Abstract
Magnesium is the metal at the center of all types of chlorophyll and is thus crucial to photosynthesis. When an element is involved in a biosynthetic pathway its isotopes are fractionated based on the difference of vibrational frequency between the different molecules. With the technical advance of multi-collectors plasma-mass-spectrometry and improvement in analytical precision, it has recently been found that two types of chlorophylls (a and b) are isotopically distinct. These results have very significant implications with regards to the use of Mg isotopes to understand the biosynthesis of chlorophyll. Here we present theoretical constraints on the origin of these isotopic fractionations through ab initio calculations. We present the fractionation factor for chlorphyll a, b, d, and f. We show that the natural isotopic variations among chlorophyll a and b are well explained by isotopic fractionation under equilibrium, which implies exchanges of Mg during the chlorophyll cycle. We predict that chlorophyll d and f should be isotopically fractionated compared to chlorophyll a and that this could be used in the future to understand the biosynthesis of these molecules.
Introduction
Magnesium is a ubiquitous element in nature and has three naturally occurring stable isotopes, 24Mg, 25Mg and 26Mg, with relative abundance of 78.99%, 10.00% and 11.01%, respectively. Magnesium also plays an essential role in a wide range of fundamental biological and non-biological reactions in the geological and biological system.
Chlorophylls are the green pigments, which allow phytoautotrophs to use light energy to produce organic matter by photosynthesis. Chlorophylls are formed of a Mg atom embedded in a porphyrin or chlorin ring1, 2. Chlorophylls can take several forms, with chlorophyll a the main pigment used by almost all oxygenic (oxygen-evolving) photosynthetic organisms. In addition to chlorophyll a (chl a), some organisms use accessory pigments: chlorophyll b (chl b) in green algae, and higher plant antennas, chlorophyll c (chl c) in certain algae, chlorophyll d (chl d) in some cyanobacteria and the recently discovered chlorophyll f (chl f) in some cyanobacteria3. In addition, there are a number of bacteriochlorophylls (BChl a through g) found in anoxygenic (non-oxygen evolving) photosynthetic bacteria2, 4.
Biological processes are widely recognized to have the ability to fractionate isotopes and create isotopic fractionation wherein the isotopic ratio of the product is different to that of the starting material (e.g. Zn, Fe, and Cu isotopic fractionation between plants and soils refs 5–10). Two studies found that Mg in cyanobacterial chl a is isotopically fractionated from the culture medium in which the cyanobacteria were grown and chl a and chl b have different isotopic compositions11, 12. They found that the 26Mg/24Mg of chl a was 0.43 ± 0.15‰ heavier than of chl b. These results have very important geochemical and biological implications: 1) Incorporating magnesium into chl a fractionates Mg isotopes and therefore the distribution of Mg isotopes in fossils may be used to search for the presence of fossil photosynthetic life and trace the origin of photosynthesis. 2) Studying the mechanism of isotopic fractionation by comparing the Mg isotopic composition measurements with ab initio calculations can help us understand the biosynthetic pathway of Mg during chlorophyll formation. In order to understand the origin of these fractionation Black et al.11 performed ab initio calculation to estimate the theoretical isotopic fractionation between chl a and chl b and predicted an isotopic fractionation of 0.66‰ for the 26Mg/24Mg ratio (compared to 0.43 ± 0.15 in the natural samples).
Other chlorophyll structures than chl a and chl b exist, including the important red-shifted chl d and chl f. These pigments have not been studied using Mg isotopic methods yet.
Here we report the molecular orbitals of a large variety of Mg-chlorophyll species (chlorophylls a, b, d, f) to obtain the reduced partition function ratio (RPFR) of isotopologues. We then use these data to test whether the Mg isotopic fractionation observed between chl a and chl b is due to chemical exchange of Mg and predict whether such effect should affect chl d and chl f. Finally, we propose that by combining our calculations with natural Mg isotopic data would permit to better understand the biosynthetic formation of chlorphyll.
Methods
Magnesium has only three stable isotopes, 24Mg, 25Mg and 26Mg. The isotopic composition of Mg is usually presented using the δ per mill (‰) notation defined as:
| 1 |
with x = 25 or 26. All the data are reported relative to the same standard (DSM-3; Black, 2006 #4698).
And we define the difference of δxMg between two species X and Y a ΔxMgX-Y, with x = 25 or 26 as:
| 2 |
Computational method
Orbital geometries, vibrational frequencies, Gibbs free energies of aqueous Mg species are computed using density functional theory (DFT) as implemented by the Gaussian03 code13. The DFT method employed is a hybrid density functional consisting of Becke’s three-parameter non-local hybrid exchange potential (B3)14 with Lee-Yang-and Parr (LYP) non-local functionals. The 6–311 + G(d,p) basis set, which is an all-electron basis set, will be chosen for H, C, O, and Mg. For the solvation effect, CPCM continuum solvation method (CPCM: conductor-like polarizable continuum model) is used.
For the structure of chlorophyll a, b, d and f we used an optimized geometry shown in the Fig. 1 (data are available as supplementary materials).
Figure 1.
Optimized geometry for chlorophyll (a,b,d, and f).
Results and Discussion
The vibrational modes v1, v2, and v3 of hexaaqua complexes are the fundamental intramolecular vibration modes. The validity of vibrational frequencies and atomic distances of Mg-O of hydrated Mg2+ ion computed is discussed in detail in Schott et al.15. The calculated vibrational frequencies of Mg(H2O)6 2+ are underestimated compared with the literature values determined by Raman and IR spectrometry. Setting 12 H2O molecules at the second coordination sphere brought the calculation results closer to the literature values. The use of conductor-like polarizable continuum model (CPCM) of solvation for Mg(H2O)6 2+ increases vibrational frequencies, but the effect is smaller than setting water molecules in the second coordination sphere.
Calculating how physicochemical properties vary with hydration of the species is a common strategy for examining the accuracy of theoretical calculations of aqueous species16, 17. In the theoretical study on the hydration enthalpy of Fe2+ and Fe3+, Li et al.18 tested a small cluster model of 6 H2O molecules as the first coordination sphere and a large cluster model of 12 additional H2O molecules as the second coordination sphere. For Fe3+, the large cluster model brought the calculated data closer to the experimental results. For Fe2+, both the small and the large cluster modes reproduced the experimental results. Similarly, the hydration enthalpies of Ni2+ and Zn2+ were appropriately reproduced by using both the small and the large cluster models. Magnesium ion is a divalent one. The small cluster model may be applicable.
The hydration enthalpy of Mg(H2O)n 2+ (n = 6 or 18) was also examined. In order to relate the calculated quantities to the experimental hydration enthalpy (ΔH°hyd) at 298 K, the following correction terms were considered18,
| 3 |
ΔE b is the total binding energy of the gas phase cluster [Mg(H2O)n]. ΔE sol represents the solvation free energy of Mg(H2O)n 2+. This term, which contains the entropic contribution to the solvation free energy for the continuum dielectric part, was neglected. ΔH vap is the heat of vaporization of water, which is 10.50 kcal/mol19. The ΔE(Cp) term arises from the difference in heat capacity of the components of the system, which corresponds to a small correction of ~1 kcal/mol at 298 K18. ΔE zp is the difference in vibrational zero-point energy in forming clusters, ΔE rel is a correction due to relativistic effects for metal centers, and ΔE geom is a correction due to geometry relaxation for H2O during the formation of the clusters. The magnitude of “−ΔE zp + ΔE rel + ΔE geom” was reported to be negligibly small (a few kcal/mol)18, and hence these corrections were not included in our calculation. The calculated value of ΔH°hyd is shown in Table 1. The hydration enthalpy of metal cations has been determined by thermochemical methods20–22, and literature values are shown for comparison. Setting 12 H2O around the small cluster Mg(H2O)6 2+ resulted in the increase of ∆H 0 hyd. For the method, O3LYP, large cluster model showed similar value compared with literature data, while B3LYP showed that small cluster model showed similar value compared with literature data. For divalent cations, even small cluster models like Mg(H2O)6 2+ can reproduce ΔH°hyd.
Table 1.
Hydration enthalpy of Mg(H2O)n 2+.
| Hydration number n | Method/Basis set | ΔH°hyd/kJ/mol | Reference |
|---|---|---|---|
| 6 | O3LYP/6-31G(d) | −1893 | This work |
| 6 | B3LYP/6-31G(d) | −1954 | This work |
| 18 | O3LYP/6-31G(d) | −2047 | This work |
| 18 | B3LYP/6-31G(d) | −2280 | This work |
| — | −1990 | Rosseinsky, 1965 | |
| — | −1921 | Smith, 1977 | |
| — | −1931 | Marcus, 1985 |
All the data are reported in Table 2 and Fig. 2 for some of the most relevant molecules discussed. The absolute values of our calculated lnβ for Mg(H2O)6 2+, Mg(H2O)18 2+, chl a and chl b are in fairly good agreement with literature data11, 23, 24 while we are using different program packages: PQS ver. 3.3. for Black et al.11 vs Gaussian09 in the present study and Schott et al.15.
Table 2.
Logarithm of the reduced partition function, ln β (‰), for the pair 26Mg-24Mg and 25Mg-24Mg of Mg(II) complexes.
| Species | Method/basic sets | lnβ25/24 298 K | lnβ25/24 A, Ba | lnβ26/24 298 K | lnβ26/24 A, Ba | Reference |
|---|---|---|---|---|---|---|
| Mg(H2O)6 2+ | O3LYP/6-31G(d) | 12.43 | 1.0425, 0.671 | 23.88 | 2.0041, 1.272 | This study |
| Mg(H2O)6 2+ | B3LYP/6-31G(d) | 13.73 | 1.1448, 0.816 | 26.37 | 2.1998, 1.556 | This study |
| Mg(H2O)18 2+ | O3LYP/6-31G(d) | 12.48 | 1.0470, 0.666 | 23.96 | 2.0104, 1.282 | This study |
| Mg(H2O)18 2+ | O3LYP/6-31G(d) | 13.18 | — | 25.22 | — | Black et al.11 |
| Mg(H2O)18 2+ | B3LYP/6-31G(d) | 14.26 | 1.1881, 0.857 | 27.40 | 2.2852, 1.615 | This study |
| Mg(H2O)18 2+ | BP86/6-31G(d) | — | — | 26.74 | — | Rustad et al.24 |
| Chlorophyll-a | O3LYP/6-31G(d) | 14.61 | 1.2077, 0.979 | 28.07 | 2.3255, 1.828 | This study |
| Chlorophyll-a | O3LYP/6-31G(d) | 15.21 | 29.27 | Black et al.11 | ||
| Chlorophyll-a | B3LYP/6-31G(d) | 15.14 | 1.2510, 1.022 | 29.08 | 2.4053, 1.944 | This study |
| Chlorophyll-b | O3LYP/6-31G(d) | 14.43 | 1.1949, 0.948 | 27.73 | 2.2995, 1.785 | This study |
| Chlorophyll-b | O3LYP/6-31G(d) | 14.88 | — | 28.64 | — | Black et al.11 |
| Chlorophyll-b | B3LYP/6-31G(d) | 14.99 | 1.2397, 1.001 | 28.79 | 2.3830, 1.905 | |
| Chlorophyll-d | O3LYP/6-31G(d) | 14.53 | 1.2028, 0.956 | 27.91 | 2.3123, 1.822 | This study |
| Chlorophyll-d | B3LYP/6-31G(d) | 15.13 | 1.2509, 1.017 | 29.08 | 2.4042, 1.949 | This study |
| Chlorophyll-f | O3LYP/6-31G(d) | 14.48 | 1.1999, 0.937 | 27.82 | 2.3063, 1.795 | This study |
| Chlorophyll-f | B3LYP/6-31G(d) | 15.03 | 1.2427, 1.010 | 28.89 | 2.3913, 1.906 | This study |
a103 ln β = 106 A T −2 + B.
Figure 2.
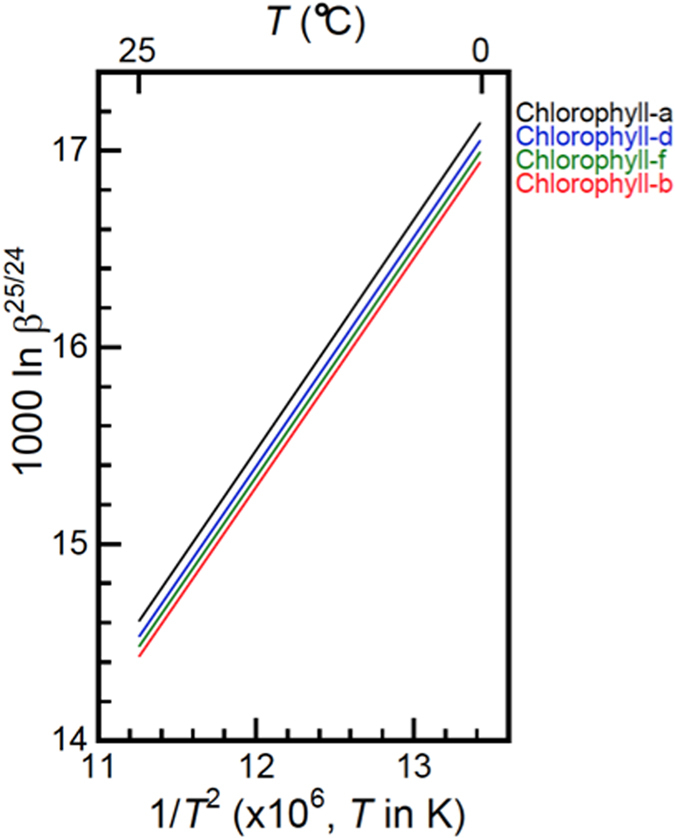
Temperature dependence of ln β. The ln β25/24 values of chlorophyll a, b, d, and f calculated by using O3LYP/6-31G(d) are shown as linear functions of T −2.
While the relative difference between the lnβ26/24Mg of chl b and chl a (Table 3) that we obtain (0.34) is slightly lower than in Black et al.11 (0.67); it is closer than the experimental data (0.43). The accuracy of our calculation using Gaussian09 for Mg isotopologues was tested by comparing our calculations with experimental data for a large set of aqueous species (sulphides, citrates, EDTA, oxalates, hydroxides; see ref. 23).
Table 3.
Theoretical isotopic fractionation between different forms of chlorophylls and chlorophyll a for 298 K.
| Species | Method | Δ25Mg | Δ26Mg | Reference |
|---|---|---|---|---|
| chl a vs. chl b | O3LYP/6-31G(d) | 0.18 | 0.34 | This study |
| chl a vs. chl b | O3LYP/6-31G(d) | 0.33 | 0.63 | Black et al.11 |
| chl d vs. chl b | O3LYP/6-31G(d) | 0.10 | 0.18 | This study |
| chl f vs. chl b | O3LYP/6-31G(d) | 0.05 | 0.09 | This study |
The calculated theoretical isotopic fractionation between the four chlorophylls species considered here (chl a, chl d and chl f compared to chl b, see Table 3) predicts resolvable isotopic variations between the different species of chlorophylls. The Mg isotopic composition of the chl f should be very close to chl b with Δ26Mg(chl f-chl b) = 0.09, chl d is slightly heavier, Δ26Mg(chl d-chl b) = 0.18 and finally chl a is the isotopically heaviest Δ26Mg(chl a-chl b) = 0.34.
Our results suggest that each type of chlorophyll should exhibit a distinct Mg isotopic composition. It is therefore possible to test whether Mg were exchanged at equilibrium via the Mg isotopic composition of the different chlorophyll. Following this approach, Black et al.11 proposed that Mg were exchanged at equilibrium between chl a and chl b based on the similarity between the measured and calculated isotopic composition, suggesting that the timescale of Mg exchanges were similar to the lifetime of the chlorophylls. Our new results show even better agreements with the measurements further suggesting that Mg were exchanged during the lifetime of the chlorophyll.
It is known that the relative abundance of chl a and chl b in plants or green algae changes as a function of the light conditions25 suggesting that the biosynthesis of chl a and chl b are closely linked. Tanaka et al.26 isolated the gene encoding for the formation of chl b by oxygenation of chl a and proposed a chlorophyll cycle with an intermediary molecule, 7-hydroxymethyl. From their model, Mg is not involved in the cycle and, therefore, chl a and chl b should have similar isotopic composition. The fact that the Mg isotopes are fractionated between isolated chl a and chl b and follow our theoretical prediction confirms that the biosynthesis of chl a and chl b are closely linked and implies the Mg is also exchanged during the chlorophyll cycle. Our results imply that the biochemical pathway of the synthesis of chl a and chl b is more complex that presently modeled.
In this study we have expended our calculations to the two most recently discovered chlorophylls, chl d and chl f. Both chl d and chl f are always found in association with chl a 27. Chlorphyll d and f are red-shifted chlorophyll that can adsorb light with wavelength up to 760 nm while chl a and b do not absorb light <700 nm. These pigments have only been discovered in a limited set of organisms so far (e.g. Acaryochloris Marina, Halomicronema Hongdechloris) and are supposed to represent the consequence of adaptation of the micro-organisms to specific ecological niches. The understanding of the biosynthesis of these novel pigments is still limited and while there is no consensus on the existence of chl d-chl a and/or chl f-chl a cycles there are hints that the biosynthesis of chl d and f are linked to chl a. Using spiked oxygen marking Schliep et al.28 suggested that chl d was formed directly from chl a via oxygenase-type reactions but the enzymes responsible for the synthesis have yet to be discovered. Our new results on the two long wave-length absorbing chlorophylls exhibit distinct isotopic composition suggesting that it would also be possible to test the kinetic of Mg exchanged compared to the lifetime of chl d and chl f.
Isotopic fractionation of Mg at equilibrium between 4 types of chlorophylls (a, b, d, and f) was demonstrated theoretically. We show that our data are consistent with previous calculations and confirms that chl a and chl b are isotopically fractionated in plants following a thermodynamic equilibrium. This implies that during the synthesis of chl b and chl a, Mg is also exchanged. We expand these calculations to the two latest discovered pigments, chl d and chl f and show that their Mg isotopes should also be isotopically fractionated during equilibrium exchanges reactions.
Electronic supplementary material
Acknowledgements
Frederic Moynier is grateful to the European Research Council under the European Community’s H2020 framework program/ERC grant agreement # 637503 (Pristine) and the Agence Nationale de la Recherche for a chaire d’Excellence Sorbonne Paris Cité (IDEX13C445), and for the UnivEarthS Labex program (ANR-10-LABX-0023 and ANR-11-IDEX-0005-02). Parts of this work were supported by IPGP multidisciplinary program PARI, and by Region Île-de-France SESAME Grant no. 12015908.
Author Contributions
F.M. and T.F. designed the project. T.F. performed the calculations. F.M. wrote the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-07305-6
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Blankenship, R. E. Molecular Mechanisms of Photosynthesis. (Blackwell, 2002).
- 2.Grimm, B., J., P. R., Rudiger, W. & H., S. Chlorophylls and Bacteriochlorophylls: Biochemistry, Biophysics, Functions, and Applications. (Springer, 2006).
- 3.Chen M, et al. A Red-Shifted Chlorophyll. Science. 2010;329:1318–1319. doi: 10.1126/science.1191127. [DOI] [PubMed] [Google Scholar]
- 4.Niedzwiedzki DM, Blankenship RE. Singlet and Triplet Excited State Properties of Natural Chlorophylls and Bacteriochlorophylls. Photosynthesis Research. 2010;106:227–238. doi: 10.1007/s11120-010-9598-9. [DOI] [PubMed] [Google Scholar]
- 5.Viers J, et al. Evidence of Zn isotopic frationation in a soil-plant system of a pristine tropical watershed (Nsimi, Cameroon) Chem. Geol. 2007;239:124–137. doi: 10.1016/j.chemgeo.2007.01.005. [DOI] [Google Scholar]
- 6.Moynier F, et al. Isotopic fractionation and transport mechanisms of Zn in plants. Chem. Geol. 2009;267:125–130. doi: 10.1016/j.chemgeo.2008.09.017. [DOI] [Google Scholar]
- 7.Weinstein, C. et al. Cu isotopic fractionation in plants. Chem. Geol (2011).
- 8.Moynier F, Fujii T, Wang K, Foriel J. Ab initio calculations of the Fe(II) and Fe(III) isotopic effects in citrates, nicotianamine, and phytosiderophore, and new Fe isotopic measurements in higher plants. Comptes Rendus Geoscience. 2013;345:230–240. doi: 10.1016/j.crte.2013.05.003. [DOI] [Google Scholar]
- 9.Guelke-Stelling M, von Blanckenburg F. Fe isotope fractionation caused by translocation of iron during growth of bean and oats as models of strategy I and II plants. Plant soil. 2012;352:217–231. doi: 10.1007/s11104-011-0990-9. [DOI] [Google Scholar]
- 10.Moynier, F., Vance, D., Fujii, T. & Savage, P. In Non-traditional stable isotopes Vol. 82 (eds F-Z Teng, J. Watkins, & N. Dauphas) 543–600 (Mineralogical Society of America, 2017).
- 11.Black JR, Yin QZ, Rustad JR, Casey WH. Magnesium isotopic equilibrium in chlorophylls. J Am Chem Soc. 2007;129:8690. doi: 10.1021/ja072573i. [DOI] [PubMed] [Google Scholar]
- 12.Black JR, Casey WH, Yin QZ. An experimental study of magnesium-isotope fractionation in chlorophyll-a photosynthesis. Geochim. Cosmochim. Acta. 2006;70:4072–4079. doi: 10.1016/j.gca.2006.06.010. [DOI] [Google Scholar]
- 13.Gaussian 09, Revision B.01, Gaussian, Inc.: Wallingford CT (2009).
- 14.Becke AD. Density-functional thermochemistry. 3. The role of exact exchange. J. Chem. Phys. 1993;98:5648–5652. [Google Scholar]
- 15.Schott J, Mavromatis V, Fujii T, Pearce CR, Oelkers E. The control of carbonate mineral Mg isotope composition by aqueous specitation: Theoritical and experimental modeling. Chem. Geol. 2016;445:120–134. doi: 10.1016/j.chemgeo.2016.03.011. [DOI] [Google Scholar]
- 16.Fujii T, Moynier F, Blichert-Toft J, Albarede F. Density functional theory estimation of isotope fractionation of Fe, Ni, Cu, and Zn among species relevant to geochemical and biological environments. Geochim. Cosmochim. Acta. 2014;140:553–576. doi: 10.1016/j.gca.2014.05.051. [DOI] [Google Scholar]
- 17.Moynier F, Fujii T. Calcium isotope fractionation between aqueous compounds relevant to low-temperature geochemistry, biology and medicine. Scientific Reports. 2017;7:44255. doi: 10.1038/srep44255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li J, Fisher CL, Chen JL, Bashford D, Noodleman L. Calculation of redox potentials and pKa values of hydrated transition metal cations by a combined density functional and continuum dielectric theory. Inorg. Chem. 1996;35:4694–4702. doi: 10.1021/ic951428f. [DOI] [Google Scholar]
- 19.Lide, D. R. CRC Handbook of Chemistry and Physics, 89th Ed., (CRC Press, 2008).
- 20.Rosseinsky DR. Electrode potentials and hydration energies. Theories and correlations. Chem. Rev. 1965;65:467–490. doi: 10.1021/cr60236a004. [DOI] [Google Scholar]
- 21.Smith DW. Ionic hydration enthalpies. J. Chem. Edu. 1977;54:540–542. doi: 10.1021/ed054p540. [DOI] [Google Scholar]
- 22.Marcus, Y. Ion Solvation. (John Wiley & Sons, 1985).
- 23.Schott J, V. M, Fujii T, Pearce CR, Oelkers E. The control of carbonate mineral Mg isotope composition by aqueous speciation: Theoretical and experimental modeling. Chem. Geol. 2014;445:120–134. doi: 10.1016/j.chemgeo.2016.03.011. [DOI] [Google Scholar]
- 24.Rustad JR, et al. Isotopic fractionation of Mg2+ (aq), Ca2+ (aq), and Fe2+ (aq) with carbonate minerals. Geochim. Cosmochim. Acta. 2010;74:6301–6323. doi: 10.1016/j.gca.2010.08.018. [DOI] [Google Scholar]
- 25.Björkman, O. et al. Carnegie Inst Washington Yearb. 1 972, 115–135 (1972).
- 26.Tanaka A, et al. Chlorophyll a oxygenase (CAO) is involved in chlorophyll b formation from chlorophyll a. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:12719–12723. doi: 10.1073/pnas.95.21.12719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Y, Chen M. Functional Plant Biology. 2015;42:493–501. doi: 10.1071/FP14350. [DOI] [PubMed] [Google Scholar]
- 28.Schliep M, Crossett B, Willows RD, Chen M. 18O Labelling of chlorophyll d in Acaryochloris marina reveals that chlorophyll a and molecular oxygen are precursors. J. Biol. Chem. 2010;37:28450–28456. doi: 10.1074/jbc.M110.146753. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


