Abstract
Platelet-rich plasma (PRP) has emerged as a new treatment modality in regenerative plastic surgery and dermatology. PRP is a simple, cost-effective and feasible treatment option with high patient satisfaction for hair loss and can be regarded as a valuable adjuvant treatment modality for androgenic alopecia and other types of non-scarring alopecias. Authors have proposed a hair model termed “Golden anchorage with ‘molecular locking’ of ectodermal and mesenchymal components for survival and integrity of hair follicle (HF)” in this article. Golden anchorage comprises of bulge stem cells, ectodermal basement membrane and bulge portion of APM. PRP with its autologous supply of millions of growth factors works on ‘Golden anchorage’ along with keratinocytes (PDGF), dermal papilla (IGF and fibroblast growth factor), vasculature (VEGF and PDGF) and neural cells (Nerve Growth Factor) in a multipronged manner serving as an ‘elixir’ for hair growth and improving overall environment.
Keywords: Platelet-rich plasma (PRP), platelet growth factors, alopecia, androgenetic alopecia
Introduction
Platelet-rich plasma (PRP) is autologous concentration of platelets contained in small volume of plasma which accelerates the rejuvenation of skin and hair follicles (HFs) due to presence of various growth factors and cellular adhesion molecules (1).
Synonyms: autologous platelet gel, plasma-rich growth factors, platelet-concentrated plasma, platelet-rich concentrate, platelet releasate (2,3).
It is the volume of plasma fraction of autologous blood with an above baseline platelet concentration (usually more than 1,000,000 platelets/µL) leading to 300–700% enrichment (4,5).
PRP therapy is a relatively new approach to tissue regeneration which is becoming a valuable adjunct to promote healing during many procedures in various medical and surgical fields, including wound healing, maxillofacial surgery, soft tissue injuries, periodontal and oral surgery, orthopaedic and trauma surgery, otolaryngology, cardiovascular surgery, gastrointestinal surgeries, burns, cosmetic and plastic surgery. PRP specifically has attracted the attention of dermatologists for management of various alopecias (6).
PRP is derived from the centrifugation of the patient’s own blood and it contains growth factors working on different target cells that influence wound healing, thereby playing an important role in tissue repair mechanisms, enhancing soft tissue healing and regeneration at various levels (6).
PRP contains more than 20 types of growth factors, such as platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), and transforming growth factor (TGF-β1). These factors regulate cell migration, proliferation, remodelling of the extracellular matrix (ECM) and promotion of angiogenesis, creating a beneficial environment for enhanced wound healing (4,7). More than 1 million/µL of platelet concentration (about four to seven times the mean levels) is therapeutically effective concentration. A bell-shaped response curve indicating a dose dependent nature is associated with PRP (4). Concentrations higher or lower than1.5 million platelets/µL inhibit the angiogenic potential in human endothelial cells. PRP should be used at the concentrations of five to ten times the mean levels.
The platelets actively secrete growth factors within 10 minutes after activation and more than ninety five percent of the presynthesized growth factors are secreted within 1 hour (8,9).
Therefore, PRP should be used within 10 minutes of activation. The viability of the concentrated platelets remains for up to 8 hours and they stay sterile if placed on a sterile surgical table (3).
The platelets remain viable for 7–10 days and the release of growth factors in the tissue continues during this period (10).
Giusti et al. found that the optimal platelet concentration for the induction of angiogenesis in human endothelial cells was 1.5 million platelets per microliter and excessively high concentrations of platelets decrease the angiogenic potential (11).
Classification
DohanEhrenfest et al. (12) proposed a classification according to which platelet concentrates can be classified into four main families depending on their cell content and fibrin architecture:
Pure PRP (P-PRP) or leucocyte-poor platelet rich plasma-preparations which are leucocyte poor and have a low-density fibrin network on activation;
Leucocyte- and platelet rich plasma (L-PRP) products-preparations with leucocytes and with formation of low-density fibrin network on activation;
Pure platelet-rich fibrin (P-PRF) or leucocyte-poor platelet-rich fibrin-preparations which are leucocyte poor and with a fibrin network which is high-density. P-PRP is mixed with an activator and a specific separator gel is used (3,12);
Leucocyte- and PRF (L-PRF) or second-generation PRP products—preparations with leucocytes and a high-density fibrin network. For the formulation of L-PRF, blood is centrifuged immediately after collection without any anticoagulant, thrombin or CaCl2 (3,12).
Our method: we use Y Cell Bio kit and REMI centrifuge for preparation of PRP. Two Vials, each containing 13.5 mL of whole blood mixed with 1.5 mL of ACD-A solution are used and centrifuged for 4 min at 3,000 rpm (revolutions per minute). Out of these two vials, a total of 5–6 mL of buffy coat along with PRP is harvested and injected as per requirement. It gives 5–7 times the concentration of the baseline platelet count.
Contraindications (13)
-
Absolute contraindications:
❖ Platelet dysfunction syndrome;
❖ Critical thrombocytopenia;
❖ Hemodynamic instability;
❖ Septicaemia;
❖ Local infection at the site;
❖ Patient unwilling to sign consent.
-
Relative contraindications:
❖ Consistent use of NSAIDs within 48 hours of procedure;
❖ Corticosteroid injection at treatment site within 1 month;
❖ Systemic use of corticosteroids within 2 weeks;
❖ Use of tobacco;
❖ Recent fever/illness;
❖ Cancer-especially hematopoietic or bone;
❖ Haemoglobin<10 g/dL;
❖ Platelet count <105/µL.
Complications
PRP is a relatively safe procedure. Though minor complications are mentioned as pain in the injected area; headache, heaviness of head, swelling, redness, infection, allergic reaction-urticarial rash, temporary skin discoloration, bruising etc. (14).
Anatomy and physiology of hair growth
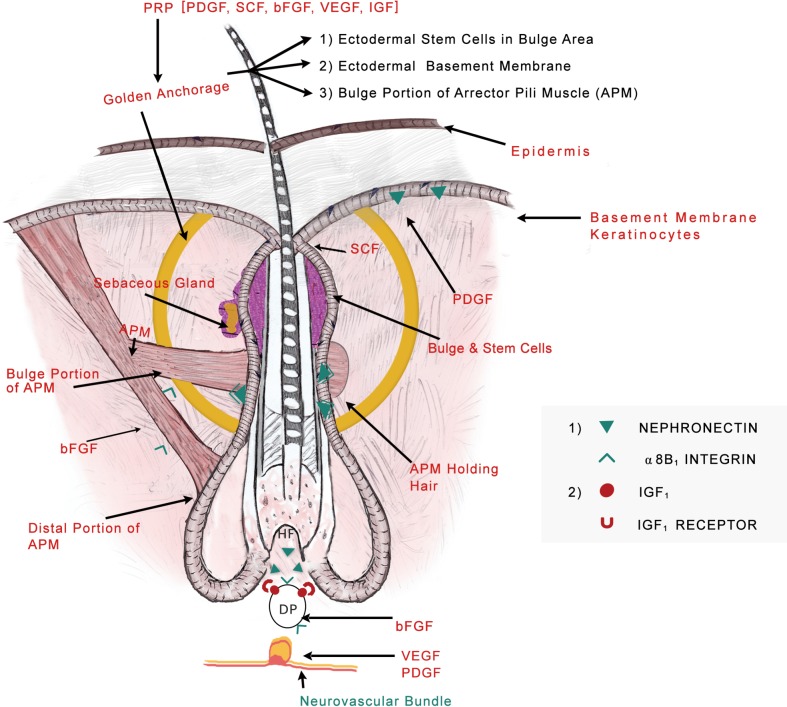
The most interesting fact in origin of hair is that it arises from amalgamation of ectodermally derived structures giving rise to follicular unit and sebaceous glands and mesoderm tissues forming dermal papillae (DP) which gives rise to arrector pili muscle (APM) and adipocytes. Dermal papilla is made up of specialized fibroblast-like cells embedded in an ECM rich in basement membrane proteins and proteoglycans (15).
In the bulge area, primitive stem cells of ectodermal origin are found, which express a number of ECM proteins, one of which is nephronectin with five EGF-like repeats. Nephronectin is considered to be mediator of epidermal interactions with the mesenchymal cells as its receptor is α8β1 integrin which is expressed in DP and APM (16).
Authors believe deposition of nephronectin in basement membrane creates a unique niche leading to attachment between bulge region of ectodermal HF and mesenchymal APM and DP and this may prove as a “golden anchorage” for HF survival and integrity.
Interactions between these two kinds of cells (DP and Bulge area) as well as with binding growth factors (PDGF, TGF-β, and VEGF) activate the proliferative phase of the hair, giving rise to the future follicular unit (17).
During anagen, HF stem cells further divide leading to formation of transient amplifying cells which forms the ORS of lower part of HF and migrate in a downward direction. On entering the hair bulb matrix, they proliferate and differentiate to form hair shaft and IRS and may also form sebaceous glands (15).
Reciprocal interactions between epidermal stem cells, dermal papilla cells and epidermal basement membrane are essential for HF formation and maintenance (16).
APM arises proximally at the HF at the bulge, which is an epithelial stem cell niche. The APM bulge connection persists throughout hair growth cycle and plays a vital role in morphogenesis and renewal of HFs. Preservation of APM may be associated with reversible hair loss while loss of attachment between APM and HF may be associated with irreversible or partially reversible hair loss (18,19).
Considering the role of APM in maintaining follicular integrity, Poblet et al. described APM as “the ribbon on a bunch of flowers” holding together HFs in a follicular unit (20,21).
We propose the extension of Poblet’s model (21) of “Ribbon on bunch of flowers” combined with “Golden Anchorage with molecular locking of ectodermal and mesenchymal components”. The hypothesis says that the epidermal stem cells underlying the APM are analogous to pebbles, while ectodermal basement membrane component is like the soil for survival of the follicular unit. The flower (HF) can only survive if held by ribbon and surrounded by soil and pebbles. Hence the “ribbon on bunch of flowers” can survive if held by “Golden Anchorage with molecular locking of ectodermal and mesenchymal components” where ‘Golden anchorage’ comprises of stem cells, ectodermal basement membrane and portion of APM attached to bulge region and also the latter ‘molecular locking’ of nephronectin with α8B1 integrin receptor needs to be maintained to hold the ectodermal and mesenchymal components together for follicular integrity and survival (Figure 1).
Figure 1.
Proposed model of “Golden anchorage and molecular locking of ectodermal and mesenchymal components for hair follicle integrity and survival”. The role of various PRP growth factors acting on different targets for hair follicle survival is also highlighted.
As soon as the nephronectin from the soil i.e., basement membrane is lost, the pebbles (stem cells) start disappearing, leading to downregulation of dermal papillary signals to APM eventually causing production of solely adipocytes. This might be an explanation for infiltration of fatty tissue in AGA as found by Torkamani et al. (22).
Overview of alopecia
Scalp hair complete the body self-image and patients who have alopecia suffer from overt disfiguration, which may cause psychosocial embarrassment and lack of self-esteem (23).
Hair loss or alopecia is one of the most common complaints amongst the patients consulting a dermatologist and can be temporary or long lasting (24).
Alopecia can be classified into two broad categories—non-cicatricial and cicatricial alopecia. The causes of non-cicatricial alopecias include androgenetic alopecia, telogen effluvium, alopecia areata, trichotillomania, anagen effluvium etc. Lichen planopilaris, frontal fibrosing alopecia, folliculitis decalvans, cutaneous discoid lupus erythematosus etc. are few of the various conditions causing cicatricial alopecia (25).
Various treatment modalities traditionally exist in the armamentarium of a clinician for management of alopecias. Therapeutic interventions for androgenetic alopecia include topical minoxidil, oral finasteride and dutasteride, peptides and hair transplant surgery (26).
High protein diet, iron supplements, exclusion of drugs which induce catagen like beta-blockers, retinoids, anticoagulants, diagnosis and treatment of catagen-inducing endocrine disorders (thyroid dysfunction, hyperandrogenism, or hyperprolactinemia) help in management of telogen effluvium (27).
An adequate evaluation including detailed dietary history and management is essential for appropriate patient care and successful treatment of alopecia (24).
Androgenetic alopecia
Epidemiology
Androgenetic alopecia is the most common type of baldness clinically characterized by progressive hair loss. Highest prevalence is found in Caucasians affecting up to 80% Caucasian men and 40% women. A population based study comprising of 1,005 Indian males aged 30–50 years showed a 58% prevalence of AGA. The frequency of AGA increases with age, even though it may start at puberty (26,28).
Pathogenesis and presentation
AGA occurs due to HF miniaturization within the follicular units. There is progressive reduction in diameter, pigmentation and length of the hair shaft. The miniaturized hair is the hallmark of AGA (24).
Currently, the Hamilton-Norwood classification system for males and the Ludwig system for females are most commonly used for the description of pattern of hair loss (29).
Female patterned hair loss (FPHL) is a non-scarring diffuse alopecia, evolving from the progressive miniaturization of HFs and subsequently leading to reduction of the number of hairs, especially in the central, frontal and parietal scalp regions (30).
Olsen observed that hair loss in women may occur in a subtle pattern which becomes apparent only when one performs a midline part as there is often a progressive decrease in hair density from vertex to the front of the scalp, also known as the “Christmas tree” distribution of loss (29).
Multiple compound follicular units comprising a primary follicle and several secondary follicles are present across the scalp. In early stages of the hair loss, patients usually complain of thinning of hair and a decrease in the pony tail volume, but there is little visible baldness. Miniaturization first occurs in the secondary follicles. APM initially loses attachment to the regressing secondary follicles in only some follicular units also known as “herald units”. The muscle still remains attached to the primary follicle at this stage (20).
With progression of the disease, miniaturization continues and the muscle completely loses attachment to the secondary follicles. Later, primary follicles in herald units are also affected by miniaturization, and eventually muscle attachment is lost. Baldness occurs when the entire follicular unit is miniaturized. Similar pattern of miniaturization and muscle loss continues until all follicular units are affected and there is visible baldness at this stage (20).
The interaction between the APM and the follicle mesenchymal might be an essential part of the HF cycle. The DP and dermal sheath include a population of mesenchymal stem cells that contribute to the follicle homeostasis (20).
There are two main schools of thoughts in pathogenesis of androgenetic alopecia. One is the coordinated follicular cycling with movement of cells between the DP and dermal sheath. This process is thought to be disrupted in AGA to cause the loss of cells from the DP and consequent follicle miniaturization. Cells of DP and dermal sheath are capable of undergoing both smooth muscle and adipose differentiation in vitro. Thus, cells from the follicle mesenchyme may also contribute to the APM maintenance. The muscle degeneration seen in AGA may be caused by the loss of progenitor cell population which is responsible for maintaining both the APM and the DP (20).
The other proposed pathogenesis is that nephronectin is an important mediator of epidermal interactions with mesenchymal cells, as the nephronectin receptor is α8β1 integrin which is expressed in the dermal papilla and APM. Loss of nephronectin or α8 integrin expression leads to delocalization of APM leading to miniaturization (16).
In their study, Torkamani et al. found that the APM was degenerated and replaced by adipose tissue in all AGA specimens. Muscle volume was decreased and fat volume increased significantly in AGA compared with controls (22).
Treatment
Treatment options for androgenic alopecia are limited and include topical minoxidil and oral finasteride (FDA approved) alone or in combination. Several reported side effects such as headache and increase in body hair are there for minoxidil whereas loss of libido has been reported with oral finasteride. Incidence of loss of libido varies from 3.1% to 5.4% (31). Finasteride also interferes with genital development in male fetus and is contraindicated in pregnant women and those likely to become pregnant (32).
PRP has emerged as a new treatment modality in regenerative plastic surgery, and dermatology and increasingly more literature suggests that it might have a beneficial role in hair regrowth (17).
PRP in androgenetic alopecia
PRP is autologous, with no potential side effects compared to pre-existing therapeutic modalities and/or high degree of compliance. Also, as the mechanism of action of PRP is different from other treatments, an additive positive effect may be there on using PRP as an adjuvant to minoxidil and finasteride (33).
Clinical evidence
Singhal et al. found that by the end of 3 months, all ten androgenetic alopecia patients treated with PRP had a good hair growth with reduction in number of pulled out hair by average 65%. New hair growth was observed in 6 patients as early as 7 days and in 4 patients in 15 days (34).
In an another study on PRP in 11 AGA patients, the hair pull test after four PRP sessions (once in two weeks) became negative in 9 patients. Moderate improvement in hair volume and coverage was reported (32).
Greco et al. in their study, involving 5 patients who were given PRP therapy and 5 patients in non PRP group (10 AGA patients in total) concluded that PRP as mesotherapy in AGA leads to a significant increase in hair diameter and hair density (35).
Uebel et al. observed a significant improvement in hair density and stimulation of growth on pre-treatment of follicular units with platelet plasma growth factors before their implantation. There was a significant difference in the yield of follicular units on comparing the experimental and control areas of the scalp (36).
Intra-operative PRP therapy just after slitting is beneficial in giving faster density, reducing the catagen loss of transplanted hair, recovering the skin faster and activating dormant follicles in FUE transplant subjects compared to placebo, as noticed by Garg et al. (37).
Our experience
At our Institute, we studied the efficacy of PRP therapy in 65 male patients with androgenetic alopecia and 50 patients with FPHL. Detailed video microscopic examination with regular follow ups was done in 10 male and 10 female patients. PRP therapy was given as three sessions repeated after every month.
Parameters which were observed on video-microscopy are hair count, diameter of hair, change in texture, multiplicity of hair, Perifollicular halo, perifollicular pigmentation, increase in telogen hair and increase in vellus hair count.
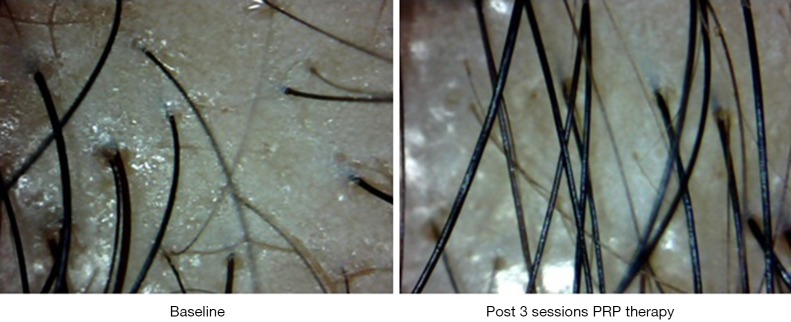
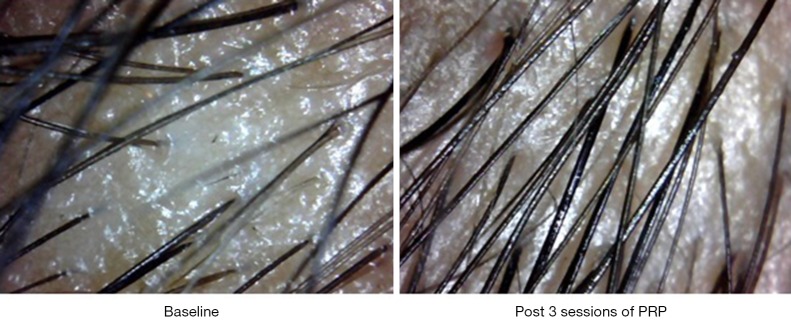
Interestingly new hair growth was seen as early as 4 weeks in male patients whereas signs of new hair growth were visualized in 6 weeks in females. Though PRP helped in increasing hair count in both groups, but the increase was statistically significant in male group with P value 0.014 for terminal hair count increase (4±5.15) and 0.003 for vellus hair count increase (2±4.15). Other findings were improved hair density, improvement in perifollicular halo and pigmentation, textural improvement of skin and multiplicity of hair in follicular units in female (Figure 2) as well as male patients (Figure 3). The comparison of median increase in hair count parameters in two groups has been highlighted in Figure 4.
Figure 2.
Video microscopic images of scalp of a patient with female patterned hair loss who underwent three sessions of PRP at an interval of one month each, depicting increase in terminal and vellus hair count, improvement in texture and reduction in perifollicular halo and evidence of new hair growth. PRP, platelet-rich plasma.
Figure 3.
Video microscopic images of scalp of a patient with androgenetic alopecia who underwent three sessions of PRP at an interval of one month each, depicting increase in terminal and vellus hair count, improvement in texture and reduction in perifollicular halo and evidence of new hair growth.
Figure 4.
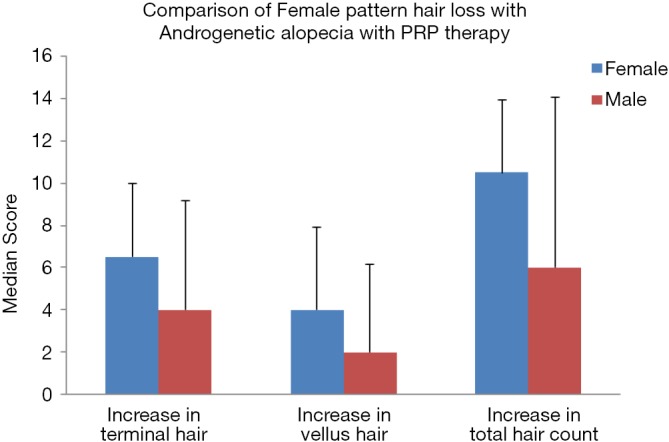
The bar chart is highlighting the comparison of response to PRP therapy with median increase in terminal/vellus/total hair count in video microscopic imaging in female pattern hair loss and androgenetic alopecia. PRP, platelet-rich plasma.
Perifollicular pigmentation is thought to be due to dermal infiltrates in AGA (38). The fact that PRP causes marked improvement in perifollicular pigmentation could be due to anti-inflammatory, vasodilator and angiogenic effects of various growth factors like VEGF and PDGF in PRP.
Authors propose that visualization of depressed haloes on video microscopy may predict a positive outcome of PRP and reversal of miniaturization as these depressed haloes probably depict fibrosed APMs which are essential for the integrity of HF.
In the published study by author, PRP therapy which was injected during FUE hair transplant in a randomized control manner just after slitting was found to play a significant role in regrowth of dormant hair, reduction in scalp redness, decrease in catagen hair loss and remarkably improved density and quality of hair growth after the FUE transplantation (37).
Effect of PRP at the molecular level
Kim et al. found that PRP increased expression of type I collagen, MMP-1, and mRNA in the human dermal fibroblasts (39). It was also shown that adding activated platelet-rich or platelet poor plasma promoted the proliferation of human adipose-derived stem cells and human dermal fibroblast significantly in the cell culture (39).
Diverse group of cells, such as endothelial cells and keratinocytes, produce platelet derived growth factor (PDGF), which is fundamental for cell growth and proliferation. PDGF induces and maintains anagen phase in mouse hair cycling. PDGF signals are involved in both epidermis-follicle interaction and the dermal mesenchymal interaction required for hair canal formation and the growth of dermal mesenchyme (4).
VEGF seems to be a major mediator of HF growth and cycling thereby, providing direct evidence that improved follicle revascularization promotes hair growth (40). PRP injections improve cutaneous ischemic conditions and increase vascular structures around HFs (17). The IGF-1, produced by DP cells acts on IGF-1 receptor on keratinocytes, promoting hair growth through stimulation of the proliferation of keratinocytes in HFs and preventing HF from developing catagen like status (4). Basic fibroblast growth factor (b FGF) promotes the proliferation of cells of papilla in vitro, and therefore, plays a key role in hair shaft elongation (14).
The activation of stem cells is cyclic which involves periodic β-catenin activity. The BMP cycle is out-of-phase with the Wnt/β-catenin cycle, thus dividing conventional telogen into two new functional phases: one refractory and the other competent for regeneration of hair; characterized by high and low Bmp signals respectively. Administration of BMP protein causes the competent region to become refractory. Bone morphogenetic proteins (BMPs) might be the “chalone” inhibitors of hair growth (41).
Li et al. suggested that activated PRP increases proliferation of human DP cells by increasing the phosphorylation of extracellular signal-regulated kinases (ERK) and Akt (42).
Activated PRP also increases levels of the anti-apoptotic protein Bcl-2, thereby preventing apoptosis. It contributes to the formation of hair epithelium and the differentiation of stem cells into HF cells, through β-catenin up regulation which is strongly expressed in the bulge region of the human anagen HF and prolongs anagen phase of hair cycle through an increase in expression of fibroblast growth factor-7.There is also increased proliferation of epidermal and HF bulge cells by PRP as revealed by an increase in Ki-67 (marker for cell proliferation) in AGA (4).
PRP might help in reversal of miniaturization by working on the vital area coined “Golden anchorage” as described previously in the article, comprising of stem cells, ectodermal basement membrane and portion of APM attached to bulge region. Also, PRP may act on rejuvenating keratinocytes (PDGF), stem cell factor (SCF), dermal papilla (IGF and fibroblast growth factor), vasculature (VEGF and PDGF) and neural cells (Nerve Growth Factor) thereby serving as an “elixir” for hair growth.
Role of PRP in other forms of alopecia
Alopecia areata
PRP was used as mesotherapy by Greco et al. in a single patient of alopecia areata, with good subjective results at 10 months of follow-up (35).
A double-blinded, placebo and active-controlled, half-head, parallel group study on 45 patients to evaluate the efficacy of PRP in Alopecia areata concluded that PRP is a safe and alternative treatment for AA. PRP was found to significantly increase hair regrowth, decrease hair dystrophy and burning or itching sensation without much side effects (43).
Singh et al. found in their study that out of 20 patients with alopecia areata treated with PRP, only one had a relapse. There were no side effects and procedure was well tolerated (44).
Donovan concluded that PRP therapy has potential to treat steroid-resistant forms of AA including ophiasis type and that PRP can be an option to treat AA patients who develop limiting side effects from steroid injections (45).
El Taieb et al. concluded in their study on 90 patients that patients treated with platelet rich plasma had an earlier response in the form of hair regrowth, reduction in short vellus hair and dystrophic hair unlike patients treated with 5% minoxidil and control. They found platelet rich plasma to be more effective in the treatment of alopecia areata than topical minoxidil 5% (46).
Telogen effluvium (TE)
There is a lack of conclusive and reliable studies on efficacy of PRP in patients with Telogen effluvium.
Our experience
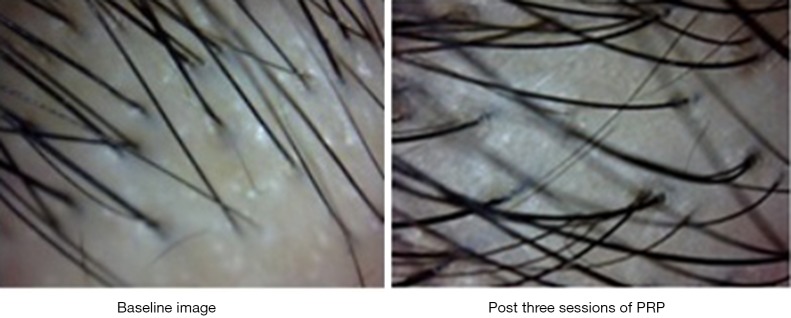
We managed a patient having telogen effluvium with high protein diet, early morning breakfast and PRP therapy offered once a month for 3 months. There was a significant increase in hair count and diameter after three sessions of PRP as shown in Figure 5 with baseline image showing hair count of 27 and post treatment image shows hair count of 30 along with visibly thicker and healthier HFs. The texture of the skin also became smoother and there was subjective improvement with a high patient satisfaction score.
Figure 5.
Video microscopic images of scalp of a female patient having telogen effluvium who was managed with PRP therapy with three sessions at one month interval. There was stoppage of active hair fall after 4–6 weeks of first session followed by increase in density of the hair follicles in subsequent sessions.
Cicatricial alopecia
Fakahany et al. found automated microneedling with platelet rich plasma effective for treating cicatricial alopecia, recalcitrant alopecia areata and traction alopecia (47).
Cicatricial alopecia patches are poor graft recipients as there is poor blood perfusion. The percentage of grafted follicles that survive depends mainly on the blood supply of the vascular bed. PRP is a rich source of anagen-maintaining factors, such as insulin-like growth factor 1 (IGF-1), basic fibroblast growth factor (bFGF), and VEGF. PRP injections improve cutaneous ischemic conditions and increase vascular structures around the HFs (48).
Saxena et al. found 1ml PRP injected intradermally into the recipient area test patch just prior to graft implantation to be efficacious for management of cicatricial lichen planus (48).
In our opinion, in cases of cicatricial alopecia, paucity of ostia might indicate less scope of reversal of destroyed HF. Hence, new follicles through FUE punch grafting can serve as source of stem cells, APM, epidermal basement membrane and nerve bundle.
Our experience
In a biopsy proven case of stable Lichen sclerosus et atrophicus (LSEA) of 4-year duration, we performed punch grafting with 2 mm diameter biopsy punches with intraoperative PRP therapy (Figure 6). Approximately twenty five grafts were transplanted. On evaluation at four months post-procedure, all the grafts were surviving well. FUE hair transplant with intraoperative PRP therapy was advocated to the patient to improve hair density further.
Figure 6.
Pre and post-procedural photographs of a patient with stable Lichen sclerosus et atrophicus (LSEA) of four years duration who was managed with punch grafting with intraoperative injectable PRP. The density can be further improved with subsequent sessions of FUE hair transplant with injectable PRP therapy. PRP, platelet-rich plasma.
Conclusion-PRP therapy works as an elixir for hair growth, as it has action on almost all the essential components required for survival of HF-keratinocytes (PDGF),stem cells (SCF),DP (IGF and bFGF), arrector pili muscle (bFGF, IGF),blood vessels (VEGF and PDGF) and neural cells (NGF).We propose the term ‘Golden anchorage’ which is formed by the confluence of stem cells, ectodermal basement membrane and bulge portion of the APM and is essential for the survival of the HF. Besides working on this vital area, PRP also works in rejuvenating melanocytes, DP and neurovascular bundle. Thus, PRP improves the overall environment for survival of HF and surrounding supportive structures and helps in reversal of miniaturization.
Acknowledgements
We would like to acknowledge Mr. Rajesh for his contribution towards statistical analysis.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Schippinger G, Prüller F, Divjak M, et al. Autologous Platelet-Rich Plasma Preparations: Influence of Nonsteroidal Anti-inflammatory Drugs on Platelet Function. Orthop J Sports Med 2015;3:2325967115588896. 10.1177/2325967115588896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banihashemi M, Nakhaeizadeh S. An introduction to application of platelet rich plasma (PRP) in skin rejuvenation. Rev Clin Med 2014;1:38-43. [Google Scholar]
- 3.Arshdeep, Kumaran MS. Platelet-rich plasma in dermatology: boon or a bane? Indian J Dermatol Venereol Leprol 2014;80:5-14. 10.4103/0378-6323.125467 [DOI] [PubMed] [Google Scholar]
- 4.Maria-Angeliki G, Alexandros-Efstratios K, Dimitris R, et al. Platelet-rich Plasma as a Potential Treatment for Noncicatricial Alopecias. Int J Trichology 2015;7:54-63. 10.4103/0974-7753.160098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marwah M, Godse K, Patil S, et al. Is there sufficient research data to use platelet-rich plasma in dermatology? Int J Trichology 2014;6:35-6. 10.4103/0974-7753.136763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Albanese A, Licata ME, Polizzi B, et al. Platelet-rich plasma (PRP) in dental and oral surgery: from the wound healing to bone regeneration. Immun Ageing 2013;10:23. 10.1186/1742-4933-10-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lian Z, Yin X, Li H, et al. Synergistic Effect of Bone Marrow-Derived Mesenchymal Stem Cells and Platelet-Rich Plasma in Streptozotocin-Induced Diabetic Rats. Ann Dermatol 2014;26:1-10. 10.5021/ad.2014.26.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dhurat R, Sukesh M. Principles and Methods of Preparation of Platelet-Rich Plasma: A Review and Author’s Perspective. J Cutan Aesthet Surg 2014;7:189-97. 10.4103/0974-2077.150734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cervelli V, Garcovich S, Bielli A, et al. The effect of autologous activated platelet rich plasma (AA-PRP) injection on pattern hair loss: clinical and histomorphometric evaluation. Biomed Res Int 2014;2014:760709. 10.1155/2014/760709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Da Silva RT, Heidrich F. Therapy with use of Platelet-rich plasma in orthopedics and sports Traumatology: Literature Review, Evidence and Personal Experience. In: Lana JF, Andrade Santana MH, Dias Belangero W, et al. editors. Platelet-Rich Plasma: Regenerative Medicine: Sports Medicine, Orthopedic, and Recovery of Musculoskeletal Injuries. 1st edition. Berlin: Springe, 2014:154. [Google Scholar]
- 11.Giusti I, Rughetti A, D’Ascenzo S, et al. Identification of an optimal concentration of platelet gel for promoting angiogenesis in human endothelial cells. Transfusion 2009;49:771-8. 10.1111/j.1537-2995.2008.02033.x [DOI] [PubMed] [Google Scholar]
- 12.DohanEhrenfest DM , Andia I, Zumstein MA, et al. Classification of platelet concentrates (Platelet-Rich Plasma-PRP, Platelet-Rich Fibrin-PRF) for topical and infiltrative use in orthopedic and sports medicine: current consensus, clinical implications and perspectives. Muscles Ligaments Tendons J 2014;4:3-9. [PMC free article] [PubMed] [Google Scholar]
- 13.Harmon K, Hanson R, Bowen J, et al. Guidelines for the Use of Platelet Rich Plasma[Internet]. The International Cellular Medical Society.2011.Version 1.0.Cited 23 March 2016.Available online: http://www.cellmedicinesociety.org/attachments/370_Section%2010%20-%20Platelet%20Rich%20Plasma%20(PRP)%20Guidelines.pdf
- 14.Godse K, Mahajan A. Platelet-rich plasma. In: Pai Ganesh S. editor. Complications in cosmetic dermatology crafting cures.1st ed. New Delhi: Jaypee publications, 2016:174-7. [Google Scholar]
- 15.Messenger A, Sinclair R, de Berker D. Disorders of hair. In: Burns T, Breathnach S, Cox N, et al. editors. Rook’s textbook of dermatology. 8th edition. West Sussex: Wiley-Blackwell, 2013:66. [Google Scholar]
- 16.Fujiwara H, Ferreira M, Donati G, et al. The basement membrane of hair follicle stem cells is a muscle cell niche. Cell 2011;144:577-89. 10.1016/j.cell.2011.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gentile P, Garcovich S, Bielli A, et al. The Effect of Platelet-Rich Plasma in Hair Regrowth: A Randomized Placebo-Controlled Trial. Stem Cells Transl Med 2015;4:1317-23. 10.5966/sctm.2015-0107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sinclair R, Torkamani N, Jones L. Androgenetic alopecia: new insights into the pathogenesis and mechanism of hair loss. F1000Res 2015;4:585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yazdabadi A, Whiting D, Rufaut N, et al. Miniaturized Hairs Maintain Contact with the Arrector Pili Muscle in Alopecia Areata but not in Androgenetic Alopecia: A Model for Reversible Miniaturization and Potential for Hair Regrowth. Int J Trichology 2012;4:154-7. 10.4103/0974-7753.100069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Torkamani N, Rufaut NW, Jones L, et al. Beyond Goosebumps: Does the ArrectorPili Muscle Have a Role in Hair Loss? Int J Trichology 2014;6:88-94. 10.4103/0974-7753.139077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poblet E, Ortega F, Jiménez F. The arrector pili muscle and the follicular unit of the scalp: A microscopic anatomy study. Dermatol Surg 2002;28:800-3. [DOI] [PubMed] [Google Scholar]
- 22.Torkamani N, Rufaut NW, Jones L, et al. Destruction of the arrector pili muscle and fat infiltration in androgenic alopecia. British Journal of Dermatology 2014;170:1291-8. 10.1111/bjd.12921 [DOI] [PubMed] [Google Scholar]
- 23.Dogra S, Sarangal R. What's new in cicatricial alopecia? Indian J Dermatol Venereol Leprol 2013;79:576-90. 10.4103/0378-6323.116726 [DOI] [PubMed] [Google Scholar]
- 24.França K, Rodrigues TS, Ledon J, et al. "Comprehensive Overview and Treatment Update on Hair Loss," Journal of Cosmetics, Dermatological Sciences and Applications 2013;3:1-8. 10.4236/jcdsa.2013.33A1001 [DOI] [Google Scholar]
- 25.Paus R, Olsen E, Messenger A. Hair growth disorders. In: Goldsmith L, Katz S, Gilchrest B, et al. editors. Fitzpatrick’s Dermatology in general medicine,8th edition. USA: McGraw-Hill Medical,2012:757. [Google Scholar]
- 26.Kaliyadan F, Nambiar A, Vijayaraghavan S. Androgenetic alopecia: An update. Indian J Dermatol Venereol Leprol 2013;79:613-25. 10.4103/0378-6323.116730 [DOI] [PubMed] [Google Scholar]
- 27.Grover C, Khurana A. Telogen effluvium. Indian J Dermatol Venereol Leprol 2013;79:591-603. 10.4103/0378-6323.116731 [DOI] [PubMed] [Google Scholar]
- 28.Gkini MA, Kouskoukis AE, Tripsianis G, et al. Study of Platelet-Rich Plasma Injections in the Treatment of Androgenetic Alopecia Through an One-Year Period. J Cutan Aesthet Surg 2014;7:213-9. 10.4103/0974-2077.150743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gupta M, Mysore V. Classifications of Patterned Hair Loss: A Review. J Cutan Aesthet Surg 2016;9:3-12. 10.4103/0974-2077.178536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramos PM, Miot HA. Female Pattern Hair Loss: a clinical and pathophysiological review. An Bras Dermatol 2015;90:529-43. 10.1590/abd1806-4841.20153370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mysore V. Finasteride and sexual side effects. Indian Dermatol Online J 2012;3:62-5. 10.4103/2229-5178.93496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khatu SS, More YE, Gokhale NR, et al. Platelet-Rich Plasma in Androgenic Alopecia: Myth or an Effective Tool. J Cutan Aesthet Surg 2014;7:107-10. 10.4103/0974-2077.138352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Borhan R, Gasnier C, Reygagne P. Autologous Platelet Rich Plasma as a Treatment of Male Androgenetic Alopecia: Study of 14 Cases. J Clin Exp Dermatol Res 2015;6:292. [Google Scholar]
- 34.Singhal P, Agarwal S, Dhot PS, et al. Efficacy of platelet-rich plasma in treatment of androgenic alopecia. Asian J Transfus Sci 2015;9:159-62. 10.4103/0973-6247.162713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Greco J, Brandt R. The effects of autologous platelet rich plasma and various growth factors on non-transplanted miniaturized hair. Hair Transplant Forum Int 2009;19:49-50. [Google Scholar]
- 36.Uebel CO, da Silva JB, Cantarelli D, et al. The role of platelet plasma growth factors in male pattern baldness surgery. Plast Reconstr Surg 2006;118:1458-66. 10.1097/01.prs.0000239560.29172.33 [DOI] [PubMed] [Google Scholar]
- 37.Garg S. Outcome of intra-operative injected platelet-rich plasma therapy during follicular unit extraction hair transplant: A prospective randomised study in forty patients. J Cutan Aesthet Surg 2016;9:157-64. 10.4103/0974-2077.191657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kibar M, Aktan Ş, Bilgin M. Scalp Dermatoscopic Findings in Androgenetic Alopecia and Their Relations with Disease Severity. Ann Dermatol 2014;26:478-84. 10.5021/ad.2014.26.4.478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim DH, Je YJ, Kim CD, et al. Can platelet-rich plasma be used for skin rejuvenation? Evaluation of effects of platelet-rich plasma on human dermal fibroblast. Ann Dermatol 2011;23:424-31. 10.5021/ad.2011.23.4.424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yano K, Brown LF, Detmar M. Control of hair growth and follicle size by VEGF-mediated angiogenesis. J Clin Invest 2001;107:409-17. 10.1172/JCI11317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Plikus MV, Mayer J, de la Cruz D, et al. Cyclic dermal BMP signaling regulates stem cell activation during hair regeneration. Nature 2008;451:340-4. 10.1038/nature06457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li ZJ, Choi HI, Choi DK, et al. Autologous platelet-rich plasma: A potential therapeutic tool for promoting hair growth. Dermatol Surg 2012;38:1040-6. 10.1111/j.1524-4725.2012.02394.x [DOI] [PubMed] [Google Scholar]
- 43.Trink A, Sorbellini E, Bezzola P, et al. A randomized, double-blind, placebo-and active-controlled, half-head study to evaluate the effects of platelet-rich plasma on alopecia areata. Br J Dermatol 2013;169:690-4. 10.1111/bjd.12397 [DOI] [PubMed] [Google Scholar]
- 44.Singh S. Role of platelet-rich plasma in chronic alopecia areata: Our centre experience. Indian J Plast Surg 2015;48:57-9. 10.4103/0970-0358.155271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Donovan J. Successful treatment of corticosteroid-resistant ophiasis-type alopecia areata (AA) with platelet-rich plasma (PRP). JAAD Case Rep 2015;1:305-7. 10.1016/j.jdcr.2015.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.El Taieb MA, Ibrahim H, Nada EA, et al. Platelets rich plasma versus minoxidil 5% in treatment of alopecia areata: A trichoscopic evaluation. Dermatol Ther 2017;30(1). [DOI] [PubMed] [Google Scholar]
- 47.Fakahany H, Raouf H, Medhat W. Using automated microneedling with platelet rich plasma for treating cicatricialalopecia, recalcitrant alopecia areata and traction alopecia, casereport. J Am Acad Dermatol 2016;74;5:140.
- 48.Saxena K, Saxena DK, Savant SS. Successful Hair Transplant Outcome in Cicatricial Lichen Planus of the Scalp by Combining Scalp and Beard Hair Along With Platelet Rich Plasma. J Cutan Aesthet Surg 2016;9:51-5. 10.4103/0974-2077.178562 [DOI] [PMC free article] [PubMed] [Google Scholar]