Abstract
Background and aims
Bleaching might affect structural properties of composite materials, and lead to monomer release. This study aimed to evaluate the effect of Laser-assisted and conventional in-office bleaching on the release of BIS-GMA, TEGDMA, and UDMA monomers from a nanohybrid and a microhybrid BIS-GMA based composite.
Materials and Methods
32 samples of each composite, were divided into 4 subgroups; subgroup 1: Conventional in-office bleaching (CIB) with the Opalescence Boost PF 38% gel, subgroup 2: Laser-assisted bleaching (LBO) with the Opalescence Boost PF 38% gel, subgroup 3: Laser-assisted bleaching (LBH) with the JW Power bleaching gel, subgroup 4: (CO) control without bleaching. All the samples were immersed in tubes of 2cc Ethanol 75% medium. The released monomers were analyzed using the high performance liquid chromatography (HPLC) method 24 h, 7, and 28 days. Data's were analyzed by Univariate Analysis of Variance test followed by Tukeys HSD.
Results
The amount of TEGDMA monomer released was not significant. However, nanohybrid composites showed significantly more monomer release than microhybrid composites (P < 0.05). For UDMA the interaction was significant only after 1 week. In microhybrid composites, the CO subgroup showed more monomer release than LBH and LBO. In nanohybrid composites, LBH showed more monomer release than CIB and CO subgroups. For BIS-GMA monomers the interaction was significant at all time periods and the LBH subgroup of nanohybrid composite had significantly more BIS_GMA release in comparison to other subgroups.
Conclusion
Bleaching by laser with JW Power Bleaching gel led to more monomer release in nanohybrid composite.
Keywords: tooth Bleaching, Diode laser, Hydrogen peroxide, composite dental resin, chromatography
Introduction
Tooth bleaching, as a means of improving the appearance of teeth, has an important position in esthetic dentistry. 1) Several bleaching techniques and products are available that may be used in-office or at home. 2)
“In-office bleaching”, has some advantages over “at-home bleaching” including dentist supervision, soft tissue protection, and more rapid results. 3) High concentration of hydrogen peroxide (35–40%) is used for “in-office bleaching”. 4) Hydrogen peroxide (HP) generates free radicals, reactive oxygen molecules and HP anions. 3) These molecules interact with carbon double bonds or breakdown pigments and transforms them into other molecules, which diffuse out of the tooth structure or absorb less light and subsequently change the color of the tooth structure. 3)
Different energy sources have been used in order to increase bleaching efficacy and reduce chairside time such as quartz-tungsten-halogen lamps, plasma-arc devices, light emitting diodes (LED) and different laser systems 5). Among these, laser systems have the ability of in-depth activation of the bleaching gels; therefore, more free radicals will be released in a shorter time 6).
The candidates for bleaching might have caries, defective restorations or non-carious lesions which must be restored immediately before bleaching, in order to protect pulp tissue from hydrogen peroxide damage. 7, 8) Microhybrid and nanohybrid composites are the main types of composites, used for anterior and posterior restorations. 9)
Composites contain monomers, which polymerize, and create a crosslinked polymer. Studies have shown that the conversion degree of monomers varies between 50–70%. 10) Subsequently un-reacted monomers get trapped between the polymer networks. The main monomers used in most composites are bisphenol A diglycidil dimethacrylate (BisGMA) and urethane dimethacrylate (UDMA), which are diluted by low viscosity monomers such as triethylene glycol dimethacrylate (TEGDMA). 11)
Some studies have been previously done to evaluate the effect of bleaching on monomer release from dental composites; however the results are conflicting. 4, 12, 13) Some of them showed that, bleaching with hydrogen peroxide might interact with c-c single or double bond and degrade the three dimensional polymer network of dental composites. 4, 14) This interaction causes; dimensional change, influences mechanical properties, and affects the clinical durability and esthetic of the restorations. 15) The degradation and softening of composite matrix induced by oxidation process, allows deeper and easier penetration of the solvents into the polymer network and facilitates diffusion of release monomers, additives and unspecific oxidative products, from composites. 12, 16)
These released monomers induce microorganism proliferation, and initiate cytotoxicity mechanism which leads to pulpal damage and gingival inflammation, and allergic reactions. 17) Additionally there are concerns about the estrogenic and mutagenic effects of some dental composite monomers. 18, 19)
As there is no data about the effect of “laser assisted bleaching” on monomer release from composites, besides there are concerns about their negative effects on dental composites, the aim of this study was to evaluate the amount of monomer release from BisGMA based nanohybrid and microhybrid composites at different storage times after bleaching.
Materials and Methods
A microhybrid and a nanohybrid composite were selected. The compositions of the composites are listed in table 1. 32 samples were made of each composite by a Teflon mold (5mm diameter and 3mm thickness).
Table 1: The compositions of the composites.
| Material | Type | Monomers Of Organic Matrix | Filler | Particle Size Range | Filler Percentage Weight | Filler Percentage Volume | Color | Density | Batch No. |
|---|---|---|---|---|---|---|---|---|---|
| CLEARFIL® AP-X | Microhybrid | Bis-GMA, TEGDMA, UDMA | Silanated barium glass filler, Silanated silica filler | 0.02 to 17 µm | 86% | 71% | A3 | 2.3 g/ml | 1236494 |
| Grandio® | Nanohybrid | Bis-GMA, TEGDMA, UDMA | Glass-Ceramic micro fillers & nano fillers | Glass-Ceramic micro fillers average particle size:1µm-Nano fillers range of particle size: 20–50nm | 87% | 71.4% | A3 | 2.425 g/ml | 01470A |
The mold was positioned on a glass plate covered by a transparent plastic strip (Frasaco, Teflon, Germany). A 1.5 mm composite was inserted in the mold and light cured for 20 seconds by a light curing unit (LED volume 2 Ultradent USA with 600 mw/cm2 output). The second layer was inserted in the mold and it was completely filled. A glass plate covered by a plastic strip was pressed on top of the composite and light cured for 20 seconds. The curing unit was directly applied on the glass surface. The LED intensity was checked before each curing using a radiometer (Bisco, IL, USA). All Samples were polished by Sof-Lex discs Pop On XT (3M ESPE), 10 strokes for 20 seconds for each disc.
Samples of each composite were divided into 4 subgroups (n=8)
Subgroup 1, CIB: the samples were bleached using the conventional office bleaching method with 38% gel (Opalescence Xtra Boost, USA Ultradent products, Inc, south Jordan, UT). The two syringes containing bleaching were completely mixed, and the gel was applied in 1 mm layers on the composite surface for 15 minutes. Then, the bleaching gel was wiped away from the samples, and completely washed.
Subgroup 2, LBO: the 38% bleaching gel (Opalescence Xtra Boost) was applied on the composite surface similar to the CIO subgroup. A diode laser (Wuhan, gigga model: DENZA, China) with a wave length of 810 nm, 1.5 watt output power, and continuous wave mode with a fixed 400 micron fiber probe tip and 6 mm distance from each tooth surface was used for three times thirty seconds irradiation and one minute interval. Five minutes after the last irradiation, the bleaching gel was wiped away and completely washed.
Subgroup 3, LBH: 30% hydrogen peroxide (J White Heydent GmbH, Germany) in 2 mm thickness and the same laser which was used in LBO subgroup was used in this group.
Subgroup 4, CO: The control subgroup, the samples were not bleached.
The output power of the laser was checked by power meter (laserpoint, Italy) before each irradiation.
After bleaching, the bleaching gel was removed completely from the samples by cotton pellet, and rinsed completely by water. Each sample was inserted in a glass tube containing 75% alcohol and 25% distilled water. The tubes were closed and the surface of the tubes was covered in order to prevent light entering, and they were stored in a dark room.
The contents of tubes was changed 24 hours, 7 days, and 28 days after bleaching. The amount of released monomer was evaluated by the HPLC 600 E waters System Controller (Waters, MA, USA) method, through The Perfect target ODS-3 column (125 mm height, 4mm width, and silica particle size of 5 µm), with a UV detector at 230 nm wave length. The mobile phase was 70% acetonitrile and 30% distilled water, at 0.8 mm flow rate, and 20µlit injection volume at room temperature.
At first, different concentrations of each monomer (0.5–50 µg/lit) were injected into the system, and a standard curve was obtained.
In order to evaluate the effect of bleaching and the type of composite, one-way ANOVA and Tukey's HSD test were used. P≤ 0.05 was considered as statistically significant.
Results
The mean and standard deviation of cumulative amount of monomers released from the two types of composites at different times and different subgroups are shown in tables 2 and 3.
Table 2: Mean and standard deviation of cumulative monomer released in microhybrid subgroups at different times.
| Monomer | TEGDMA | UDMA | BIS-GMA | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Time | 24h | 7d | 28d | 24h | 7d | 28d | 24h | 7d | 28d | |
| Subgroups | ||||||||||
| CIO | 6.49 (±1.17) | 13.43 (±3.36) | 18.77 (±3.60) | 31.91 (±12.22) | 65.95 (±12.55) | 122.29 (±30.07) | 14.86 (±3.76) | 25.96 (±4.32) | 42.90 (±9.23) | |
| LBO | 6.66 (±.78) | 16.88 (±2.76) | 24.24 (±4.97) | 26.48 (±4.48) | 59.55 (±6.03) | 128.60 (±37.09) | 13.81 (±4.48) | 41.28 (±32.07) | 57.98 (±29.17) | |
| LBH | 6.22 (±1.75) | 11.28 (±3.45) | 21.03 (±4.56) | 33.12 (±7.45) | 62.07 (16.11) | 126.79 (±31.00) | 15.37 (±2.63) | 24.96 (±4.30) | 40.76 (±9.53) | |
| CO | 5.67 (±1.48) | 13.65 (±4.15) | 19.73 (±4.48) | 32.95 (±8.86) | 80.73 (±32.31) | 147.87 (±31.12) | 11.92 (5.30) | 23.82 (±6.58) | 43.69 (±8.71) | |
Table 3: Mean and standard deviation of cumulative monomer released in nanohybrid subgroups at different time's intervals.
| Monomer | TEGDMA | UDMA | BIS-GMA | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Time | 24h | 7d | 28d | 24h | 7d | 28d | 24h | 7d | 28d | |
| Subgroups | ||||||||||
| CIO | 10.50 (±3.00) | 22.40 (±4.60) | 30.44 (±64.78) | 1.79 (±3.59) | 5.70 (±5.43) | 55.38 (±14.34) | 24.98 (±7.91) | 34.52 (±9.68) | 41.29 (±10.41) | |
| LBO | 11.69 (±2.67) | 24.05 (±5.06) | 33.34 (±6.09) | 2.77 (±3.97) | 6.48 (±5.92) | 68.16 (±20.34) | 26.82 (±3.48) | 37.55 (±6.21) | 44.81 (±12.32) | |
| LBH | 13.62 (±1.80) | 25.18 (±3.96) | 33.06 (±5.24) | 21.92 (±9.96) | 30.83 (±18.60)) | 78.24 (±30.65) | 34.79 (±4.74) | 131.88 (±20.70) | 142.24 (±21.99) | |
| CO | 12.53 (±1.66) | 25.33 (±7.67) | 35.05 (±9.39) | 5.41 (±10.82) | 5.98 (±10.73) | 54.12 (±14.26) | 32.47 (±4.95) | 43.97 (±8.08) | 51.38 (±11.03) | |
HPLC analysis showed that TEGDMA, UDMA and BisGMA monomers were released from all type of composites with or without bleaching. Maximum release was detected in the first 24 hours for all types of monomers, and significantly decreased over time.
TEGDMA
The amount of TEGDMA released after 24 h, 1 week, and 28 days did not differ significantly between the three methods of bleaching. However, in evaluating the type of composites, nanohybrid composites showed higher TEGDMA release than microhybrid composites (P < 0.001). Figure 1
Figure 1:
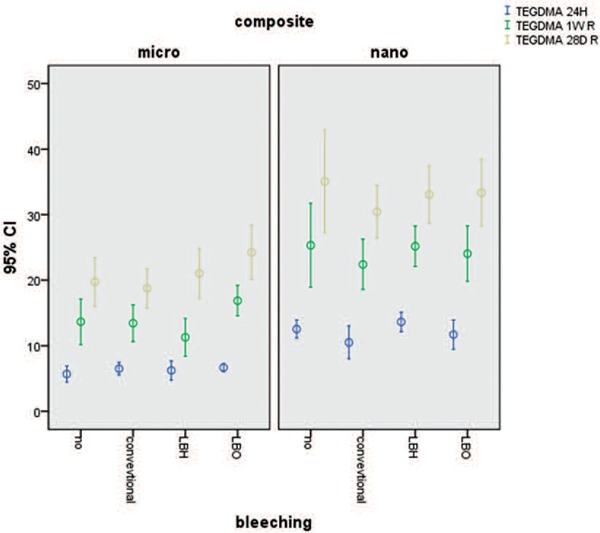
Mean and standard deviation cumulative release of TEGDMA at differen times from two types composites.
UDMA
With respect to UDMA, the interaction was significant after one week. In microhybrid composites the CO subgroup showed more monomer release than LBH and LBO subgroups (P < 0.001). In nanohybrid composites, LBH showed more monomer release than CIB and CO subgroups (P < 0.001). Figure 2
Figure 2:
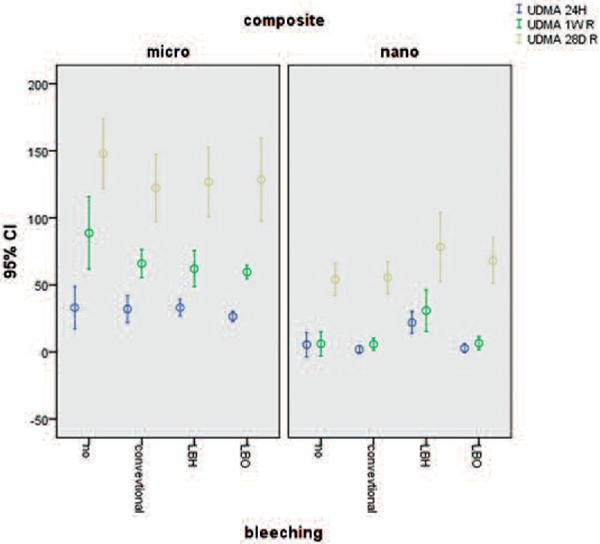
Mean and standard deviation cumulative release of UDMA at differen times from two types composites.
BISGMA
For the BISGMA the interaction was significant after 24 hours, 7 days, and 28 days for nanohybrid composites and the LBH subgroup had more monomer release and the difference was significant (P ≤ 0.035). Figure 3
Figure 3:
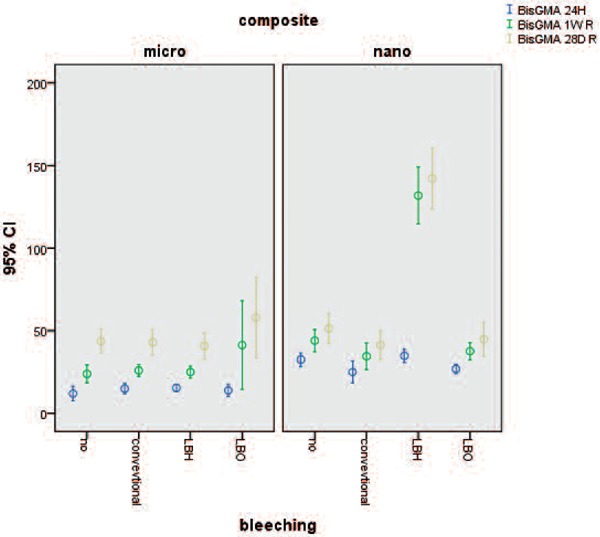
Mean and standard deviation cumulative release of BisGMA at differen times from two types composites.
Discussion
In this study effect of three in-office bleaching protocols were evaluated on monomer release from two types of BIS-GMA based composites (microhybrid and nanohybrid). The bleaching gels selected for this study had the ability of diode laser beam absorption, Opalescence Boost PF 38% contains red carotene 20), and JW Power Bleaching gel 30% contains titanium oxide. 21, 22)
For the elution of monomers the samples were transferred to 75% ethanol. This solution is accepted as a food and vegetable simulator by the Food and Drug Association (FDA) 23), and the monomer released in this solution is close to the clinical situation. It also has a solubility parameter similar to the monomers used in dental composites, therefore diffuses into the composite matrix, swells its structure and simplifies monomer release from the composite structure. 24) Additionally, it is quickly removed from the HPLC system and does not retard the analysis results. 24)
It is obvious that hydrogen peroxide induces different free radicals and ions. These molecules interact with single and double carbon bonds, and esteric bonds in the three dimensional structure of composites. 25) These effects cause cracks in the polymeric network, and subsequently more uncured monomers can be released. Furthermore, the deeper layers of composites come in contact with hydrogen peroxide and more carbon bond might be destroyed, and more monomer will be released subsequently. 25)
This study found that conventional and laser-assisted in- office bleaching by opalescence, did not increase monomer release. These findings are consistent with Polydorou et.al study showing no increase in monomer release after bleaching nanohybrid and to ormocer composites. 12)
The amount of TEGDMA monomer release was significantly higher in nanohybrid composites than microhybrid ones. However, bleaching did not affect the TEGDMA release. There are two explanations for this finding: 1-TEGDMA has lower molecular weight compared with other monomers, and it would be released immediately from the samples; washing the samples after bleaching probably removed the released TEGDMA as a result of bleaching 26) 2- TEGDMA might decompose to other molecules because of the oxidative process of bleaching. 27)
UDMA monomer released in the CO subgroup of microhybrid composite was significantly higher than nanohybrid composite. In microhybrid composite (Clearfil AP-X) one week after bleaching the release of UDMA monomer decreased compared with the CO subgroup.
Laser-assisted bleaching by Hydent bleaching gel increased the release of BIS-GMA in nanohybrid (Grandio) composite and UDMA in some situations.
The diode laser at the wave lengths used in this study had a photothermal effect, and the ability of in-depth activation, which affects the total depth of the bleaching gel at once. At the same time, more hydroxyl radicals will release from the gel, and the bleaching process can be done in a shorter duration. 6) Heydent JW power bleaching gel contains titanium dioxide particles that are special adjuvant for the laser beam, promoting the laser effect in a shorter time. 21) Theoretically, titanium dioxide may increase hydroxyl radical release from the bleaching gels, leading to more severe structural change and deeper penetration into nanohybrid composites that have a lower conversion degree, leading to higher monomer release. 4, 28)
Grandio and Clearfil AP-X have the same percentage of filler load (87 vs 86 wt %). Theoretically, the interface of matrix and filler is the most likely site for water accumulation. The more surface area in nanohybrid filled composites (due to smaller filler size) leads to more water accumulation between the filler and polymeric matrix. 29) The absorbed water consequently decomposes the composite structure and debonds the filer from the polymeric matrix, leading to more monomer release. 29, 30)
Durned and colleagues found that conventional bleaching led to more monomer release from dental composites. The difference in the results of their study compared with this study might be due to different composites, bleaching time, and method of removal of the bleaching agent from the samples. They removed the bleaching gel by a cotton, while we completely washed the samples in order to simulate the clinical situation, and evaluated the amount of monomer released after bleaching. 14)
Wiping the composites might lead to incomplete removal of the bleaching gel, and the remnants of hydrogen peroxide continue their oxidative activity, and more monomers will be released from the composite. Washing the samples might remove monomers released immediately after bleaching.
Polydura and co-workers found that bleaching did not increase monomer release from composites. They concluded that, washing the composite removes the released monomers, and effect of hydrogen peroxide would not remain after its removal. 24)
Sorption ability and monomer leakage from dental composites depends on many factors including the chemical structure of the resin, the type and percentage of each monomer, type of filler, monomer/filler proportion, etc. 31) More release of Bis-GMA in the LBH group, of nanohybrid composite is the result of higher absorption ability of this type of composites. Since the molecular structure of hydrogen peroxide is close to water, it can be expected that Grandio absorbs more hydrogen peroxide which induces effective oxidative cracks in the composite structure. The cumulative effect of these two factors results in more UDMA and BIS-GMA release. BIS-GMA is a viscous molecule with high glass transition temperature, and has the lowest degree of conversion, and it might be released from the composite. 24) Different active molecules produced by bleaching gels may convert monomers to other molecules and the results of this study might not show the exact effect of bleaching on dental composites.
The exact percent of each monomer, the size and arrangement of fillers, might be responsible for differences, observed in monomer release between two types of composites. Though there is no data about the exact structure of the composites, comparing the difference in the monomer release of these composites is impossible. 29) It should be noted that, according to manufacturer's brochure none of the composites contain UDMA, while the HPLC analysis of both of them UDMA was detected.
This is the first study evaluating the effect of laser bleaching on monomer release from dental composites. Difference in bleaching material, time of immersion, type of composites, and lack of data about effect of laser bleaching on dental material structure complicates the explanation of the results of this study. Bleaching process might break the released monomers to other molecules which have not been evaluated in this study.
According to limitation of this study using laser in combination with bleaching agents containing titanium oxide on nanohybrid composites might increase the monomer release after bleaching.
[Acknowledgements]
This article has been supported by Dental School of Tehran University of Medical Science (Grant No 93-53-69-25556)
References
- 1: Taher N. M. (2005). The effect of bleaching agents on the surface hardness of tooth colored restorative materials. J Contemp Dent Pract, 6 (2), 18-26. [PubMed] [Google Scholar]
- 2: Féliz-Matos L., Hernández L. M., Abreu N. (2014). Dental bleaching techniques; hydrogen-carbamide peroxides and light sources for activation, an update. Mini review article. The open dentistry journal, 8, 264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3: Shim Y. S. (2015). Effect of In-Office Bleaching Application on the Color, Microhardness and Surface Roughness of Five Esthetic Restorative Materials. Indian Journal of Science and Technology, 8 (S1), 420-425. [Google Scholar]
- 4: Durner J., Stojanovic M., Urcan E., Spahl W., Haertel U., Hickel R., Reichl F. X. (2011). Effect of hydrogen peroxide on the three-dimensional polymer network in composites. dental materials, 27 (6), 573-580. [DOI] [PubMed] [Google Scholar]
- 5: Gurgan S., Cakir F. Y., Yazici E. (2010). Different light-activated in-office bleaching systems: a clinical evaluation. Lasers in medical science, 25 (6), 817-822. [DOI] [PubMed] [Google Scholar]
- 6: Mirzaie M., Yassini E., Ganji S., Moradi Z., Chiniforush N. (2016). A Comparative Study of Enamel Surface Roughness after Bleaching with Diode Laser and Nd: YAG Laser. Journal of Lasers in Medical Sciences, 7 (3), 197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7: Wu T. T., Li L. F., Du R., Jiang L., Zhu Y. Q. (2013). Hydrogen peroxide induces apoptosis in human dental pulp cells via caspase-9 dependent pathway. Journal of endodontics, 39 (9), 1151-1155. [DOI] [PubMed] [Google Scholar]
- 8: Schuster L., Reichl F. X., Rothmund L., He X., Yang Y., Van Landuyt K. L., Högg C. (2016). Effect of Opalescence® bleaching gels on the elution of bulk-fill composite components. Dental Materials, 32 (2), 127-135. [DOI] [PubMed] [Google Scholar]
- 9: Ilie N., Hickel R. (2011). Resin composite restorative materials. Australian dental journal, 56 (s1), 59-66. [DOI] [PubMed] [Google Scholar]
- 10: Durner J., Obermaier J., Draenert M., Ilie N. (2012). Correlation of the degree of conversion with the amount of elutable substances in nanohybrid dental composites. Dental Materials, 28 (11), 1146-1153. [DOI] [PubMed] [Google Scholar]
- 11: Gajewski V. E., Pfeifer C. S., Fróes-Salgado N. R., Boaro L. C., Braga R. R. (2012). Monomers used in resin composites: degree of conversion, mechanical properties and water sorption/solubility. Brazilian dental journal, 23 (5), 508-514. [DOI] [PubMed] [Google Scholar]
- 12: Polydorou O., Beiter J., Konig A., Hellwig E., Kummerer K. (2009). Effect of bleaching on the elution of monomers from modern dental composite materials. dental materials, 25 (2), 254-260. [DOI] [PubMed] [Google Scholar]
- 13: Tabatabaee M. H., Arami S., Ghavam M., Rezaii A. (2014). Monomer release from nanofilled and microhybrid dental composites after bleaching. Journal of dentistry (Tehran, Iran), 11 (1), 56. [PMC free article] [PubMed] [Google Scholar]
- 14: Durner J., Obermaier J., Ilie N. (2014). Investigation of different bleaching conditions on the amount of elutable substances from nanohybrid composites. Dental Materials, 30 (2), 192-199. [DOI] [PubMed] [Google Scholar]
- 15: Örtengren U., Wellendorf H., Karlsson S., Ruyter I. E. (2001). Water sorption and solubility of dental composites and identification of monomers released in an aqueous environment. Journal of oral rehabilitation, 28 (12), 1106-1115. [DOI] [PubMed] [Google Scholar]
- 16: Goldberg M. (2008). In vitro and in vivo studies on the toxicity of dental resin components: a review. Clinical oral investigations, 12 (1), 1-8. [DOI] [PubMed] [Google Scholar]
- 17: Tanaka K., Taira M., Shintani H., Wakasa K., Yamaki M. (1991). Residual monomers (TEGDMA and Bis–GMA) of a set visible–light–cured dental composite resin when immersed in water. Journal of oral rehabilitation, 18 (4), 353-362. [DOI] [PubMed] [Google Scholar]
- 18: Volk J., Leyhausen G., Dogan S., Geurtsen W. (2007). Additive effects of TEGDMA and hydrogen-peroxide on the cellular glutathione content of human gingival fibroblasts. dental materials, 23 (8), 921-926. [DOI] [PubMed] [Google Scholar]
- 19: Reichl F. X., Simon S., Esters M., Seiss M., Kehe K., Kleinsasser N., Hickel R. (2006). Cytotoxicity of dental composite (co) monomers and the amalgam component Hg2+ in human gingival fibroblasts. Archives of toxicology, 80 (8), 465-472. [DOI] [PubMed] [Google Scholar]
- 20: Omrani L. R., Taher A., Albujeer A., Parvin M., Daryakenari G., Gorgani-Firuzjaee S., Chiniforush V. (2016). Penetration of hydrogen peroxide into the pulp chamber after conventional and laser-assisted bleaching. South African Dental Journal, 71 (2), 58-61. [Google Scholar]
- 21: Goharkhay K., Schoop U., Wernisch J., Hartl S., De Moor R., Moritz A. (2009). Frequency doubled neodymium: yttrium–aluminum–garnet and diode laser-activated power bleaching—pH, environmental scanning electron microscopy, and colorimetric in vitro evaluations. Lasers in medical science, 24 (3), 339-346. [DOI] [PubMed] [Google Scholar]
- 22: Basir M. M., Rezvani M. B., Chiniforush N., Moradi Z. (2016). Effect of CO2, Nd: YAG and Er: YAG Lasers on Microtensile Bond Strength of Composite to Bleached-Enamel. The open dentistry journal, 10, 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23: Sideridou I. D., Achilias D. S., Karabela M. M. (2007). Sorption kinetics of ethanol/water solution by dimethacrylate–based dental resins and resin composites. Journal of Biomedical Materials Research Part B: Applied Biomaterials, 81(1), 207-218. [DOI] [PubMed] [Google Scholar]
- 24: Polydorou O., Rogatti P., Bolek R., Wolkewitz M., Kümmerer K., Hellwig E. (2013). Elution of monomers from three different bonding systems and their antibacterial effect. Odontology, 101 (2), 170-176. [DOI] [PubMed] [Google Scholar]
- 25: Goldberg M., Grootveld M., Lynch E. (2010). Undesirable and adverse effects of tooth-whitening products: a review. Clinical oral investigations, 14 (1), 1-10. [DOI] [PubMed] [Google Scholar]
- 26: Sideridou I. D., Achilias D. S. (2005). Elution study of unreacted Bis–GMA, TEGDMA, UDMA, and Bis–EMA from light–cured dental resins and resin composites using HPLC. Journal of Biomedical Materials Research Part B: Applied Biomaterials, 74 (1), 617-626. [DOI] [PubMed] [Google Scholar]
- 27: Schuster L., Reichl F. X., Rothmund L., He X., Yang Y., Van Landuyt K. L., Hogg C. (2016). Effect of Opalescence® bleaching gels on the elution of bulk-fill composite components. Dental Materials, 32 (2), 127-135. [DOI] [PubMed] [Google Scholar]
- 28: Sakai K., Kato J., Kurata H., Nakazawa T., Akashi G., Kameyama A., Hirai Y. (2007). The amounts of hydroxyl radicals generated by titanium dioxide and 3.5% hydrogen peroxide under 405-nm diode laser irradiation. Laser Physics, 17 (8), 1062-1066. [Google Scholar]
- 29: Leprince J., Palin W. M., Mullier T., Devaux J., Vreven J., Leloup G. (2010). Investigating filler morphology and mechanical properties of new low–shrinkage resin composite types. Journal of oral rehabilitation, 37 (5), 364-376. [DOI] [PubMed] [Google Scholar]
- 30: Silva E. M. D., Almeida G. S., Poskus L. T., Guimarães J. G. A. (2008). Relationship between the degree of conversion, solubility and salivary sorption of a hybrid and a nanofilled resin composite. Journal of Applied Oral Science, 16 (2), 161-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31: Tabatabaei M. H., Sadrai S., Bassir S. H., Veisy N., Dehghan S. (2013). Effect of food stimulated liquids and thermocycling on the monomer elution from a nanofilled composite. The open dentistry journal, 7, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]


