Abstract
The catabolism of hyaluronan in articular cartilage remains unclear. The aims of this study were to investigate the effects of hyaluronidase 2 (Hyal2) knockdown in articular cartilage on the development of osteoarthritis (OA) using genetic manipulated mice. Destabilization of the medial meniscus (DMM) model of Col2a promoter specific conditional Hyal2 knockout (Hyal −/−) mice was established and examined. Age related and DMM induced alterations of articular cartilage of knee joint were evaluated with modified Mankin score and immunohistochemical staining of MMP-13, ADAMTS-5, KIAA11199, and biotinylated- hyaluronan binding protein staining in addition to histomorphometrical analyses. Effects of Hyal2 suppression were also analyzed using explant culture of an IL-1α induced articular cartilage degradation model. The amount and size of hyaluronan in articular cartilage were higher in Hyal2 −/− mice. Hyal2 −/− mice exhibited aggravated cartilage degradation in age-related and DMM induced mice. MMP-13 and ADAMTS-5 positive chondrocytes were significantly higher in Hyal2 −/− mice. Articular cartilage was more degraded in explant cultures obtained from Hyal2 −/− mice. Knockdown of Hyal2 in articular cartilage induced OA development and progression possibly mediated by an imbalance of HA metabolism. This suggests that Hyal2 knockdown exhibits mucopolysaccharidosis-like OA change in articular cartilage similar to Hyal1 knockdown.
Introduction
Hyaluronan (HA) is a major constituent of extracellular matrices (ECMs) of various tissues and belongs to the glycosaminoglycan (GAG) family of polysaccharides. Its structure is a nonsulfated GAG composed of repeating disaccharides; beta-(1,3)-linked-D-glucuronic acid and beta-(1,4)-linked-N-acetyl-D-glucosamine1–5. HA is abundant particularly in connective tissues including articular cartilage, synovial fluid of the joint and the vitreous humor of the eye6, and the turnover rate of HA is high in most connective tissues7. The balance of anabolism and catabolism in HA is crucial for maintenance of connective tissue quality. Synthesis of HA in articular cartilage, is regulated mainly by hyaluronan synthase 2 (HAS2) in man, and effects of anabolic or catabolic cytokines on HA synthesis and HAS mRNA expression have been revealed in previous studies8–10. On the other hand, the catabolic activity of crucial enzymes for HA, particularly in articular cartilage has not been clarified yet.
There are six human hyaluronidase-like genes that are localized in two chromosomal regions, 3p21.3 (HYAL1, HYAL2, HYAL3), and 7q31.3 (HYAL4, PH-20, and HYALP1) 2. The tissue-specific, restricted expression patterns of HYAL4, PH-20, and HYALP1 have been reported11, with HYAL1, HYAL2, and HYAL3 seeming to play critical roles in the degradation of HA.
Among the three putative active hyaluronidases, the ability to degrade HA by HYAL3 is questionable12. Therefore, two hyaluronidases (HYAL1 and HYAL2) and cell surface HA receptor (CD44) have been surmised to play key roles in HA degradation2, 5. Chow et al. demonstrated HYAL2 to be the predominant mRNA transcript expressed in articular chondrocytes13. Csoka et al. suggested collaboration between HYAL1 with HYAL2 to degrade HA11. Initial cleavage of HA might occur at the cell surface or within the acidic endocytic vesicles by HYAL214. Intermediate size of cleaved HA is transported to the lysosomes for further degradation by HYAL115.
A previous report analyzed only the effects of deficiency of HYAL1, not HYAL2 on the development of osteoarthritis16, because knockdown of Hyal2 in mice is associated with cardiopulmonary dysfunction and pre-weaning lethality3, 4.
Deficiency of Hyal1 causes mucopolysaccharidosis (MPS) IX, a lysosomal storage disorder characterized by joint abnormalities due to HA accumulation and osteoarthritis16. In contrast, Hyal3 knockout mice exhibited no gross phenotypic changes, nor histologic abnormalities of knee joints17. Hyal2 conditional knockout mice were non-lethal, and displayed craniofacial and cervical vertebral abnormalities, but the effect of this mutation on articular cartilage and development of osteoarthritis has not been reported3.
The aims of the current study were to analyze the roles of Hyal2 in the development and progression of osteoarthritis using non-treated aged mice (natural course) and joint instability OA models of mice, in addition to an explant culture inflammation model of articular cartilage. To achieve these aims, we generated Col2a1-dependent conditional knockout mice using the Cre-loxP system focusing on the Hyal2 deficiency in articular cartilage.
Results
Natural course of conditional Hyal2−/− mice
Macroscopically, Hyal2 +/Flox, Hyal2 +/ and Hyal2 −/− neonates exhibited no obvious gross phenotypic abnormalities. Soft X-ray analyses of 3- and 9-month-old mice revealed no significant differences in skeletal formation between Hyal2 −/− and WT mice. Although tibia bone length of Hyal2 −/− mice at 9 months was slightly shorter than that of WT, the difference is statistically significant (Hyal2:−/− 17.5 ± 0.2 mm, WT: 17.9 ± 0.1mm, P < 0.001). There was no statistical difference between the body weight of Hyal2 −/− and WT mice at 9 months of age (Hyal2:−/− 34.4 ± 3.5 g, WT: 32.4 ± 3.5).
Results of Safranin-O staining demonstrated that there was no significant difference in stainable proteoglycan of articular cartilage between Hyal2 −/− and WT mice at 3 months of age (Fig. 1A, G). However, at 9 months, there was a marked reduction in Hyal2 −/− mice compared with WT mice (Fig. 1H and B, respectively). Total modified Mankin score in Hyal2 −/− was significantly higher than that in WT, Hyal2 +/Flox, Hyal2 +/ mice at 9 months (P < 0.01, Fig. 1M). Modified Mankin score indicated that only minimal OA change occurred in WT mice, similar to that in Hyal2 +/Flox, Hyal2 +/ mice at 9 months. It should be noted that the growth plate physiology differs between humans and mice, since the growth plates do not close directly after sexual maturation in mice. The growth plate heights were slightly reduced in Hyal2 −/− mice compared with those in WT mice at 9 months. In comparison with the 3-month-old ones, the heights in both 9-month-old Hyal2 −/− and WT mice were reduced probably due to their sexual maturation (Supplementary Fig. S1). HABP positivity in the growth plate was higher in Hyal2 −/− mice compared with WT mice. HABP staining revealed a difference in HA accumulation in articular cartilage between WT and Hyal2 −/− mice (Fig. 1C and I, respectively).
Figure 1.
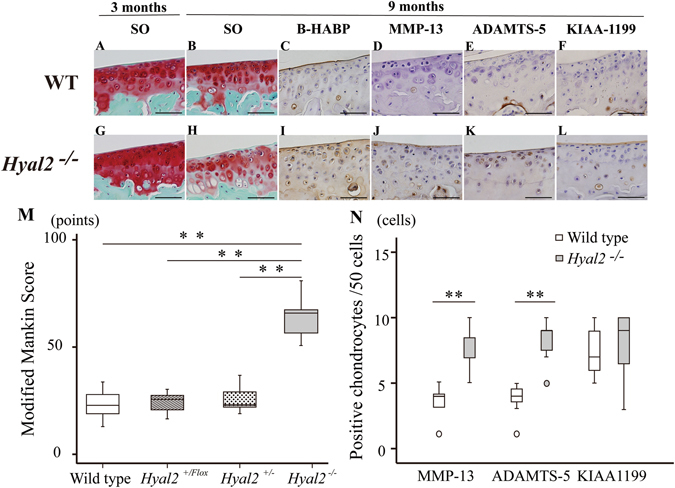
Histologic analysis of structural damage of articular cartilage in natural course of WT and Hyal2 −/− mice at 3 and 9 months of age. Safranin-O staining (SO): WT and Hyal2 −/− mice at 3 months (A and G, respectively) and 9 months (B and H, respectively). B-HABP staining: WT and Hyal2 −/− mice at 9 months (C and I, respectively). Immunohistochemical staining for MMP-13: WT and Hyal2 −/− mice at 9 months (D and J, respectively). Immunohistochemical staining for ADAMTS-5: WT and Hyal2 −/− mice at 9 months (E and K, respectively). Immunohistochemical staining for KIAA1199: WT and Hyal2 −/− mice at 9 months (F and L, respectively). Scale bars depict 50 μm in length. Modified Mankin score of medial tibia in WT and Hyal2 −/− mice at 9 months (**P < 0.01) (M). The numbers of immunostaining positive chondrocytes for MMP-13, and ADAMTS-5 in Hyal2 −/− (K/O) mice were significantly elevated compared to that in WT mice (**P < 0.01, respectively) (N). The number of positive chondrocytes for KIAA1199 in the articular cartilage was not statistically different between the two groups. Small circle represented outlier.
MMP-13 positive chondrocytes were observed in the area where safranin O staining was decreased in 9-month-old Hyal2 −/− mice (Fig. 1J). In contrast, fewer MMP-13 positive chondrocytes were observed in articular cartilage of WT mice (Fig. 1D), and statistically significant (P < 0.01, Fig. 1N). More ADAMTS-5-positive chondrocytes were observed in Hyal2 −/− articular cartilage compared with those in WT cartilage (Fig. 1K and E, respectively) (P < 0.01, Fig. 1N). There was no significant difference in the ratio of articular chondrocytes positive for KIAA1199 between the two groups (Hyal2:−/− Fig. 1L, WT: Fig. 1F, P > 0.05: Fig. 1N). Micro CT did not show significant differences between the two groups (Supplementary Table S1).
Results of RT-PCR analyses revealed that Hyal2 mRNA expression was specifically reduced in articular cartilage of Hyal2 −/− mice compared with that of WT mice (Fig. 2). There was no significant difference in mRNA expression of Hyal1, KIAA1199, and HAS1–3 between Hyal2 −/− and WT mice (Fig. 3A–E). On the other hand, expression levels of MMP-13, ADAMTS-5 mRNA were significantly increased in articular cartilage of Hyal2 −/− mice compared with that of WT mice (Fig. 3F and G, respectively), which was consistent with the results of immunohistochemistry.
Figure 2.

Hyal2 mRNA expression in cartilage, muscle and liver of 9-month-old male Hyal2 −/− and WT mice. Expression levels of Hyal2 mRNA were determined with RT-PCR, and normalized with those of Gapdh. (n = 6, respectively). **P < 0.01.
Figure 3.
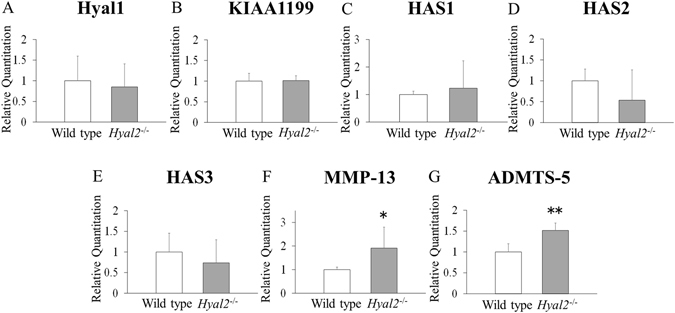
mRNA expression levels of hyaluronan-related molecules and proteolytic enzyme of extracellular matrix. mRNA expression of Hyal1(A), KIAA1199(B), HAS1(C), HAS2(D), HAS3(E), MMP-13(F), ADAMTS-5(G) in articular cartilage from 9-month-old male Hyal2 −/− and WT mice as determined by real-time PCR amplification (n = 6, respectively). The data presented are the average ± S.D of relative mRNA expression values standardized by Gapdh mRNA expression. *P < 0.05, **P < 0.01.
Effect of Hyal2 deficiency in the amount and size of cartilage hyaluronan
The obtained glycosaminoglycans were subjected to HA quantification using competitive ELISA, which is more sensitive for low molecular weight HA than sandwich ELISA18. The yields of joint HA from Hyal2 −/− and WT mice were 55.3 ± 9.7 and 33.7 ± 17.5 ng/mg wet joint, respectively. Obviously, Hyal2 deficiency has significantly caused the accumulation of hyaluronan in joint cartilage (p = 0.025, n = 6).
We then further examined whether the molecular size of HA also altered. The glycosaminoglycan preparations from 6 mice were pooled and subjected to Sephacryl S-1000 gel filtration chromatography. As shown in Fig. 4, HA molecules with a molecular mass bigger than 280 kDa are greatly increased in Hyal2 −/− mice, while the low molecular weight HA molecules are comparable between Hyal2 −/− and WT mice.
Figure 4.
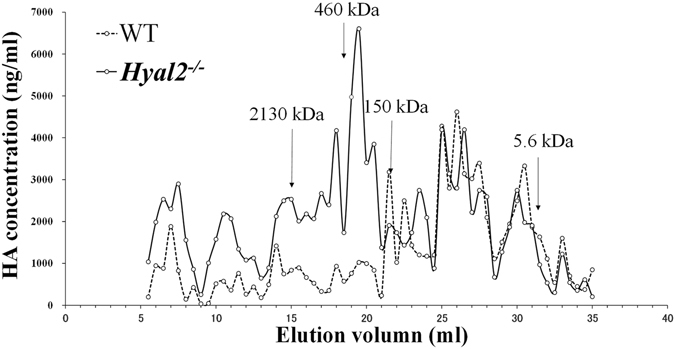
Hyal2 deficiency in cartilage causes the accumulation of high molecular weight hyaluronan. Hyaluronan extracted from knee joints and femoral heads of 9-month-old male mice and hyaluronan references with known molecular sizes were subjected to Sephacryl S-1000 gel filtration chromatography (0.7 × 40 cm). Hyaluronan contents in each fraction were determined using a competitive ELISA. The arrows indicate the positions of peaks of hyaluronan references.
Collectively, the data clearly indicate a role of Hyal2 in the turnover of cartilage HA. In Hyal2 −/− mice, HA content increases by about 60%, most of which are high molecular weight molecules with molecular masses bigger than 280 kDa.
OA development in mice subjected to destabilization of the medial meniscus (DMM) surgery
There was no significant difference between the body weight of Hyal2 −/− and WT mice at 6 or 8 weeks after DMM surgery. (Hyal2:−/− 29.8 ± 2.8 g and 31.6 ± 2.4 g, WT: 28.0 ± 1.1 g and 29.6 ± 1.4 g, respectively. P = 0.144 and 0.072) However, at 10 weeks after surgery, the average body weight of Hyal2 −/− mice was greater than that of WT mice (Hyal2:−/− 32.3 ± 2.4 g; WT: 28.6 ± 1.2 g, p < 0.05)
As for differences between sham-treated Hyal2 −/− and WT, Safranin O staining revealed no marked reduction of stainable proteoglycan in articular cartilage of either sham-treated Hyal2 −/− or WT mice at 6, 8, or 10 weeks after surgery (Fig. 5B and H, D and J, F and L, respectively). There were no significant differences in modified Mankin scores at 6 or 8 weeks between Hyal2 −/− (sham) and WT (sham) mice. In contrast, the difference was significant at 10 weeks (P = 0.038) (Fig. 5M). As expected, the results of HABP staining revealed greater accumulation of HA in articular cartilage of Hyal2 −/− mice (sham) (Fig. 6H) compared with WT (sham) (Fig. 6A). Interestingly, lacunae of the deeper cells of articular cartilage of the Hyal2 −/− mice (sham) (Fig. 5H,J and L) were so large and odd-looking compared to those of WT mice (sham) (Fig. 5B,D and F). Considering that these large lacunae exhibited strong positive staining for HABP (Fig. 6H), suggesting the HA accumulation by Hyal2 knockout might cause the enlargement of lacunae, particularly of deeper cells.
Figure 5.
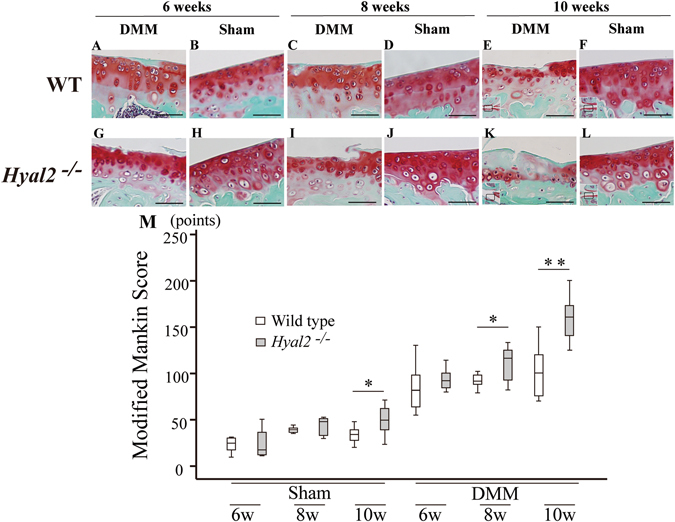
Histological findings of articular cartilage in DMM and sham treated WT and Hyal2 −/− mice. Safranin O staining of articular cartilage in sham treated (B, D, F, H, J, L) and DMM treated (A, C, E, G, I, K) mice. Bars depict 50μm in length. Modified Mankin score (M) of mice articular cartilage (n = 8 per group). * P < 0.05, ** P < 0.01.
Figure 6.
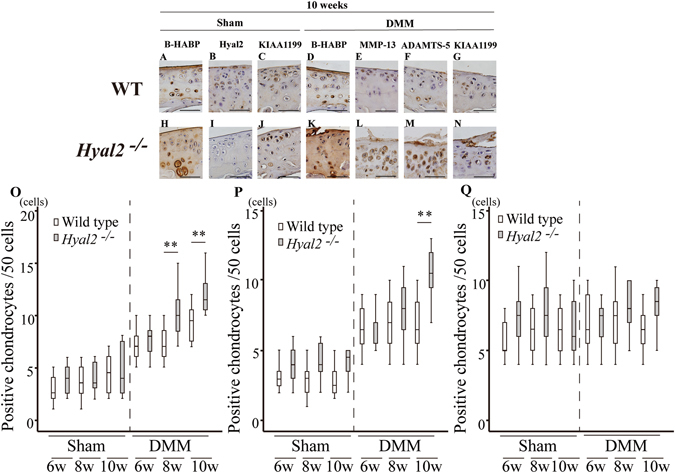
Results of b-HABP and immunohistochemical staining. Staining of articular cartilage in medial tibia at 10 weeks after surgery. B-HABP staining of sham operated side (A; WT, H; Hyal2 −/−) and DMM operated side (D; WT, K; Hyal2 −/−). Hyal2 (B; WT sham, I; Hyal2 −/− sham), MMP-13 (E; WT DMM, L; Hyal2 −/− DMM), ADAMTS-5 (F; WT DMM, M; Hyal2 −/− DMM), and KIAA1199 (C; WT sham, J; Hyal2 −/− sham, G; WT DMM, N; Hyal2 −/− DMM) at 10 weeks after surgery. The numbers of positive chondrocytes for MMP-13 (O), ADAMTS-5 (P), and KIAA1199 (Q) were graphed (** P < 0.01).
Dark staining of HA was observed in and around the chondrocytes in addition to the territorial area of ECM of Hyal2 −/−sham (Fig. 6H). There was no difference in the staining positivity for KIAA1199 between Hyal2 −/− (sham) (Fig. 6J) and WT mice (sham) (Fig. 6C). Micro CT analysis at 6 weeks after surgery showed no significant difference in any of the parameters between Hyal2 −/− (sham) and WT (sham) mice (Supplementary Table S2), whereas, at 8 weeks, subchondral bone plate thickness (SbTh) and trabecular thickness (TbTh) in Hyal2 −/− (43.0 ± 4.1 and 54.6 ± 5.3 μm, respectively) were significantly lower than those in WT (SbTh: 48.5 ± 5.2, TbTh: 61.6 ± 6.0 μm). At 10 weeks after sham surgery, almost all parameters in Hyal2 −/− were significantly lower than those in WT (Table 1). The differences noted in the parameters of bone morphometry might be partly attributable to abnormal HA accumulation during endochondral bone formation, particularly in the growth plate.
Table 1.
Micro CT analysis at 10 weeks after DMM surgery.
| Parameter | WT DMM (n = 8) | WT Sham (n = 8) | Hyal2 −/− DMM (n = 8) | Hyal2 −/− Sham (n = 8) | WT relative change (%) | Hyal2 −/− relative change (%) |
|---|---|---|---|---|---|---|
| Sb BV/TV (%) | 57.2 ± 8.6* | 44.1 ± 7.5 | 52.0 ± 5.5† | 32.7 ± 10.2‡ | 25.1 ± 4.0 | 59.9 ± 14.0§ |
| Sb Th (μm) | 60.4 ± 3.1* | 52.5 ± 3.5 | 51.3 ± 5.7†¶ | 42.9 ± 7.4‡ | 12.8 ± 7.0 | 24.1 ± 8.7 |
| Tb BV/TV (%) | 41.1 ± 4.2 | 43.7 ± 2.7 | 30.5 ± 5.8¶ | 31.3 ± 7.6‡ | −5.5 ± 7.0 | −2.3 ± 2.6 |
| Tb Th (μm) | 58.2 ± 2.7 | 61.0 ± 6.6 | 50.1 ± 6.5¶ | 52.0 ± 3.6‡ | −3.8 ± 4.6 | −4.9 ± 3.6 |
| Tb Sp (μm) | 101.6 ± 18.0* | 85.2 ± 5.4 | 119.9 ± 14.0†¶ | 99.3 ± 12.7‡ | 19.3 ± 11.2 | 17.9 ± 4.0 |
| Tb.N (/mm) | 7.1 ± 0.8 | 7.9 ± 0.6 | 6.1 ± 1.0¶ | 6.8 ± 1.6‡ | −9.8 ± 8.2 | −11.1 ± 6.4 |
Relative increase of parameters was calculated as follows [(value of DMM – value of Sham) ÷ value of sham × 100%]
*Statistically significant difference between Wild DMM and Wild Sham.
†Statistically significant difference between Hyal2 −/− DMM and Hyal2 −/− Sham.
‡Statistically significant difference between Hyal2 −/− Sham and Wild Sham.
§Statistically significant difference between relative increase Hyal2 −/− and Wild.
¶Statistically significant difference between Hyal2 −/− DMM and Wild DMM.
Articular cartilage in DMM joints showed a significantly higher modified Mankin score than that in Sham joints (Fig. 5M). These results confirmed the adequacy of the DMM model used in the present study. Regarding the difference between DMM surgery of Hyal2 −/− and WT cartilage, the results of Safranin O staining at 10 weeks revealed that stainable proteoglycan was reduced in Hyal2 −/− (DMM) mice compared to WT (DMM) mice (Fig. 5K and E, respectively). At 8 and 10 weeks after DMM surgery, articular cartilage in Hyal2 −/− (Fig. 5I and K) mice showed osteoarthritic structural change as compared with WT mice (Fig. 5C and E).
HA deposition detected by HABP staining was increased in the pericellular area and in and around the chondrocytes in Hyal2 −/− mice compared with WT mice with DMM surgery at 10 weeks (Fig. 6K and D, respectively). More MMP-13 positive (Fig. 6L) and ADAMTS-5 positive chondrocytes (Fig. 6M) were observed in Hyal2 −/− mice (DMM) at 10 weeks than in WT mice (Fig. 6E and F, respectively). HA deposition in Hyal2 −/− mice was also increased compared with WT mice with DMM surgery at 6 and 8 weeks. MMP-13 positive chondrocytes were significantly more observed Hyal2 −/− mice compared with WT mice with DMM surgery at 8 and 10 weeks (P < 0.01, Fig. 6O), while there was no difference at 6 weeks. The number of ADAMTS-5 positive chondrocytes was significantly higher in articular cartilage of Hyal2 −/− (DMM) mice at 10 weeks than in WT (DMM) mice (P < 0.01, Fig. 6P), while there was no statistical difference at 6 or 8 weeks. The number of KIAA1199 positive chondrocytes (Fig. 6G and N) was not statistically different between the two groups (Hyal2 −/− with DMM vs WT with DMM) (Fig. 6Q).
Micro CT analysis did not show any significant differences in the bone morphometric parameters between Hyal2 −/− and WT DMM mice at 6 weeks after surgery (Supplementary Table S2). SbTh and TbTh at 8 and 10 (Table 1) weeks were lower in Hyal2 −/− than in WT (Hyal2 −/− DMM vs WT DMM; p < 0.05) (Hyal2 −/− Sham vs WT Sham; p < 0.05). In order to analyze the effects of Hyal2 knockout with or without DMM surgery on the changes in morphometric parameters, relative changes in parameters from sham to DMM was were calculated as previously described19. Relative change in the parameters was calculated as follows: [(“value of DMM” minus “value of sham”) / “value of sham”] × 100. The relative increase of SbTh and TbTh at 8 weeks was not significantly different between Hyal2 −/− and WT mice. Scores of Sb BV/TV, SbTh and TbSp of both Hyal2 −/− and WT were significantly higher in joints with DMM than those with the sham procedure at 10 weeks after surgery (Table 1). Relative change of Sb BV/TV was significantly increased in Hyal2 −/− DMM as compared with WT DMM (P < 0.05), indicating the Hyal2 knockout accelerates the sclerotic change of subchondral bone.
Effects of IL-1α on Hyal2 deficient cartilage explant
Three days’ culture with IL-1α resulted in a marked reduction of Safranin O positive staining in Hyal2 −/− mice cartilage compared to that in WT mice cartilage (Fig. 7F and A, respectively). Results of immunohistochemistry showed that HA was abundantly deposited in and around the chondrocytes in Hyal2 −/− mice (Fig. 7G) compared with WT mice (Fig. 7B). Dark positive staining with MMP-13 was observed in chondrocytes of Hyal2 −/− cartilage (Fig. 7H), where stainable proteoglycan was reduced with safranin O staining, compared with that in WT cartilage (Fig. 7C). More MMP-13-positive chondrocytes were observed in Hyal2 −/− articular cartilage (P < 0.01, Fig. 7K) Also, higher positive staining for ADAMTS-5 was observed in chondrocytes of Hyal2 −/− cartilage (Fig. 7I) compared with those of WT (Fig. 7D). Staining pattern with anti-KIAA1199 antibody was similar in Hyal2 −/− and WT cartilage (Fig. 7J and E, respectively). There was no significant difference in the ratio of articular chondrocytes positive for KIAA1199 between the two groups. (Fig. 7K).
Figure 7.
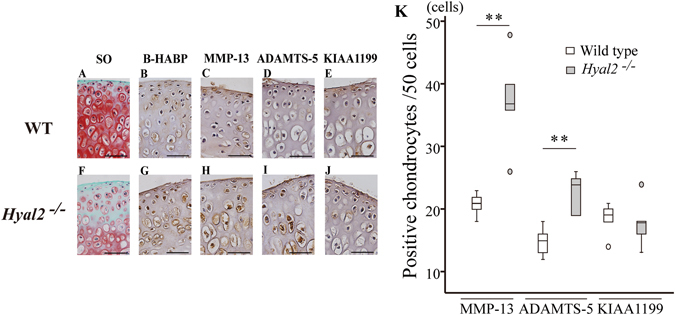
Articular cartilage explant culture with IL-1α of WT and Hyal2 −/− mice. Pieces of articular cartilage was cultured for 72 h in serum free DMEM containing 10 ng/ml mouse IL-1α, and were subjected to Safranin O staining (SO) (A; WT, F; Hyal2 −/−), B-HABP staining (B; WT, G; Hyal2 −/−), MMP-13 staining (C; WT, H; Hyal2 −/−), ADAMTS-5 staining (D; WT, I; Hyal2 −/−), and KIAA1199 staining (E; WT, J; Hyal2 −/−). The numbers of positive stainable cells were graphed (** = P < 0.01) (K). Small circle represented outlier.
Discussion
The results of the present study indicated that Hyal2 knockdown selectively in cartilage accelerates OA development not only in the natural course of aged mice, but also in a DMM induced OA model. Effects of Hyal2 knockdown on neonatal or young mice knee joints were minimal, but were pronounced under conditions more prone to OA including increased age and in a DMM model.
A previous study investigating Hyal1 knockout mice demonstrated that knee joints of the mice showed an increased positive pericellular and/or cytoplasmic HA staining and a reduction of proteoglycans at as early as 3 months of age, and showed progression at 12 months16. The OA change was observed earlier in their study compared with the present one, possibly because of differences in HA catabolic activity between Hyal1 and Hyal2. The enzymatic activity of Hyal2 is reported to be lower than that of Hyal15. Another reason might be some difference in the cell population, which was Hyal-knocked down. In their study, another OA-related cell population was also Hyal1-knocked down, including synovial cells, macrophages, and osteoblasts/osteoclasts. Whereas, in the present study, Hyal2 knockdown is Col2a1 promoter specific. One possibility would be that Hyal2 depletion does not cause marked osteoarthritic change in mice. We have preliminarily examined the expression levels of HYAL2 mRNA in human articular cartilage. Interestingly, in non-OA cartilage evaluated with HE and safranin-O staining, levels of HYAL2 mRNA differed among cases, suggesting that HYAL2 levels might not directly be associated with OA development (Supplementary protocol 1 and Supplementary Fig. S2). Because human and mouse articular chondrocytes express the three most broadly distributed HYAL1/Hyal1, HYAL2/Hyal2 and HYAL3/Hyal320, 21, the roles of Hyal3 in the development of OA require investigation.
Atumuri et al. analyzed Hyal3 knockout mice. Hyal3 −/− mice were viable, fertile and exhibited no gross phenotypic abnormalities. Moreover, investigations of knee joint tissues from Hyal3 −/− mice at 12–14 months of age exhibited no difference between the knee joint tissues from the Hyal3 −/− and Hyal3 +/+ mice17. These findings suggest that Hyal3 does not have crucial roles in the extracellular matrix degradation of articular cartilage. A previous study established and analyzed Hyal2 −/− mice using PGK-Cre system, and revealed that they exhibit localized congenital defects in frontonasal and vertebral bone formation3. Compared with the present study using Col2a1-Cre system, they used relatively young mice, and articular cartilage was not investigated. Moreover, a disease model such as DMM in our study was not used.
MMP-13 and ADAMTS-5 are thought to be one of the crucial enzymes responsible for collagen and aggrecan degradation in articular cartilage22–25. MMP-13 is thought to play very important roles in the degradation of collagen within the cartilage, due to its preferential digestion of type II collagen over types І and III22, 24, 25. In three different OA conditions (age-associated, instability-induced, and explant culture with IL-1α), Hyal2 −/− mice displayed increased expression of MMP-13 and ADAMTS-5 when compared to WT mice. The mechanisms by which excessive storage of HA induces the catabolic cytokines are unknown. Simonaro et al. investigated the articular cartilage and cultured articular chondrocytes from rats and cats with MPS VI. In their study an age-related increase in the number of apoptotic chondrocytes was identified possibly due to the accumulation of dermatan sulfate, and articular chondrocytes from MPS VI animals released more nitric oxide and tumor necrosis factor alpha26. The accumulation of HA within the articular cartilage of the Hyal2 −/− mice at 9 months, and DMM mice of Hyal2 −/− might induce the secretion of inflammatory cytokines like in MPS VI models. Precise mechanisms should be analyzed further.
Recently, KIAA1199, a deafness gene of unknown function, has been shown to have HA degrading activity independent of CD44 and Hyal enzymes, particularly in dermal fibroblasts in normal skin and synovial cells in inflamed joints27. In the present study, the expression of KIAA1199 in the articular chondrocytes did not increase with OA progression, or in Hyal2 -/- compared with WT mice. These findings suggest that HA degrading activity by Hyal enzymes and KIAA1199 acts differently.
OA change in subchondral bone has been reported as a two-stage process. A recent review has proposed that micro damage to the subchondral bone results in increased resorption and a decrease in the thickness in the early stage of OA. Subsequently subchondral trabecular sclerosis occurs and calcified cartilage thickness increases in the late stage of OA28, 29.
In our study, Hyal2 was expressed in growth plate cartilage, which is consistent with a previous report describing that both Hyal1 and Hyal2 mRNAs were highly expressed at the proliferative and hypertrophic layers of rabbit growth plate21. Hyal2 knockout in growth plate cartilage resulted in the accumulation of HA. Hyal2 deficiency has significantly caused the accumulation of hyaluronan in joint cartilage, high molecular weight HA content increases compared with WT mice.
Because a previous report indicated that high molecular weight hyaluronic acid (HMW-HA) prevented osteoclast differentiation and inhibited osteoclastogenesis30, HMW-HA, which accumulated in the growth plate cartilage because of Hyal2 deficiency, might have affected subchondral bone formation. In fact, bone histomorphometric parameters of Hyal2 −/− sham side at 10 weeks were lower compared to those of WT mice. However, at 9 months of age, the difference in bone histomorphometric parameters was reduced between Hyal2 −/− and WT mice. These findings suggest that Hyal2 deficiency in growth plate cartilage might affect epiphyseal bone formation just slightly.
It has been reported that Hyal2 knockout mice do not alter HAS expression such as HAS1 and HAS231, and these results were similar to the results of this study. Whereas, Hyal2 knockout mice had higher Hyal1 expression in the kidneys than wild type mice31. In this study, there was no significant difference in mRNA expression of Hyal1 between Hyal2 −/− and WT mice. This may have represented the difference of HA catabolism between cartilage and kidney.
This study has several limitations. First, the precise mechanism of OA development in Hyal2 deficient animals is not fully understood. As was observed in other MPS animals, which were characterized by accumulation of glycosaminoglycans (GAG), articular cartilage exhibits OA change16 and articular chondrocytes became apoptotic and release more inflammatory cytokines26. Second, we evaluated mRNA expression of MMP-13, ADAMTS-5 and KIAA1199, and the amounts and molecular sizes of HA in the cartilage of Hyal2 −/− and WT mice. A tolerable level of error may be expected in these analyses because of the minimum amount of bone contamination. Furthermore, we analyzed the amounts and molecular sizes of HA in the cartilage of 9-month-old male Hyal2 −/− and WT mice. The results might be influenced by osteoarthritic change of knee joints.
Other limitations include the lack of investigation as to why there was a difference in body weight at 10 weeks after DMM surgery between Hyal2 −/− and WT mice. Although gait analyses of these mice were not performed in this study, progression of osteoarthritic change in knees might have exerted a negative influence on the locomotion of Hyal2 −/− mice, leading to the increase noted in their body weight.
In conclusion, the current study demonstrated that the absence of Hyal2 in articular cartilage was associated with accumulation of HA and stimulation of OA development and progression. It was previously reported that Hyal1 deficiency in a mouse model of MPS IX exhibited OA. Hyal2 deficiency showed similar OA features, suggesting a novel MPS-like disorder of HA storage.
Methods
Mice
All animal experiments were performed in accordance with the Institute for Laboratory Animal Research, National Research Council of the National Academies Guide for the Care and Use of Laboratory Animals under the approval of the Institutional Animal Care and Use Committee at Nagoya University (approved number: 28068). FLP mice in C57BL/6 J background were provided by Unitech Co., Ltd (Chiba, Japan). Age matched male C57BL/6 J WT mice were used as a control. All mice were housed in a temperature and humidity controlled environment under 12 – h light / 12 h dark cycle and fed a standard rodent diet.
Generation of cartilage specific conditional Hyal2−/− mice
Design of the replacement vector (Supplementary Figure S3), Generation of the homologous recombination ES cells, Generation of Hyal2 +/Flox mice, and Generation of Col2a1 promoter inducible conditionally Hyal2 −/− mice were described in Supplementary protocol 2.
Natural course and instability-induced OA model of Hyal2−/− mice
Initially, to investigate the roles of Hyal2 in the development of OA in non-treated mice, knee joints of 3- and 9-month-old male WT and Col2a1 conditional Hyal2 −/− mice were removed as specimens of the natural course32. We evaluated the macroscopic phenotype of WT and Hyal2 −/− including body weight, and a soft radiograph of the skeleton was obtained using a Softex apparatus (Type EMB).
Destabilization of the medial meniscus (DMM) surgery was performed as previously described33–35 using 10-week-old male WT and Hyal2 −/− mice. Briefly, mice were anesthetized with a 0.1 ml intraperitoneal injection of pentobarbital sodium (10 mg/ml). Under sterile conditions, the skin was incised followed by medial parapatellar arthrotomy, and the anterior fat pad was dissected to expose the anterior medial meniscotibial ligament, which was severed. A skin incision and medial parapatellar arthrotomy were performed in the left knees (sham side) of each mouse. Hyal2 −/− (n = 24) and WT (n = 24) mice were subjected to this procedure. To investigate the time course of OA development after DMM or sham surgery, mice (n = 8 each) were sacrificed at 6, 8, 10 weeks after surgery, and the knee joints were excised and subjected to histologic analyses.
Explant culture of femoral head cartilage
Since previous studies reported the use of IL-1α36, 37, to investigate the effects of this catabolic mediator on Hyal2 deficient articular cartilage, femoral head cartilage (2 mm × 2 mm) of Hyal2 −/− and WT mice was obtained, and subjected to explant cultures.
The femoral head cartilage was harvested from 4-week-old mice (n = 5) and pre-cultured for 48 h at 37 °C in a humidified atmosphere of 5% CO2 and 95% air, using Dulbecco’s modified Eagle’s medium (DMEM) containing 1% antibiotic solution (penicillin-streptomycin, Sigma-Aldrich), 2 mM glutamine, 10 mM 4-(2-HydroxyEthyl)−1-PiperazineEthaneSulfonicacid (HEPES), 50 µg/ml ascorbate (Sigma-Aldrich), and 10% fetal bovine serum (FBS). The explants were washed three times with serum free DMEM and cultured for an additional 72 h in serum free DMEM containing 10 ng/ml mouse IL-1α (Cell Signaling Technology, Danvers, MA, USA).
Histologic evaluation of OA
Mice were trans-cardially perfused with cold 4% paraformaldehyde (PFA), and the knee joint was post-fixed in 4% PFA at 4 °C for 12 hours. After washing with phosphate buffered saline (PBS), the samples were decalcified in 10% EDTA solution at 4 °C for 3 weeks and then embedded in paraffin. Coronal sections (5 µm) were cut and mounted onto silicone coated slides.
OA change was analyzed in coronal sections of knee joints and graded according to a modified Mankin histologic score for the tibial articular side in all weight bearing areas of the femoro-tibial joint22, 38. Each sample was stained with Safranin O. Ten coronal sections around the midline of the medial tibia were examined in each mouse. A total modified Mankin score representing the overall state of the cartilage in the joint was summed and averaged from the scores of ten individual sections. This grade of OA was determined by 2 observers (EK and DM), who were blinded to the experimental details to avoid observer bias. Explant cultures of mice femoral head were also subjected to staining with HE or Safranin O to assess the presence of proteoglycan.
Immunohistochemistry
Tissue sections adjacent to those evaluated with modified Mankin score and femoral head were subjected to immunohistochemical analyses according to the method previously described22. To unmask the extracellular matrix around the epitope, the sections were incubated with chondroitinase ABC (0.25 units/ml, pH 8.0, Sigma-Aldrich) overnight at 4 °C. Primary antibodies used were rabbit anti-Hyal2 polyclonal antibody (1:100 dilution; Abcam, Cambridge, MA, USA), mouse anti-MMP-13 polyclonal antibody (1:100 dilution; Proteintech, Chicago, IL, USA), rabbit anti-ADAMTS-5 polyclonal antibody (1:100 dilution; Abcam), rabbit anti-KIAA1199 polyclonal antibody (1:100 dilution; Proteintech), and biotinylated HABP (1:100 dilution; SEIKAGAKU BIOBUSINESS, Tokyo, Japan). All antibodies were confirmed to cross-react mice antigens as shown in our previous study39 and datasheet provided by manufacturers. After washing, sections were subjected to the usual staining procedures. For negative controls, tissue sections were incubated with IgG from non-immunized rabbit as a substitute for the first antibody.
The number of positively stained cells was counted in articular cartilage. Each section was observed under a light microscope at 400× magnification. The mean number of positive cells/50 cells was calculated from 8 mice in each group. All slides were evaluated independently by 2 blinded observers (EK and DM).
Real-time reverse transcriptase-polymerase chain reaction (RT-PCR)
To evaluate the mRNA expression of HAS1, HAS2, HAS3, Hyal1, Hyal2, KIAA1199, MMP-13, and ADAMTS-5, cartilage samples from 9-month-old male Hyal2 −/− and WT mice were obtained and subjected to the RT-PCR analyses according to a previous report40. Liver and triceps surae muscle from the Hyal2 −/− and WT mice were retrieved and subjected to RT-PCR as a positive control for Hyal2 expression and to confirm the cartilage specificity of the Col2a1 promoter Hyal2 −/− mice. The relative levels of mRNA in a sample were expressed after normalization with those of glyceraldehyde-3-phosphate dehydrogenase (Gapdh). Details of the primers used are listed in Supplementary Table S3.
Gel filtration of cartilage glycosaminoglycan
Methods for the preparation of cartilage glycosaminoglycans is provided as Supplementary protocol 3. Aliquots of cartilage glycosaminoglycan preparations from Hyal2 −/− or WT mice (n = 6, respectively) were pooled and applied on to an analytical Sephacryl S-1000 column (0.7 × 40 cm). The column was eluted with PBS solution at a flow rate of 0.5 ml/min, and 0.5 ml fractions were collected. Hyaluronan contents in each cartilage glycosaminoglycan preparation and the column fractions were determined using a competitive ELISA18. Hyaluronan references with known molecular masses (2130, 460, 150 and 5.6 kDa, a courtesy from Seikagaku Corp., Tokyo, Japan) were used to calibrate the column.
Bone histomorphometry
To analyze the trabecular and cortical structure of the proximal tibia (epiphyses) in the natural course of Hyal2 −/− and WT mice, and DMM Hyal2 −/− and WT mice, at first, knee joints were scanned using the Skyscan 1072 micro-CT scanner (Skyscan, Kontich, Belgium) with 5 µm/pixel and reconstructed with software (NRecon software, SkyScan). To evaluate the trabecular and subchondral bone of the epiphyses, the epiphysis of the medial tibia was chosen as the region of interest. The target region in the epiphysis was selected using 3D data analysis software (CTA anlyser, Skyscan). The thickness of the medial side of the subchondral bone plate and trabecular bone was measured. An area (0.5 mm mediolateral width, 1.0 mm ventro-dorsal length) of the weight bearing region of the medial tibial plateau was taken as the region of interest19, and 394 slices of 9 µm thickness were reconstructed.
Bone morphometric parameters of targeted region in the tibial bone were determined using the freely available software package, 3D-Calculator. The indices examined included trabecular bone volume ratio (Tb BV/TV), trabecular thickness (TbTh), trabecular separation (TbSp), trabecular number (TbN), the bone volume ratio of subchondral plate (Sb BV/TV) and subchondral plate thickness (SbTh). These parameters were determined as previously described41.
Statistical analysis
All data are expressed as the mean ± SD. Group means were compared using Student’s t-test or Mann-Whitney U test. Analysis of variance followed by Bonferroni correction or Dunn’s post hoc test was used to assess differences among multiple groups. These statistical analyses were performed using SPSS version 21 (IBM Corp., Armonk, NY). A p value < 0.05 was considered statistically significant.
Data Availability
All data generated or analyzed during this study are included in this published article (and its Supplementary Information files. Associated protocols and data of this study are available for readers. Knockout mice could be available with material transfer agreement in our institution.
Electronic supplementary material
Acknowledgements
The authors thank Ms. Eri Ishihara for secretarial assistance, and Dr. Naohisa Futamura for technical support. This work was supported in part by the Ministry of Education, Culture, Sports, Science and Technology of Japan [Grant-in-Aid 20591751 for Scientific Research (C)] (Y.N.), and [Grant-in-Aid 26293334 for Scientific Research (B)] (Y.N.).
Author Contributions
Y.N., Y.H., and N.I. designed the study, interpreted the data and wrote the manuscript. Y.H., E.K., L.Z., E.A., S.H., D.M., K.I., and K.K. performed the study experiments. H.Y., L.Z., K.K., and T.U. prepared Figure. 4. Y.N., E.A., S.H., K.I., and N.I. contributed to the establishment Hyal2 knockout mice. All authors reviewed the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-07376-5
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Stern R. Hyaluronan catabolism: a new metabolic pathway. Eur J Cell Biol. 2004;83:317–25. doi: 10.1078/0171-9335-00392. [DOI] [PubMed] [Google Scholar]
- 2.Csoka AB, Frost GI, Stern R. The six hyaluronidase-like genes in the human and mouse genomes. Matrix Biol. 2001;20:499–508. doi: 10.1016/S0945-053X(01)00172-X. [DOI] [PubMed] [Google Scholar]
- 3.Jadin L, et al. Skeletal and hematological anomalies in HYAL2-deficient mice: a second type of mucopolysaccharidosis IX? FASEB J. 2008;22:4316–26. doi: 10.1096/fj.08-111997. [DOI] [PubMed] [Google Scholar]
- 4.Chowdhury B, Hemming R, Hombach-Klonisch S, Flamion B, Triggs-Raine B. Murine hyaluronidase 2 deficiency results in extracellular hyaluronan accumulation and severe cardiopulmonary dysfunction. J Biol Chem. 2003;288:520–8. doi: 10.1074/jbc.M112.393629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harada H, Takahashi M. CD44-dependent intracellular and extracellular catabolism of hyaluronic acid by hyaluronidase-1 and -2. J Biol Chem. 2007;282:5597–607. doi: 10.1074/jbc.M608358200. [DOI] [PubMed] [Google Scholar]
- 6.Fraser JR, Laurent TC, Laurent UB. Hyaluronan: its nature, distribution, functions and turnover. J Intern Med. 1997;242:27–33. doi: 10.1046/j.1365-2796.1997.00170.x. [DOI] [PubMed] [Google Scholar]
- 7.Laurent TC, Fraser JR. Hyaluronan. FASEB J. 1992;6:2397–404. [PubMed] [Google Scholar]
- 8.Nishida Y, Knudson CB, Nietfeld JJ, Margulis A, Knudson W. Antisense inhibition of hyaluronan synthase-2 in human articular chondrocytes inhibits proteoglycan retention and matrix assembly. J Biol Chem. 1999;274:21893–9. doi: 10.1074/jbc.274.31.21893. [DOI] [PubMed] [Google Scholar]
- 9.Nishida Y, Knudson CB, Eger W, Kuettner KE, Knudson W. Osteogenic protein 1 stimulates cells-associated matrix assembly by normal human articular chondrocytes: up-regulation of hyaluronan synthase, CD44, and aggrecan. Arthritis Rheum. 2000;43:206–14. doi: 10.1002/1529-0131(200001)43:1<206::AID-ANR25>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 10.Nishida Y, D’Souza AL, Thonar EJ, Knudson W. Stimulation of hyaluronan metabolism by interleukin-1alpha in human articular cartilage. Arthritis Rheum. 2000;43:1315–26. doi: 10.1002/1529-0131(200006)43:6<1315::AID-ANR14>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 11.Csóka AB, Scherer SW, Stern R. Expression analysis of six paralogous human hyaluronidase genes clustered on chromosomes 3p21 and 7q31. Genomics. 1999;60:356–61. doi: 10.1006/geno.1999.5876. [DOI] [PubMed] [Google Scholar]
- 12.Lee TH, Wisniewski HG, Vilcek J. A novel secretory tumor necrosis factor-inducible protein (TSG-6) is a member of the family of hyaluronate binding proteins, closely related to the adhesion receptor CD44. J Cell Biol. 1992;116:545–57. doi: 10.1083/jcb.116.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chow G, Knudson CB, Knudson W. Human hyaluronidase-2 is localized intracellularly in articular chondrocytes and other cultured cell lines. Osteoarthritis Cartilage. 2006;14:1312–4. doi: 10.1016/j.joca.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tammi R, et al. Hyaluronan enters keratinocytes by a novel endocytic route for catabolism. J B iol Chem. 2001;276:35111–22. doi: 10.1074/jbc.M103481200. [DOI] [PubMed] [Google Scholar]
- 15.Stern R, et al. Devising a pathway for hyaluronan catabolism: are we there yet? Glycobiology. 2003;13:105R–115R. doi: 10.1093/glycob/cwg112. [DOI] [PubMed] [Google Scholar]
- 16.Martin DC, et al. A mouse model of human mucopolysaccharidosis IX exhibits osteoarthritis. Hum Mol Genet. 2008;17:1904–15. doi: 10.1093/hmg/ddn088. [DOI] [PubMed] [Google Scholar]
- 17.Atmuri V, et al. Hyaluronidase 3 (HYAL3) knockout mice do not display evidence of hyaluronan accumulation. Matrix Biol. 2008;27:653–60. doi: 10.1016/j.matbio.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 18.Zhuo, L., Kimata, K. GlycoPODhttp://jcggdb.jp/GlycoPOD. Web.30,1, (2017).
- 19.Botter SM, et al. ADAMTS5−/− mice have less subchondral bone changes after induction of osteoarthritis through surgical instability: implications for a link between cartilage and subchondral bone changes. Osteoarthritis Cartilage. 2009;17:636–45. doi: 10.1016/j.joca.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 20.Bastow ER, et al. Hyaluronan synthesis and degradation in cartilage and bone. Cell Mol Life Sci. 2008;65:395–413. doi: 10.1007/s00018-007-7360-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanimoto K, et al. Hyaluronidase expression in cultured growth plate chondrocytes during differentiation. Cell Tissue Res. 2004;318:335–42. doi: 10.1007/s00441-004-0966-7. [DOI] [PubMed] [Google Scholar]
- 22.Kozawa E, et al. Osteoarthritic change is delayed in a Ctsk-knockout mouse model of osteoarthritis. Arthritis Rheum. 2012;64:454–64. doi: 10.1002/art.33398. [DOI] [PubMed] [Google Scholar]
- 23.Stanton H, et al. ADAMTS5 is the major aggrecanase in mouse cartilage in vivo and in vitro. Nature. 2005;434:648–52. doi: 10.1038/nature03417. [DOI] [PubMed] [Google Scholar]
- 24.Knäuper V, et al. Cellular mechanisms for human procollagenase-3 (MMP-13) activation. Evidence that MT1-MMP (MMP-14) and gelatinase a (MMP-2) are able to generate active enzyme. J Biol Chem. 1996;271:17124–31. doi: 10.1074/jbc.271.29.17124. [DOI] [PubMed] [Google Scholar]
- 25.Matsui Y, et al. Accelerated development of aging-associated and instability-induced osteoarthritis in osteopontin-deficient mice. Arthritis Rheum. 2009;60:2362–2371. doi: 10.1002/art.24705. [DOI] [PubMed] [Google Scholar]
- 26.Simonaro CM, Haskins ME, Schuchman EH. Articular chondrocytes from animals with a dermatan sulfate storage disease undergo a high rate of apoptosis and release nitric oxide and inflammatory cytokines: a possible mechanism underlying degenerative joint disease in the mucopolysaccharidoses. Lab Invest. 2001;81:1319–28. doi: 10.1038/labinvest.3780345. [DOI] [PubMed] [Google Scholar]
- 27.Yoshida H, et al. KIAA1199, a deafness gene of unknown function, is a new hyaluronan binding protein involved in hyaluronan depolymerization. Proc Natl Acad Sci USA. 2013;110:5612–7. doi: 10.1073/pnas.1215432110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li G, et al. Subchondral bone in osteoarthritis: insight into risk factors and microstructural changes. Arthritis Res Ther. 2013;15:223–35. doi: 10.1186/ar4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Camacho A, et al. Iron overload in a murine model of hereditary hemochromatosis is associated with accelerated progression of osteoarthritis under mechanical stress. Osteoarthritis Cartilage. 2016;24:494–502. doi: 10.1016/j.joca.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 30.Ariyoshi W, Okinaga T, Knudson CB, Knudson W, Nishihara T. High molecular weight hyaluronic acid regulates osteoclast formation by inhibiting receptor activator of NF-κB ligand through Rho kinase. Osteoarthritis Cartilage. 2014;22:111–20. doi: 10.1016/j.joca.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 31.Colombaro V, et al. Lack of hyaluronidases exacerbates renal post-ischemic injury, inflammation, and fibrosis. Kidney Int. 2015;88:61–71. doi: 10.1038/ki.2015.53. [DOI] [PubMed] [Google Scholar]
- 32.Poole R, et al. Recommendations for the use of preclinical models in the study and treatment of osteoarthritis. Osteoarthritis Cartilage. 2010;18:S10–S16. doi: 10.1016/j.joca.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 33.Xu C, et al. The Therapeutic Effect of rhMK on Osteoarthritis in Mice, Induced by Destabilization of the Medial Meniscus. Biol Pharm Bull. 2014;37:1803–10. doi: 10.1248/bpb.b14-00470. [DOI] [PubMed] [Google Scholar]
- 34.Glasson SS, Blanchet TJ, Morris EA. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthritis Cartilage. 2007;15:1061–9. doi: 10.1016/j.joca.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 35.Das NBP, Forte aE, Vincent TL, Dini D, Marenzana M. Rapid, automated imaging of mouse articular cartilage by microCT for early detection of osteoarthritis and finite element modelling of joint mechanics. Osteoarthritis Cartilage. 2014;22:1419–28. doi: 10.1016/j.joca.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sasazawa F, et al. Depletion of gangliosides enhances cartilage degradation in mice. Osteoarthritis Cartilage. 2014;22:313–22. doi: 10.1016/j.joca.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 37.Glasson SS, et al. Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature. 2005;434:644–648. doi: 10.1038/nature03369. [DOI] [PubMed] [Google Scholar]
- 38.Furman BD, et al. Joint degeneration following closed intraarticular fracture in the mouse knee: a model of posttraumatic arthritis. J Orthop Res. 2007;25:578–92. doi: 10.1002/jor.20331. [DOI] [PubMed] [Google Scholar]
- 39.Yoshioka Y, et al. Suppression of hyaluronan synthesis alleviates inflammatory responses in murine arthritis and in human rheumatoid synovial fibroblasts. Arthritis Rheum. 2013;65:1160–70. doi: 10.1002/art.37861. [DOI] [PubMed] [Google Scholar]
- 40.Chan DD, et al. Deficiency of hyaluronan synthase1 (HAS1) results in chronic joint inflammation and widespread intra-articular fibrosis in a murine model of knee joint cartilage damage. Osteoarthritis Cartilage. 2015;23:1879–1889. doi: 10.1016/j.joca.2015.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bouxsein ML, et al. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res. 2010;25:1468–86. doi: 10.1002/jbmr.141. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article (and its Supplementary Information files. Associated protocols and data of this study are available for readers. Knockout mice could be available with material transfer agreement in our institution.


