Abstract
Background and Aims
Pandrug-resistant Acinetobacter baumannii (PDRAB) are including colistin resistant starins (CoRAB) which cause infections potentially untreatable infections. Recently, incidence of these strains are increasing worldwide. Therefore, new approaches, methods and strategies are urgently needed for treatment and eradication of infections due to PDRAB. So the aim of this study was to evaluate the efficacy of photodynamic therapy (PDT) in combination treatment with colistin against PDRAB.
Materials and Methods
PDRAB which was isolated from burn patients was used as a test strain. PDT carried out in which toluidine blue O (TBO) and light-emitting diode (LED) were used as photosensitizer and radiation source, respectively. Then, the effect of PDT plus colistin was evaluated on CoRAB and the colony-forming units of each tested groups calculated. Finally, confirmation of antibacterial activity of combination therapy was carried out using scanning electron microscope.
Results
PDT declined bacterial count in comparing with control group by 83.7% of killing percentage, in other words, less than one log reduction. While PDT in combination with colistin showed high synergetic effect against A. baumannii in all concentrations of colistin tested by 100% of killing percentage with 9-log reduction.
Conclusions
According to our results, PDT alone couldn't eliminate all of the treated bacterial cells. But when combined with colistin, it killed all of the treated bacterial cells in all tested concentrations. Also PDT decreased the minimal inhibitory concentration of colistin against PDRAB by more than 11 fold.
Keywords: Acinetobacter baumannii, colistin, photodynamic therapy, toluidine blue O, wound infection, pandrug resistance
Introduction
Burn and wound infections due to Acinetobacter baumannii are the major threat to global health. The treatment is remarkably difficult, not only developing extensive antimicrobial resistance but it can also form biofilms that resistant to host defense and antimicrobial treatment. Such causative factors, biofilm formation and resistance to antibiotics can lead to non-healing wounds 1). Also these infections can develope and cause systemic infections such as pneumonia, meningitis, and bloodstream infections 1–3). Therefore, burn infection due to A. baumannii has a high mortality rate 4, 5).
Multidrug-resistant (MDR) of A. baumannii has increased over the years and has become a major problem worldwide 6). A. baumannii is defined as MDR when the organism is resistant to one or more than one agent in three or more antimicrobial categories that would otherwise serve as treatments for A. baumannii infections 7). Because of development of resistance to most available antibiotics including betalactams, carbapenems, fluoroquinolones, and aminoglycosides among A. baumannii strains and lacks of development of new antimicrobial agents against this pathogen medical community have prompted to reuse of colistin for treatment of A. baumannii infections in many health care centers around the world. So, it is recommended to use colistin for treating XDR Acinetobacter infections 8, 9). The XDR-A. baumannii is non-susceptible to one or more than one agent in all but two or less than two antimicrobial categories.
The rise in colistin use for treatment of infections due to A. baumannii causes emergence of colistin resistance strains worldwide. Unfortunately, colistin resistant strains of A. baumannii several times have been reported 10, 11). A. baumannii is explained as pandrug-resistant (PDR) when it is non-susceptible to all antimicrobial agents 7). In this regard, PDR-A. baumannii (PDRAB) are including colistin resistant A. baumannii (CoRAB) strains whose infections are potentially untreatable. Recently, incidence of these strain are increasing worldwide 12). The highest resistance rates were seen in Asia, followed by Europe and America, and also the regions in which colistin resistance rates are continually increasing 12). In conclusion resistance to most antibiotics for treateatment of A. baumannii infections, this makes the treatment more difficult and cost ineffective. Therefore, new approaches, methods and strategies are urgently needed for treatment and eradication of severe infections due to highly drug resistant bacteria such as A. baumannii 13).
One of these strategies is photodynamic therapy (PDT) as a novel antimicrobial approach. This method commonly used for cancer and ophthalmological treatments. Today PDT has been used clinically for the treatment of skin infections caused by a variety of pathogens 14) such as treatment of ulcer and wound infection due to Pseudomonas aeruginosa 15) and acne bacteria-induced inflammation 16).
PDT works with generation of reactive oxygen species (ROS) through the use of a combination of oxygen, visible or near infrared light, and a photosensitizer (PS), a nontoxic dye that is photo reactive 17).
The advantages of this method are that interact with bacterial membrane, and it does not make selection of resistant strains after repeated cycles of treatment 17). Also, this method can target bacterial cells rather than host tissue with appropriate chemical design of the PS 17).
PSs are almost photo bactericidal dyes that form metachromatic complexes with lipopolysaccharides (LPS) in Gram negative bacteria. Interactions between the PSs and LPS might contribute in the mechanism of photo killing of Gram negative bacteria 18).
Also the target of colistin is LPS of Gram negative bacteria. Resistance to the colistin in A. baumannii has two molecular mechanisms. These include: mutations in the PmrAB which lead to alterations of the lipid A component of LPS, latter mutations in the lpxA, lpxC and lpxD genes resulting complete loss of LPS production 19). Commonly the cationic PSs are used against Gram negative bacteria which interact with LPS of bacterial cells. In colistin resistant strains with complete loss of the LPS or modified LPS structures, anionic polymers on the outer membrane can be a major target site for interaction with cationic PSs. Notably outer membrane proteins (OMPs) presented in outer membrane more than LPS. Therefore, significantly high amount of protein such as porins in the outer membrane of Gram negative bacteria 18) can be targeted by PDT. Therefore, our hypothesis was treatment of colistin resistant strains with PDT can increase sensitivity towards antibiotics (such as colistin). So the aim of this study was evaluation of PDT in combination with colistin against CoRAB.
Materials and methods
Photosensitizer and light source
TBO (Merck, Frankfurter, and Germany) was dissolved in dH2O to obtain a final concentration of 0.4 mg/mL. Then this solution was sterilized by 0.22-micron syringe filter and subsequently kept in the dark at 4 °C 20). The light-emitting diode (LED) (FotoSan 630 nm LAD, CMS dental, Denmark), at a wavelength of 635 nm with output power of 220 mW was used as a light source.
Bacterial strain
In this study a PDRAB isolated from burn patient was used 21). This strain is resistant to amikacin, ampicillinsulbactam, cefepime, ceftazidime, ciprofloxacin, colistin, gentamicin, imipenem, levofloxacin, meropenem, minocycline, piperacillin, piperacillintazobactam, rifampicin, tetracycline, tobramicin, and trimethoprim-sulfamethoxazole. The minimal inhibitory concentration (MIC) value of colistin was > 32 µg/mL. For further analysis A. baumannii strain was cultured in tripticase soy broth (TSB) (Himedia, India) and was stored in −70 °C, and then freshly sub-cultured on brain heart infusion (BHI) (Himedia, India) agar prior to assay.
In our experiment, a study sample is divided into four groups including: 1. A. baumannii which grown on Cation-Adjusted Mueller Hinton broth (CAMHB; Himedia, India,), 2. A. baumannii which grown on CAMHB containing colistin (Sigma-Aldrich, Germany) (0.03 to 32 µg/mL), 3. A. baumannii which treated with PDT, then grown on CAMHB, 4. A. baumannii which treated with PDT, then grown on CAMHB containing colistin (0.03 to 32 µg/mL) (Table 1) .
Table 1: Tested group for each assay.
| Groups | Description |
|---|---|
| 1 | MHBa + A. baumannii b |
| 2 | MHB + Colc + A. baumannii |
| 3 | MHB + A. baumannii treated with PDTd |
| 4 | MHB + Col + A. baumannii treated with PDT |
Mueller hinton broth
Acinetobacter baumannii
Colistin
Photodynamic therapy
For PDT experiments, one colony of CoRAB isolate was used for inoculation in 5 mL of CAMHB. The culture was incubated for 6 h at 37 °C with aeration at 200 rpm. Following incubation, then the number of cells in culture was adjusted to 1.5×108 CFU/mL as verified by spectrophotometry (optical density [OD] 600: 0.08–0.11).
PDT experiments
Aliquots of 100 µL of bacterial suspensions (2.0×106 CFU/mL) were placed in a 96-well microtiter plate (TPP, Trasadingen, Switzerland), then incubated with 100 µL TBO at a final concentration of 0.1 mg/mL in the dark and at room temperature for 5 min and exposed to LED for 3min 20).
For determination of MIC value of colistin (Sigma-Aldrich) against treated group (PDT with/ without colistin and vice versa) (Table 1) of A. baumannii, broth microdilution testing was carried out according to Clinical and Laboratory Standards Institute (CLSI) procedures using CAMHB (0.03 to 32 µg/mL) 22).
In this regard, five groups were used in present study including: 1. The wells in column 1 which contain CAMHB and inoculated by CoRAB; 2. The wells in column 2 which contain CAMHB and colistin (0.03 to 32 µg/mL) then inoculated by CoRAB as a control group; 3. The wells in column 3 which contain CAMHB and inoculated by PDT treated CoRAB; 4. The wells in column 4 which contain CAMHB and colistin (0.03 to 32 µg/mL), then inoculated by PDT treated CoRAB as a combination therapy; 5. The wells in column 5 which contain CAMHB and colistin (0.03 to 32 µg/mL) without bacteria as negative control. The colony-forming units (CFUs)/mL of test wells was calculated using Miles and Misra Method 23).
Killing percentage and log reduction analysis
Eq. [1] was used to calculate the killing efficacy as kill percentage (%). Also, the log population reduction of the test organism was obtained using Eq [2]. Regarding the standard method, at least a one log reduction of bacterial load is required to consider an antibacterial property. The wells in column 2 which contain colistin serial dilution and inoculated by CoRAB was used as control group 24). All experiments were repeated three times.
 |
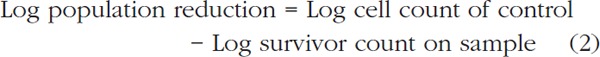 |
Scanning electron microscope (SEM) analysis
For confirmation of efficacy of combination therapy SEM was carried out as described previously 25). Briefly, the specimen was coated with gold-palladium by a sputter coater (Bal-Tec SCD 005, Netherland) and examined with a SEM (5800LV, JEOL, Japan).
Statistical analysis
All data analyzed by SPSS v.22. One-way ANOVA was used for significant differences between groups. A P value < 0.05 was accepted as statistically significant.
Result
Our analysis showed that colistin couldn't decrease significantly the CoRAB CFU/mL in comparing with media which without colistin and inoculated by CoRAB (without colistin: 8×108 CFU/mL and colistin treated A. baumannii: 6.0×108 CFU/mL) (pv≈0.5). Killing percentage of colistin against A. baumannii was 25% but in the last concentration (32 µg/mL). PDT decreased bacterial CFU/mL in compering to group 1 (13×107 CFU/mL), and decreased CFU/mL more than colistin alone (group 2), but significant differences were not seen (pv≈0.14 and pv≈0.28, respectively). Killing percentage of PDT was 83.7% whereas the log population reduction was less than one log reduction (Fig. 1, 2). But PDT in combination with colistin showed high synergistic effect against A. baumannii in all tested concentrations of colistin (killing percentage 100%). Moreover PDT decreased the MIC value of colistin by more than 11 fold. In this regard, MIC value decreased from > 32 to < 0.03 µg/mL, which it's the colistin susceptible pattern according to CLSI guideline 22).
Figure 1:

Killing percentage of different treatments against A. baumannii. (colistin: treatment of colistin aginst A. baumannii, PDT: photodynamic therapy against A. baumannii, PDT+ colistin: combination therapy of PDT and colistin against A. baumannii).
Figure 2:
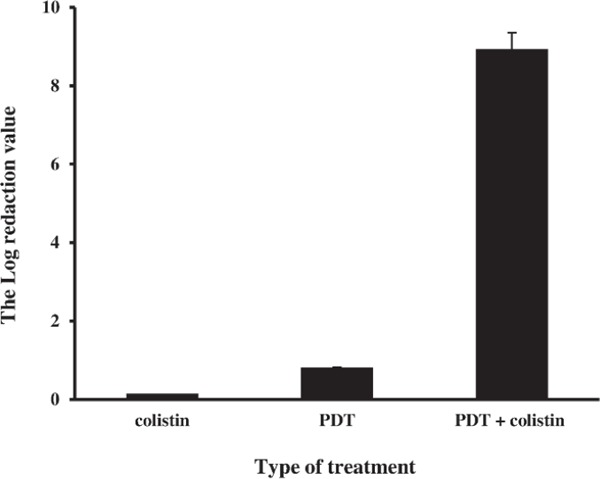
Population reduction of each assay against A. baumannii. (colistin: treatment of colistin aginst A. baumannii, PDT: photodynamic therapy against A. baumannii, PDT+ colistin: combination therapy of PDT and colistin against A. baumannii).
SEM analysis showed that any bacterial growth were not seen in PDT plus colistin treated group in all tested concentration, while the colistin treated group produced thin layer of biofilm (Fig. 3).
Figure 3:

Scanning electron microscope images of A. baumannii. a) Control biofilm growth without treatment, b) PDT+ colistin: combination therapy of PDT and colistin against A. baumannii, c) colistin: treatment of colistin aginst A. baumannii.
Discussion
In present study, we evaluated the effect of PDT in combination with antibiotic therapy (colistin) for treatment of PDRAB which isolated from burn patients. Today, colistin is used as the last line of drug for treatment of A. baumannii infections. Nevertheless, infections caused by strains with resistance to this antibiotic have increased worldwide 7–10).
According to our results, PDT alone couldn't eliminate all of the treated A. baumannii. But when combined with colistin, it killed all of the treated bacterial cells in all of colistin concentrations CoRAB. The mechanisms that how PDT has synergy effects with colistin yet unknown.
Pourhajibagher et al. has been reported that PDT effects similar to EDTA, which cause alternation of permeability of the OMPs of A. baumannii, these proteins are responsible for nutrient, drugs and ions selective permeability and also responsible for resistance against variety of drugs 25). It has been demonstrated that cationic PSs interact with LPS own of its negative charge. Also it has been showed that in CoRAB, LPS not expressed 19). According to Usacheva et al. revealed that, OMPs presented in outer membrane more than LPS. In this situation anionic polymer on the outer membrane besides LPS such porins are also capable to interact with the cationic PSs 18). Also PSs can enter the bacterial cytosol by other mechanisms including the self-promoted uptake pathway and protein transport machineries which present in the bacterial cells envelope. In this regard the ‘porin’ class of protein transporters facilitate the uptake of low molecular weight (600–700 Da) hydrophilic compounds like PSs 26). Therefore it is probable that inactivation of OMPs and porins due to PDT, may lead to passing antibiotics across the bacterial membrane, resulting reduction in the MIC value of these materials.
As described above, colistin causes bacterial cells death directly through membrane lysis 27). Sampson et al. showed that, killing effect of colistin was increased in presence of ROS even in colistin resistant strains 27). Also they showed that colistin is able to both induce hydroxyl radicals and kill A. baumannii through hydroxyl radical production 27). On the other hand, PDT works with ROS production 17). Also another effect of PDT on bacterial cells is mediating membrane disruption and increasing permeability 17). In this situation colistin molecules can direct in to bacterial cells easily and interact and inactive its targets through oxidative cell death pathway 27). Also Hood et al. showed when A. baumannii treated with colistin and efflux pump inhibitor, caused bacterial death more than colistin alone 28). Therefore, in addition to LPS, efflux pump also is another mechanism for resistance to colistin. It has been reported that PDT cause disruption of efflux pump integrity and prevents effluxing of certain antimicrobials, such as minocycline, tetracycline, and tigecycline which lead to bacterial cells death.
Finally, when A. baumannii is treated with PDT, LPS and efflux pumps destabilize and colistin can penetrate in to the bacterial cells. Importantly, the type of PS is a major factor in PDT. For example, cationic dyes are effective against Gram negative bacteria than anionic dyes. In this regard TBO has a greater interaction with LPS than other dyes. Because TBO unlike other dyes forms higher aggregates on the surface of LPS. Among cationic dyes TBO not only have a greater affinity for LPS than other cationic dyes, but it also causes more bacterial photo damage 18). Also it has been demonstrated that TBO-mediated photo killing through the OMPs which probably are involved in photo killing 18). Photodynamic inactivation of Gram negative bacteria was overcome either by using cationic PS, or by combining the PS with positively charged antibiotics such colistin 26). In this regard cationic PS can interact with LPS, anionic proteins, porins and efflux pumps and destabilize the bacterial membrane 18, 26) which lead to PS and colistin entering into the cells and makes cell death.
Recently, effects and mechanisms of action of PDT in combination with antibiotics are described 17). For example, Cahan et al. showed that treating of Gram negative and Gram positive bacteria with PS-antibiotic conjugates has a good bactericidal activity 29).
Almeida et al. indicated that PDT in combination with antibiotics can kill MDR bacteria in hospital wastewaters 30). Barra et al. showed that PDT in combination with gentamycin is highly effective against pathogenic bacteria, also in low dose of gentamycin is effective strategy against biofilms 31). These data support our hypothesis which says PDT disrupts membrane integrity that allowes entering antibiotics into bacterial cells.
Also the researches that studied PDT effect in combination with antibiotics to fight bacterial biofilms published recently 32, 33). In present study SEM analysis showed that PDT prevents biofilm formation of A. baumannii. Kashef et al. conducted the study that sub lethal PDT and some antibiotics cause resistance to erythromycin, amoxicillin-clavulanate and amikacin. But our study result was contrary to this study 34). Recently, it has been reported that PDT dose has no influence on enzymatic resistant mechanisms, because it interact with selective OMPs more than enzymatic activities 26).
Conclusion
In conclusion, our analysis showed that combination of PDT and colistin is highly effective against PDRAB. Therefore, it seems that this strategy can be used against drug resistant bacteria in localized infections. But further studies should conduct for determination of its efficacy in clinics.
References
- 1: Mody L, Gibson KE, Horcher A, Prenovost K, McNamara SE, Foxman B, et al. (2015): Prevalence of and risk factors for multidrug-resistant Acinetobacter baumannii colonization among high-risk nursing home residents. Infection Control & Hospital Epidemiology, 36:1155 - 1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2: Opazo A, Vali L, Al Obaid K, Dashti AA, Amyes SG. (2014): Novel genetic structure harbouring blaPER-1 in ceftazidime-resistant Acinetobacter baumannii isolated from Kuwait. International journal of antimicrobial agents, 43:383 - 384. [DOI] [PubMed] [Google Scholar]
- 3: Potron A, Poirel L, Nordmann P. (2015): Emerging broad-spectrum resistance in Pseudomonas aeruginosa and Acinetobacter baumannii: mechanisms and epidemiology. International journal of antimicrobial agents, 45:568 - 585. [DOI] [PubMed] [Google Scholar]
- 4: Pourhajibagher M, Hashemi FB, Pourakbari B, Aziemzadeh M, Bahador A. (2016): Antimicrobial Resistance of Acinetobacter baumannii to Imipenem in Iran: A Systematic Review and Meta-Analysis. Open Microbiology journal, 10:32 - 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5: Opazo A, Sonnevend A, Lopes B, Hamouda A, Ghazawi A, Pal T, et al. (2012): Plasmid-encoded PER-7 β-lactamase responsible for ceftazidime resistance in Acinetobacter baumannii isolated in the United Arab Emirates. Journal of antimicrobial chemotherapy, 671619 - 1622. [DOI] [PubMed] [Google Scholar]
- 6: Moradi J, Hashemi FB, Bahador A. (2015): Antibiotic resistance of Acinetobacter baumannii in Iran: a systemic review of the published literature. Osong public health and research perspectives, 30:79 - 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7: Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. (2012): Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect, 18: 268 - 281. [DOI] [PubMed] [Google Scholar]
- 8: Liu Q, Li W, Feng Y, Tao C. (2014): Efficacy and safety of polymyxins for the treatment of Acinectobacter baumannii infection: a systematic review and meta-analysis. PloS one, 9:e98091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9: Kassamali Z, Jain R, Danziger LH. (2015): An update on the arsenal for multidrug-resistant Acinetobacter infections: polymyxin antibiotics. International Journal of Infectious Diseases, 30:125- 132. [DOI] [PubMed] [Google Scholar]
- 10: Qureshi ZA, Hittle LE, O'Hara JA, Rivera JI, Syed A, Shields RK, et al. (2015): Colistin-resistant Acinetobacter baumannii: beyond carbapenem resistance. Clinical Infectious Diseases, 60:1295 - 1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11: Beceiro A, Moreno A, Fernández N, Vallejo JA, Aranda J, Adler B, et al. (2014): Biological cost of different mechanisms of colistin resistance and their impact on virulence in Acinetobacter baumannii. Antimicrobial agents and chemotherapy, 58:518 - 526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12: Bialvaei AZ, Samadi Kafil H. (2015): Colistin, mechanisms and prevalence of resistance. Current medical research and opinion, 31:707 - 721. [DOI] [PubMed] [Google Scholar]
- 13: Singh R, Smitha MS, Singh SP. (2014): The role of nanotechnology in combating multi-drug resistant bacteria. Journal of nanoscience and nanotechnology, 14:4745 - 4756. [DOI] [PubMed] [Google Scholar]
- 14: Morton CO, Chau M, Stack C. (2014): In vitro combination therapy using low dose clotrimazole and photodynamic therapy leads to enhanced killing of the dermatophyte Trichophyton rubrum. BMC microbiology, 14:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15: Lei X, Liu B, Huang Z, Wu J. (2015): A clinical study of photodynamic therapy for chronic skin ulcers in lower limbs infected with Pseudomonas aeruginosa. Archives of dermatological research, 307:49 - 55. [DOI] [PubMed] [Google Scholar]
- 16: Jeon YM, Lee HS, Jeong D, Oh HK, Ra KH, Lee MY. (2015): Antimicrobial photodynamic therapy using chlorin e6 with halogen light for acne bacteria-induced inflammation. Life sciences, 124:56 - 63. [DOI] [PubMed] [Google Scholar]
- 17: García-Quintanilla M, Pulido MR, López-Rojas R, Pachón J, McConnell MJ. (2013): Emerging therapies for multidrug resistant Acinetobacter baumannii. Trends in microbiology2, 1:157-163. [DOI] [PubMed] [Google Scholar]
- 18: Usacheva MN, Teichert MC, Sievert CE, Biel MA. (2006): Effect of Ca+ on the photobactericidal efficacy of methylene blue and toluidine blue against gram–negative bacteria and the dye affinity for lipopolysaccharides. Lasers in surgery and medicine, 38:946 - 954. [DOI] [PubMed] [Google Scholar]
- 19: Cai Y, Chai D, Wang R, Liang B, Bai N. (2012): Colistin resistance of Acinetobacter baumannii: clinical reports, mechanisms and antimicrobial strategies. Journal of antimicrobial chemotherapy, 67: 1607 - 1615. [DOI] [PubMed] [Google Scholar]
- 20: Pourhajibagher M, Chiniforush N, Raoofian R, Ghorbanzadeh R, Shahabi S, Bahador A. (2016): Effects of sub-lethal doses of photo-activated disinfection against Porphyromonas gingivalis for pharmaceutical treatment of periodontal-endodontic lesions. Photodiagnosis and Photodynamic Therapy, 16:50 - 53. [DOI] [PubMed] [Google Scholar]
- 21: Bahador A, Raoofian R, Farshadzadeh Z, Beitollahi L, Khaledi A, Rahimi S, et al. (2015): The Prevalence of ISAba1 and ISAba4 in Acinetobacter baumannii Species of Different International Clone Lineages Among Patients With Burning in Tehran, Iran. Jundishapur journal of microbiology, 8: e17167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22: Clinical and Laboratory Standards Institute (2014): In Performance standards for antimicrobial susceptibility testing; 16th informational supplement. Clinical and laboratory institute, editor. wayne, Penncylvania. [Google Scholar]
- 23: Miles AA, Misra SS, Irwin JO. (1938): The estimation of the bactericidal power of the blood. Journal of Hygiene, 38:732 - 749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24: Doulabi AH, Mirzadeh H, Imani M, Samadi N. (2013): Chitosan/polyethylene glycol fumarate blend film: Physical and antibacterial properties. Carbohydrate polymers, 92:48 - 56. [DOI] [PubMed] [Google Scholar]
- 25: Pourhajibagher M, Boluki E, Chiniforush N, Pourakbari B, Farshadzadeh Z, Ghorbanzadeh R, et al. (2016): Modulation of virulence in Acinetobacter baumannii cells surviving photodynamic treatment with toluidine blue. Photodiagnosis and Photodynamic Therapy, 15:202 - 212. [DOI] [PubMed] [Google Scholar]
- 26: George S, Hamblin MR, Kishen A. (2009): Uptake pathways of anionic and cationic photosensitizers into bacteria. Photochemical & Photobiological Sciences, 8:788 - 795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27: Sampson TR, Liu X, Schroeder MR, Kraft CS, Burd EM, Weiss DS. (2012): Rapid killing of Acinetobacter baumannii by polymyxins is mediated by a hydroxyl radical death pathway. Antimicrobial agents and chemotherapy, 56:5642 - 5649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28: Hood MI, Jacobs AC, Sayood K, Dunman PM, Skaar EP. (2010): Acinetobacter baumannii increases tolerance to antibiotics in response to monovalent cations. Antimicrobial agents and chemotherapy, 54:1029 - 1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29: Cahan R, Swissa N, Gellerman G, Nitzan Y. (2010): Photosensitizer–antibiotic conjugates: A novel class of antibacterial molecules. Photochemistry and photobiology, 86:418 - 425. [DOI] [PubMed] [Google Scholar]
- 30: Almeida J, Tomé JP, Neves MG, Tomé AC, Cavaleiro JA, Cunha Â, et al. (2014): Photodynamic inactivation of multidrug-resistant bacteria in hospital wastewaters: influence of residual antibiotics. Photochemical & Photobiological Sciences, 13:626 - 633. [DOI] [PubMed] [Google Scholar]
- 31: Barra F, Roscetto E, Soriano AA, Vollaro A, Postiglione I, Pierantoni GM, et al. (2015): Photodynamic and antibiotic therapy in combination to fight biofilms and resistant surface bacterial infections. International journal of molecular sciences, 16:20417 - 20430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32: Vatansever F, de Melo WC, Avci P, Vecchio D, Sadasivam M, Gupta A, et al. (2013): Antimicrobial strategies centered around reactive oxygen species–bactericidal antibiotics, photodynamic therapy, and beyond. FEMS microbiology reviews, 37:955 - 989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33: Almeida J, Tomé JP, Neves MG, Tomé AC, Cavaleiro JA, Cunha Â, et al. (2014): Photodynamic inactivation of multidrug-resistant bacteria in hospital wastewaters: influence of residual antibiotics. Photochemical & Photobiological Sciences, 13:626 - 633. [DOI] [PubMed] [Google Scholar]
- 34: Kashef N, Akbarizare M, Kamrava SK. (2013): Effect of sub-lethal photodynamic inactivation on the antibiotic susceptibility and biofilm formation of clinical Staphylococcus aureus isolates. Photodiagnosis and photodynamic therapy, 10:368 - 373. [DOI] [PubMed] [Google Scholar]


