Abstract
Fistulas still represent one of the most important complications in patients with Crohn’s disease (CD). At least one third of CD patients suffer from fistulas during their disease course and amongst them longstanding remission of complex fistulas occurs only in about one third. So far, fistula pathogenesis is only partially understood. From a histopathological view, a fistula is a tube covered by flat epithelial cells. Current research suggests that the driving force for fistula development is epithelial-to-mesenchymal transition (EMT). Around the fistula, high levels of tumor necrosis factor (TNF), IL-13, and TGFβ can be detected and recent studies indicated an involvement of the intestinal microbiota. Fistula diagnosis requires clinical and surgical assessment, radiologic investigations, e.g., magnet resonance imaging and endoscopy. Routine medical treatment of fistulas includes antibiotics, immunosuppressives, and anti-TNF antibodies. There is no well-established role for calcineurin inhibitors in fistula treatment, corticosteroids appear to be even contra-productive. A promising novel approach might be the application of adipose tissue-derived or bone marrow-derived mesenchymal stem cells that have been studied recently. Due to insufficient efficacy of medical treatment and recurrence of fistulas, surgical interventions are frequently necessary. Further research is needed to better understand fistula pathogenesis aiming to develop novel treatment option for our patients.
Introduction
The first description of perianal fistulas as a typical complication of terminal ileitis was published in 1938.1 Population-based studies indicate that one third of Crohn’s disease (CD) patients will develop fistulas at least once during the disease course. Hereby, perianal fistulas are most common.2 At time of CD diagnosis, two third of patients present with inflammatory disease and only up to one-third of the patients reveal stricturing or penetrating complications in the gastrointestinal tract.3, 4 Nevertheless, during a longstanding and relapsing disease course, frequently a shift from the inflammatory disease phenotype towards a stricturing and/or penetrating phenotype is observed. Newer epidemiological data suggests that the risk of developing a stricturing or penetrating phenotype over time has somewhat decreased within the last years, specifically in those patients with elderly onset of CD.5 Population-based studies indicate that longer disease duration increases the cumulative incidence of perianal fistulas. The cumulative frequency in year 1 is 12%, after 5 years it is 15%, after 10 years 21, and after 20 years 26%.2 Further, the incidence of perianal fistulas depends on disease location. Perianal fistulas are most common in patients suffering from colonic CD with rectal involvement (92% of patients) but are rare in patients with isolated ileal disease (12% of patients).6 About 10% of CD patients present with perianal fistulas as first disease manifestation either together with luminal inflammation or not. In some patients, perianal fistulas may even occur years before the onset of luminal inflammation.2, 6 However, in the vast majority of patients (95%) perianal disease activity is paralleled by luminal disease activity and only in 5% of the patients perianal disease activity is detectable in patients without luminal inflammation.7 Besides fistulas, a significant number of CD patients develops intestinal fibrosis and strictures. Together, fistulas and stenosis affect ~70% of CD patients during life time and presence of stenosis frequently results in the onset of intestinal obstruction.8 Bowel resections reduce the risk for developing fistulas.9
Classification and predictive factors
Fistulas can be discriminated into simple and complex fistulas. A simple fistula is a low fistula with only a single external opening and is not associated with abscess formation, rectovaginal fistula, or an anorectal stricture. However, simple fistulas might be associated with active and severe rectal disease.10 A low fistula is characterized by a tract that penetrates the lower one-third of the external anal sphincter. After a follow-up period of 10 years about one third of patients suffer from persistent perianal fistulas according to a study from 1980. The remaining two third of patients either underwent surgery or experienced spontaneous healing.11 A more recent study revealed that recurrence of clinically healed fistulas is 44% within 18 months.12 The chance of fistula healing depends on fistula location. Superficial and low fistulas have a higher healing rate when treated by fistulotomy, especially in the absence of proctitis.13 In contrast, in patients with high fistulas or presence of proctitis a considerably lower healing rate and risk for postoperative incontinence has been reported.14, 15 In line with this, absence of proctitis independently predicts both, enhanced healing and reduced recurrence rates.12 In contrast, in patients with perianal CD and rectal involvement proctectomy is more frequent.16
In a single center study involving 232 patients with perianal CD longstanding remission for complex fistulas was seen in only 37% of patients after a 10-year follow-up compared with almost 67% for simple fistulas.17 A recent systematic review concluded that a combination of medical and surgical treatment approaches is superior to either single treatment alone. The importance of a multidisciplinary patient care is highlighted by superior rates of complete remission (52%) in the combination vs. single therapy (43%) group.18 In female patients of child-bearing age with established CD the cumulative probability of developing perianal fistulas following delivery is 8% after 1 year, 12% after 2 years, and 21% after 5 years. This probability is lower as in the general CD population. However, perianal disease is also associated with fewer pregnancies.19
An important and worrysome aspect of fistulizing CD is the occurrence of malignant transformation of perianal fistulas. Although this event is rare, it nevertheless is of crucial importance for the affected patients. In a systematic review from 2010, 61 cases of carcinomas arising in perineal fistulas in CD patients have been described, the majority in female patients (61%). Interestingly, females were significantly younger at time of cancer diagnosis, had shorter duration of CD, and suffered from fistula formation for a shorter duration prior to cancer transformation as compared to males. The most frequent histological subtype was adenocarcinoma (59%), followed by squamous cell carcinoma (31%). Fistulas were most frequently from rectal origin.20 In a Dutch multicenter study containing more than over 6,000 CD patients, only four cases of fistula-associated adenocarcinoma were detected. This observation confirms that carcinomas arising from fistula tracts are a rare event in CD patients.21
Pathogenesis
Fistulas are thought to arise as consequence of an acute inflammatory process paralleled by infection and suppuration.22 From a morphological point of view, a fistula represents a tract between two surfaces that is lined by epithelial cells and filled with cell debris, erythrocytes, and inflammatory cells.23 A chronic inflammatory infiltrate and fibrosis are common findings surrounding the fistula tract. In ~30% of intestinal or perianal CD fistulas the tracts are covered by flattened intestinal epithelial cells or squamous epithelial cells. In contrast, ~70% of CD fistula tracts are covered by a thin layer of myofibroblast-like cells, so-called “transitional cells”, forming a new basal membrane. CD fistulas show a central infiltration by CD45R0+ T-cells, an underlying band of CD68+ macrophages and a dense CD20+ B-cell infiltrate in the outer wall.23 In perianal fistulas accumulation of CD4+ CD161+ T-cells with a Th17, Th17/Th1, and Th1 phenotype has been described.24 Kirkegaard et al. found a strong expression of matrix remodeling enzymes, namely matrix metallo-proteinase (MMP)-3 and MMP-9 around CD fistulas while expression of tissue inhibitors of MMP (TIMP)-1, TIMP-2 and TIMP-3 was lower as compared to normal colon tissue. This indicates an altered balance between MMPs and TIMPs in and around CD fistulas resulting in aberrant extra-cellular matrix degradation.25
Current hypothesis suggests that epithelial-to-mesenchymal transition (EMT) is the driving force behind the development of fistulas in CD patients. Though EMT is involved in important mechanisms such as embryogenesis, organ development, wound healing, and tissue remodeling, EMT plays also a major role for pathological processes such as tissue fibrosis and cancer progression.26, 27 During EMT epithelial cells lose epithelial-specific characteristics, such as apico-basal polarity and epithelial-specific cell contacts, but reacquire a mesenchymal cell shape and exhibit enhanced motility and cell spreading.26 On a molecular level, EMT is characterized by down-regulation of epithelial cell specific proteins, such as E-cadherin or claudin-4, and upregulation of mesenchymal proteins, such as alpha smooth muscle actin and vimentin.26 EMT-associated transcription factors, such as SNAIL, SLUG, and ETS-1, as well as markers for cell proliferation and migration, such as β6-integrin are expressed in or around CD fistula tracts.28, 29
Presence of fistulas correlates with elevated serum levels of the proinflammatory cytokines tumor necrosis factor (TNF) and IL-6. In rectal mucosa expression of IL-1β and IL-6 is higher in patients with perianal CD than in those with small bowel CD and in healthy controls.30 Upregulation of TNF and IL-12 in resected intestinal tissue from patients with CD was correlated with fistula development. Analysis of cytokine expression patterns in the epithelial lining of CD fistula tracts revealed that TNF and TNF receptor I are highly expressed by epithelial cells as well as immune cells surrounding the fistula tract and also by epithelial cells of the adjacent crypts.29 Further, IL-13, IL-13α1, and transforming growth factor (TGF)-β, the strongest inducer of EMT, are detectable in fistula lining cells.28, 31
Data from the IBDchip European Project including 1528 Caucasian patients suggest that genetic factors differently modulate the risk for perianal disease and internal fistulas.32 On the one hand, variations within the genes encoding PRDM1, NOD2, IL-23 receptor, ATG16L1 are associated with onset of internal fistulas and internal penetrating disease. On the other hand, significant associations between the NOD2 variant rs72796353 as well as variations within the gene loci encoding TNFSF15, OCTN, IBD5 locus on 5q31, IRGM, DLG5 (in pediatric CD) and NCF4 and the onset of perianal fistulas have been found.33, 34, 35, 36, 37, 38
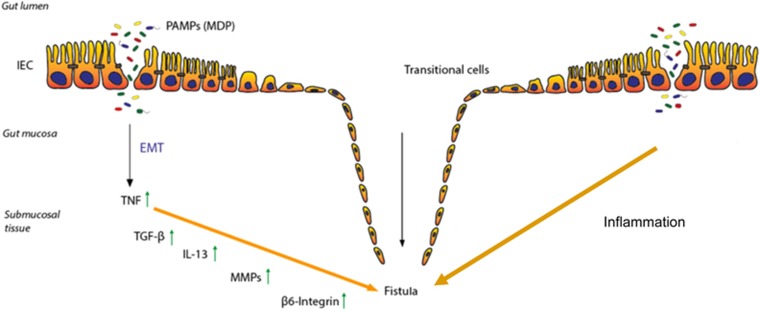
Recent data also suggest a role for the intestinal microbiota in fistula pathogenesis. By analyzing microbiota from the distal part of surgically removed fistula tracts, bowel- and/or skin-derived bacteria, but no mycobacteria were identified. Peptidoglycans were detected in ~90% of those patient samples suggesting that peptidoglycan might contribute to the ongoing inflammation and therefore development of perianal fistulas.39 This assumption would be supported by the fact that peptidoglycan is also able to induce the expression of EMT-associated molecules.40 In a study that assessed the microbiota within fistula tracts of CD patients a predominance of Gram-positive bacteria (staphylococci and streptococci) over Gram-negative bacteria was found41 (Figure 1).
Figure 1.
Pathogenesis of Crohn’s disease-associated fistulae. Due to an epithelial barrier defect several pathogen-associated molecular patterns (PAMPs), like e.g., muramyl-dipeptide (MDP), as well as bacteria are able to enter the gut mucosa. The resulting inflammatory response induces the event of epithelial-to-mesenchymal transition (EMT). First, an increased expression of tumor necrosis factor (TNF) is initiated, resulting in an upregulation of TGF-β production. This triggers a signaling cascade of molecules associated with matrix remodeling, in particular enhanced activity of matrix metalloproteinases (MMPs) and cell invasiveness, such as β6-Integrin. These events favor the transformation of the intestinal epithelial cells (IECs) towards invasive myofibroblast like cells, what finally results in fistula formation.
Diagnosis
The accurate diagnosis is the prerequisite for and the first step in the optimal management of patients with perianal fistulizing CD. To obtain the correct diagnosis of fistulizing disease a number of different techniques have been described, such as examination under anesthesia (EUA) and imaging by endoscopic ultasonography (EUS) or magnet resonance imaging (MRI).
The current standard of care guidelines on the diagnosis and treatment of perianal fistulizing CD have been published by the European Crohn’s and Colitis Organisation (ECCO) in 2016.42 Contrast-enhanced pelvic MRI should be the initial procedure for assessment of perianal fistulizing CD which has an accuracy of 76–100% for fistulas and might also provide additional information, such as presence of stenosis.42, 43 If a rectal stenosis has been excluded, a good alternative to MRI might be an endoscopic anorectal ultrasound (EUS) which has an accuracy of 56–100%. EUS should ideally performed using hydrogen peroxide or simple carbonated water (which is less painful) enhancement.42, 44 Endoscopy plays also a key role in the initial diagnosis of perianal disease. Since the extent of inflammation in the affected parts of the intestinal tract has prognostic and therapeutic relevance and also aids in deciding whether medical therapy should be combined with surgical therapy, rectosigmoidoscopy should routinely be performed during initial assessment of perianal fistulizing CD.42, 45
According to current literature, EUA is the most sensitive diagnostic procedure with an accuracy of 90%. On the one hand, it can enhance the sensitivity and specificity of MRI and EUS. On the other hand, EUA provides also the advantage for performing concomitant surgical procedures. When an abscess is suspected, EUA should immediately be performed and abscess drainage conducted even before MRI examination. According to the ECCO guidelines from 2016, once a perianal fistula is detected, EUA is considered to be the gold standard when performed by an experienced surgeon.2, 42, 43, 46, 47 Fistulography is no longer recommended by the ECCO,42 due to clear-cut superiority of MRI in depicting fistula tract anatomy in higher resolution including the tract-surrounding space, for example, abscess formation and extension thereof or muscular penetration and destruction.
Treatment
In terms of limited ongoing basic research initiatives, it becomes evident that available treatment options and high-class evidence clinical trials are in a striking discrepancy to the relatively high burden of fistula in CD patients. Fortunately, this deficit has received more attention within the last few years. However, it remains to be stressed, that dedicated clinical trials with healing of fistula or reduction in the amount of secreting fistula tracts are still limited. Therefore, most of the evidence on the following treatment options is derived from subgroup analyses or ill-defined secondary outcome measures. As a consequence, any interpretation of the effectiveness of agents (above all comparative statements) have to be made with great caution. The latter is underlined by a recent meta-analysis of placebo response rates in fistulizing CD, revealing that almost one in six patients receiving placebo reported fistula closure.48 Indeed, there are hardly any topics in the field of IBD, where the almost omnipresent statement—“further studies are needed to better…”—is that much justified as in fistulizing CD. In the following a brief overview of available treatment options will be provided. Any interventions with course of fistulizing CD investigated as primary objective/endpoint are highlighted with a plus symbol (+).
Antibiotics
The role of antibiotics in fistulizing CD rather founds on decades of clinical experience than robust scientific evidence49, 50 (+51, 52). As antibiotics do not induce fistula healing in the vast majority of cases, their primary role is to address penetrating complications, improve symptoms including drainage on the short- to midterm and act as an adjunctive treatment in surgical management, such as for instance seton placement. Antibiotics should not be considered and used as a sole therapeutic concept in itself in fistulizing disease,45 but as an adjunct in combination with surgical management or medical treatment, especially anti-TNF53, 54) and to a lesser extent with azathioprine.55
Aminosalicylates
To date, there is no evidence for the efficacy of 5-ASA agents for the treatment of fistulizing CD, neither for orally nor rectally applied formulations. Therefore, these agents cannot be recommended for this indication. However, there might be a role especially for rectal applied 5-ASA formulations to address clinical symptoms of active rectal inflammation, such as fecal urgency or lower abdominal pain and potentially also to act synergistically with other topical or systemic agents to reduce inflammatory activity in the rectum. Although such a potential synergistic effect has never been systematically studied, addressing inflammation in the rectum as profound as possible by means of medical treatment remains an important prerequisite for subsequent successful surgical treatment.
Corticosteroids
There is no data to support the use of corticosteroids in fistulizing CD. In fact, older therapeutic observations have reported on a worsening of clinical symptoms upon treatment with steroids,56 which is why both topical and systemic corticosteroids, do not have any place in this indication.
Thiopurines and methotrexate
The evidence for thiopurines is considerably limited, including a subgroup analysis from a randomized trial of some 80 patients with CD from the 1980s with an impressive complete fistula healing rate of 31%57 as well as metaanalyses with conflicting results on presence58 or absence59 of overall effectiveness. In a small study, a “sonic-like” increase in fistula closure rates in combined thiopurine/infliximab treatment was suggested (+60). With the exception of a small case series with some thirty CD patients suggesting a benefit with partial or complete fistula closure in more than 50% of patients,61 there are no studies on the potential role of Methotrexate in this indication.
Calcineurin inhibitors
There are only observational studies with cyclosporine in fistulizing CD. The bottom line of these investigations may be subsumed as a robust effectiveness in the short-term with however a high rate of relapse after withdrawal of cyclosporine.62, 63, 64 In contrast, a trial with tacrolimus dedicated to investigate fistula healing revealed a significant effect in the amount of patients achieving of at least 50% of their fistulas to be closed (+65). In contrast, although a beneficial effect regarding inflammatory activity in the rectum was observed by the topical administration of tacrolimus, there was no effect on fistula closure.66
Anti-TNF
By far, the data with anti-TNF in fistulizing CD are the most robust in terms of both, efficacy and scientific quality. Infliximab (IFX) revealed to achieve impressively high complete (55 vs. 13% placebo) and partial (i.e., reduction of 50% or more of draining fistula; 68 vs. 26% placebo) fistula closure rates (+67). Patients with maintenance IFX treatment were found to show significantly higher rates of durable response68). It remains unclear however, why the efficacy rates in this trial in the higher induction dose arm (10 m/kg) revealed to be somewhat lower. In any case, a recent multicenter trial (currently only published in abstract form, DDW 2016, +69) with 117 patients revealed an incremental gain in efficacy rates with higher doses and IFX trough levels with fistula healing rates (defined as absence of draining fistula) in up to 80% in the patients with very high trough levels (IFX≥20, 2 μg/ml). In a small prospective study including 32 patients the combination of surgical management (EUA and seton placement if indicated) revealed that antecedent surgical intervention increases initial and long-term response rates (+70). Achieving initial response (radiological healing as assessed by MRI) was found to be a strong predictor of long-term response and concomitant treatment with thiopurines appeared to be beneficial (+71, 72). In the long-term, about two out of three patients appeared to achieve persistent fistula closure, whereas one out of three patients suffered from recurrence in a retrospective analysis of 156 CD patients with perianal disease receiving IFX.72 Regarding adalimumab, there are no trials investigating fistula closure as primary endpoint. In the major trials evaluating efficacy of adalimumab for induction of remission in previously anti-TNF naïve (CLASSIC-I73 and exposed patients (GAIN74), no difference in fistula closure rates to placebo were observed. In contrast, a higher rate of complete fistula healing (33 vs. 13% in the placebo group) was observed in a subgroup analysis of the maintenance trial (CHARM75), including an open label extension phase, revealing high rates of sustained fistula healing in responders.76 The major Certolizumab pegol trials in CD (PRECiSE 177 and 278)—both not designed to investigate this issue, however—did not reveal superiority of the verum compared with placebo. A subgroup analysis of patients with a response on active fistulizing CD indicated a benefit in those patients with ongoing drug treatment.79
Anti-integrin
Up to present, there is no specific clinical trial investigating a potential effect of vedolizumab on fistula closure in CD. However, a study with fistula healing at week 30 as primary endpoint appears to be currently recruiting patients (NCT02630966). In the GEMINI 2 study,80 fistula closure was a pre-specified endpoint. The maintenance population with continuous vedolizumab exposure achieved fistula closure at week 52 in a higher percentage (30.8%) as compared to those patients being re-randomized to placebo (11.1%).81 However, patient numbers were extremely small (higher fraction of responders 7 out of 18, 41.2% in the 8 week vs. the 4 week treatment interval group, 5 out of 22 patients, 22.7%), and therefore, results of the above-mentioned study have to be awaited until any verdict on the potential effectiveness of this agent in fistulizing CD can be reached.
Emerging local cell-based treatment options
First small studies about 7 years ago using topically administered mesenchymal stem cells in fistulizing CD revealed promising results (+82, 83). A recent study in the Netherlands with 21 refractory fistulizing CD patients investigated the effect of locally administered bone marrow-derived mesenchymal stromal cells in three different dosings (group 1–3) in a placebo-controlled, double-blind trial, with fistula healing (no discharge, no fluid collection >2 cm—assessed by physical examination and MRI, respectively, +84). Although the results may be considered highly promising, it remains difficult to interpret, whether a virtual absence of efficacy in the highest dosing group compared to the placebo group rather is indicative of a statistical problem due to small numbers (i.e., coincidence) or should rather raise concern on the potential overall efficacy of this intervention. A large randomized trial in Europe compared the effect of a one-time intralesional injection of 120 million allogenic, expanded, adipose-derived stem cells (applied by an unblinded surgeon) with placebo (1:1) in 212 patients. A blinded gastroenterologists assessed closure of all treated external fistula openings with baseline drainage and absence of collections >2cm, the primary endpoint, at 24 weeks (+85). This endpoint was achieved in a significantly higher proportion of patients (50%, ITT) in the verum group as compared to placebo (34%). No relevant adverse events were observed, aside from a higher rate of local events indicative of treatment failure (e.g., anal abscess) in the placebo group.
Varia
A variety of other treatment options have been suggested in fistulizing CD, including oral adsorptive carbon (AST -120, with highly promising initial results in a trial from Japan (+86), which however could not be confirmed in a subsequent very large trial (+87)), hyperbaric oxygen (currently only human pilot data in abstract form available, ECCO 2017, P576) or thalidomide.88 None of these options can, however, be recommended according to at present highly limited or conflicting data, availability of the drug or side effects.
Surgical treatment options
A detailed overview on the surgical treatment options of fistulizing CD is beyond the scope of this review and we would like to refer the reader to excellent available compilations on surgical management of this condition.89, 90, 91, 92 Amongst the interventions, that have been investigated in detail are surgical drainages of perianal abscesses in combination with IFX to prevent worsening septic complications,93 seton placement,94, 95 fistulotomy for superficial, and low intersphincteric but not higher transsphincteric fistula,14 fistula plug (success rates considerably lower in follow-up as opposed to initial trials96, 97), potentially also as a second consecutive treatment option after seton removal98), ligation of the intersphincteric fistula tract99 and fibrin glue.100, 101 It should be underlined that an important prerequisite for the success of any surgical intervention is a best possible control of inflammatory activity in the rectum by means of medical treatment options.16
Conclusion
Fistulas remain one of the major unmet needs in the treatment of CD patients. To date, only a limited number of effective therapies has been established. Thus, the number of patients with severe and recurrent problems arising from CD fistulas is considerably high while surgery, though often required, does not always provide a definitive cure. As also our understanding of fistula pathogenesis is limited to date, further research is warranted to on the one hand obtain a better understanding of the complex mechanisms underlying fistula development and, on the other hand, for identifying and establishing novel and hopefully more effective treatment options for fistula therapy.
Footnotes
Guarantor of the article: Michael Scharl, MD.
Specific author contributions: All authors participated sufficiently, intellectually, or practically in the work to take public responsibility for the content of the article, including the conception, design, data interpretation, and writing of the manuscript. The final version of the manuscript was approved by all authors.
Financial support: No study sponsors had any involvement in study design, data collection, interpretation, or writing of the manuscript.
Potential competing interests: Gerhard Rogler has consulted to Abbot, Abbvie, Augurix, Boehringer, Calypso, FALK, Ferring, Fisher, Genentech, Essex/MSD, Novartis, Pfizer, Phadia, Roche, UCB, Takeda, Tillots, Vifor, Vital Solutions, and Zeller; received speaker’s honoraria from Astra Zeneca, Abbott, Abbvie, FALK, MSD, Phadia, Tillots, UCB, and Vifor; received educational grants and research grants from Abbot, Abbvie, Ardeypharm, Augurix, Calypso, Essex/MSD, FALK, Flamentera, Novartis, Roche, Takeda, Tillots, UCB, and Zeller. Michael Scharl has received speaker’s honoraria from FALK.
References
- Penner A, Crohn BB. Perianal fistulae as a complication of regional ileitis. Ann Surg 1938; 108: 867–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz DA et al. The natural history of fistulizing Crohn's disease in Olmsted County, Minnesota. Gastroenterology 2002; 122: 875–880. [DOI] [PubMed] [Google Scholar]
- Peyrin-Biroulet L et al. The natural history of adult Crohn's disease in population-based cohorts. Am J Gastroenterol 2010; 105: 289–297. [DOI] [PubMed] [Google Scholar]
- Cosnes J et al. Long-term evolution of disease behavior of Crohn's disease. Inflamm Bowel Dis 2002; 8: 244–250. [DOI] [PubMed] [Google Scholar]
- Gower-Rousseau C et al. Epidemiology of inflammatory bowel diseases: new insights from a French population-based registry (EPIMAD). Dig Liver Dis 2013; 45: 89–94. [DOI] [PubMed] [Google Scholar]
- Hellers G et al. Occurrence and outcome after primary treatment of anal fistulae in Crohn's disease. Gut 1980; 21: 525–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gecse KB et al. Results of the Fifth Scientific Workshop of the ECCO II. Clinical Aspects of Perianal Fistulising Crohn's Disease-the Unmet Needs. J Crohns Colitis 2016; 10: 758–765. [DOI] [PubMed] [Google Scholar]
- Bernstein CN et al. Hospitalisations and surgery in Crohn's disease. Gut 2012; 61: 622–629. [DOI] [PubMed] [Google Scholar]
- Kruis W et al. Risikofaktoren fur die Entstehung von Fisteln bei Morbus Crohn. Z Gastroenterol 1989; 27: 313–316. [PubMed] [Google Scholar]
- Sandborn WJ et al. AGA technical review on perianal Crohn's disease. Gastroenterology 2003; 125: 1508–1530. [DOI] [PubMed] [Google Scholar]
- Buchmann P et al. Natural history of perianal Crohn's disease. Ten year follow-up. A plea for conservatism. Am J Surg 1980; 140: 642–644. [DOI] [PubMed] [Google Scholar]
- Makowiec F, Jehle EC, Starlinger M. Clinical course of perianal fistulas in Crohn's disease. Gut 1995; 37: 696–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks AG, Gordon PH, Hardcastle JD. A classification of fistula-in-ano. Br J Surg 1976; 63: 1–12. [DOI] [PubMed] [Google Scholar]
- Williams JG et al. Fistula-in-ano in Crohn's disease. Results of aggressive surgical treatment. Dis Colon Rectum 1991; 34: 378–384. [DOI] [PubMed] [Google Scholar]
- Nordgren S, Fasth S, Hulten L. Anal fistulas in Crohn's disease. Incidence and outcome of surgical treatment. Int J Colorectal Dis 1992; 7: 214–218. [DOI] [PubMed] [Google Scholar]
- Kamm MA, Ng SC. Perianal fistulizing Crohn's disease. A call to action. Clin Gastroenterol Hepatol 2008; 6: 7–10. [DOI] [PubMed] [Google Scholar]
- Molendijk I et al. Disappointing durable remission rates in complex Crohn's disease fistula. Inflamm Bowel Dis 2014; 20: 2022–2028. [DOI] [PubMed] [Google Scholar]
- Yassin NA et al. Systematic review. The combined surgical and medical treatment of fistulising perianal Crohn's disease. Aliment Pharmacol Ther 2014; 40: 741–749. [DOI] [PubMed] [Google Scholar]
- Grouin A et al. Perianal Crohn's disease results in fewer pregnancies but is not exacerbated by vaginal delivery. Dig Liver Dis 2015; 47: 1021–1026. [DOI] [PubMed] [Google Scholar]
- Thomas M et al. Malignant transformation in perianal fistulas of Crohn's disease. A systematic review of literature. J Gastrointest Surg 2010; 14: 66–73. [DOI] [PubMed] [Google Scholar]
- Baars JE et al. Malignant transformation of perianal and enterocutaneous fistulas is rare: results of 17 years of follow-up from The Netherlands. Scand J Gastroenterol 2011; 46: 319–325. [DOI] [PubMed] [Google Scholar]
- Odze RD, Goldblum JR. Odze and Goldblum Surgical Pathology Of The GI Tract, Liver, Biliary Tract, And Pancreas. Elsevier: Saunders, Philadelphia, PA, USA, 2015. [Google Scholar]
- Bataille F et al. Morphological characterisation of Crohn's disease fistulae. Gut 2004; 53: 1314–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi L et al. CD4+CD161+ T lymphocytes infiltrate Crohn's disease-associated perianal fistulas and are reduced by anti-TNF-alpha local therapy. Int Arch Allergy Immunol 2013; 161: 81–86. [DOI] [PubMed] [Google Scholar]
- Kirkegaard T et al. Expression and localisation of matrix metalloproteinases and their natural inhibitors in fistulae of patients with Crohn's disease. Gut 2004; 53: 701–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest 2009; 119: 1420–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest 2003; 112: 1776–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bataille F et al. Evidence for a role of epithelial mesenchymal transition during pathogenesis of fistulae in Crohn's disease. Inflamm Bowel Dis 2008; 14: 1514–1527. [DOI] [PubMed] [Google Scholar]
- Scharl M et al. Potential role for SNAIL family transcription factors in the etiology of Crohn's disease-associated fistulae. Inflamm Bowel Dis 2011; 17: 1907–1916. [DOI] [PubMed] [Google Scholar]
- Ruffolo C et al. Cytokine network in chronic perianal Crohn's disease and indeterminate colitis after colectomy. J Gastrointest Surg 2007; 11: 16–21. [DOI] [PubMed] [Google Scholar]
- Scharl M et al. Interleukin-13 and transforming growth factor β synergise in the pathogenesis of human intestinal fistulae. Gut 2013; 62: 63–72. [DOI] [PubMed] [Google Scholar]
- Cleynen I et al. Genetic factors conferring an increased susceptibility to develop Crohn's disease also influence disease phenotype: results from the IBDchip European Project. Gut 2012; 62: 556–565. [DOI] [PubMed] [Google Scholar]
- Schnitzler F et al. The NOD2 p.Leu1007fsX1008 mutation (rs2066847) is a stronger predictor of the clinical course of Crohn's disease than the FOXO3A intron variant rs12212067. PLoS ONE 2014; 9: e108503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D-H et al. TNFSF15 is an independent predictor for the development of Crohn's disease-related complications in Koreans. J Crohns Colitis 2014; 8: 1315–1326. [DOI] [PubMed] [Google Scholar]
- Vermeire S et al. Association of organic cation transporter risk haplotype with perianal penetrating Crohn's disease but not with susceptibility to IBD. Gastroenterology 2005; 129: 1845–1853. [DOI] [PubMed] [Google Scholar]
- Latiano A et al. Polymorphism of the IRGM gene might predispose to fistulizing behavior in Crohn's disease. Am J Gastroenterol 2009; 104: 110–116. [DOI] [PubMed] [Google Scholar]
- Eglinton TW et al. Clinical and genetic risk factors for perianal Crohn's disease in a population-based cohort. Am J Gastroenterol 2012; 107: 589–596. [DOI] [PubMed] [Google Scholar]
- de Ridder L et al. Genetic susceptibility has a more important role in pediatric-onset Crohn's disease than in adult-onset Crohn's disease. Inflamm Bowel Dis 2007; 13: 1083–1092. [DOI] [PubMed] [Google Scholar]
- van Onkelen RS et al. Assessment of microbiota and peptidoglycan in perianal fistulas. Diagn Microbiol Infect Dis 2013; 75: 50–54. [DOI] [PubMed] [Google Scholar]
- Frei SM et al. A role for tumor necrosis factor and bacterial antigens in the pathogenesis of Crohn's disease-associated fistulae. Inflamm Bowel Dis 2013; 19: 2878–2887. [DOI] [PubMed] [Google Scholar]
- Karban A et al. Risk factors for perianal Crohn's disease: the role of genotype, phenotype, and ethnicity. Am J Gastroenterol 2007; 102: 1702–1708. [DOI] [PubMed] [Google Scholar]
- Gionchetti P et al. 3rd European Evidence-based Consensus on the Diagnosis and Management of Crohn’s Disease 2016: Part 2: Surgical Management and Special Situations. J Crohn's Colitis 2017; 11: 135–149. [DOI] [PubMed] [Google Scholar]
- Orsoni P et al. Prospective comparison of endosonography, magnetic resonance imaging and surgical findings in anorectal fistula and abscess complicating Crohn's disease. Br J Surg 1999; 86: 360–364. [DOI] [PubMed] [Google Scholar]
- Sloots CE et al. Assessment and classification of never operated and recurrent cryptoglandular fistulas-in-ano using hydrogen peroxide enhanced transanal ultrasound. Colorectal Dis 2001; 3: 422–426. [DOI] [PubMed] [Google Scholar]
- Gecse KB et al. A global consensus on the classification, diagnosis and multidisciplinary treatment of perianal fistulising Crohn's disease. Gut 2014; 63: 1381–1392. [DOI] [PubMed] [Google Scholar]
- Haggett PJ et al. Pelvic and perineal complications of Crohn's disease. Assessment using magnetic resonance imaging. Gut 1995; 36: 407–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bodegraven AA et al. Endosonographic evidence of persistence of Crohn's disease-associated fistulas after infliximab treatment, irrespective of clinical response. Dis Colon Rectum 2002; 45: 39–45 discussion 45-6. [DOI] [PubMed] [Google Scholar]
- Ford AC et al. Placebo response rate in clinical trials of fistulizing Crohn's disease: systematic review and meta-analysis. Clin Gastroenterol Hepatol 2014; 12: 1981–1990. [DOI] [PubMed] [Google Scholar]
- Bernstein LH et al. Healing of perineal Crohn's disease with metronidazole. Gastroenterology 1980; 79: 357–365. [PubMed] [Google Scholar]
- Khan KJ et al. Antibiotic therapy in inflammatory bowel disease: a systematic review and meta-analysis. Am J Gastroenterol 2011; 106: 661–673. [DOI] [PubMed] [Google Scholar]
- Maeda Y et al. Randomized clinical trial of metronidazole ointment versus placebo in perianal Crohn's disease. Br J Surg 2010; 97: 1340–1347. [DOI] [PubMed] [Google Scholar]
- Thia KT et al. Ciprofloxacin or metronidazole for the treatment of perianal fistulas in patients with Crohn's disease. A randomized, double-blind, placebo-controlled pilot study. Inflamm Bowel Dis 2009; 15: 17–24. [DOI] [PubMed] [Google Scholar]
- Dewint P et al. Adalimumab combined with ciprofloxacin is superior to adalimumab monotherapy in perianal fistula closure in Crohn's disease: a randomised, double-blind, placebo controlled trial (ADAFI). Gut 2013; 63: 292–299. [DOI] [PubMed] [Google Scholar]
- West RL et al. Clinical and endosonographic effect of ciprofloxacin on the treatment of perianal fistulae in Crohn's disease with infliximab. A double-blind placebo-controlled study. Aliment Pharmacol Ther 2004; 20: 1329–1336. [DOI] [PubMed] [Google Scholar]
- Dejaco C et al. Antibiotics and azathioprine for the treatment of perianal fistulas in Crohn's disease. Aliment Pharmacol Ther 2003; 18: 1113–1120. [DOI] [PubMed] [Google Scholar]
- Jones JH, Lennard-Jones JE. Corticosteroids and corticotrophin in the treatment of Crohn's disease. Gut 1966; 7: 181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Present DH et al. Treatment of Crohn's disease with 6-mercaptopurine. A long-term, randomized, double-blind study. N Engl J Med 1980; 302: 981–987. [DOI] [PubMed] [Google Scholar]
- Pearson DC et al. Azathioprine and 6-mercaptopurine in Crohn disease. A meta-analysis. Ann Intern Med 1995; 123: 132–142. [DOI] [PubMed] [Google Scholar]
- Prefontaine E, Macdonald JK, Sutherland LR. Azathioprine or 6-mercaptopurine for induction of remission in Crohn's disease. Cochrane Database Syst Rev 2010: CD000545. [DOI] [PubMed]
- Ochsenkuhn T, Goke B, Sackmann M. Combining infliximab with 6-mercaptopurine/azathioprine for fistula therapy in Crohn's disease. Am J Gastroenterol 2002; 97: 2022–2025. [DOI] [PubMed] [Google Scholar]
- Mahadevan U, Marion JF, Present DH. Fistula response to methotrexate in Crohn's disease. A case series. Aliment Pharmacol Ther 2003; 18: 1003–1008. [DOI] [PubMed] [Google Scholar]
- Egan LJ, Sandborn WJ, Tremaine WJ. Clinical outcome following treatment of refractory inflammatory and fistulizing Crohn's disease with intravenous cyclosporine. Am J Gastroenterol 1998; 93: 442–448. [DOI] [PubMed] [Google Scholar]
- Hanauer SB, Smith MB. Rapid closure of Crohn's disease fistulas with continuous intravenous cyclosporin A. Am J Gastroenterol 1993; 88: 646–649. [PubMed] [Google Scholar]
- Present DH, Lichtiger S. Efficacy of cyclosporine in treatment of fistula of Crohn's disease. Dig Dis Sci 1994; 39: 374–380. [DOI] [PubMed] [Google Scholar]
- Sandborn WJ et al. Tacrolimus for the treatment of fistulas in patients with Crohn's disease. A randomized, placebo-controlled trial. Gastroenterology 2003; 125: 380–388. [DOI] [PubMed] [Google Scholar]
- Hart AL, Plamondon S, Kamm MA. Topical tacrolimus in the treatment of perianal Crohn's disease. Exploratory randomized controlled trial. Inflamm Bowel Dis 2007; 13: 245–253. [DOI] [PubMed] [Google Scholar]
- Present DH et al. Infliximab for the treatment of fistulas in patients with Crohn's disease. N Engl J Med 1999; 340: 1398–1405. [DOI] [PubMed] [Google Scholar]
- Sands BE et al. Infliximab maintenance therapy for fistulizing Crohn's disease. N Engl J Med 2004; 350: 876–885. [DOI] [PubMed] [Google Scholar]
- Yarur A et al. 514 Higher infliximab trough levels are associated with a higher rate of perianal fistula healing in patients with Crohn's disease. Aliment Pharmacol Ther 2017; 45: 933–940. [DOI] [PubMed] [Google Scholar]
- Regueiro M, Mardini H. Treatment of perianal fistulizing Crohn's disease with infliximab alone or as an adjunct to exam under anesthesia with seton placement. Inflamm Bowel Dis 2003; 9: 98–103. [DOI] [PubMed] [Google Scholar]
- Tozer P et al. Long-term MRI-guided combined anti-TNF-α and thiopurine therapy for Crohn's perianal fistulas. Inflamm Bowel Dis 2012; 18: 1825–1834. [DOI] [PubMed] [Google Scholar]
- Bouguen G et al. Long-term outcome of perianal fistulizing Crohn's disease treated with infliximab. Clin Gastroenterol Hepatol 2013; 11: 975. [DOI] [PubMed] [Google Scholar]
- Hanauer SB et al. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn's disease: the CLASSIC-I trial. Gastroenterology 2006; 130: 323. [DOI] [PubMed] [Google Scholar]
- Sandborn WJ et al. An open-label study of the human anti-TNF monoclonal antibody adalimumab in subjects with prior loss of response or intolerance to infliximab for Crohn's disease. Am J Gastroenterol 2004; 99: 1984–1989. [DOI] [PubMed] [Google Scholar]
- Colombel J-F et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn's disease: the CHARM trial. Gastroenterology 2007; 132: 52–65. [DOI] [PubMed] [Google Scholar]
- Colombel J-F et al. Adalimumab for the treatment of fistulas in patients with Crohn's disease. Gut 2009; 58: 940–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandborn WJ et al. Certolizumab pegol for the treatment of Crohn's disease. N Engl J Med 2007; 357: 228–238. [DOI] [PubMed] [Google Scholar]
- Schreiber S et al. Maintenance therapy with certolizumab pegol for Crohn's disease. N Engl J Med 2007; 357: 239–250. [DOI] [PubMed] [Google Scholar]
- Schreiber S et al. Randomised clinical trial: certolizumab pegol for fistulas in Crohn's disease—subgroup results from a placebo-controlled study. Aliment Pharmacol Ther 2011; 33: 185–193. [DOI] [PubMed] [Google Scholar]
- Sandborn WJ et al. Vedolizumab as induction and maintenance therapy for Crohn's disease. N Engl J Med 2013; 369: 711–721. [DOI] [PubMed] [Google Scholar]
- Feagan BG et al. Sa1261 Vedolizumab for the treatment of fistulizing Crohn's disease. An exploratory analysis of data from GEMINI 2. Gastroenterology 2015; 148: S–274. [Google Scholar]
- Garcia-Olmo D et al. Expanded adipose-derived stem cells for the treatment of complex perianal fistula. A phase II clinical trial. Dis Colon Rectum 2009; 52: 79–86. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R et al. Autologous bone marrow-derived mesenchymal stromal cells in the treatment of fistulising Crohn's disease. Gut 2011; 60: 788–798. [DOI] [PubMed] [Google Scholar]
- Molendijk I et al. Allogeneic bone marrow-derived mesenchymal stromal cells promote healing of refractory perianal fistulas in patients with Crohn's disease. Gastroenterology 2015; 149: 918–27.e6. [DOI] [PubMed] [Google Scholar]
- Panes J et al. Expanded allogeneic adipose-derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn's disease. A phase 3 randomised, double-blind controlled trial. Lancet 2016; 388: 1281–1290. [DOI] [PubMed] [Google Scholar]
- Fukuda Y et al. Oral spherical adsorptive carbon for the treatment of intractable anal fistulas in Crohn's disease. A multicenter, randomized, double-blind, placebo-controlled trial. Am J Gastroenterol 2008; 103: 1721–1729. [DOI] [PubMed] [Google Scholar]
- Reinisch W et al. AST-120 (spherical carbon adsorbent) in the treatment of perianal fistulae in mild-to-moderate Crohn's disease. FHAST-1, a phase 3, multicenter, placebo-controlled study. Inflamm Bowel Dis 2014; 20: 872–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramuzzo M et al. Thalidomide for inflammatory bowel disease: systematic review. Medicine 2016; 95: e4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis RT, Bleier JIS. Surgical treatment of anorectal crohn disease. Clin Colon Rectal Surg 2013; 26: 90–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellino G, Selvaggi F. Surgical treatment of perianal fistulizing Crohn's disease: from lay-open to cell-based therapy—an overview. ScientificWorldJournal 2014; 2014: 146281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atienza P, Ksiaa M. Particular aspects of proctology for anoperineal lesions in Crohn's disease. J Visc Surg 2015; 152: S45–S53. [DOI] [PubMed] [Google Scholar]
- Harb WJ. Crohn's disease of the colon, rectum, and anus. Surg Clin North Am 2015; 95: 1195–1210 vi. [DOI] [PubMed] [Google Scholar]
- Hyder SA et al. Fistulating anal Crohn's disease. Results of combined surgical and infliximab treatment. Dis Colon Rectum 2006; 49: 1837–1841. [DOI] [PubMed] [Google Scholar]
- Buchanan GN et al. Long-term outcome following loose-seton technique for external sphincter preservation in complex anal fistula. Br J Surg 2004; 91: 476–480. [DOI] [PubMed] [Google Scholar]
- Hamalainen KP, Sainio AP. Cutting seton for anal fistulas. High risk of minor control defects. Dis Colon Rectum 1997; 40: 1443–1446 discussion 1447. [DOI] [PubMed] [Google Scholar]
- Champagne BJ et al. Efficacy of anal fistula plug in closure of cryptoglandular fistulas. Long-term follow-up. Dis Colon Rectum 2006; 49: 1817–1821. [DOI] [PubMed] [Google Scholar]
- Owen G et al. Plugs unplugged. Anal fistula plug. The Concord experience. ANZ J Surg 2010; 80: 341–343. [DOI] [PubMed] [Google Scholar]
- Senéjoux A et al. Fistula plug in fistulising ano-perineal Crohn’s disease. A randomised controlled trial. J Crohns Colitis 2016; 10: 141–148. [DOI] [PubMed] [Google Scholar]
- Rojanasakul A et al. Total anal sphincter saving technique for fistula-in-ano; the ligation of intersphincteric fistula tract. J Med Assoc Thai 2007; 90: 581–586. [PubMed] [Google Scholar]
- Grimaud J-C et al. Fibrin glue is effective healing perianal fistulas in patients with Crohn's disease. Gastroenterology 2010; 138: 2275. [DOI] [PubMed] [Google Scholar]
- Lindsey I et al. A randomized, controlled trial of fibrin glue vs. conventional treatment for anal fistula. Dis Colon Rectum 2002; 45: 1608–1615. [DOI] [PubMed] [Google Scholar]