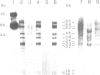
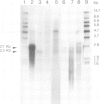
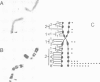
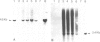Abstract
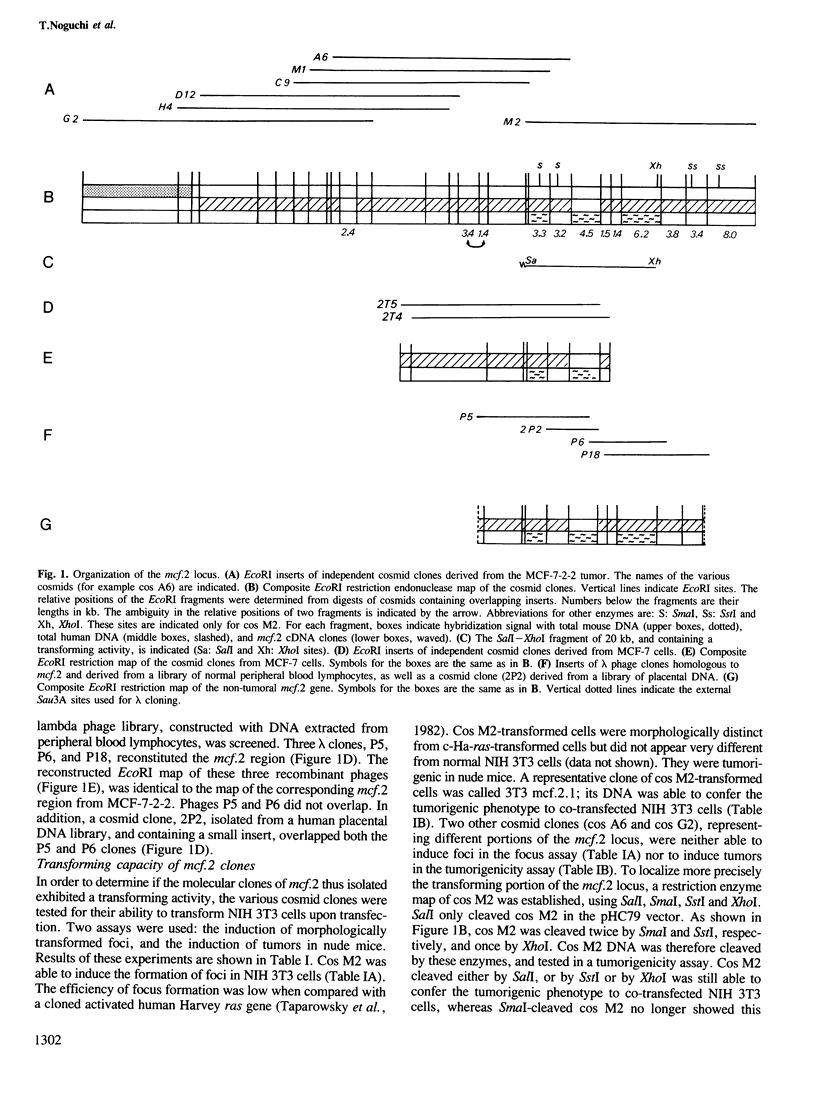
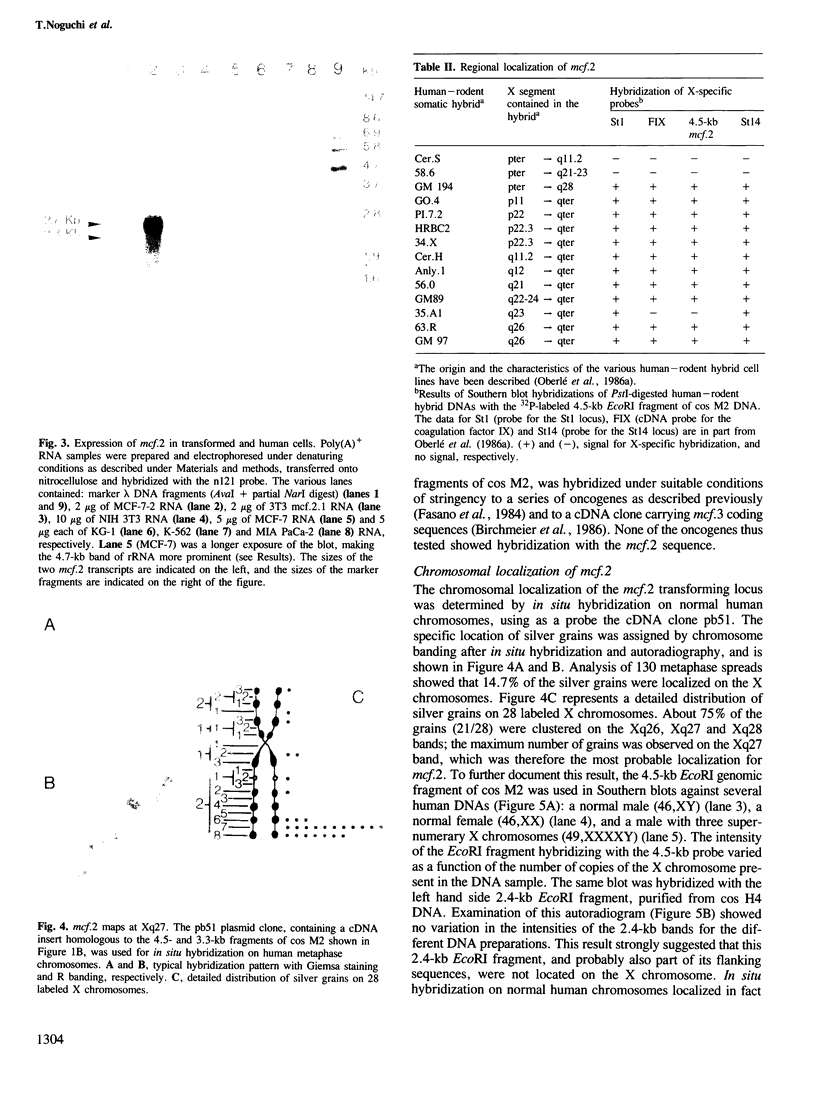
A transforming sequence was identified using co-transfection of DNA from the human mammary carcinoma cell line MCF-7 and of a G418 resistance gene into NIH 3T3 cells, followed by tumor formation in athymic mice. This sequence, named mcf.2, was molecularly cloned. A transforming activity resides in a cosmid clone of 42 kb. mcf.2 did not cross-hybridize with the known oncogenes tested. In situ hybridization localized it on the X chromosome, probably at q27. This localization was confirmed by hybridization to a panel of human--rodent cell line DNAs.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey J. M., Davidson N. Methylmercury as a reversible denaturing agent for agarose gel electrophoresis. Anal Biochem. 1976 Jan;70(1):75–85. doi: 10.1016/s0003-2697(76)80049-8. [DOI] [PubMed] [Google Scholar]
- Birchmeier C., Birnbaum D., Waitches G., Fasano O., Wigler M. Characterization of an activated human ros gene. Mol Cell Biol. 1986 Sep;6(9):3109–3116. doi: 10.1128/mcb.6.9.3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochran B. H., Zullo J., Verma I. M., Stiles C. D. Expression of the c-fos gene and of an fos-related gene is stimulated by platelet-derived growth factor. Science. 1984 Nov 30;226(4678):1080–1082. doi: 10.1126/science.6093261. [DOI] [PubMed] [Google Scholar]
- Cooper C. S., Park M., Blair D. G., Tainsky M. A., Huebner K., Croce C. M., Vande Woude G. F. Molecular cloning of a new transforming gene from a chemically transformed human cell line. Nature. 1984 Sep 6;311(5981):29–33. doi: 10.1038/311029a0. [DOI] [PubMed] [Google Scholar]
- Eva A., Aaronson S. A. Isolation of a new human oncogene from a diffuse B-cell lymphoma. Nature. 1985 Jul 18;316(6025):273–275. doi: 10.1038/316273a0. [DOI] [PubMed] [Google Scholar]
- Fasano O., Birnbaum D., Edlund L., Fogh J., Wigler M. New human transforming genes detected by a tumorigenicity assay. Mol Cell Biol. 1984 Sep;4(9):1695–1705. doi: 10.1128/mcb.4.9.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Hohn B., Collins J. A small cosmid for efficient cloning of large DNA fragments. Gene. 1980 Nov;11(3-4):291–298. doi: 10.1016/0378-1119(80)90069-4. [DOI] [PubMed] [Google Scholar]
- Huebner K., ar-Rushdi A., Griffin C. A., Isobe M., Kozak C., Emanuel B. S., Nagarajan L., Cleveland J. L., Bonner T. I., Goldsborough M. D. Actively transcribed genes in the raf oncogene group, located on the X chromosome in mouse and human. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3934–3938. doi: 10.1073/pnas.83.11.3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeffler H. P., Golde D. W. Acute myelogenous leukemia: a human cell line responsive to colony-stimulating activity. Science. 1978 Jun 9;200(4346):1153–1154. doi: 10.1126/science.306682. [DOI] [PubMed] [Google Scholar]
- Lozzio C. B., Lozzio B. B. Human chronic myelogenous leukemia cell-line with positive Philadelphia chromosome. Blood. 1975 Mar;45(3):321–334. [PubMed] [Google Scholar]
- Martin-Zanca D., Hughes S. H., Barbacid M. A human oncogene formed by the fusion of truncated tropomyosin and protein tyrosine kinase sequences. 1986 Feb 27-Mar 5Nature. 319(6056):743–748. doi: 10.1038/319743a0. [DOI] [PubMed] [Google Scholar]
- Mattei M. G., Philip N., Passage E., Moisan J. P., Mandel J. L., Mattei J. F. DNA probe localization at 18p113 band by in situ hybridization and identification of a small supernumerary chromosome. Hum Genet. 1985;69(3):268–271. doi: 10.1007/BF00293038. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi J., Kagimoto M., Soeda E., Sakaki Y. The human c-Ha-ras2 is a processed pseudogene inactivated by numerous base substitutions. Nucleic Acids Res. 1984 Feb 24;12(4):1821–1828. doi: 10.1093/nar/12.4.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller R., Müller D. Co-transfection of normal NIH/3T3 DNA and retroval LTR sequences: a novel strategy for the detection of potential c-onc genes. EMBO J. 1984 May;3(5):1121–1127. doi: 10.1002/j.1460-2075.1984.tb01939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien S. J., Nash W. G., Goodwin J. L., Lowy D. R., Chang E. H. Dispersion of the ras family of transforming genes to four different chromosomes in man. Nature. 1983 Apr 28;302(5911):839–842. doi: 10.1038/302839a0. [DOI] [PubMed] [Google Scholar]
- Oberlé I., Camerino G., Kloepfer C., Moisan J. P., Grzeschik K. H., Hellkuhl B., Hors-Cayla M. C., Van Cong N., Weil D., Mandel J. L. Characterization of a set of X-linked sequences and of a panel of somatic cell hybrids useful for the regional mapping of the human X chromosome. Hum Genet. 1986 Jan;72(1):43–49. doi: 10.1007/BF00278816. [DOI] [PubMed] [Google Scholar]
- Oberlé I., Heilig R., Moisan J. P., Kloepfer C., Mattéi G. M., Mattéi J. F., Boué J., Froster-Iskenius U., Jacobs P. A., Lathrop G. M. Genetic analysis of the fragile-X mental retardation syndrome with two flanking polymorphic DNA markers. Proc Natl Acad Sci U S A. 1986 Feb;83(4):1016–1020. doi: 10.1073/pnas.83.4.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padhy L. C., Shih C., Cowing D., Finkelstein R., Weinberg R. A. Identification of a phosphoprotein specifically induced by the transforming DNA of rat neuroblastomas. Cell. 1982 Apr;28(4):865–871. doi: 10.1016/0092-8674(82)90065-4. [DOI] [PubMed] [Google Scholar]
- Padua R. A., Barrass N., Currie G. A. A novel transforming gene in a human malignant melanoma cell line. Nature. 1984 Oct 18;311(5987):671–673. doi: 10.1038/311671a0. [DOI] [PubMed] [Google Scholar]
- Perucho M., Goldfarb M., Shimizu K., Lama C., Fogh J., Wigler M. Human-tumor-derived cell lines contain common and different transforming genes. Cell. 1981 Dec;27(3 Pt 2):467–476. doi: 10.1016/0092-8674(81)90388-3. [DOI] [PubMed] [Google Scholar]
- Reddy E. P., Reynolds R. K., Santos E., Barbacid M. A point mutation is responsible for the acquisition of transforming properties by the T24 human bladder carcinoma oncogene. Nature. 1982 Nov 11;300(5888):149–152. doi: 10.1038/300149a0. [DOI] [PubMed] [Google Scholar]
- Shimizu K., Birnbaum D., Ruley M. A., Fasano O., Suard Y., Edlund L., Taparowsky E., Goldfarb M., Wigler M. Structure of the Ki-ras gene of the human lung carcinoma cell line Calu-1. Nature. 1983 Aug 11;304(5926):497–500. doi: 10.1038/304497a0. [DOI] [PubMed] [Google Scholar]
- Shimizu K., Goldfarb M., Suard Y., Perucho M., Li Y., Kamata T., Feramisco J., Stavnezer E., Fogh J., Wigler M. H. Three human transforming genes are related to the viral ras oncogenes. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2112–2116. doi: 10.1073/pnas.80.8.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland G. R., Ashforth P. L. X-linked mental retardation with macro-orchidism and the fragile site at Xq 27 or 28. Hum Genet. 1979 Apr 17;48(1):117–120. doi: 10.1007/BF00273283. [DOI] [PubMed] [Google Scholar]
- Tabin C. J., Bradley S. M., Bargmann C. I., Weinberg R. A., Papageorge A. G., Scolnick E. M., Dhar R., Lowy D. R., Chang E. H. Mechanism of activation of a human oncogene. Nature. 1982 Nov 11;300(5888):143–149. doi: 10.1038/300143a0. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Ritz J., Cooper G. M. Activation of a novel human transforming gene, ret, by DNA rearrangement. Cell. 1985 Sep;42(2):581–588. doi: 10.1016/0092-8674(85)90115-1. [DOI] [PubMed] [Google Scholar]
- Taparowsky E., Suard Y., Fasano O., Shimizu K., Goldfarb M., Wigler M. Activation of the T24 bladder carcinoma transforming gene is linked to a single amino acid change. Nature. 1982 Dec 23;300(5894):762–765. doi: 10.1038/300762a0. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young D., Waitches G., Birchmeier C., Fasano O., Wigler M. Isolation and characterization of a new cellular oncogene encoding a protein with multiple potential transmembrane domains. Cell. 1986 Jun 6;45(5):711–719. doi: 10.1016/0092-8674(86)90785-3. [DOI] [PubMed] [Google Scholar]
- Yunis A. A., Arimura G. K., Russin D. J. Human pancreatic carcinoma (MIA PaCa-2) in continuous culture: sensitivity to asparaginase. Int J Cancer. 1977 Jan;19(1):128–135. doi: 10.1002/ijc.2910190118. [DOI] [PubMed] [Google Scholar]