Abstract
Objectives:
Patient-reported outcomes such as health-related quality of life (HRQOL) are impaired in cirrhosis due to under-treated mood and sleep disorders, which can adversely impact their caregivers. Mindfulness-based stress reduction (MBSR) can improve patient-reported outcomes (PRO) in non-cirrhotic patients but their impact in cirrhosis is unclear. To evaluate the effect of MBSR and supportive group therapy on mood, sleep and HRQOL in cirrhotic patients and their caregivers.
Methods:
Cirrhotic outpatients with mild depression (Beck Depression Inventory (BDI)>14) on screening with an adult caregiver were enrolled. At baseline, BDI, sleep (Pittsburgh sleep quality index PSQI, Epworth Sleepiness Scale, ESS), anxiety (Beck Anxiety inventory) and HRQOL (Sickness Impact Profile, SIP) for both patients/caregivers and caregiver burden (Zarit Burden Interview Short-form, ZBI-SF and perceived caregiver burden, PCB) and patient covert HE(CHE) status were measured. Patients who had BDI>14 at baseline, along with their caregivers then underwent a structured MBSR program with four weekly hour-long group sessions interspersed with home practice using CDs. After the last group, all questionnaires were repeated.
Results:
20 patient/caregiver dyads were included. All patients were men (60±8 years MELD 12.9±5.7, 14 prior hepatic encephalopathy (HE)) while most caregivers (n=15) were women (55±12 years, 23±14 years of relationship, 65% spouses). There was no change in patient BDI between screening and baseline (20.1±11.2 vs. 19.0±10.6, P=0.81). All dyads were able to complete the four MBSR+supportive group therapy sessions. There was a significant improvement in BDI (19.0±10.6 vs.15.6±8.2 P=0.01), PSQI (7.2±3.7 vs. 5.5±3.7, P<0.001) and overall HRQOL (25.0±13.2 vs. 17.7±14.0,P=0.01) but not in anxiety or CHE rates in patients. Similarly caregiver burden (ZBI-SF13.0±9.0 vs. 9.8±6.9,P=0.04, Perceived burden 72.1±29.9 vs. 63.0±14.5,P=0.05) and depression reduced (BDI 9.1±7.8 vs. 5.9±6.0,P=0.03) while caregiver sleep quality (7.2±3.7 vs. 5.5±3.7,P<0.001) improved. Prior HE did not affect PRO change after MBSR+supportive groups but the ZBI-SF of caregivers taking care of HE patients improved to a greater extent (delta −1.1±6.5 vs. 7.4±5.3 HE, P=0.04).
Conclusion:
A short program of mindfulness and supportive group therapy significantly improves PRO and caregiver burden in cirrhotic patients with depression. This non-pharmacological method could be a promising approach to alleviate psychosocial stress in patients with end-stage liver disease and their caregivers.
Introduction
Cirrhosis is associated with impaired patient-reported outcomes such as depression, and sleep disorders.1, 2, 3 This can be modulated by cognitive impairment that ranges from covert to overt hepatic encephalopathy (HE).4 However, even after cognitive recovery persistent cognitive deficits can remain which exacerbate this psychological burden.5 These disorders of mood and sleep are often inadequately treated in cirrhosis because of the role of the liver in clearing psychotropic medications and the fact that the therapeutic-toxic threshold of these medications is very narrow in cirrhosis.6 Therefore, strategies that do not depend on psychotropic medications are needed to relieve this immense burden on the patients and their families.7
An attractive approach is mindfulness training, which can modify the cognitive, affective and physiological mechanisms implicated in alcohol, substance abuse dependence and overeating/obesity.8, 9, 10 Mindfulness has also been shown to reduce the stress levels of caregivers of those with significant medical conditions (e.g., Alzheimer’s disease).11 The primary focus is to cultivate attention and a mental capacity termed “mindfulness,” which reflects a nonjudgmental awareness of the present moment, without cognitive elaboration.12 The practice involves sustained attention to internal and external sensory stimuli. Formal practice often involves a meditative body scan which involves moving a spotlight of attention from one body part to another. However, this approach has not been formally studied in the setting of chronic liver disease and cirrhosis for patients or caregivers.
The primary objective of the study was to determine whether a structured mindfulness treatment, Mindfulness-Based Stress Reduction (MBSR), plus Supportive Group Therapy is effective at reducing depression, anxiety, and sleep disturbances, and improving health-related quality of life in depressed patients with cirrhosis and to reduce the perceived burden on their caregivers.
Methods
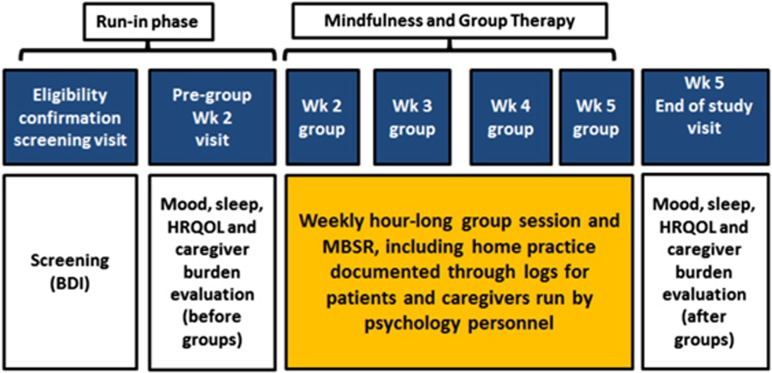
We only included cirrhotic patients (proven by radiology, endoscopic evidence of varices in chronic liver disease, or biopsy) who were able to provide informed consent and had a co-dwelling family member or caregiver who was willing to participate. We excluded those who were unable to consent, had active (within 3 months) alcohol/illicit drug abuse, active psychosis, bipolar disorder, current suicidal ideation and those without a family member or caregiver who could participate. For caregivers, we required them to share the same dwelling as the patient for at least one year, be >21 years, able to consent independently and be willing and able to participate in the study. After determining eligibility, the medical history, complications of cirrhosis and concomitant medications were recorded. The trial is registered at www.clinicaltrials.gov NCT02944643 and was approved by the IRB at the McGuire VA Medical Center. The trial design is in Figure 1.
Figure 1.
Study design showing the flow of patients throughout the trial.
After informed consent separately for the patients and the caregivers, cirrhotic patients underwent screening analysis at baseline with the Beck Depression Inventory-II (BDI-II); patients were allowed to continue if the score was ≥14 (ref. 13). A run-in period of 2 weeks was allowed during which the patients and caregivers were evaluated and administered the following questionnaires: (A) Mood: BDI-II and Beck Anxiety Inventory (BAI),14 (B) Sleep: Pittsburgh Sleep Quality Index (PSQI) and Epworth Sleepiness Scale (ESS)15 and (C) HRQOL: Sickness Impact Profile (SIP).16 In addition, the caregivers were administered the Zarit Burden Interview Short Version (ZBI-SF)17 and Perceived Caregiver Burden (PCB) survey18 and and the patients were administered the Psychometric hepatic encephalopathy score (PHES) to diagnose covert HE (CHE) based on local population norms.19 Both dyads then underwent the groups led by doctoral clinical psychologists over 4 weeks which had the following structure. Patients who attended at least three of the four groups were considered to have completed the study.
Group intervention format
Group therapy sessions were an hour long and were conducted on a weekly basis over a four-week period. Each group began with a review of what was learned in the group before and a review of the concrete goals/homework that the participants set from the last group. The group worked together to identify barriers and strategies. The second portion of the group focused on acquiring specific skills. These skills include Qigong (gentle movements), body scan, progressive relaxation, and loving kindness meditation.20 At the conclusion of the session, subjects, family members or caregiver core skills to be targeted as detailed below were evaluated by the psychologists running the group. The MBSR program was modified from a curriculum developed for parents of special-needs children for chronic liver disease21 and the group sessions lasted for one hour.
The core skills that were targeted during the mindfulness study groups were (A) Stress Management: The concept of stress was introduced with regards to the psychological and physical response of the body that occurs when we must adapt to changing conditions. The effect of stress on everyday functioning, disruption of daily routines, and physical and emotional functioning was discussed. Participants were taught basic stress management techniques to trigger the relaxation response such as deep breathing, progressive muscle relaxation and guided imagery (B) Dealing with Depression: Concepts related to how changes in thoughts and behaviors can improve one’s ability to cope with stressful situations were discussed. Participants were taught to recognize how their thoughts and behaviors influence their experience of situations and their accompanying emotional reactions. Participants were also taught to identify alternative ways of thinking and reacting to situations in order to modulate their emotional experiences. (C) Adjusting to Anxiety: In addition to discussion of relationship among thoughts, behaviors and emotional experiences to situations, the focus included physiologic components associated with anxiety. Participants were taught to recognize how their thoughts and behaviors influence their experience of situations and their accompanying emotional reactions. Participants were taught to identify alternative ways of thinking and reacting to situations in order to modulate their emotional experiences and (D) Family Health and Changes in Roles: Discussion focused on the common health experiences, the change in family roles, and the related stress that the group members may share. There was also education about how family roles change during the illness experience. Participants were taught to identify alternative ways of thinking and reacting to situations in order to modulate their emotional experiences
To use between groups, both caregivers and patients were given CDs with guided mindfulness exercises to listen to daily. In the spirit of supportive group therapy, individuals were also allowed to discuss matters that were important to them in the group sessions in addition to the structured interventions.
At the end of the four sessions, the psychological questionnaires were re-administered to both patients and caregivers.
Sample size
We assumed that there would not be any significant change in BDI-II over the run-in period. In depressed non-cirrhotic subjects, a systematic review found that BDI-II was reduced by a mean of 4.7 points with treatment (95% CI 2.1–7.3).22 Assuming that the reduction is relatively lower in cirrhotic subjects who have other co-morbid conditions to 4.0 points with a power of 0.80, we would require 16 patients.
Statistical analysis
Paired t-tests and Wilcoxon signed rank paired tests were performed between the baseline and the end-of-study assessments for each of the questionnaires. A P<0.05 was considered significant.
Results
We considered 36 dyads for this study; seven were not interested and six could not commit to all visits. Ultimately 23 dyads were enrolled, of which three did not have BDI-II >14 on screening. Ultimately 20 dyads were included in the study.
We enrolled twenty cirrhotic men with a mean age of 60.4±8.3 years. The most prevalent cirrhosis etiology was hepatitis C (n=7) followed by alcohol (n=6), alcohol+hepatitis C (n=3), NASH (n=3) and others (n=1). The mean MELD score was 12.9±5.7, 14 patients had prior hepatic encephalopathy (HE) of whom 12 were on rifaximin in addition to lactulose and five patients had prior variceal bleeding, all of whom were on beta-blockers. Two patients were on opioids (for chronic back pain), three were on anti-depressants and one was on anti-anxiety medications. All these were stable doses for at least six months prior to enrollment and none of the patients or caregivers were started on new treatments during the trial. Four patients were active on the liver transplant waiting list but were not expected to receive a transplant within the trial timeframe given their MELD score trajectories.
The caregivers were mostly women (n=15) with a mean age of 54.9±12.6 years. Most caregivers were spouses (n=13), while the remaining were unmarried partners (n=4) or adult children (n=3). The relationship between caregiver and patients was a mean of 23.1±14.2 years long. None of the caregivers were on any psychoactive medications.
There was no significant change in the BDI-II between the screening and the pre-group visits (20.1±11.2 vs. 19.0±10.6, P=0.81), all patients continued to have BDI-II >14 in both the administrations and none of the patients indicated suicidal ideation at any of these administrations.
Course during the sessions
The groups consisted of an average of 2–3 dyads per session and all dyads completed the four assigned sessions. Two dyads had difficulty with listening to the CDs with the mindfulness data in between the first and second groups but this was corrected by the end of the second group. At the end of four weeks, two patients were hospitalized for cirrhosis-related conditions within one week of the last visit, one for pneumonia and one for hepatic encephalopathy (HE). The patients and caregivers reported listening to the CDs at least one hour daily >90% of the time when questioned by the team during the return visits.
Change in scores
As shown in Table 1, there were significant improvements in depression, sleep quality and overall HRQOL in patients. The majority (55%) of patients changed from depressed to not-depressed on the BDI-II category after the intervention. There was no change in anxiety, but there was a trend towards improvement in daytime sleepiness. When we studied the relative change in specific questionnaires in patients who had prior HE over the study, there was no significant differences between the groups. Delta was calculated as pre minus post group values. This was similar for BDI (4.8±5.4 vs. 2.9±5.5 HE, P=0.49), BAI (−6.0±7.9 vs. 0.6±8.9 HE, P=0.13), ESS (0.8±3.5 vs. 1.5±3.2 HE, P=0.69), PSQI (2.0±2.8 vs. 1.0±2.6, P=0.47), total SIP (2.6±9.3 vs. 9.3±11.9 HE, P=0.20), physical SIP (−2.9±10.6 vs. 8.9±13.4 HE, P=0.07), psychosocial SIP (3.9±14.9 vs. 4.3±16.8 HE, P=0.96).
Table 1. Change in Patient Questionnaires.
| Patients | Pre-group | Post-group | P-value |
|---|---|---|---|
| MELD score | 12.9±5.7 | 12.5±5.5 | 0.48 |
| Beck Depression Inventory | 19.0±10.6 | 15.6±8.2 | 0.012 |
| % with depression | 20 (100%) | 9 (45%) | 0.0001 |
| Beck Anxiety Inventory | 11.9±10.1 | 12.3±10.4 | 0.51 |
| Total SIP | 25.0±13.2 | 17.7±14.0 | 0.005 |
| Psychosocial SIP | 25.1±15.9 | 17.3±13.2 | 0.01 |
| Physical SIP | 18.5±17.4 | 13.1±12.5 | 0.001 |
| Pittsburgh Sleep Quality Index | 7.2±3.7 | 5.5±3.7 | <0.001 |
| Epworth Sleepiness Scale | 7.1±3.5 | 5.7±4.4 | 0.13 |
| PHES score median (IQR) | −7 (−10 to −4) | −6 (−8 to −3) | 0.42 |
| Covert HE by PHES (%) | 55% | 50% | 0.75 |
HE, hepatic encephalopathy; IQR, interquartile range; PHES, psychometric hepatic encephalopathy score; SIP, sickness Impact Profile.
Data is presented as mean±s.d. unless stated otherwise. A high score on all these values indicates worse functioning except on PHES.
Caregivers demonstrated a significant change in burden scores with improvement in their sleep quality and depression with a trend towards improvement in the other aspects (Table 2). Caregiver burden levels (ZBI-SF) improved overall in most caregivers, but in three patients lifestyle burden scores actually increased. Two of these were from patients who were ultimately hospitalized for their cirrhosis complications within one week of the end of the groups. Delta calculation showed the ZBI-SF improvement (pre minus post) was greater in caregivers of patients with prior HE (−1.1±6.5 vs. 7.4±5.3 HE, P=0.04) but other measures were similar. Specifically, delta SIP (0.71±4.0 vs. 0.35±2.6 HE, P=0.84), delta SIP physical (−0.5±5.4 vs. 0.5±3.3 HE, P=0.69), delta SIP psychosocial (2.2±5.0 vs. −3.3±11.6 HE, P=0.17), delta BDI-II (2.0±8.3 vs. 3.8±5.8 HE, P=0.65), delta BAI (−1.7±4.3 vs. 1.1±4.4 HE, P=0.3), delta ESS (1.5±3.3 vs. 1.5±4.3 HE, P=1.0) and delta PSQI (1.8±1.2 vs. 1.6±1.9 HE, P=0.79).
Table 2. Change in Caregiver Questionnaires.
| Caregivers | Pre-group | Post-group | P-value |
|---|---|---|---|
| Zarit Burden Interview-SF | 13.0±9.0 | 9.8±6.9 | 0.04 |
| Perceived Caregiver Burden | 72.1±29.9 | 63.0±14.5 | 0.05 |
| Beck Depression Inventory | 9.1±7.8 | 5.9±6.0 | 0.03 |
| Beck Anxiety Inventory | 5.5±5.2 | 5.2±7.1 | 0.80 |
| Total SIP | 6.5±9.7 | 6.1±9.1 | 0.52 |
| Psychosocial SIP | 6.4±9.6 | 8.0±12.6 | 0.51 |
| Physical SIP | 4.9±9.8 | 4.7±9.4 | 0.82 |
| Pittsburgh Sleep Quality Index | 7.2±3.7 | 5.5±3.7 | <0.001 |
| Epworth Sleepiness Scale | 7.2±3.4 | 5.7±4.4 | 0.11 |
SIP, Sickness Impact Profile.
Data is presented as mean±s.d. unless stated otherwise. A high score on all these values indicates worse functioning.
Discussion
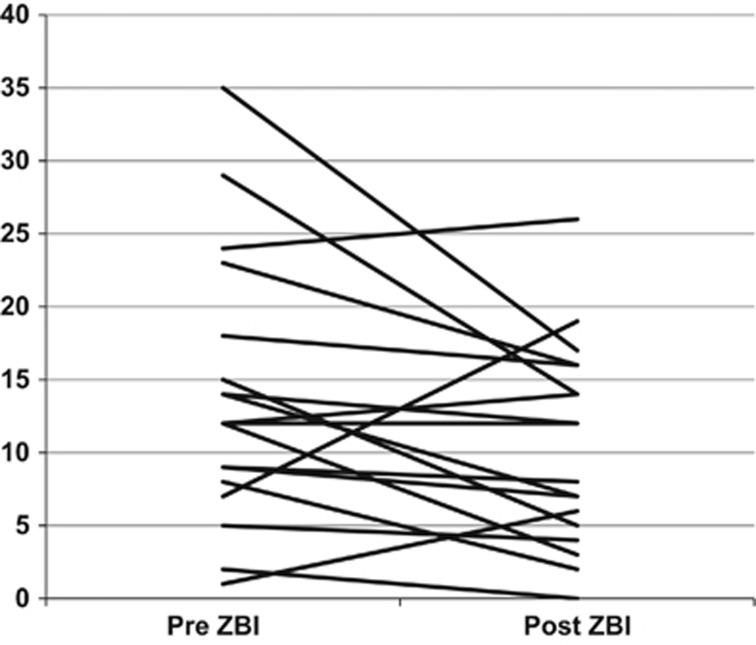
Our current study shows that caregivers and patients with cirrhosis and depression have a favorable response to mindfulness-based stress reduction and supportive group therapy. This therapeutic approach is also accompanied by improvement in sleep and overall HRQOL in patients while a reduction in perceived caregiver burden is found in caregivers (Figure 2).
Figure 2.
Zarit Burden Interview Short form of caregivers pre (baseline) and post (after the study ended). This shows that the perceived burden reduced in most caregivers (lower score indicates lower burden, P=0.04). However three caregivers actually experienced a higher burden. Two of these were taking care of patients who were hospitalized within a week of study end, therefore indicating that the caregivers could sense a deterioration in patient performance which was not improved with mindfulness and supportive group therapy.
Prior studies have defined the high prevalence of mood and sleep disorders in cirrhotic patients which have an adverse impact on HRQOL.1, 23 Given the severity of cirrhosis impacting the metabolism of most hypnotic and psychotropic medications, most of these mood and sleep disorders are not adequately treated.6 This was reflected in our patient population, where the minority was on anti-depressant medications. While liver transplant can alleviate these problems, most patients with cirrhosis are not candidates and even if on the transplant list, continue to suffer from these mood and sleep issues.24 In addition to the patients themselves, these issues impact the caregivers and the patient’s families.7 Given the time constraints of most hepatology practices, the impact on the caregivers is often not addressed, which has the potential to compound the challenges facing this patient-caregiver dyad.
Therefore non-pharmacological alternatives focused at the patient-caregiver dyad, rather than the patients only; and which can potentially be carried out at home are required. Our approach of mindfulness-based stress reduction (MBSR) with supportive group therapy was offered only to dyads in whom the patients had depression. This selected for the subjects that could potentially benefit from techniques which have been previously shown to improve mood and sleep in non-cirrhotic individuals.20 We found that even a brief duration of MBSR was efficacious in addressing depression and sleep quality. It is possible that the sleep quality improved as a concomitant of improved depression.1, 23 This was accompanied by a trend towards improved daytime sleepiness, which could be due to a contribution of better sleep hygiene, reduced depression, and cirrhosis-related factors. Enhancement of sleep quality and depression contributed to an improvement in both the psychosocial and physical aspects of HRQOL. This improvement in HRQOL is especially relevant given that cirrhotic patients are living longer and have greater psychosocial needs that prevent them from being productive members of society.3, 25 Interestingly, the improvement in depression and HRQOL did not translate into an enhancement of CHE testing indices. These PROs are not necessarily reflective of cognitive function, and are modulated by mood disorders and cognitive reserve.26, 27
The lack of change in anxiety in this study could be related to a floor effect. At study initiation most subjects’ anxiety levels were not clinically elevated. This also replicates a prior study with PROMIS criteria that demonstrated that anxiety, in contrast to depression, was not a major differentiator between cirrhotic patients and controls.2
The results of the current study extend prior studies of mindfulness-based strategies in alleviating caregiver burden performed in Alzheimer’s disease and other dementias into the field of cirrhosis.11 Given that patients and caregivers were treated as dyads and as a single functioning unit, the improvements in PROs were accompanied also by improvement in caregiver outcomes such as their burden interviews, as well as the depression and personal sleep quality. This change profile was similar to what seen in patients, even though none of the caregivers were ever beyond the mild depression stage on the BDI-II. The sleep quality improvement could again reflect the contribution of depressive symptoms in caregivers that was related to the patients’ mood disorders. Another possibility is that MBSR techniques used upon awakening from sleep may serve to lessen excessive non-productive rumination, and replace potentially negative emotionally-laden thoughts, with ones of loving kindness.28, 29 These findings reflect the importance of treating both patients and their caregivers, especially in end-stage liver disease, together. Despite the challenges in perceived burden, caregivers at study initiation did not have significant HRQOL impairment (i.e., a “floor effect”), which could explain the lack of change of these scores following study sessions.
The majority of our patients had prior HE controlled on rifaximin. We found similar rates of improvement in patient-reported outcomes in HE compared to those without prior HE. However, the improvement in caregiver burden (as measured by the ZBI-SF) was higher in the significant other caring for HE patients. This likely reflects additional caregiver lifestyle demands (burden) associated with increasing illness severity.7 This is encouraging because it demonstrates that with MBSR and supportive group therapy, even this relatively higher perceived burden can be ameliorated. From the patient perspective, HE is one of the major determinants of decline in HRQOL and associated mood and sleep disorders can confound, or complicate the treatment and prognosis of HE patients.1, 30 Therefore, the relatively similar improvement in PROs after MBSR and supportive group therapy, regardless of prior HE, underscores the power of this therapeutic approach.
While the psychosocial burden of disease is immense in cirrhosis, the underlying focus of clinicians has always been to mitigate illness progression and prevent need for hospitalization. Indeed in this study, the underlying cirrhosis-related disease process resulted in worsening of the caregiver burden and lack of improvement in patient-reported outcomes in at least three dyads, of whom two were subsequently hospitalized. This extends a prior study in which HRQOL reduced in patients who then developed breakthrough HE several weeks before that episode occurred.31 This reiterates the importance of tracking PROs since these patients and their caregivers were able to sense a clinical deterioration that resisted improvement with MBSR and supportive group therapy. Therefore a balanced approach to both medical and psychosocial aspects of cirrhosis care is important.
The current sample size of patients without HE was limited, therefore conclusions regarding these patients may require further studies. All patients were men with majority women caregivers; it is possible that different spousal/gender dynamics in other populations may have a different experience. Since we used both mindfulness and supportive group therapy together, the current study does not permit us to evaluate the effect of these techniques separately. We also did not perform a randomized trial, which could have influenced the results given that these are patient-reported outcomes. However, the primary outcome, BDI-II was performed in the run-in period and only improved after the intervention. Our follow-up was limited, but we wanted to evaluate the relatively proximate impact of these therapies on PROs, although prior studies have shown that benefit in non-cirrhotic patients can last up to several months after discontinuation of the therapy.21, 32
While our groups were in-person and led by psychologists with a home-based CD, mindfulness can also be practiced using smartphone Apps as well as internet-based delivery systems for care.29, 33 These options may be more attractive for younger patients and family members as well as in clinical practices where psychology specialists are not available for extended periods of time.
We conclude that a short program of mindfulness and supportive group therapy significantly improves patient-reported outcomes and caregiver burden in men with cirrhosis and depression. This non-pharmacological method could be a promising approach to alleviate psychosocial stress in patients with end-stage liver disease and their caregivers.
Study Highlights

Footnotes
Guarantor of the article: Jasmohan S. Bajaj, MD.
Specific Author contributions: JSB, ME and JBW conceptualized and designed the study, ME, JBW, TA and TB were responsible for conducting and designing the groups, AF and EAG were responsible for study coordination, and DMH, BJ and MF helped with recruitment.
Financial Support: This was partly supported by VA Merit Review I0CX001076, NIH RO1DK089713 and McGuire Research Institute funds to JSB.
Potential Competing Interests: None.
References
- Nardelli S, Pentassuglio I, Pasquale C et al. Depression, anxiety and alexithymia symptoms are major determinants of health related quality of life (HRQoL) in cirrhotic patients. Metab Brain Dis 2013; 28: 239–243. [DOI] [PubMed] [Google Scholar]
- Bajaj JS, Thacker LR, Wade JB et al. PROMIS computerised adaptive tests are dynamic instruments to measure health-related quality of life in patients with cirrhosis. Aliment Pharmacol Ther 2011; 34: 1123–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwal F. Patient-reported outcomes of cirrhosis. Clin Gastroenterol Hepatol 2013; 11: 1043–1045. [DOI] [PubMed] [Google Scholar]
- Vilstrup H, Amodio P, Bajaj J et al. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology 2014; 60: 715–735. [DOI] [PubMed] [Google Scholar]
- Bajaj JS, Schubert CM, Heuman DM et al. Persistence of cognitive impairment after resolution of overt hepatic encephalopathy. Gastroenterology 2010; 138: 2332–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JH, Stine JG. Review article: prescribing medications in patients with cirrhosis—a practical guide. Aliment Pharmacol Ther 2013; 37: 1132–1156. [DOI] [PubMed] [Google Scholar]
- Bajaj JS, Wade JB, Gibson DP et al. The multi-dimensional burden of cirrhosis and hepatic encephalopathy on patients and caregivers. Am J Gastroenterol 2011; 106: 1646–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann SG, Sawyer AT, Witt AA et al. The effect of mindfulness-based therapy on anxiety and depression: A meta-analytic review. J Consult Clin Psychol 2010; 78: 169–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantzios M, Wilson JC. Mindfulness, eating behaviours, and obesity: a review and reflection on current findings. Curr Obes Rep 2015; 4: 141–146. [DOI] [PubMed] [Google Scholar]
- Bowen S, Witkiewitz K, Dillworth TM et al. Mindfulness meditation and substance use in an incarcerated population. Psychol Addict Behav 2006; 20: 343–347. [DOI] [PubMed] [Google Scholar]
- Epstein-Lubow GP, Miller IW, McBee L. Mindfulness training for caregivers. Psychiatr Serv 2006; 57: 421. [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn J, Wheeler E, Light T et al. Influence of a mindfulness meditation-based stress reduction intervention on rates of skin clearing in patients with moderate to severe psoriasis undergoing phototherapy (UVB) and photochemotherapy (PUVA). Psychosom Med 1998; 60: 625–632. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Beck Depression Inventory-Second Edition Manual. The Psychological Corportation: San Antonio, TX, USA, 1996. [Google Scholar]
- Beck AT, Epstein N, Brown G et al. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol 1988; 56: 893–897. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF 3rd, Monk TH et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 1989; 28: 193–213. [DOI] [PubMed] [Google Scholar]
- Bergner M, Bobbitt RA, Carter WB et al. The Sickness Impact Profile: development and final revision of a health status measure. Med Care 1981; 19: 787–805. [DOI] [PubMed] [Google Scholar]
- Bedard M, Molloy DW, Squire L et al. The Zarit Burden Interview: a new short version and screening version. Gerontologist 2001; 41: 652–657. [DOI] [PubMed] [Google Scholar]
- Stommel M, Given M, Given E. Depression as an overriding variable explaining caregiver burdens. J Aging Health 1990; 2: 81–102. [Google Scholar]
- Allampati S, Duarte-Rojo A, Thacker LR et al. Diagnosis of minimal hepatic encephalopathy using stroop encephalapp: A Multicenter US-Based, Norm-Based Study. Am J Gastroenterol 2016; 111: 78–86. [DOI] [PubMed] [Google Scholar]
- Ludwig DS, Kabat-Zinn J. Mindfulness in medicine. JAMA 2008; 300: 1350–1352. [DOI] [PubMed] [Google Scholar]
- Dykens EM, Fisher MH, Taylor JL et al. Reducing distress in mothers of children with autism and other disabilities: a randomized trial. Pediatrics 2014; 134: e454–e463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JM, Russell I, Russell D. Mindfulness-based cognitive therapy: further issues in current evidence and future research. J Consult Clin Psychol 2008; 76: 524–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barboza KC, Salinas LM, Sahebjam F et al. Impact of depressive symptoms and hepatic encephalopathy on health-related quality of life in cirrhotic hepatitis C patients. Metab Brain Dis 2016; 31: 869–880. [DOI] [PubMed] [Google Scholar]
- Ahluwalia V, Wade JB, White MB et al. Liver transplantation significantly improves global functioning and cerebral processing. Liver Transplant 2016; 22: 1379–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwal F. Decreasing mortality in patients hospitalized with cirrhosis. Gastroenterology 2015; 148: 897–900. [DOI] [PubMed] [Google Scholar]
- Patel AV, Wade JB, Thacker LR et al. Cognitive reserve is a determinant of health-related quality of life in patients with cirrhosis, independent of covert hepatic encephalopathy and model for end-stage liver disease score. Clin Gastroenterol Hepatol 2015; 13: 987–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wunsch E, Szymanik B, Post M et al. Minimal hepatic encephalopathy does not impair health-related quality of life in patients with cirrhosis: a prospective study. Liver Int 2011; 31: 980–984. [DOI] [PubMed] [Google Scholar]
- Eisenlohr-Moul TA, Peters JR, Pond RS Jr. et al. Both trait and state mindfulness predict lower aggressiveness via anger rumination: a multilevel mediation analysis. Mindfulness 2016; 7: 713–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Querstret D, Cropley M, Fife-Schaw C. Internet-based instructor-led mindfulness for work-related rumination, fatigue, and sleep: assessing facets of mindfulness as mechanisms of change. A randomized waitlist control trial. J Occup Health Psychol 2016; 22: 153–169. [DOI] [PubMed] [Google Scholar]
- Arguedas MR, DeLawrence TG, McGuire BM. Influence of hepatic encephalopathy on health-related quality of life in patients with cirrhosis. Dig Dis Sci 2003; 48: 1622–1626. [DOI] [PubMed] [Google Scholar]
- Sanyal A, Younossi ZM, Bass NM et al. Randomised clinical trial: rifaximin improves health-related quality of life in cirrhotic patients with hepatic encephalopathy—a double-blind placebo-controlled study. Aliment Pharmacol Ther 2011; 34: 853–861. [DOI] [PubMed] [Google Scholar]
- Teasdale JD, Segal ZV, Williams JM et al. Prevention of relapse/recurrence in major depression by mindfulness-based cognitive therapy. J Consult Clin Psychol 2000; 68: 615–623. [DOI] [PubMed] [Google Scholar]
- Coulon SM, Monroe CM, West DS. A systematic, multi-domain review of mobile smartphone apps for evidence-based stress management. Am J Prev Med 2016; 51: 95–105. [DOI] [PubMed] [Google Scholar]