotech, Eatontown, New Jersey) as a bone graft extender in posterolateral lumbar fusion surgery.
The use of DBM products is not well investigated in trauma surgery, with only case series that mainly describe the use of Allomatrix (Wright Medical, London, United Kingdom), DBX (DePuy Synthes, Zuchwil, Switzerland), Grafton DBM, and OrthoBlast (Citagenix Laval, Canada) as bone graft extenders in trauma surgery (level III/IV evidence).
Strengths and limitations
Product-specific overview of DBM used for specific trauma- and orthopaedic-related indications.
Inclusion of a large number of papers describing the clinical outcomes of DBM products used in trauma and orthopaedic surgery
Not all DBM products that are commercially available worldwide could be included.
Introduction
Bone grafting is a common procedure in trauma and orthopaedic surgery, with more than two million procedures being performed worldwide each year.1 Autologous bone is the gold standard grafting material and it is mostly harvested from the iliac crest. However, this harvesting procedure has a considerable morbidity rate (8% to 39%),2 and the amount of bone that can be harvested is not always sufficient. These issues resulted in the development of alternative bone graft materials. These alternative bone graft materials have been used as a bone graft extender (reinforcing autologous bone) or as a bone graft substitute (replacing autologous bone).
Demineralised bone matrix (DBM) is processed allogeneic bone that has been demineralised by extensive decalcification procedures. These procedures include chemical and radiation steps to minimise immunogenic response and the risk of infection.3 The resulting material consists of matrix proteins containing certain quantities of osteo-inductive growth factors (e.g. bone morphogenetic proteins). In 1965, Urist4 showed that DBM maintains its osteo-inductive potential, since subcutaneous implantation led to de novo bone formation in rabbits This was later confirmed by Geesink et al5 in a small human series, where five out of six critical fibular bone defects were successfully treated with DBM. However, whether these osteo-inductive properties can be consistently reproduced in a DBM product remains heavily debated.6 The preserved amount of bone morphogenetic protein (BMP) after demineralisation merely lies within the nanogram range and, moreover, the absolute amount of BMP per DBM product varies up to fourfold between various batches of the same DBM product.7-9
Nevertheless, many trauma and orthopaedic surgeons consider DBM to be a useful bone graft substitute for a wide range of clinical indications in trauma and orthopaedic surgery. Subsequently, the number of commercially available DBM products is constantly increasing, which is possible due to favourable regulatory pathways that allow quick access of new products onto the clinical market (i.e. DBMs are not regulated under 510(k) regulation, but are considered minimally manipulated tissue for transplantation). Nevertheless, there is no evidence-based guideline available that assists trauma and orthopaedic surgeons in making evidence-based decisions regarding the use of DBM products in different clinical indications. Therefore, the aim of this systematic review was to provide the clinical level of evidence that supports the use of DBM products in trauma- and orthopaedic-related surgery.
Materials and Methods
Literature search
Product names of commercially available DBM products in the Netherlands (Table I) were used as search terms in two available online databases: PubMed and Embase. The search key used for PubMed was: “(product name) AND bone[Title/Abstract]” and for Embase, it was: “product name AND bone:ab,ti”. The databases were searched from the earliest date available until 01 January 2017. Independently, two researchers (JvdS and KAH) performed the search. Manuscripts describing original studies on the use of DBM products written in English, German and Dutch were considered eligible. In order to select manuscripts primarily concerning the clinical use of DBM products for trauma- and orthopaedic-related indications, manuscripts were excluded if they only contained in vitro data or animal experiments or reported on the use of DBM products in non-orthopaedic-related indications (e.g. dental or maxillofacial surgery). References in the selected manuscripts were reviewed in order to ensure that no papers were missed with the chosen search strategy. All included manuscripts were assigned a level of evidence by two agreeing reviewers (JvdS and KAH) for their use as a bone graft extender or bone graft substitute, as described by Wright et al10 (Table II).
Table I.
Product specifications of commercially available demineralised bone matrix (DBM)
| Product | Manufacturer | DBM % | Carrier | Form | FDA | Indication |
|---|---|---|---|---|---|---|
| Accell Connexus | Citagenix, Laval, Canada | 70 | Reverse phase medium | Paste | 510(k) | Bone void filler/bone graft extender |
| Accell TBM | Integra, Irvine, California | 100 | - | Strips | 510(k) | Bone void filler/bone graft extender |
| AlloCraft | Stryker, Mahwah, New Jersey | 80 | Acellular matrix | Paste | 510(k) | Bone void filler |
| Allomatrix | Wright Medical, London, United Kingdom | 40 to 86 | Calcium sulphate | Paste | 510(k) | Bone void filler |
| AlphaGRAFT | Alphatech, Carlsbad, California | 80 | Acellular matrix | Paste | 510(k) | Bone void filler |
| Altiva | Exactech, Gainesville, Florida | ND | Gelatin | Paste | 510(k) | Bone void filler |
| BioSet | Penta Biomedical, Verona, Italy | 24 | Gelatin | Paste/strips | 510(k) | Bone void filler |
| DBX | DePuy Synthes, Zuchwil, Switzerland | 32 | Hyaluronic acid | Paste/strips | 510(k) | Bone graft extender/bone void filler |
| Grafton DBM | Osteotech, Eatontown, New Jersey | 17 to 31 | Glycerol | Paste/strips | 510(k) | Bone graft substitute/bone graft extender/bone void filler |
| InterGro | Zimmer Biomet., Westminster, California | 40 | Lecithin | Paste | 510(k) | Bone graft extender/bone void filler |
| Optefil | Exactech | 24 | Gelatin | Paste | 510(k) | Bone void filler |
| Opteform | Exactech | ND | Cortical and cancellous bone chips suspended in collagen-gelatin | Paste | 510(k) | Bone void filler |
| Optium DBM | DePuy, Warsaw, Indiana | ND | Glycerol | Paste | 510(k) | Bone void filler |
| OrthoBlast | Citagenix | ND | Reverse phase medium | Paste | 510(k) | Bone void filler/bone graft extender |
| OrthoBlast II | Citagenix | 20 | Reverse phase medium | Paste | 510(k) | Bone void filler/bone graft extender |
| Osteofil | Medtronic, Minneapolis, Minnesota | 24 | Collagen | Paste/strips | 510(k) | Bone void filler |
| VIAGRAF | Smith & Nephew, London, United Kingdom | ND | Glycerol | Paste/strips | 510(k) | Bone void filler |
FDA, Food and Drug Administration; ND, no data available
Table II.
Level of evidence as described by Wright et al10
| Level of evidence | Type of study |
|---|---|
| I | 1. RCT 2. Systematic review of level I RCT |
| II | 1. Prospective cohort study 2. Poor-quality RCT 3. Systematic review level II studies |
| III | 1. Case-control study 2. Retrospective cohort study 3. Systematic review of level III studies |
| IV | Case series (no, or historical, control group) |
| V | Expert opinion |
RCT, randomised control trial
Results
Literature search
The initial literature search in PubMed and Embase resulted in a total of 823 papers. After screening all titles and abstracts, 101 manuscripts from PubMed and 140 manuscripts from Embase were considered eligible. Exclusion of duplicate articles resulted in a total of 149 eligible manuscripts. Of these 149 eligible manuscripts, 93 were excluded based on the exclusion criteria described, and eight manuscripts were added based upon the reference list. This resulted in a final number of 64 manuscripts that fulfil all selection criteria (Fig. 1). A detailed overview specified per product is provided in Table III.
Fig. 1.
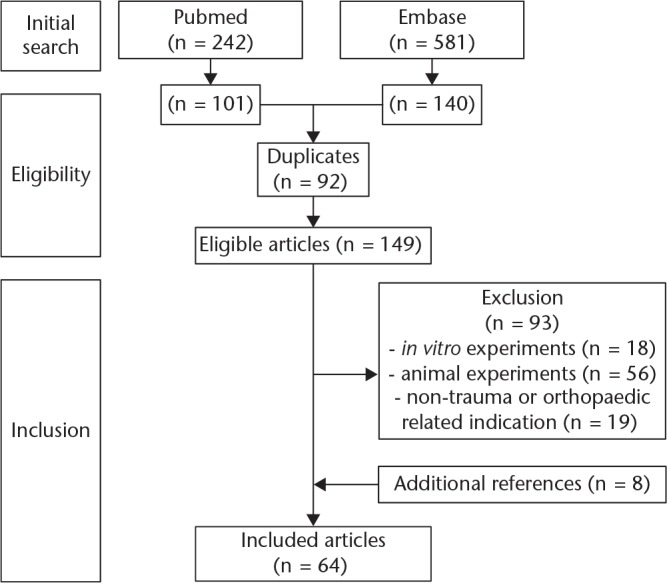
Flowchart of manuscript selection.
Table III.
Search results of systematic literature search
| Products | Inclusion | Exclusion | Final | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PubMed | Embase | In vitro experiments | Animal experiments | Non-trauma- or orthopaedic-related indication | Additional references | ||||||
| papers (n) | Eligible | papers (n) | Eligible | Duplicates | Total | ||||||
| Accell Connexus (Citagenix, Laval, Canada) | 2 | 2 | 7 | 3 | 1 | 4 | 1 | 2 | 0 | 0 | 1 |
| Accell TBM (Integra, Irvine, California) | 0 | 0 | 3 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 |
| AlloCraft (Stryker, Mahwah, New Jersey) | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 |
| Allomatrix (Wright Medical, London, United Kingdom) | 13 | 13 | 52 | 24 | 13 | 24 | 3 | 6 | 1 | 4 | 18 |
| AlphaGRAFT (Alphatech, Carlsbad, California) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Altiva (Exactech, Gainesville, Florida) | 1 | 1 | 3 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 |
| BioSet (Penta Biomedical, Verona, Italy) | 2 | 2 | 5 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | 0 |
| DBX (DePuy Synthes, Zuchwil, Switzerland) | 25 | 24 | 66 | 31 | 20 | 35 | 6 | 18 | 6 | 0 | 5 |
| Grafton DBM (Osteotech, Eatontown, New Jersey) | 181 | 46 | 371 | 57 | 42 | 61 | 5 | 20 | 6 | 3 | 33 |
| InterGro (Zimmer Biomet, Westminster, California) | 1 | 1 | 5 | 2 | 1 | 2 | 1 | 1 | 0 | 0 | 0 |
| Optefil (Exactech) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Opteform (Exactech) | 0 | 0 | 16 | 2 | 0 | 2 | 0 | 1 | 0 | 1 | 2 |
| Optium DBM (DePuy. Warsaw, Indiana) | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| OrthoBlast (Citagenix) | 7 | 4 | 20 | 5 | 4 | 5 | 1 | 0 | 1 | 0 | 3 |
| OrthoBlast II (Citagenix) | 4 | 4 | 4 | 4 | 4 | 4 | 0 | 0 | 4 | 0 | 0 |
| Osteofil (Medtronic. Minneapolis, Minnesota) | 5 | 4 | 25 | 8 | 4 | 8 | 1 | 5 | 0 | 0 | 2 |
| VIAGRAF (Smith & Nephew. London, United Kingdom) | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Orthopaedic-related indications
DBM products have been used as a bone graft extender or as a bone graft substitute for a wide range of trauma- and orthopaedic-related indications (Table IV). Most studies were case series (n = 49); there were only four randomised control trials (RCTs) and eight cohort studies. The remaining three studies reported the random use of DBM products among different study groups without reporting outcomes related to the use of DBM.11-13 The number of clinical studies per DBM product varies extensively. The most frequently examined DBM products, Grafton DBM (Osteotech. Eatontown, New Jersey) and Allomatrix (Wright Medical, London, United Kingdom), were described in 33 and 18 studies, respectively. No clinical studies were found for ten of the 15 remaining DBM products.
Table IV.
Clinical level of evidence of commercially available demineralised bone matrix (DBM)
| Products | Spine surgery | Trauma surgery | Orthopaedic surgery | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Lumbar | Cervical | Thoracic | Acute fractures | Nonunions | Arthrodesis | Avascular necrosis | Reconstructive surgery | Tumour surgery | |
| Accell Connexus (Citagenix, Laval, Canada) | Ext: II14 | ||||||||
| Accell TBM (Integra, Irvine, California) | |||||||||
| AlloCraft (Stryker, Mahwah, New Jersey) | |||||||||
| Allomatrix (Wright Medical, London, United Kingdom) | Ext: III15, Ext: IV16 | *Sub: I17, Sub: IV18-20 | Ext: IV21, Sub: IV22,23 | Ext: IV24 | Ext: IV25,26 | Ext: IV27, Sub: IV23,28,29 | |||
| AlphaGRAFT (Alphatech, Carlsbad, California) | |||||||||
| Altiva (Exactech, Gainesville, Florida) | |||||||||
| BioSet (Penta Biomedical, Verona, Italy) | |||||||||
| DBX (DePuy Synthes, Zuchwil, Switzerland) | Sub: IV30,31 | Sub: IV32 | Sub: IV33,34 | ||||||
| Grafton DBM (Osteotech, Eatontown, New Jersey) | Ext: I35, Ext: II36, Ext: III37, Ext: IV38-40 | *Ext: I41, Ext: IV42-44, Sub: IV45 | Sub: III46 | Ext: II47, Ext: III48, Ext: IV21,49-52 | Ext: II47, Ext: III53, Ext: IV21, Sub: III54, Sub: IV49 | Ext: IV55,56 | Ext: IV57 | Ext58 | Ext: IV59, Sub: IV60-62 |
| InterGro (Zimmer Biomet, Westminster, California) | |||||||||
| Optefil (Exactech) | |||||||||
| Opteform (Exactech) | |||||||||
| Optium DBM (DePuy. Warsaw, Indiana) | |||||||||
| OrthoBlast (Citagenix) | Ext: III48, Ext: IV49 | Ext: IV55 | |||||||
| OrthoBlast II (Citagenix) | |||||||||
| Osteofil (Medtronic, Minneapolis, Minnesota) | Ext: IV63 | Ext: IV64 | |||||||
| VIAGRAF (Smith & Nephew, London, United Kingdom) | |||||||||
Information presented as bone graft type followed by level of evidence number as described by Wright et al10
DBM performance inferior to that of control group used
Ext, bone graft extender; Sub, bone graft substitute
Spine surgery
Four DBM products have been used in spinal surgery: Accell Connexus (Citagenix, Laval, Canada); Allomatrix; Grafton DBM; and Osteofil (Medtronic, Minneapolis, Minnesota). These DBM products were used as bone graft extenders in most studies; only in two studies45,46 was Grafton DBM used as a bone graft substitute.
Lumbar spinal fusion
Accell Connexus was used as a bone graft extender in instrumented posterolateral lumbar fusions in 33 patients.14 In this randomised study, iliac crest bone was augmented with a mixture of Accell Connexus and autologous bone marrow aspirate, and compared with the use of iliac crest bone alone. After one year, grafting had resulted in similar fusion rates (70% versus 76%). There was no difference in pain or duration of surgery on the visual analogue scale (VAS). This study provides level II evidence that Accell Connexus may be used as a bone graft extender for lumbar spinal fusion.
Allomatrix has been used in posterolateral lumbar fusions. Fu et al15 showed in a case-control study that Allomatrix or autologous bone resulted in comparable fusion rates when used with hydroxyapatite/tricalciumphosphate granules: 81% fusion with Allomatrix and 86% fusion with autologous bone respectively. Girardi and Cammisa16 described a retrospective case series of 65 patients where Allomatrix was mixed (1:1) with iliac crest bone. Radiological follow-up showed an improvement in the Lenke scores, 3.7 after one month to 1.6 after 12 months. In another retrospective case series13 following 32 patients who underwent posterior lumbar interbody fusion for 36 months (18 to 42), clinical and radiological scores improved significantly. At the latest follow-up, the mean Oswestry Disability Index improved from 52% to 22%. The mean Roland-Morris Disability Questionnaire improved from 52% to 29%, while > 90% of the operated levels were fused. These studies provide level III evidence that Allomatrix may be used as a bone graft extender for lumbar spinal fusion.
Grafton DBM was studied as a bone graft extender for posterolateral spinal fusion in a RCT by Cammisa et al in 2004.35 In 120 patients, posterolateral lumbar fusions were carried out with pedicle screw fixation and one side of the spine was grafted with autograft (17.2 standard deviation (sd) 9.7 mL), while the contralateral side was grafted with autograft and Grafton DBM (17.2 sd 9.7 mL, mixed 1:2). After two years, autograft with Grafton DBM resulted in fusion in 42 cases (52%) and autograft alone resulted in fusion of 44 cases (54%). In another prospective cohort study,36 patients undergoing instrumented posterolateral lumbosacral spinal fusion were grafted with Grafton DBM and aspirated bone marrow (19 cases), Grafton DBM and autologous bone (27 cases), or autologous bone alone (27 cases). All groups showed similar fusion rates after two years’ follow-up (63%, 70% and 67%, respectively). Use of Grafton DBM as a bone graft extender in posterolateral lumbar fusions is further described in three other studies37-39 encompassing 138 patients in total, where fusion rates after more than two years’ follow-up ranged from 86% (Kang et al37) to 93% (Thalgott et al38). These studies include level I evidence that Grafton DBM can be used as a bone graft extender for lumbar spinal fusion.
Osteofil was used as a bone graft extender for one-level (95 cases) and two-level (45 cases) posterolateral spinal fusions in a study from Epstein and Epstein.63 In this study, Osteofil was mixed (1:1) with autologous bone, and patient outcome was assessed by the SF-36 Questionnaire. One year post-operatively, clinical improvement was observed on six out of eight health scales of the SF-36 Questionnaire. In one-level fusion, 2D-CT showed fusion rates of 93% after an average of 5.2 (sd)1.8 months. Two patients required secondary surgery to treat nonunion or instability. In two-level fusion, 2D-CT showed fusion rates of 92% after an average of 6.1 sd 1.9 months. Again, two patients required secondary surgery due to nonunion or instability.63 These studies provide level IV evidence that Osteofil may be used as a bone graft extender for lumbar spinal fusion.
Cervical spinal fusion
DBM products used in anterior cervical fusions include Allomatrix, Grafton DBM and Osteofil.
Allomatrix was used as a bone graft substitute in a study that included 29 patients suffering from craniocervical spine instability requiring occipitocervical fusion (OCF) due to trauma, rheumatoid arthritis or neoplasms.65 Two instrumentation techniques were compared (screw-rod instrumentation versus hook-and-screw-rod instrumentation), although the authors mention that Allomatrix was used in most cases. In fact, neither graft-specific outcome, nor graft-related complications were mentioned. This study provides no graft-specific outcomes on the use of Allomatrix as a bone graft substitute for cervical spinal fusions.
Grafton DBM was used in a RCT by An et al41 which included 77 patients undergoing anterior cervical fusion. Grafton DBM was combined with tricortical allografts and this was compared with tricortical autografts alone. Nonunion developed in 46% of patients who were grafted with Grafton DBM and tricortical allografts, compared with only 26% of patients who received a tricortical autograft (p = 0.11), suggesting that the combination of Grafton DBM and allograft results in a higher rate of nonunion. Grafton DBM was also used to fill, partially42 or fully,45 polyetheretherketone (PEEK) cages used for cervical fusion. Park et al42 used PEEK cages containing autologous bone chips and Grafton DBM for cervical fusion of 42 levels in 31 patients. After one year, a fusion rate of 97% was observed. Both the VAS score for neck and arm pain and the modified Japanese Orthopaedic Association (JOA) scoring system for myelopathy were significantly improved. Elsawaf et al45 describe complete filling of the PEEK cage with Grafton DBM in anterior cervical discectomy and fusion in a case series of 20 patients; the mean Cobb angle improved (3.4° pre-operatively vs 14.5° post-operatively) and JOA myelopathy scores and neck disability index also subsequently improved. These studies include level I evidence that Grafton DBM is not useful as a bone graft extender for cervical spinal fusion.
Osteofil was mixed with autologous bone (n = 11) in a study by Epstein,64 which also included 24 patients in which Vitoss (β-tricalcium phosphate) was mixed with autologous bone. Radiological follow-up showed that all levels were fused after an average of 5.2 months. Less than 50% of the original fusion mass remained visible on 2D-CT scans after six months in 64% of fusions grafted with Osteofil, compared with 21% of fusions grafted with Vitoss (Stryker, Mahwah, New Jersey), which suggested a quicker resorption rate of Vitoss. This study provides level IV evidence that Osteofil may be used as a bone graft extender with autologous bone for cervical spinal fusion.
Thoracic spinal fusion
Grafton DBM was the only product also used for thoracic fusions. In a retrospective cohort, Weinzapfel et al46 used Grafton DBM in patients who underwent anterior thoracic discectomies and compared their results with using morsellised cancellous allografts. On the final radiographs, 82% of the allograft group and 92% of the Grafton DBM group were rated as fused. There was no significant difference between the two groups. This study provides level III evidence that Grafton DBM may be used as a bone graft substitute for thoracic spinal fusion.
Trauma surgery
Four DBM products have been used to treat bone defects that accompany fractures or were the result of nonunion: Allomatrix, DBX (DePuy Synthes, Zuchwil, Switzerland), Grafton DBM and OrthoBlast (Citagenix). They are used as a graft extender in most cases, however, they may also be used as a graft substitute.
Fractures
Allomatrix was used to treat distal radial fractures. In a RCT,17 unstable distal radial fractures were treated by operative fixation with Kirschner-wires, with (n = 24) or without (n = 26) augmentation of the fracture site with Allomatrix. The physical and radiological outcomes did not show any significant difference between wrist function, speed to recovery, union rate, or complication rate during a one-year follow-up. Allomatrix has also been used for primary treatment of fresh bone defects caused by small-calibre gunshot wounds in the hand. In a retrospective case series of 12 patients, 11 bone defects healed without further intervention and one defect required a second bone grafting procedure.20 In another case report, Allomatrix was used to fill metaphyseal bone defects after elevation of the impressed osteochondral fragments in the treatment of reverse Hill-Sachs lesions of the humeral head.18 These studies include level I evidence that Allomatrix is not a useful bone graft substitute to treat unstable distal radial fractures already fixed with Kirschner-wires.
DBX has been used to treat sternal segment dislocations.30,31 Sternal segment dislocations in eight patients were treated with titanium screws and DBX. Titanium screws and DBX reduced the length of hospitalisation, and led to rapid functional recovery and excellent aesthetic results according to the authors.31 This study provides level IV evidence that DBX may be used as a bone graft substitute to treat fractures of the sternum.
Grafton DBM and OrthoBlast were applied as a bone graft extender by Cheung et al.48 Grafton DBM or OrthoBlast were mixed with cancellous allografts and used to graft bone defects encountered in periarticular fractures of the tibia, fibula, femur, humerus, forearm and acetabulum. Fracture healing occurred in 69% of the patients who received Grafton DBM (n = 13) compared with 100% in patients who received OrthoBlast (n = 15). Grafton DBM mixed with cancellous allograft was also used to graft bone defects to stimulate bone healing in smokers and non-smokers.66 After an average follow-up of 32 months, treatment was successful in 68% of the smokers compared with 88% of the non-smokers. Four patients with foot and ankle fractures (tibial/fibular fracture, fifth metatarsal fracture) were grafted with Grafton DBM and autografts. No complications were described.56 Grafton DBM was also used to enhance cancellous allografts in two tibial stress fractures treated by drilling and bone grafting,52 and for reconstructing large segmental bone defects of the tibia (n = 2)50 and humerus (n = 1),51 using a titanium mesh cage filled with Grafton DBM and cancellous allograft chips. These studies include level III evidence that both Grafton DBM and OrthoBlast may be used in combination with allograft as a bone graft substitute to treat bone defects during fracture surgery.
Nonunion
Allomatrix, mixed with cancellous allograft chips, was used to treat 41 atrophic or avascular nonunions. These nonunions were located in the femur, radius, tibia, and humerus. In 13 cases the nonunion recurred (32%), and revision surgery was necessary in 19 cases.21 Additionally, Allomatrix has been used to graft bone defects that resulted after nonunion (n = 35).23 Allomatrix was mixed (1:3) with calcium sulphate pellets, and after seven months, 85% of the grafted nonunions were healed. These studies provide level IV evidence that Allomatrix may be used as a bone graft substitute to treat nonunion.
DBX has been described in a case report, showing the successful treatment of a subtrochanteric nonunion of an 11-year-old patient with an adult proximal humeral locking plate and additional grafting with DBX.32 This study provides level IV evidence that DBX may be used as a bone graft substitute to treat nonunion.
Grafton DBM was used in a cohort study by Hierholzer et al.53 Treatment of 33 humeral nonunions with Grafton DBM were compared with 45 nonunions that received autologous bone grafts. Autologous bone grafts resulted in union in all cases, whereas Grafton DBM resulted in union in 97% in a mean time to union of 4.2 and 4.5 months, respectively. Ziran et al21,49 described the use of Grafton DBM to treat nonunion in two studies. First, Grafton DBM was mixed with cancellous allograft to stimulate bone healing in smokers and non-smokers.66 After an average follow-up of 32 months, treatment was successful in 68% of the smokers versus 88% of the non-smokers. Subsequently, treatment of nonunion in smokers with Grafton DBM (n = 25) was compared with OrthoBlast (n = 13).49 Treatment with Grafton DBM was only successful in 52%, whereas treatment with OrthoBlast was successful in 85%, which was not a statistically significant difference. In a case report,54 Grafton DBM was used to treat a non-displaced coracoid fracture. After screw fixation, the nonunion site was debrided and successfully grafted with Grafton DBM. These studies include level III evidence that Grafton DBM may be used as a bone graft substitute to treat nonunion and level IV evidence that Grafton DBM or OrthoBlast may be used as a bone graft extender to treat nonunion.
Orthopaedic surgery
Four DBM products, Allomatrix, DBX, Grafton DBM, and OrthoBlast, have been described for other clinical indications that included the treatment of arthrodesis, avascular necrosis, and tumour treatment.
Allomatrix was added to a calcium sulphate bone substitute (OsteoSet) in a small case series by Deheshi, Allen and Kim,12 to be used as a graft to restore retroacetabular bone stock in seven patients who showed retroacetabular osteolysis after primary total hip arthroplasty with cementless acetabular sockets. Specific graft-related outcomes were not reported.12 These studies provide level IV evidence that Allomatrix can be used as a bone graft extender to treat acetabular bone defects.
Allomatrix has been used to treat benign bone tumours in the tibia (n = 17), humerus (n = 11), fibula (n = 3), and radius (n = 2). Defects were filled with a mixture of Allomatrix and a calcium sulphate bone substitute (OsteoSet, 1:3). After seven months, 93% of the bone defects were healed, tumour recurrence was seen in three cases, and one wound infection required antibiotic treatment.23 In addition, in a study that investigated the treatment of 98 benign bone tumours located in the tibia, humerus, femur and pelvis with various bone grafts, Allomatrix was used in 34 of these grafting procedures but no Allomatrix-specific outcomes were reported.28 These studies provide level IV evidence that Allomatrix may be used as a bone graft substitute with or without a calcium sulphate bone substitute to treat benign bone tumours.
DBX was used as a graft in the treatment of enchondromas of the hand. Kwon and Wong,34 and Dietz, Kachar and Nagle,33 reported small case series of five and two patients, respectively. No recurrence or pathological fractures were reported. These studies provide level IV evidence that DBX may be used as a bone graft substitute to treat hand enchondromas.
Grafton DBM has been described in ankle and foot surgery. In a case series by Kado, Gambetta and Perlman, Grafton56 DBM was combined with autologous or allogenic bone for arthrodesis (n = 18) or osteotomies (n = 6). In addition, Grafton DBM (n = 37) and OrthoBlast (n = 26) were also used for complex ankle or hindfoot arthrodesis.55 Nonunions developed in five patients who had Grafton DBM (14%) and in two patients who had OrthoBlast (8%). Furthermore, Grafton DBM was used as a bone graft after core decompression of asymptomatic avascular necrosis of the hip in 37 patients,57 but it did not reduce the need for total hip arthroplasty compared with treatment of symptomatic avascular necrosis during the two years of follow-up. Finally, Grafton DBM was used in combination with autologous bone marrow for percutaneous injection of unicameral bone cysts in 33 patients. After six weeks, patients returned to everyday activity, and bone healing was confirmed radiologically after six months.59 Furthermore, solitary bone cysts in children could be treated with Grafton DBM.60,62 After filling the defects with Grafton DBM in seven cases, a continuous decrease in radiographic bone transparency was observed over a period of two years.60 These studies include level IV evidence that Grafton DBM can be used as a bone graft substitute with or without autologous bone marrow to treat benign bone tumours.
Discussion
The aim of this systematic review was to provide the clinical level of evidence for using DBM products in trauma and orthopaedic surgery. This review reveals that the clinical level of evidence is mainly limited to level IV studies: case series in which DBM products are described for numerous indications with or without additional autografts, allografts or calcium-based bone substitutes. Combined with the fact that the composition and amount of osteo-inductive growth factors may vary extensively between and within DBM products,8,67 it is not possible to point out specific indications where DBM products have added value in trauma and orthopaedic surgery. Formulating an evidence-based guideline to assist trauma and orthopaedic surgeons requires more well designed clinical studies that prove a beneficial effect of DBM compared with treatment with a bone graft substitute (negative control) or treatment including the current gold standard autografts (positive control).
The clinical use of DBM is most thoroughly described for cervical and lumbar spinal fusion surgery.68 In a systematic review by Zadegan et al69 which included 12 studies, the fusion rate of DBM in cervical fusion surgery was comparable with that of other graft materials. However, most studies did not specify which DBM product was used, which may possibly lead to false claims of the effect of specific DBM products. This review shows that there is only one level I study available that determines the effect of Grafton DBM in cervical fusion surgery.41 However, this study shows a higher rate of nonunion compared with that found in autologous bone grafting when it is used in cervical spine fusions. In another RCT, Grafton DBM was used in lumbar spinal fusion,35 showing that similar fusion rates were achieved when Grafton DBM was added to autologous bone versus autologous bone alone. Except for Allomatrix, there is hardly any evidence for using other DBM products in spinal fusion surgery.
The clinical use of DBM products in trauma surgery is not well supported. For example, the use of DBX is only described in a small case series of sternum fractures30 and a single case report of nonunion.32 Furthermore, the case-control study that used Grafton DBM or OrthoBlast in fracture treatment48 included only 13 and 15 cases and did not have a control group. The RCT by Hierholzer et al53 which concludes that grafting humeral nonunions with Grafton DBM is as effective as using autologous bone reported union rates of 97% and 100%, respectively, after four months. These high union rates in both groups raise the question as to whether the re-osteosynthesis performed has not been the most critical factor in achieving union. This is in line with recent publications regarding the treatment of nonunion70 that indicate that optimising mechanical stability is often the key factor in achieving union. Although using autologous bone grafts for treatment of post-traumatic bone defects seems to be effective,71 this cannot yet be concluded for using DBM or specific DBM products and therefore warrants further investigation.
The clinical use of DBM in other orthopaedic indications, such as arthrodesis, avascular necrosis or tumour treatment, is described in surprisingly few studies. For example, there are only two case series found that describe the use of DBM in foot or ankle arthrodesis.55,56 Foot and ankle arthrodesis are known to have a high nonunion rate leading to poor functional outcomes,72 however, whether DBM is capable of reducing the nonunion rate, similar to autologous bone grafts,73 remains unknown.
In addition to the limited clinical evidence for using DBM products in trauma- and orthopaedic-related indications, several other important drawbacks of DBM have to be considered. The most significant drawback is the possible difference of growth factor concentrations between and within DBM products. Bae et al8 showed significant lot-to-lot variability of single DBM products with regard to BMP concentrations and in vivo fusion rates in rats. Another study by Bae et al67 showed that the variability of BMP concentrations among different lots of the same product exceeded the variability of BMP concentrations among different products. The different BMP concentrations might be explained through donor-related factors or preparation methods. Donor variability in terms of age and gender affect the osteo-inductive potential of derived DBM products.74,75 More importantly, preparation methods such as sterilisation through gamma irradiation or hydrogen peroxide exposure reduce the osteo-inductive potential in in vitro and in vivo rat models.76,77 Also, the carrier materials used can affect its potential. Reduced osteo-inductive potential has been observed for glycerol carriers which generate a highly acidic environment for host tissues.78 All of these results underline the necessity of studying the clinical effect of DBM in a product-specific manner.
The limitations of this systematic review that should be taken into account are related to the search method. First, the inclusion of DBM products within this review is based on the commercial availability within the Netherlands. However, the authors believe that the most commonly used products (Allomatrix, DBX, and Grafton DBM) are included. Furthermore, there is no official international database where all available DBM products are registered. This makes it difficult to include every possible DBM product. Secondly, despite the search terms used here, articles may have been overlooked if the use of a specific DBM product was not clearly stated within the methods section. This cannot be avoided by adjusting the search strategy. Instead, it is the author’s responsibility to state clearly which DBM product they have used in their study. Finally, the broad inclusion criteria resulted in heterogeneity among the included studies. This heterogeneity made it impossible to perform a meta-analysis on the overall clinical effect of DBM in trauma and orthopaedic surgery.
In conclusion, preclinical studies have shown that the in vitro and in vivo efficacy of DBM depends upon the preparation methods and carrier materials used. Due to these differences, it is important for trauma and orthopaedic surgeons to make evidence-based decisions based on available literature that specifies the DBM product used for each indication. This review provides an up-to-date overview of the currently available clinical evidence for 17 different DBM products. Overall, the level of evidence of the studies available to date is generally low, mainly including retrospective case series. The highest clinical level of evidence is available for using DBM products in spinal surgery where its performance is comparable with that of autologous bone grafting for lumbar spinal fusions only (level I). The clinical level of evidence for using DBM products in trauma surgery is minimal (mainly level IV). Thus, no evident benefit is proven for treatment of fractures or nonunion. The same holds for orthopaedic indications such as arthrodesis, avascular necrosis, and tumour surgery. Therefore, there is a need for more level I (RCTs or prospective case-cohort) studies in order to understand clearly the clinical effects of DBM in trauma and orthopaedic surgery, and to stimulate the discussion regarding the effectivity of using DBM in trauma and orthopaedic surgery.
Footnotes
Author Contribution: J. van der Stok: Study design, Data acquisition, Writing.
K. A. Hartholt: Data acquisition, Writing.
D. A. L. Schoenmakers: Writing, Editing.
J. J. C. Arts: Study design, Writing.
Conflicts of Interest Statement: None declared.
Funding Statement
None declared.
References
- 1. Brinker MR, O’Connor DP. The incidence of fractures and dislocations referred for orthopaedic services in a capitated population. J Bone Joint Surg [Am] 2004;86-A:290-297. [PubMed] [Google Scholar]
- 2. Cypher TJ, Grossman JP. Biological principles of bone graft healing. J Foot Ankle Surg 1996;35:413-417. [DOI] [PubMed] [Google Scholar]
- 3. Gruskin E, Doll BA, Futrell FW, Schmitz JP, Hollinger JO. Demineralized bone matrix in bone repair: history and use. Adv Drug Deliv Rev 2012;64:1063-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Urist MR. Bone: formation by autoinduction. Science 1965;150:893-899. [DOI] [PubMed] [Google Scholar]
- 5. Geesink RG, Hoefnagels NH, Bulstra SK. Osteogenic activity of OP-1 bone morphogenetic protein (BMP-7) in a human fibular defect. J Bone Joint Surg [Br] 1999;81-B:710-718. [DOI] [PubMed] [Google Scholar]
- 6. Schwartz Z, Mellonig JT, Carnes DL, Jr, et al. Ability of commercial demineralized freeze-dried bone allograft to induce new bone formation. J Periodontol 1996;67:918-926. [DOI] [PubMed] [Google Scholar]
- 7. Blokhuis TJ, Arts JJ. Bioactive and osteoinductive bone graft substitutes: definitions, facts and myths. Injury 2011;42(Suppl 2):S26-S29. [DOI] [PubMed] [Google Scholar]
- 8. Bae HW, Zhao L, Kanim LE, et al. Intervariability and intravariability of bone morphogenetic proteins in commercially available demineralized bone matrix products. Spine (Phila Pa 1976) 2006;31:1299-1306. [DOI] [PubMed] [Google Scholar]
- 9. Wildemann B, Kadow-Romacker A, Haas NP, Schmidmaier G. Quantification of various growth factors in different demineralized bone matrix preparations. J Biomed Mater Res A 2007;81:437-442. [DOI] [PubMed] [Google Scholar]
- 10. Wright JG, Swiontkowski MF, Heckman JD. Introducing levels of evidence to the journal. J Bone Joint Surg [Am] 2003;85-A:1-3. [PubMed] [Google Scholar]
- 11. Kamath AF, Lewallen DG, Hanssen AD. Porous tantalum metaphyseal cones for severe tibial bone loss in revision knee arthroplasty: a five to nine-year follow-up. J Bone Joint Surg [Am] 2015;97-A:216-223. [DOI] [PubMed] [Google Scholar]
- 12. Deheshi BM, Allen DJ, Kim PR. Treatment of retroacetabular osteolysis with calcium sulfate and retention of original components. J Arthroplasty 2008;23:1240.e9-1240.e12. [DOI] [PubMed] [Google Scholar]
- 13. Sapkas GS, Mavrogenis AF, Themistocleous GS, et al. Posterior lumbar interbody fusion versus circumferential fusion using the B-Twin expandable spinal system. J Long Term Eff Med Implants 2007;17:217-227. [DOI] [PubMed] [Google Scholar]
- 14. Schizas C, Triantafyllopoulos D, Kosmopoulos V, Tzinieris N, Stafylas K. Posterolateral lumbar spine fusion using a novel demineralized bone matrix: a controlled case pilot study. Arch Orthop Trauma Surg 2008;128:621-625. [DOI] [PubMed] [Google Scholar]
- 15. Fu TS, Wang IC, Lu ML, et al. The fusion rate of demineralized bone matrix compared with autogenous iliac bone graft for long multi-segment posterolateral spinal fusion. BMC Musculoskelet Disord 2016;17:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Girardi FP, Cammisa FP., Jr The effect of bone graft extenders to enhance the performance of iliac crest bone grafts in instrumented lumbar spine fusion. Orthopedics 2003;26(Suppl):s545-s548. [DOI] [PubMed] [Google Scholar]
- 17. D’Agostino P, Barbier O. An investigation of the effect of AlloMatrix bone graft in distal radial fracture: a prospective randomised controlled clinical trial. Bone Joint J 2013;95-B:1514-1520. [DOI] [PubMed] [Google Scholar]
- 18. Banerjee S, Singh VK, Das AK, Patel VR. Anatomical reconstruction of reverse hill-sachs lesions using the underpinning technique. Orthopedics 2012;35:e752-e757. [DOI] [PubMed] [Google Scholar]
- 19. Bibbo C, Patel DV. The effect of demineralized bone matrix-calcium sulfate with vancomycin on calcaneal fracture healing and infection rates: a prospective study. Foot Ankle Int 2006;27:487-493. [DOI] [PubMed] [Google Scholar]
- 20. Nguyen V, Wollstein R. Civilian gunshot wounds to the fingers treated with primary bone grafting. J Plast Reconstr Aesthet Surg 2009;62:e551-e555. [DOI] [PubMed] [Google Scholar]
- 21. Ziran BH, Smith WR, Morgan SJ. Use of calcium-based demineralized bone matrix/allograft for nonunions and posttraumatic reconstruction of the appendicular skeleton: preliminary results and complications. J Trauma 2007;63:1324-1328. [DOI] [PubMed] [Google Scholar]
- 22. Neel M. Successful treatment of a persistent humeral shaft nonunion using open reduction internal fixation, electrical stimulation, and AlloMatrix Custom Bone Putty. Orthopedics 2004;27(Suppl):s109-s111. [DOI] [PubMed] [Google Scholar]
- 23. Wilkins RM, Kelly CM. The effect of allomatrix injectable putty on the outcome of long bone applications. Orthopedics 2003;26(Suppl):s567-s570. [DOI] [PubMed] [Google Scholar]
- 24. Goitz HT. Minimally invasive treatment of non-steroid induced knee osteonecrosis of the lateral femoral condyle. Orthopedics 2004;27(Suppl):s129-s130. [DOI] [PubMed] [Google Scholar]
- 25. Neel M. The use of a periosteal replacement membrane for bone graft containment at allograft-host junctions after tumor resection and reconstruction with bulk allograft. Orthopedics 2003;26(Suppl):s587-s589. [DOI] [PubMed] [Google Scholar]
- 26. Beaulé PE, Antoniades J. Patient selection and surgical technique for surface arthroplasty of the hip. Orthop Clin North Am 2005;36:177-185, viii-ix. [DOI] [PubMed] [Google Scholar]
- 27. Ozger H, Eralp L, Sungur M, Atalar AC. Surgical management of sacral chordoma. Acta Orthop Belg 2010;76:243-253. [PubMed] [Google Scholar]
- 28. Gitelis S, Virkus W, Anderson D, Piasecki P, Yao TK. Functional outcomes of bone graft substitutes for benign bone tumors. Orthopedics 2004;27(Suppl):s141-s144. [DOI] [PubMed] [Google Scholar]
- 29. Kelly CM, Wilkins RM. Treatment of benign bone lesions with an injectable calcium sulfate-based bone graft substitute. Orthopedics 2004;27(Suppl):s131-s135. [DOI] [PubMed] [Google Scholar]
- 30. Divisi D, Di Leonardo G, Crisci R. Surgical management of traumatic isolated sternal fracture and manubriosternal dislocation. J Trauma Acute Care Surg 2013;75:824-829. [DOI] [PubMed] [Google Scholar]
- 31. Divisi D, Crisci R. Use of demineralized bone matrix and plate for sternal stabilization after traumatic dislocation. Gen Thorac Cardiovasc Surg 2011;59:52-56. [DOI] [PubMed] [Google Scholar]
- 32. Cortes LE, Triana M, Vallejo F, Slongo TF, Streubel PN. Adult proximal humerus locking plate for the treatment of a pediatric subtrochanteric femoral nonunion: a case report. J Orthop Trauma 2011;25:e63-e67. [DOI] [PubMed] [Google Scholar]
- 33. Dietz JF, Kachar SM, Nagle DJ. Endoscopically assisted excision of digital enchondroma. Arthroscopy 2007;23:678.e1-678.e4. [DOI] [PubMed] [Google Scholar]
- 34. Kwon TY, Wong HK. Evolving treatment modality of hand enchondroma in a local hospital: from autograft to artificial bone substitutes. J Orthop Traum Rehabil 2016;20:19-23. [Google Scholar]
- 35. Cammisa FP, Jr, Lowery G, Garfin SR, et al. Two-year fusion rate equivalency between Grafton DBM gel and autograft in posterolateral spine fusion: a prospective controlled trial employing a side-by-side comparison in the same patient. Spine (Phila Pa 1976) 2004;29:660-666. [DOI] [PubMed] [Google Scholar]
- 36. Vaccaro AR, Stubbs HA, Block JE. Demineralized bone matrix composite grafting for posterolateral spinal fusion. Orthopedics 2007;30:567-570. [DOI] [PubMed] [Google Scholar]
- 37. Kang J, An H, Hilibrand A, et al. Grafton and local bone have comparable outcomes to iliac crest bone in instrumented single-level lumbar fusions. Spine (Phila Pa 1976) 2012;37:1083-1091. [DOI] [PubMed] [Google Scholar]
- 38. Thalgott JS, Giuffre JM, Fritts K, Timlin M, Klezl Z. Instrumented posterolateral lumbar fusion using coralline hydroxyapatite with or without demineralized bone matrix, as an adjunct to autologous bone. Spine J 2001;1:131-137. [DOI] [PubMed] [Google Scholar]
- 39. Bose B. Safety and efficacy of demineralized bone matrix used in posterolateral lumbar spine fusion. Neurosurg Q 2006;16:35-39. [Google Scholar]
- 40. Acosta FL, Cloyd JM, Aryan HE, Ames CP. Patient satisfaction and radiographic outcomes after lumbar spinal fusion without iliac crest bone graft or transverse process fusion. J Clin Neurosci 2009;16:1184-1187. [DOI] [PubMed] [Google Scholar]
- 41. An HS, Simpson JM, Glover JM, Stephany J. Comparison between allograft plus demineralized bone matrix versus autograft in anterior cervical fusion. A prospective multicenter study. Spine (Phila Pa 1976) 1995;20:2211-2216. [PubMed] [Google Scholar]
- 42. Park HW, Lee JK, Moon SJ, et al. The efficacy of the synthetic interbody cage and Grafton for anterior cervical fusion. Spine (Phila Pa 1976) 2009;34:E591-E595. [DOI] [PubMed] [Google Scholar]
- 43. Herrmann AM, Geisler FH. Geometric results of anterior cervical plate stabilization in degenerative disease. Spine (Phila Pa 1976) 2004;29:1226-1234. [DOI] [PubMed] [Google Scholar]
- 44. Christodoulou A, Ploumis A, Terzidis I, et al. Combined interbody cage and anterior plating in the surgical treatment of cervical disc disease. Acta Orthop Belg 2004;70:461-465. [PubMed] [Google Scholar]
- 45. Elsawaf A, Mastronardi L, Roperto R, et al. Effect of cervical dynamics on adjacent segment degeneration after anterior cervical fusion with cages. Neurosurg Rev 2009;32:215-224. [DOI] [PubMed] [Google Scholar]
- 46. Weinzapfel B, Son-Hing JP, Armstrong DG, et al. Fusion rates after thoracoscopic release and bone graft substitutes in idiopathic scoliosis. Spine (Phila Pa 1976) 2008;33:1079-1083. [DOI] [PubMed] [Google Scholar]
- 47. Lindsey RW, Wood GW, Sadasivian KK, Stubbs HA, Block JE. Grafting long bone fractures with demineralized bone matrix putty enriched with bone marrow: pilot findings. Orthopedics 2006;29:939-941. [DOI] [PubMed] [Google Scholar]
- 48. Cheung S, Westerheide K, Ziran B. Efficacy of contained metaphyseal and periarticular defects treated with two different demineralized bone matrix allografts. Int Orthop 2003;27:56-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ziran B, Cheung S, Smith W, Westerheide K. Comparative efficacy of 2 different demineralized bone matrix allografts in treating long-bone nonunions in heavy tobacco smokers. Am J Orthop (Belle Mead NJ) 2005;34:329-332. [PubMed] [Google Scholar]
- 50. Cobos JA, Lindsey RW, Gugala Z. The cylindrical titanium mesh cage for treatment of a long bone segmental defect: description of a new technique and report of two cases. J Orthop Trauma 2000;14:54-59. [DOI] [PubMed] [Google Scholar]
- 51. Attias N, Lehman RE, Bodell LS, Lindsey RW. Surgical management of a long segmental defect of the humerus using a cylindrical titanium mesh cage and plates: a case report. J Orthop Trauma 2005;19:211-216. [DOI] [PubMed] [Google Scholar]
- 52. Miyamoto RG, Dhotar HS, Rose DJ, Egol K. Surgical treatment of refractory tibial stress fractures in elite dancers: a case series. Am J Sports Med 2009;37:1150-1154. [DOI] [PubMed] [Google Scholar]
- 53. Hierholzer C, Sama D, Toro JB, Peterson M, Helfet DL. Plate fixation of ununited humeral shaft fractures: effect of type of bone graft on healing. J Bone Joint Surg [Am] 2006;88-A:1442-1447. [DOI] [PubMed] [Google Scholar]
- 54. Skedros JG, Mears CS, Phippen CM. Glenohumeral instability and coracoid fracture nonunion corrected without coracoid transfer or nonunion takedown. J Shoulder Elbow Surg 2014;23:e166-e169. [DOI] [PubMed] [Google Scholar]
- 55. Thordarson DB, Kuehn S. Use of demineralized bone matrix in ankle/hindfoot fusion. Foot Ankle Int 2003;24:557-560. [DOI] [PubMed] [Google Scholar]
- 56. Kado KE, Gambetta LA, Perlman MD. Uses of Grafton for reconstructive foot and ankle surgery. J Foot Ankle Surg 1996;35:59-66. [DOI] [PubMed] [Google Scholar]
- 57. Hsu JE, Wihbey T, Shah RP, Garino JP, Lee GC. Prophylactic decompression and bone grafting for small asymptomatic osteonecrotic lesions of the femoral head. Hip Int 2011;21:672-677. [DOI] [PubMed] [Google Scholar]
- 58. Hamadouche M, Karoubi M, Dumaine V, Courpied JP. The use of fibre-based demineralised bone matrix in major acetabular reconstruction: surgical technique and preliminary results. Int Orthop 2011;35:283-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Rougraff BT, Kling TJ. Treatment of active unicameral bone cysts with percutaneous injection of demineralized bone matrix and autogenous bone marrow. J Bone Joint Surg [Am] 2002;84-A:921-929. [DOI] [PubMed] [Google Scholar]
- 60. Hass HJ, Krause H, Kroker S, Wagemann W, Meyer F. Bone formation using human demineralised bone matrix (Grafton) for the treatment of bone cysts in children. Eur J Pediatr Surg 2007;17:45-49. [DOI] [PubMed] [Google Scholar]
- 61. Dawe EJ, Jukes CP, Gougoulias N, Wee A. Successful arthroscopic decompression and synthetic grafting of a posterior talar cyst: a case report. Foot Ankle Surg 2014;20:e35-e36. [DOI] [PubMed] [Google Scholar]
- 62. Hass HJ, Krause H, Kroker S, Wagemann W. Implantation of human demineralized bone matrix (DBM) for the treatment of juvenile bone cysts. Oper Orthop Traumatol 2006;18:19-33. [DOI] [PubMed] [Google Scholar]
- 63. Epstein NE, Epstein JA. SF-36 outcomes and fusion rates after multilevel laminectomies and 1 and 2-level instrumented posterolateral fusions using lamina autograft and demineralized bone matrix. J Spinal Disord Tech 2007;20:139-145. [DOI] [PubMed] [Google Scholar]
- 64. Epstein NE. An argument for traditional posterior cervical fusion techniques: evidence from 35 cases. Surg Neurol 2008;70:45-51. [DOI] [PubMed] [Google Scholar]
- 65. Sapkas G, Papadakis SA, Segkos D, et al. Posterior instrumentation for occipitocervical fusion. Open Orthop J 2011;5:209-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ziran BH, Hendi P, Smith WR, Westerheide K, Agudelo JF. Osseous healing with a composite of allograft and demineralized bone matrix: adverse effects of smoking. Am J Orthop (Belle Mead NJ) 2007;36:207-209. [PubMed] [Google Scholar]
- 67. Bae H, Zhao L, Zhu D, et al. Variability across ten production lots of a single demineralized bone matrix product. J Bone Joint Surg [Am] 2010;92-A:427-435. [DOI] [PubMed] [Google Scholar]
- 68. Tilkeridis K, Touzopoulos P, Ververidis A, et al. Use of demineralized bone matrix in spinal fusion. World J Orthop 2014;5:30-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zadegan SA, Abedi A, Jazayeri SB, Vaccaro AR, Rahimi-Movaghar V. Demineralized bone matrix in anterior cervical discectomy and fusion: a systematic review. Eur Spine J 2017;26:958-974. [DOI] [PubMed] [Google Scholar]
- 70. Mills L, Tsang J, Hopper G, Keenan G, Simpson AH. The multifactorial aetiology of fracture nonunion and the importance of searching for latent infection. Bone Joint Res 2016;5:512-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Azi ML, Aprato A, Santi I, et al. Autologous bone graft in the treatment of post-traumatic bone defects: a systematic review and meta-analysis. BMC Musculoskelet Disord 2016;17:465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Krause F, Younger AS, Baumhauer JF, et al. Clinical outcomes of nonunions of hindfoot and ankle fusions. J Bone Joint Surg [Am] 2016;98-A:2006-2016. [DOI] [PubMed] [Google Scholar]
- 73. Lareau CR, Deren ME, Fantry A, Donahue RM, DiGiovanni CW. Does autogenous bone graft work? A logistic regression analysis of data from 159 papers in the foot and ankle literature. Foot Ankle Surg 2015;21:150-159. [DOI] [PubMed] [Google Scholar]
- 74. Traianedes K, Russell JL, Edwards JT, et al. Donor age and gender effects on osteoinductivity of demineralized bone matrix. J Biomed Mater Res B Appl Biomater 2004;70:21-29. [DOI] [PubMed] [Google Scholar]
- 75. Alaribe FN, Razwinani M, Maepa MJ, Bierman F, Motaung SC. Osteoinductive activity of selected demineralized bone matrix products from donors of different ages. Iran Biomed J 2016;Pii-IBJ-A-10-1994-1. [Epub ahead of print] [PubMed] [Google Scholar]
- 76. Han B, Yang Z, Nimni M. Effects of gamma irradiation on osteoinduction associated with demineralized bone matrix. J Orthop Res 2008;26:75-82. [DOI] [PubMed] [Google Scholar]
- 77. Carpenter EM, Gendler E, Malinin TI, Temple HT. Effect of hydrogen peroxide on osteoinduction by demineralized bone. Am J Orthop (Belle Mead NJ) 2006;35:562-567. [PubMed] [Google Scholar]
- 78. Wang JC, Kanim LE, Nagakawa IS, et al. Dose-dependent toxicity of a commercially available demineralized bone matrix material. Spine (Phila Pa 1976) 2001;26:1429-1435. [DOI] [PubMed] [Google Scholar]


