Abstract
OBJECTIVES
The study objective was to analyze the impact of prognostic factors, including treatment modality, on outcome in patients with advanced-stage uterine serous carcinoma (USC).
METHODS
A retrospective review of patients diagnosed with stage III or IV USC between 1993 and 2012 was performed. Summary statistics were used to describe demographic and clinical characteristics. Overall survival (OS) and recurrence free survival (RFS) were estimated by Kaplan-Meier analysis. Cox proportional hazards regression was used to model the association of potential prognostic factors with OS and RFS.
RESULTS
The study included 260 patients with median follow-up of 26.6 months (range 1–172.8). Median age was 63 years (range 30–88) and 52.3% had stage III disease. In all, 60% were treated with surgery followed by chemotherapy, 18.1% received surgery, chemotherapy, and radiotherapy, 11.5% had surgery and radiotherapy, and 10.4% had neoadjuvant chemotherapy. The overall complete response rate was 68.9%, and the cumulative incidence of recurrence was 82.7%. Treatment that included surgery, chemotherapy, and radiation and stage III disease were associated with improved RFS on multivariate analysis. For OS, therapy with surgery, chemotherapy, and radiation, mixed histology, and stage III disease were associated with better OS on multivariate analysis.
CONCLUSIONS
Patients with advanced-stage USC have a poor prognosis, regardless of clinical factors or treatment received. However, combination therapy that includes chemotherapy and radiation appears to be associated with improved survival in these women.
Keywords: Endometrial Neoplasm, Prognosis, Survival, Radiotherapy, Chemotherapy
Introduction
Of the more than 60,000 new cases of endometrial cancer in 2016, approximately 10% were diagnosed with uterine serous carcinoma (USC).[1, 2] USC is a high-grade histologic subtype of endometrial adenocarcinoma characterized by its aggressive nature. While only 16% of endometrioid endometrial cancer cases are advanced stage at diagnosis, up to 38% of women with USC are stage III or IV. Even in cases with little or no myometrial invasion, extrauterine metastases are found at the time of surgery in up to 37% of patients.[3, 4] For these reasons, this relatively rare tumor accounts for up to 50% of all endometrial cancer-related deaths.[5, 6]
Though women with USC are typically included in prospective endometrial cancer trials, they tend to make up only a small number of patients. This can lead to difficulty in making meaningful conclusions regarding USC from the results of these studies. Additionally, the vast majority of USC-only studies are retrospective, with small numbers that include patients with all stages of disease. However, as with most malignancies, the prognosis for women with USC varies widely by stage. While the 5-year survival for patients with stage IA disease is 81.5%, it is only 19.9% for stage IV disease.[3]
Several authors have attempted to evaluate how various clinical variables affect survival among women with advanced stage USC.[7–9] However, the majority of these studies were either small or included all stages of USC. The aim of the present study was to retrospectively assess potential prognostic factors among women with stage III or IV USC.
Materials and Methods
A retrospective review was conducted of women with USC treated at The University of Texas MD Anderson Cancer Center between March 1993 and January 2012. This study was approved by the Institutional Review Board (IRB) of MD Anderson Cancer Center. As no data were collected prospectively, a waiver of informed consent was granted by the IRB.
To be included in the study, women must have had stage III or IV USC as defined by 2009 criteria published by the International Federation of Gynecology and Obstetrics (FIGO).[10] For the purposes of this study, patients who were diagnosed prior to 2009 had their cancer re-staged. All pathology had been previously confirmed by a gynecologic pathologist at MD Anderson Cancer Center. Any tumor with more than 5% serous component was included. Women with inadequate data in the medical record, synchronous primary tumors, or carcinosarcoma were excluded.
Demographic information and medical history were abstracted from the medical record. Patients were divided into one of four groups based upon the primary treatment they received: 1) neoadjuvant chemotherapy, 2) surgery and chemotherapy, 3) surgery and radiotherapy, or 4) surgery, radiotherapy, and chemotherapy. Neoadjuvant chemotherapy was defined as women who received any chemotherapy immediately after diagnosis, with or without interval tumor reductive surgery. To be included in the surgery and chemotherapy group, women must have had primary tumor reductive surgery followed by any chemotherapy. Women in the surgery and radiotherapy group underwent primary tumor reductive surgery followed by radiation treatment with or without concurrent chemotherapy. The radiation may have included vaginal brachytherapy, whole pelvic radiation, or both. The surgery, radiotherapy, and chemotherapy group had primary surgery followed by treatment with any radiation with or without concurrent chemotherapy and adjuvant chemotherapy. A small number of women did not fall into any of the defined treatment groups as they did not receive any treatment (n = 2), underwent surgery alone (n = 18), or received radiation alone (n = 2) and were lost to follow-up. These women were excluded from the analysis. Patients were considered to have a recurrence if it was documented from physical examination or imaging findings. Complete response was defined as no evidence of disease by exam or imaging at the completion of therapy.
Summary statistics were utilized to describe demographic and clinical characteristics. Fisher’s exact test was used to compare treatment groups with respect to categorical demographic and clinical characteristics. The Kruskal-Wallis test was used to compare medians of groups with respect to demographic and clinical characteristics measured on a continuous scale.
Time to recurrence (TTR) was defined as the time from the start of treatment to the date of recurrence. Patients were censored on the date of their last clinic visit, and death was considered a competing event. Cumulative incidence of recurrence was estimated using the methods of Gooley and colleagues.[11] The methods of Fine and Gray were used to model the cumulative incidence of recurrence as a function of treatment, with death as a competing event.[12] Overall survival (OS) was defined as the time from the date treatment started to the date of death or last contact. Patients alive at last contact were censored on that date. Recurrence-free survival (RFS) was defined as the time from the date treatment started to the date of recurrence, date of the last clinic visit, or the date of death. Patients were censored on the date of the last clinic visit. For RFS, recurrence and death from any cause were considered events. OS and RFS were estimated with the product-limit estimator of Kaplan and Meier.[13] Cox proportional hazards regression was used to model the association of potential prognostic factors with OS and RFS in a univariate fashion.[14] All factors with a p-value < 0.25 were then used in a saturated, multivariate model and backward elimination was used to remove factors one at a time until only those with a p-value of ≤0.05 remained. Type of adjuvant chemotherapy, CA125 at diagnosis, and platelet level at diagnosis were not considered in the multivariate models as there were too many missing values. All analyses were performed with SAS 9.3 for Windows (Copyright© 2002–2010 by SAS Institute Inc., Cary, NC), STATATM 11.0 for Windows (Copyright© 1985–2009, StataCorp LP, College Station, TX), and S-PLUS© 8.0 for Windows (Copyright© 1988, 2007 Insightful Corp., Seattle, WA).
Results
Inclusion criteria were met by 260 women with a median follow-up of 30 months (range 1.1–172.8 months). The median age was 63.5 years (range 30–88), 176 (67.7%) women were white, and 248 (95.4%) women were postmenopausal at the time of diagnosis. Most women had stage IVB disease (n=116, 44.6%). On pathology, 98 women (37.7%) were noted to have pure USC. Of those women with mixed histology, 98 (60.5%) had endometrioid adenocarcinoma with USC, 26 (16%) had clear cell adenocarcinoma with USC, and 18 (11.1%) had all three histologic subtypes present. The remainder of women (n=20) had various combinations of undifferentiated, endometrioid, and clear cell carcinoma mixed with USC. In regards to the endometrioid component of the mixed tumors, 93.8% (n=121) were grade 2 or grade 3.
In terms of primary treatment, 27 (10.4%) women received neoadjuvant chemotherapy, 156 (60%) received surgery followed by adjuvant chemotherapy, 30 (11.5%) received surgery followed by radiation, and 47 (18.1%) received surgery followed by radiation and chemotherapy. In the neoadjuvant group, 17 received interval cytoreductive surgery (63%). The remainder progressed after neoadjuvant chemotherapy. Table 1 details the demographics and clinical characteristics of the women in each treatment group. Though median age and body mass index (BMI) were the same across all four groups, there were statistically significant differences in terms of race, stage, and histology. Among patients with mixed histology, the majority were treated with surgery and chemotherapy (62 or 63.3% of those with endometrioid and USC, 15 or 57.7% of those with clear cell and USC, 4 or 80% of those with undifferentiated and USC, and 18 or 54.5% of those with three histologic subtypes).
Table 1. Participant Characteristics by Treatment Group.
Demographics and tumor characteristics for patients in each treatment group.
| Neoadjuvant Chemo % (n = 27) | Surgery/ Chemo % (n = 156) | Surgery/ Radiation % (n = 30) | Surgery/Radiation/ Chemo % (n = 47) | p | |
|---|---|---|---|---|---|
|
| |||||
| Age | 0.279 | ||||
| Median (Range) | 66.3 (46–81) | 63.2 (30–82) | 65.5 (38–88) | 60.0 (40–78) | |
|
| |||||
| Race | 0.008 | ||||
| White | 29.6 (8) | 71.2 (111) | 76.7 (23) | 72.3 (34) | |
| African-American | 48.2 (13) | 16.7 (26) | 13.3 (4) | 12.8 (6) | |
| Hispanic | 14.8 (4) | 6.4 (10) | 10 (3) | 8.5 (4) | |
| Asian | 7.4 (2) | 5.1 (8) | 0 (0) | 6.4 (3) | |
| Other | 0 (0) | 0.6 (1) | 0 (0) | 0 (0) | |
|
| |||||
| BMI | 0.216 | ||||
| Median (Range) | 29.2 (18.1–68.8) | 29.6 (17.5–105) | 26.1 (18.7–39.9) | 29.6 (17.8–45) | |
|
| |||||
| Stage | <0.001 | ||||
| IIIA | 0 (0) | 6.4 (10) | 13.3 (4) | 19.2 (9) | |
| IIIB | 0 (0) | 1.3 (2) | 0 (0) | 8.5 (4) | |
| IIIC1 | 0 (0) | 14.7 (23) | 40 (12) | 36.2 (17) | |
| IIIC2 | 0 (0) | 21.2 (33) | 30 (9) | 27.7 (13) | |
| IVA | 7.4 (2) | 1.9 (3) | 0 (0) | 0 (0) | |
| IVB | 81.5 (22) | 54.5 (85) | 16.7 (5) | 8.5 (4) | |
| Unstaged | 11.1 (3) | 0 (0) | 0 (0) | 0 (0) | |
|
| |||||
| Histology | 0.002 | ||||
| Pure USC | 70.4 (19) | 36.5 (57) | 23.3 (7) | 31.9 (15) | |
| Mixed | 29.6 (8) | 63.5 (99) | 76.7 (23) | 68.1 (32) | |
The complete response (CR) rate after primary therapy for all women was 68.9% (95% CI 62.8–74.4), while the cumulative incidence of recurrence was 81.1% (95% CI 74.4–86.3). However, the CR rates and cumulative incidence of recurrence varied widely by treatment group (Table 2). When each treatment group was compared with respect to the cumulative incidence of recurrence, women who received neoadjuvant chemotherapy were statistically significantly more likely to recur than women who received treatment with primary surgery, radiation, and chemotherapy (p=0.005). There was no statistical difference in cumulative incidence of recurrence among women in the neoadjuvant chemotherapy and the primary surgery and chemotherapy treatment group (p=0.567) or with the primary surgery and radiation group (p=0.064). Additionally, women who received surgery and chemotherapy were more likely to recur than either the group who received surgery and radiation (p=0.038) or those who received surgery, radiation, and chemotherapy (p=0.001). However, the cumulative incidence of recurrence was similar among the two groups who were treated with radiation during their primary therapy (p=0.467). With respect to CR rates, women in the neoadjuvant chemotherapy group had a significantly lower CR rate than all other groups (vs surgery and chemotherapy, p=0.0001; vs surgery and radiation, p<0.0001; vs primary surgery, radiation, and chemotherapy, p=0.0002). However, there were no differences in CR rates among any other groups (all p>0.05).
Table 2. Complete Response Rate and Cumulative Incidence of Recurrence by Treatment Group.
Rate of complete response to primary therapy and rate of recurrence for each treatment group.
| Treatment Group | Complete Response% (n) | 95% CI | Cumulative Incidence of Recurrence% (n) | 95% CI |
|---|---|---|---|---|
| Neoadjuvant Chemo | 29.6 (8) | 13.8–50.2 | 81.5 (22) | 61.1–91.8 |
| Surgery/Chemotherapy | 69.9 (109) | 62.0–77.0 | 87.6 (128) | 79.8–92.5 |
| Surgery/Radiation | 86.7 (26) | 69.3–96.2 | 70.0 (21) | 50.3–83.1 |
| Surgery/Radiation/Chemotherapy | 76.6 (36) | 62.0–87.7 | 63.0 (27) | 45.8–76.0 |
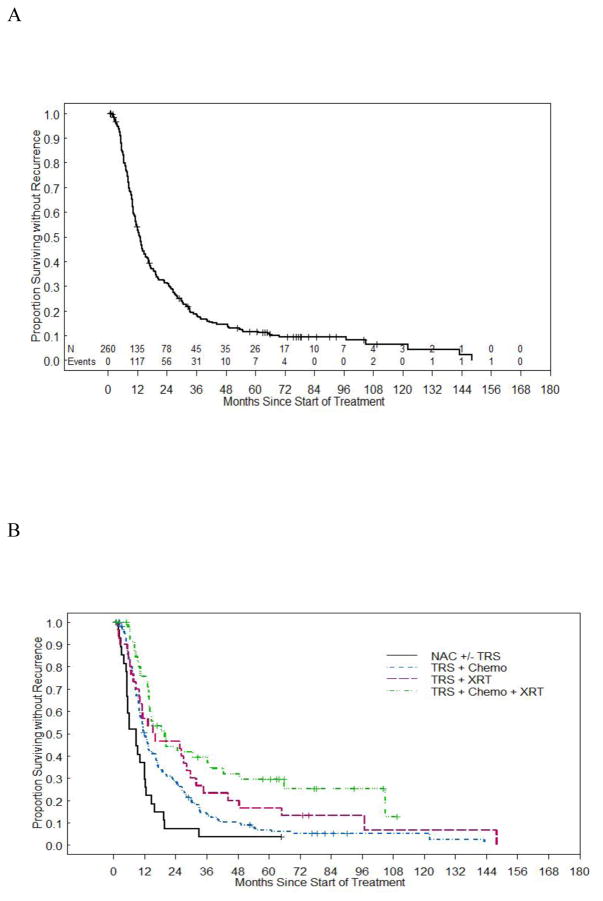
The median RFS for all groups was 13.0 months (95% CI 11.3 –14.7), while the five-year RFS was 11.6% (95% CI 8.0% 16.0%). As demonstrated in Figure 1, women who received neoadjuvant chemotherapy had the shortest median RFS at 8.7 months. Women in the surgery and radiation and in the surgery, radiation, and chemotherapy groups had the longest median RFS at 15.8 months and 19.9 months, respectively. Multiple clinical factors were analyzed to determine how they might play a role in predicting RFS (Table 3). On univariate analysis, combination therapy, earlier stage, platinum sensitivity, optimal tumor reductive surgery, and platinum and taxane chemotherapy were associated with improved RFS. However, on multivariate analysis, only stage III disease and combination therapy that included surgery, chemotherapy, and radiation were associated with improved RFS.
Figure 1. Overall RFS and RFS by Treatment Group.
(a) Median RFS for all groups. (b) Median RFS stratified by treatment group.
Table 3. Factors Prognostic of RFS.
Multivariate analysis of clinical factors associated with recurrence free survival.
| Univariate | Multivariate | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Factor | N Median (events) | Median (Months) | HR | 95% CI | p | HR | 95% CI | p |
|
| ||||||||
| Treatment Group | ||||||||
| Neoadjuvant | 27 (26) | 8.7 | REF | REF | ||||
| Surgery/Chemo | 156 (143) | 12.0 | 0.55 | 0.36–0.84 | 0.005 | 0.66 | 0.42–1.05 | 0.079 |
| Surgery/Radiation | 30 (28) | 15.8 | 0.40 | 0.23–0.69 | 0.001 | 0.60 | 0.32–1.11 | 0.102 |
| Surgery/Chemo/Radiation | 47 (33) | 19.9 | 0.28 | 0.17–0.48 | <0.001 | 0.48 | 0.26–0.87 | 0.015 |
|
| ||||||||
| BMI | ||||||||
| < 25 | 50 (43) | 11.9 | REF | |||||
| 25–29.9 | 47 (39) | 11.7 | 0.85 | 0.55–1.31 | 0.455 | |||
| ≥30 | 87 (75) | 17.8 | 0.77 | 0.53–1.12 | 0.170 | |||
|
| ||||||||
| Tumor Reductive Surgery | ||||||||
| No Gross Residual | 144 (120) | 16.6 | REF | |||||
| ≤0.5 cm | 11 (11) | 20.5 | 1.28 | 0.69–2.38 | 0.437 | |||
| >0.5 cm and < 1 cm | 14 (14) | 9.4 | 1.90 | 1.09–3.32 | 0.023 | |||
| >1 cm | 26 (25) | 9.9 | 2.63 | 1.69–4.10 | <0.001 | |||
|
| ||||||||
| Stage | ||||||||
| III | 136 (111) | 17.0 | REF | REF | ||||
| IV | 121 (116) | 10.3 | 2.06 | 1.57–2.70 | <0.001 | 1.79 | 1.31–2.45 | <0.001 |
|
| ||||||||
| Histology | ||||||||
| Pure USC | 98 (87) | 11.3 | REF | |||||
| Mixed | 162 (143) | 14.2 | 0.80 | 0.61–1.05 | 0.106 | |||
|
| ||||||||
| Platinum Sensitive | ||||||||
| No | 78 (78) | 7.3 | REF | |||||
| Yes | 116 (116) | 16.4 | 0.16 | 0.11–0.23 | <0.001 | |||
|
| ||||||||
| Number of Chemo Cycles | ||||||||
| 0 | 29 (27) | 16.2 | REF | |||||
| 1–5 | 52 (43) | 11.4 | 1.23 | 0.75–2.00 | 0.416 | |||
| ≥ 6 | 167 (148) | 12.8 | 1.27 | 0.84–1.94 | 0.258 | |||
|
| ||||||||
| Type of Chemo | ||||||||
| Platinum Alone | 16 (16) | 8.3 | REF | |||||
| Taxane Alone | 21 (21) | 12.8 | 0.61 | 0.31–1.18 | 0.142 | |||
| Platinum + Taxane | 35 (35) | 19.9 | 0.44 | 0.24–0.80 | 0.008 | |||
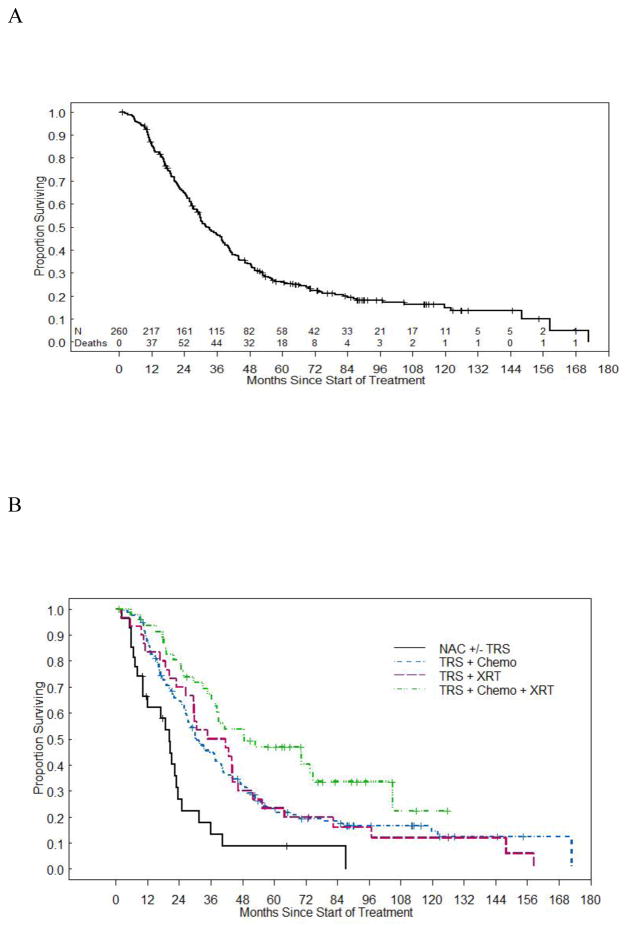
Median OS was 32.1 months (95% CI 28.7–37.9) for all women. The five-year OS was 26.2% (95% CI 20.9–31.9). As with RFS, OS varied widely by treatment group (Figure 2). For example, while women who received neoadjuvant chemotherapy had a median OS of 20.4 months, women in the surgery/radiation group had a median OS of 38.3 months. On univariate analysis, combination therapy, mixed histology, stage III disease, optimal tumor reductive surgery, and platinum sensitivity were significantly associated with OS (Table 4). However, the multivariate analysis revealed that only mixed histology, stage III disease, and treatment with surgery, radiation, and chemotherapy or surgery and chemotherapy, were associated with improved OS in these women.
Figure 2. Overall OS and OS by Treatment Group.
(a) Median OS for all groups. (b) Median OS stratified by treatment group.
Table 4. Factors Prognostic of OS.
Multivariate analysis of clinical factors associated with overall survival.
| Univariate | Multivariate | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Factor | N (deaths) | Median (months) | HR | 95% CI | p | HR | 95% CI | p |
|
| ||||||||
| Treatment Group | ||||||||
| Neoadjuvant | 27 (23) | 20.4 | REF | REF | ||||
| Surgery/Chemo | 156 (125) | 30.8 | 0.44 | 0.28–0.68 | <0.001 | 0.65 | 0.40–1.08 | 0.095 |
| Surgery/Radiation | 30 (28) | 38.3 | 0.44 | 0.25–0.76 | 0.004 | 0.86 | 0.44–1.67 | 0.656 |
| Surgery/Chemo/Radiation | 47 (29) | 48.8 | 0.26 | 0.15–0.45 | <0.001 | 0.49 | 0.26–0.93 | 0.029 |
|
| ||||||||
| BMI | ||||||||
| < 25 | 50 (36) | 37.8 | REF | |||||
| 25–29.9 | 47 (36) | 33.3 | 1.01 | 0.64–1.61 | 0.957 | |||
| ≥ 30 | 87 (66) | 37.7 | 0.99 | 0.66–1.49 | 0.962 | |||
|
| ||||||||
| Tumor Reductive Surgery | ||||||||
| No Gross Residual | 144 (110) | 40.6 | REF | |||||
| ≤ 0.5 cm | 11 (8) | 29.7 | 1.16 | 0.57–2.38 | 0.684 | |||
| >0.5 cm and < 1 cm | 14 (11) | 17.9 | 1.78 | 0.96–3.31 | 0.070 | |||
| >1 cm | 26 (23) | 26.0 | 1.75 | 1.11–2.76 | 0.015 | |||
|
| ||||||||
| Stage | ||||||||
| III | 136 (100) | 43.7 | REF | REF | ||||
| IV | 121 (103) | 26.0 | 1.93 | 1.45–2.55 | <0.001 | 1.80 | 1.29–2.51 | <0.001 |
|
| ||||||||
| Histology | ||||||||
| Pure USC | 98 (79) | 29.8 | REF | REF | ||||
| Mixed | 162 (126) | 37.7 | 0.72 | 0.54–0.96 | 0.025 | 0.74 | 0.55–0.99 | 0.047 |
|
| ||||||||
| Platinum Sensitive | ||||||||
| No | 78 (69) | 19.0 | REF | |||||
| Yes | 116 (102) | 39.1 | 0.39 | 0.28–0.53 | <0.001 | |||
|
| ||||||||
| Number of Chemo Cycles | ||||||||
| 0 | 29 (27) | 41.7 | REF | 0.455 | ||||
| 1 – 5 | 52 (41) | 26.5 | 1.21 | 0.74–1.97 | 0.649 | |||
| ≥ 6 | 167 (126) | 33.3 | 0.91 | 0.60–1.38 | ||||
|
| ||||||||
| Type of Chemo | ||||||||
| Platinum Alone | 16 (14) | 25.9 | REF | |||||
| Taxane Alone | 21 (20) | 34.9 | 1.02 | 0.51–2.06 | 0.953 | |||
| Platinum + Taxane | 35 (30) | 40.6 | 0.75 | 0.40–1.42 | 0.374 | |||
At our institution, women with node-positive USC are often treated with a combination of chemotherapy and radiation in the post-operative setting. A post-hoc subset analysis of the patients with stage IIIC disease (n=86) was therefore performed to determine if radiation provided a survival advantage over chemotherapy in this population. However, there was no difference in RFS (17.3 vs. 14.0 months, p=0.99) nor OS (43.7 vs. 48.6 months, p=0.84) between the group treated with surgery and chemotherapy and the group who received surgery, chemotherapy, and radiation.
Discussion
The current study confirms the dismal prognosis associated with advanced stage USC. Though almost 70% of women had a complete response after primary therapy, three-fourths of patients subsequently recurred. This finding is consistent with what has been previously reported in the literature.[3, 8, 15] The lower 5-year OS in our patient population (26%), may be attributed to the inclusion of only stage III/IV USC compared to other studies that included all stages of disease.[16, 17]
While a variety of treatment regimens were used in our cohort, the majority of women received a combination of chemotherapy and surgery. Furthermore, more than 60% of patients in this group received platinum-based chemotherapy with a taxane. Prior studies have confirmed that USC is highly responsive to this chemotherapy combination.[15, 18–20] In spite of this, the majority of these patients will recur.
Our multivariate analysis found that treatment modality has a significant impact on survival. Specifically, we noted that RFS and OS were improved among women who received combination therapy that included radiation. It is important to note that there may be imbalances in clinical factors that were not included in the multivariate analysis that may account for this finding. However, several studies, including large, prospective trials, have also described a survival advantage among endometrial cancer patients treated with both radiation and chemotherapy.[21–23] However, most of these reports excluded patients with serous histology. Few authors have attempted to determine the role of radiation therapy among women with advanced stage USC. In their retrospective investigation of 135 women with stage I–IVA USC, Viswanathan and colleagues found that treatment with chemotherapy and external beam radiation were associated with longer RFS (HR=0.44, p=0.004).[17] A subsequent study by the same group reported that women with stage IVB USC treated with adjuvant chemotherapy and external beam radiation also have decreased rates of disease recurrence or progression.[24]
The combination of radiation and adjuvant chemotherapy has also been reported to improve survival among women with node-positive non-serous endometrial cancer.[25] Furthermore, it appears that when these patients are treated with chemotherapy alone, there is an increased rate of locoregional recurrence.[26] However, there are little data regarding the impact of combination therapy on survival in node-positive USC patients.[27] In our subset analysis of women with stage IIIC disease, we found no difference in RFS or OS between patients treated with surgery and chemotherapy and those treated with surgery, chemotherapy, and radiation. Further studies are warranted to confirm this finding and better delineate patterns of recurrence and survival in women with node-positive USC.
In the present study, patients who had at least 5% serous component of their tumor were included in the analysis. Multiple studies have determined that even a small amount of serous histology confers a poorer prognosis than pure type I endometrial cancers. Quddus and colleagues reported that tumors with 5% to 30% serous component have poorer survival.[28] Other authors have found that survival is negatively impacted by the presence of any serous carcinoma component to the tumor.[29] Furthermore, molecular analysis of mixed USC and endometrioid tumors has found them to more closely resemble pure USC than pure endometrioid malignancies.[30] In our study, however, mixed histology was associated with improved OS as compared with pure USC. These findings highlight the importance of careful pathologic characterization of these tumors, rather than simply defining them as USC.
While we found that the type of treatment received and stage are some of the strongest predictors of survival for advanced stage USC, optimal tumor reductive surgery was not a predictor of either RFS or OS in the multivariate setting. This is in contrast to previously published studies which have consistently found a significant correlation between survival and the amount of tumor at the conclusion of primary surgery.[9, 31–33] For example, in their study of 70 patients with advanced stage USC, Thomas and colleagues reported that patients with no gross residual disease at the conclusion of tumor reductive surgery had a median survival of 52 months, compared with 16 months for women with residual disease (p<0.001).[32] Though one study found no clear difference in survival based upon the amount of disease after cytoreduction, there was a trend towards improved survival in women who underwent an optimal debulking.[34] Of note, most of these studies included only patients with at least stage IIIC disease, often did not report the number of patients with mixed histology, and had smaller numbers of patients in comparison with our study. These differences may provide an explanation for the conflicting results.
The major limitation of this study is its retrospective nature. Specifically, the manner in which women were assigned treatment is likely affected by selection bias and cannot be controlled for in this type of study. However, given the relatively few number of USC cases each year, it is a difficult disease to study in a prospective fashion. Furthermore, though all pathology had been reviewed by a gynecologic pathologist at the time of diagnosis, the pathology was not re-reviewed for this study. While this is a large study of advanced stage USC patients, another limitation is that the numbers in some of the treatment groups were small and there were significant clinical differences between these groups. This may make it difficult to make comparisons across specific treatment groups. Also, there is likely wide variation in practice patterns regarding the treatment of USC as well as technological advances with radiation that occurred during the study period, which could alter the results.
In conclusion, women with advanced stage USC have a poor prognosis regardless of treatment received. The most significant predictors of RFS and OS are disease stage and treatment modality. Specifically, combination therapy that includes radiation may have a positive impact on survival. Prospective studies of USC are needed, though difficult to perform due to the rarity of the tumor.
Research Highlights.
Retrospective review of 260 patients with stage III or IV uterine serous carcinoma.
Treatment group and stage were predictors of survival on multivariate analysis.
The prognosis for women with advanced USC is poor regardless of treatment received.
Acknowledgments
Funding Source: This study was supported by funds from Grant Number T32 CA101642 from the NIH National Research Service Award, Grant Number K12CA088084 from the NIH K12 Calabresi Scholar Award, Grant Number 2P50CA098258-06 from the NIH SPORE in Uterine Cancer, Grant Number P30CA016672 from the NIH MD Anderson Cancer Center Support Grant and the Andrew Sabin Family Fellowship. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66:271–89. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- 2.Hendrickson M, Ross J, Eifel PJ, Cox RS, Martinez A, Kempson R. Adenocarcinoma of the endometrium: analysis of 256 cases with carcinoma limited to the uterine corpus. Pathology review and analysis of prognostic variables. Gynecol Oncol. 1982;13:373–92. doi: 10.1016/0090-8258(82)90076-2. [DOI] [PubMed] [Google Scholar]
- 3.Slomovitz BM, Burke TW, Eifel PJ, Ramondetta LM, Silva EG, Jhingran A, et al. Uterine papillary serous carcinoma (UPSC): a single institution review of 129 cases. Gynecol Oncol. 2003;91:463–9. doi: 10.1016/j.ygyno.2003.08.018. [DOI] [PubMed] [Google Scholar]
- 4.Cirisano FD, Jr, Robboy SJ, Dodge RK, Bentley RC, Krigman HR, Synan IS, et al. Epidemiologic and surgicopathologic findings of papillary serous and clear cell endometrial cancers when compared to endometrioid carcinoma. Gynecol Oncol. 1999;74:385–94. doi: 10.1006/gyno.1999.5505. [DOI] [PubMed] [Google Scholar]
- 5.del Carmen MG, Birrer M, Schorge JO. Uterine papillary serous cancer: a review of the literature. Gynecol Oncol. 2012;127:651–61. doi: 10.1016/j.ygyno.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 6.Moore KN, Fader AN. Uterine papillary serous carcinoma. Clin Obstet Gynecol. 2011;54:278–91. doi: 10.1097/GRF.0b013e318218c755. [DOI] [PubMed] [Google Scholar]
- 7.Huang CY, Tang YH, Chiang YC, Wang KL, Fu HC, Ke YM, et al. Impact of management on the prognosis of pure uterine papillary serous cancer - a Taiwanese Gynecologic Oncology Group (TGOG) study. Gynecol Oncol. 2014;133:221–8. doi: 10.1016/j.ygyno.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 8.Gadducci A, Cosio S, Landoni F, Maggino T, Zola P, Fuso L, et al. Analysis of treatment failures and survival of patients with uterine papillary serous carcinoma: a Cooperation Task Force (CTF) Study. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 2012;22:1355–60. doi: 10.1097/IGC.0b013e318267f7a0. [DOI] [PubMed] [Google Scholar]
- 9.Rauh-Hain JA, Growdon WB, Schorge JO, Goodman AK, Boruta DM, McCann C, et al. Prognostic determinants in patients with stage IIIC and IV uterine papillary serous carcinoma. Gynecol Oncol. 2010;119:299–304. doi: 10.1016/j.ygyno.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 10.Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet. 2009;105:103–4. doi: 10.1016/j.ijgo.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 11.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Statistics in medicine. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 12.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 13.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. Journal of the American Statistical Association. 1958;53:457– 81. [Google Scholar]
- 14.Cox D. Regression models and life tables (with discussion) Journal of the Royal Statistical Society. 1972;34:187–220. [Google Scholar]
- 15.Vaidya AP, Littell R, Krasner C, Duska LR. Treatment of uterine papillary serous carcinoma with platinum-based chemotherapy and paclitaxel. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 2006;16(Suppl 1):267–72. doi: 10.1111/j.1525-1438.2006.00413.x. [DOI] [PubMed] [Google Scholar]
- 16.Wang W, Do V, Hogg R, Wain G, Brand A, Bull C, et al. Uterine papillary serous carcinoma: patterns of failure and survival. The Australian & New Zealand journal of obstetrics & gynaecology. 2009;49:419–25. doi: 10.1111/j.1479-828X.2009.01016.x. [DOI] [PubMed] [Google Scholar]
- 17.Viswanathan AN, Macklin EA, Berkowitz R, Matulonis U. The importance of chemotherapy and radiation in uterine papillary serous carcinoma. Gynecol Oncol. 2011;123:542–7. doi: 10.1016/j.ygyno.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 18.Zanotti KM, Belinson JL, Kennedy AW, Webster KD, Markman M. The use of paclitaxel and platinum-based chemotherapy in uterine papillary serous carcinoma. Gynecol Oncol. 1999;74:272–7. doi: 10.1006/gyno.1999.5444. [DOI] [PubMed] [Google Scholar]
- 19.Fader AN, Drake RD, O'Malley DM, Gibbons HE, Huh WK, Havrilesky LJ, et al. Platinum/taxane-based chemotherapy with or without radiation therapy favorably impacts survival outcomes in stage I uterine papillary serous carcinoma. Cancer. 2009;115:2119–27. doi: 10.1002/cncr.24247. [DOI] [PubMed] [Google Scholar]
- 20.Fader AN, Nagel C, Axtell AE, Zanotti KM, Kelley JL, Moore KN, et al. Stage II uterine papillary serous carcinoma: Carboplatin/paclitaxel chemotherapy improves recurrence and survival outcomes. Gynecol Oncol. 2009;112:558–62. doi: 10.1016/j.ygyno.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 21.Greven K, Winter K, Underhill K, Fontenesci J, Cooper J, Burke T. Final analysis of RTOG 9708: adjuvant postoperative irradiation combined with cisplatin/paclitaxel chemotherapy following surgery for patients with high-risk endometrial cancer. Gynecol Oncol. 2006;103:155–9. doi: 10.1016/j.ygyno.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 22.Hogberg T, Signorelli M, de Oliveira CF, Fossati R, Lissoni AA, Sorbe B, et al. Sequential adjuvant chemotherapy and radiotherapy in endometrial cancer--results from two randomised studies. European journal of cancer. 2010;46:2422–31. doi: 10.1016/j.ejca.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alvarez Secord A, Havrilesky LJ, Bae-Jump V, Chin J, Calingaert B, Bland A, et al. The role of multi-modality adjuvant chemotherapy and radiation in women with advanced stage endometrial cancer. Gynecol Oncol. 2007;107:285–91. doi: 10.1016/j.ygyno.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 24.Lee LJ, Demaria R, Berkowitz R, Matulonis U, Viswanathan AN. Clinical predictors of long-term survival for stage IVB uterine papillary serous carcinoma confined to the abdomen. Gynecol Oncol. 2014;132:65–9. doi: 10.1016/j.ygyno.2013.10.035. [DOI] [PubMed] [Google Scholar]
- 25.Lee LJ, Viswanathan AN. Combined chemotherapy and radiation improves survival for node-positive endometrial cancer. Gynecol Oncol. 2012;127:32–7. doi: 10.1016/j.ygyno.2012.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klopp AH, Jhingran A, Ramondetta L, Lu K, Gershenson DM, Eifel PJ. Node-positive adenocarcinoma of the endometrium: outcome and patterns of recurrence with and without external beam irradiation. Gynecol Oncol. 2009;115:6–11. doi: 10.1016/j.ygyno.2009.06.035. [DOI] [PubMed] [Google Scholar]
- 27.Lee LJ, Bu P, Feltmate C, Viswanathan AN. Adjuvant chemotherapy with external beamradiation therapy for high-grade, node-positive endometrial cancer. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 2014;24:1441–8. doi: 10.1097/IGC.0000000000000248. [DOI] [PubMed] [Google Scholar]
- 28.Quddus MR, Sung CJ, Zhang C, Lawrence WD. Minor serous and clear cell components adversely affect prognosis in ''mixed-type'' endometrial carcinomas: a clinicopathologic study of 36 stage-I cases. Reproductive sciences. 2010;17:673–8. doi: 10.1177/1933719110368433. [DOI] [PubMed] [Google Scholar]
- 29.Fader AN, Starks D, Gehrig PA, Secord AA, Frasure HE, O'Malley DM, et al. An updated clinicopathologic study of early-stage uterine papillary serous carcinoma (UPSC) Gynecol Oncol. 2009;115:244–8. doi: 10.1016/j.ygyno.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 30.Lawrenson K, Pakzamir E, Liu B, Lee JM, Delgado MK, Duncan K, et al. Molecular Analysis of Mixed Endometrioid and Serous Adenocarcinoma of the Endometrium. PloS one. 2015;10:e0130909. doi: 10.1371/journal.pone.0130909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bristow RE, Duska LR, Montz FJ. The role of cytoreductive surgery in the management of stage IV uterine papillary serous carcinoma. Gynecol Oncol. 2001;81:92–9. doi: 10.1006/gyno.2000.6110. [DOI] [PubMed] [Google Scholar]
- 32.Thomas MB, Mariani A, Cliby WA, Keeney GL, Podratz KC, Dowdy SC. Role of cytoreduction in stage III and IV uterine papillary serous carcinoma. Gynecol Oncol. 2007;107:190–3. doi: 10.1016/j.ygyno.2007.05.039. [DOI] [PubMed] [Google Scholar]
- 33.Memarzadeh S, Holschneider CH, Bristow RE, Jones NL, Fu YS, Karlan BY, et al. FIGO stage III and IV uterine papillary serous carcinoma: impact of residual disease on survival. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 2002;12:454–8. doi: 10.1046/j.1525-1438.2002.01149.x. [DOI] [PubMed] [Google Scholar]
- 34.Moller KA, Gehrig PA, Van Le L, Secord AA, Schorge J. The role of optimal debulking in advanced stage serous carcinoma of the uterus. Gynecol Oncol. 2004;94:170–4. doi: 10.1016/j.ygyno.2004.03.040. [DOI] [PubMed] [Google Scholar]