Abstract
On the 400th anniversary of Harvey's Lumleian lectures, this review focuses on “hemodynamic” forces associated with the movement of blood through arteries in humans and the functional and structural adaptations that result from repeated episodic exposure to such stimuli. The late 20th century discovery that endothelial cells modify arterial tone via paracrine transduction provoked studies exploring the direct mechanical effects of blood flow and pressure on vascular function and adaptation in vivo. In this review, we address the impact of distinct hemodynamic signals that occur in response to exercise, the interrelationships between these signals, the nature of the adaptive responses that manifest under different physiological conditions, and the implications for human health. Exercise modifies blood flow, luminal shear stress, arterial pressure, and tangential wall stress, all of which can transduce changes in arterial function, diameter, and wall thickness. There are important clinical implications of the adaptation that occurs as a consequence of repeated hemodynamic stimulation associated with exercise training in humans, including impacts on atherosclerotic risk in conduit arteries, the control of blood pressure in resistance vessels, oxygen delivery and diffusion, and microvascular health. Exercise training studies have demonstrated that direct hemodynamic impacts on the health of the artery wall contribute to the well-established decrease in cardiovascular risk attributed to physical activity.
I. INTRODUCTION: EXERCISE AND ARTERY HEALTH IN HUMANS
Recent technological “advances” have fundamentally altered the vocational and lifestyle behaviors of humans in the space of a few generations. Profound changes associated with ubiquitous exposure to television, mobile communication devices, and the internet have rapidly accelerated an underlying trend in sedentary behavior related to urbanization, automation, and widespread use of the automobile (272). In global terms, it was recently estimated that physical inactivity caused 6-10% of all deaths from major noncommunicable diseases (coronary disease, type 2 diabetes, breast and colon cancers), or more than 5.3 of the 57 million deaths that occurred worldwide. This equates to the number of deaths attributable to tobacco (112).
Approximately one-third of the global population do not meet minimum physical activity (PA) requirements to sustain health (112). In the West, the impact of technological change on PA levels and cardiovascular health is occurring on a background of unprecedented demographic shifts associated with population ageing, raising the spectre of individuals experiencing more years of frailty and compromised life quality, with associated increases in healthcare costs (229). There has never been a more sedentary population of humans than the 21st century Western society, prompting some to suggest that the positive historical trend in life expectancy may soon be threatened (231). These observations reinforce the critical importance of increasing physical activity levels, and primary prevention is now a global policy agenda (137).
Although exercise programs may be regarded as an effective strategy to “compensate” for loss of routine physical activity, better insight is required into the physiological adaptations to distinct stimuli associated with exercise. This review focuses on the impact of exercise on the vasculature, in particular, the direct effects mediated by physical, mechanical, and/or hemodynamic forces on arterial function, structure, and adaptation in humans.
A. Impact of Exercise and Physical Activity on Cardiovascular Risk
Retrospective studies strongly suggested that regular physical activity is associated with lower risk for cardiovascular (CV) mortality and morbidity (197, 241). Prospective studies provide direct evidence that adopting a physically active lifestyle delays all-cause mortality, extends longevity (242), and reduces risk for CV mortality by 42–44%, compared with persistently unfit men (28, 180). Furthermore, the relationship between PA and CV risk exhibits a curvilinear dose-response pattern (319) with increasing, but diminishing, returns at higher activity levels (210). It is important to acknowledge that, while fitness has been regarded as a surrogate for habitual physical activity, these factors have independent and overlapping roles in the prevention of cardiovascular disease (63). In those with heart disease, exercise-based rehabilitation is associated with a reduction in CV mortality and fewer hospital admissions (9). These benefits, in the context of both primary and secondary prevention of cardiovascular disease (CVD), approximate and may exceed those associated with antihypertensive (308) or lipid-lowering drugs (47, 203). Indeed, meta-epidemiological evidence (205 randomized controlled trials, n = 339,274) found equal effectiveness of exercise training and contemporary drug interventions (220), in terms of mortality reduction.
B. The Risk Factor Gap: Traditional Risk Factors Do Not Fully Explain Risk Reduction
Until recently, the rationale for the promotion of exercise, and methods of prescribing it, were based on the assumption that exercise exerted its benefits by virtue of “secondary” effects. That is, exercise benefit was judged by its capacity to modify CV risk factors such as blood pressure (BP), lipids, insulin resistance, smoking, and obesity (303). Indeed, studies linking exercise to changes in CV risk factors report significant improvement in individual CV risk factors (106, 155), although the magnitude of such change is typically modest compared with pharmacological interventions (303). Importantly, the cardioprotective effects of exercise training remain after statistical correction for traditional and novel CV risk factors (28, 159). Mora et al. (211) assessed the contribution of changes in CV risk factors as a result of physical activity to the occurrence of CVD in 27,055 women (10.9 year follow-up) and reported that established and novel risk factors explained only part of the beneficial impact of exercise on CVD risk. Others have reported that CV risk factors explained only 27–41% of the cardioprotective benefits of exercise training (48, 120, 286). The beneficial impacts of exercise on CV risk therefore exceed that expected from changes in CV risk factors alone: a risk factor gap exists in explaining the benefits of exercise in humans (106).
Exercise exerts direct effects on the vasculature via the impact of repetitive exposure to hemodynamic stimuli, such as shear stress and transmural pressure. Consequently, exercise transduces functional and structural adaptations in the vascular wall, providing a plausible contribution to the risk factor gap described above (106). Improvements in flow-mediated dilation (FMD), a validated surrogate for CV health and disease risk (103, 145, 252, 287), can occur as a result of exercise training in the absence of changes in CV risk factors (109), reinforcing the notion (196) that exercise exerts some of its benefit by virtue of impacts distinct from those on traditional risk factors (104, 296). This proposition is supported by epidemiological evidence, presented above, that ∼50% or more of the beneficial impact of exercise on CV end points cannot be explained by risk factor modification (211). Although alternative explanations exist (32), including modulation of autonomic tone (106, 155), there is a strong basis to propose that exercise-induced hemodynamic changes induce anti-atherogenic adaptations in vascular function and structure that contribute to the CV benefits of exercise training.
C. Evidence for a Role of Direct Impacts of Hemodynamic Forces on Vascular Health
At the end of the 19th century, Thoma (301) noted, in observations of chick embryos, that many branches developed in blood vessels in which blood flow was rapid, while no branches developed in blood vessels where blood flow was slower. This early observation suggested that hemodynamic forces, broadly defined as mechanical forces associated with flowing blood (i.e., shear stress and/or pressure), were important in adaptation of the vasculature. More recently, the endothelium has provided a focus for research, given its strategic placement between the flowing blood and artery wall and crucial role in the progression and development of atherosclerosis (201). It is now understood that vascular adaptation is dependent on an intact, functional endothelium (257, 326) and that hemodynamic stimuli induce functional and structural changes in the arterial wall via endothelial cell signal transduction.
D. Integrative Aspects of Vascular Adaptation to Training
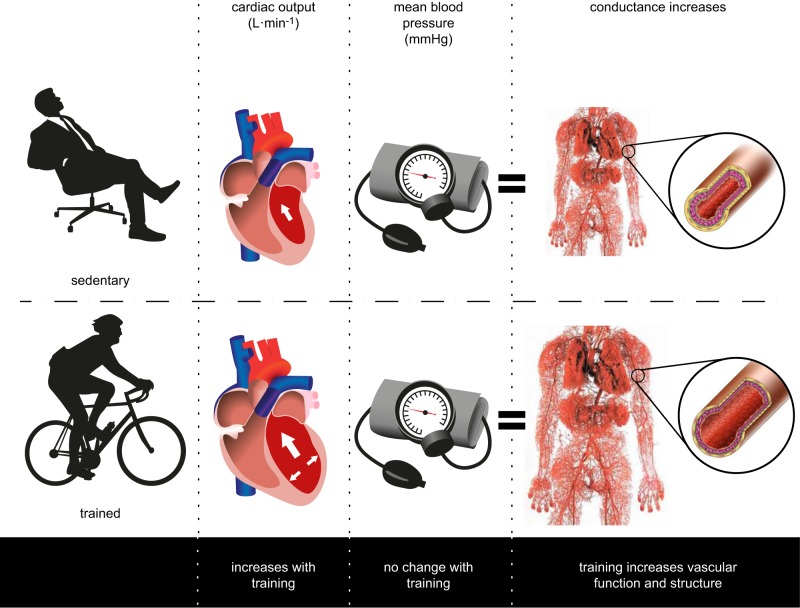
Clausen (50) noted nearly four decades ago in this journal that exercise training improves oxygen uptake and cardiac output during maximal exercise (Figure 1), whereas mean arterial pressure remains unaffected. These findings infer that the increase in cardiac output as a result of exercise training is accommodated by a corresponding rise in vascular conductance, the latter mediated by functional and/or structural adaptations in conduit, resistance, and microvessels. Changes in the vasculature are associated with decreased cardiac afterload at rest and during submaximal exercise, which enhances ventricular function and myocardial oxygen demand (118). This integrative physiological perspective emphasizes the key role played by changes in the vasculature in response to exercise training.
FIGURE 1.
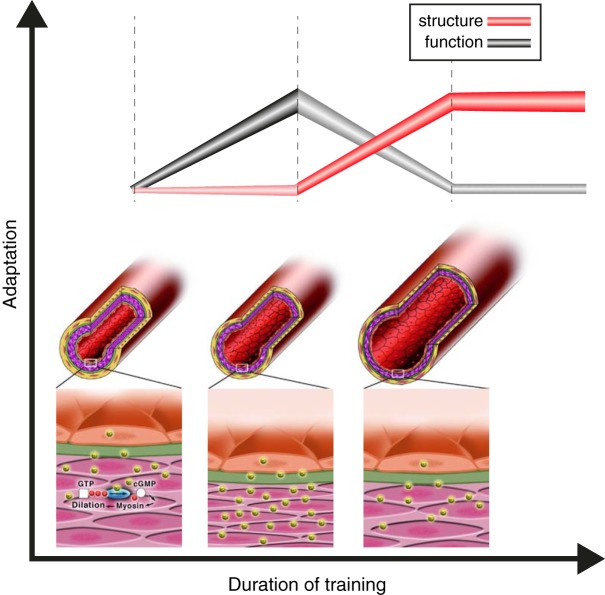
Using data from a series of exercise training studies, Clausen (50) demonstrated that oxygen uptake improved with training (by 0.34 l/min on average), cardiac output also significantly increased (by 2.1 l/min), yet blood pressure did not change, or slightly decreased. This insight highlights the relevance of the peripheral vasculature in accommodating the increase in cardiac output that accompanies training. Vascular adaptations encompass both functional and structural changes, which may occur along distinct time courses (see Figure 9). [Redrawn from Clausen (50).]
II. WHAT HEMODYNAMIC FORCES ARE RELEVANT IN THE VASCULATURE?
A. Pressure Effects
Exercise increases systolic pressure, while diastolic pressures remain at resting levels or may decrease (127). As arterial pressure waves propagate, pulse pressure changes due to interactions between the segmental arterial compliance and pressure wave harmonics, such that systolic and diastolic pressures in peripheral arteries (brachial, femoral) can be significantly different from those measured in the aorta (260).
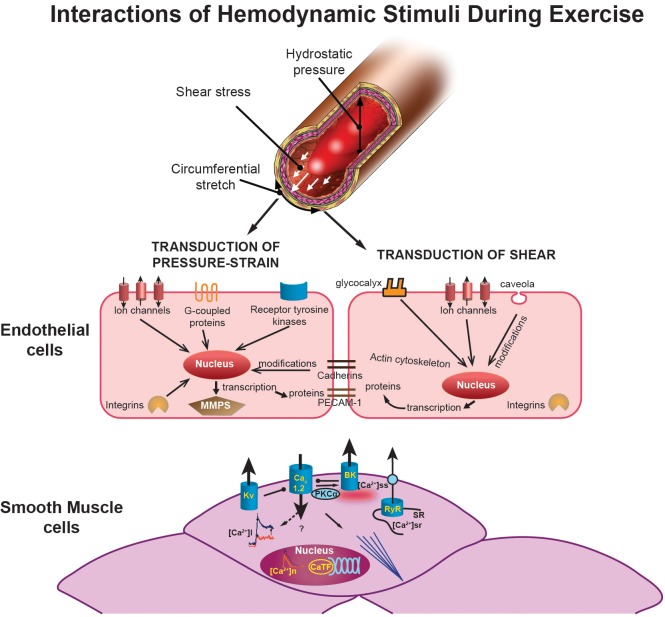
Blood pressure can influence vascular cells in at least two ways. First, cell culture experiments have demonstrated that exposure of endothelial cells to pressure affects their growth rate; pressures of 20–100 mmHg increase growth compared with no pressure (173). Second, pressure distends arteries, thereby stretching vascular cells in the wall. Because arteries are compliant, changes in pressure consequently produce circumferential stress (i.e., strain). Because of the pulsatile nature of arterial blood pressure, this circumferential strain results in cyclic circumferential strain (Figure 2).
FIGURE 2.
Illustration showing the interactions of hemodynamic signals (top) (hydrostatic pressure, shear stress, and circumferential stretch) that modulate vascular adaptation to exercise. The effects of pressure and/or stretch on the endothelial cells are shown in the middle as described in the text. At the bottom, the figure illustrates exercise-induced adaptations of smooth muscle cells. The middle left of the smooth muscle figure shows calcium transients with decreased intracellular calcium ([Ca2+]i) response to selective agonists (e.g., endothelin) in exercise-trained cells in red (which produces a reduced Ca2+-dependent activation of contraction). This decreased [Ca2+]i occurs despite an increased Ca2+ influx through L-type Ca2+ channels (Cav1.2). Nuclear Ca2+ responses ([Ca2+]n) are also reduced by exercise training, which may affect Ca2+-dependent transcription factors (CaTF; e.g., CREB, NFAT) and target gene expression. Also illustrated is the increased spontaneous, slow-Ca2+ release from the SR into the subscarolemmal space ([Ca2+]ss) which may contribute to the increased activation of large-conductance, Ca2+-activated (BK) potassium channels caused by exercise. Voltage-gated (Kv) potassium channels are also activated by exercise training. [Bottom panel modified from Bowles and Laughlin (32) and Langille and O'Donnell (167), with permission from American Heart Association.]
1. Detection of cyclic circumferential strain by the endothelium
In response to cyclic circumferential strain, endothelial cells respond morphologically with alignment of cells perpendicular to the force vector, subsequently followed by phenotypic changes (52). The mechanism by which the endothelium recognizes and transduces mechanical stimuli involves various signaling systems (i.e., integrins, ion channels, G protein-coupled receptors, and receptor tyrosine kinases). This complex system of mechanosensors converts mechanical stimuli into chemical signals that lead to the activation of intracellular signaling cascades (Figure 2). The latter can alter transcription factors and activation of genes that regulate the fate of the endothelial cells and smooth muscle cells, i.e., proliferation, migration, and/or apoptosis. In this process, cyclic circumferential strain eventually modifies matrix proteins [i.e., metalloproteinases (MMPs)] that can affect components of the extracellular matrix, but also influence nonmatrix substrates (e.g., growth factors and receptors) (52).
2. Relevance of the pattern of cyclic circumferential strain
Increases in arterial pressure that distend arteries and increase transmural pressure also induce increased cyclic circumferential strain on endothelial cells (67). Cyclic circumferential strain can also increase as a result of relaxation of vascular smooth muscle, which induces vasodilation and stretching of the endothelial cell lining (Figure 2). Endothelial cells experience cyclic circumferential strain across the cardiac cycle in vivo. In humans, cyclic circumferential strain has been measured in the aorta using MRI, with peak strain occurring after peak flow (67). During exercise, the distension in the aorta increases because of increases in heart rate and systolic pressure. Increased exposure to cyclic circumferential strain has been reported to alter vascular cell gene expression, such as increased expression/activity of the endothelium-dependent dilator pathways, endothelial nitric oxide synthase (eNOS) and endothelium-derived hyperpolarizing factor (EDHF) synthase (CYP450), as well as increased release of reactive oxygen species (ROS), expression of adhesion molecules such as intercellular adhesion molecule (ICAM), selectin, and monocyte chemoattractant protein-1 (MCP-1) (173). Consistent with these observations, chronic increases in blood pressure are associated with impaired endothelial function and progression of atherosclerosis (71) (Figure 2).
The effects of cyclic circumferential strain are complex and variable. Because cyclic circumferential strain can have direct effects on endothelial cell gene expression, but also increases superoxide, other forms of ROS and adhesion molecules (e.g., VCAM-1), the direct effects of stretch on gene expression are not easily predicted (173). ROS produced by cyclic circumferential strain may indirectly alter vascular cell phenotypes. The major effect of increased cyclic circumferential strain on endothelial cells appears to be pro-atherogenic, even though the changes in eNOS expression alone would be anti-atherogenic. The pro-atherogenic effect of increased cyclic circumferential strain is likely explained because the increased ROS production and expression of adhesion molecules override the effects of increased eNOS expression (124). Increased eNOS expression, although not sufficient to preserve function, may compensate for the loss of bioactive nitric oxide (NO) caused by superoxide and other ROS. While exercise bouts are of usually <2-h duration, many studies of the effects of cyclic circumferential strain on endothelial cell phenotype have used 24 h/day exposure to a similar level of pressure. These data suggest that the pattern of change in cyclic circumferential strain is relevant as transient increases in blood pressure and ROS, associated with exercise bouts, may increase eNOS expression and other beneficial effects of exercise, whereas chronic increases in blood pressure may chronically elevate ROS, causing maladaptations.
It is clear that circumferential strain can influence vascular smooth muscle cell phenotype through effects of stretch on these cells. While not the focus of this review, Figure 2 illustrates many known effects of exercise on vascular smooth muscle cells. The classic pressure-induced myogenic response in smooth muscle is initiated by a stretch-induced depolarization due to activation of cation channels (167). This depolarization activates Ca2+ influx through Cav1.2 and subsequent Ca-induced contraction. In addition, both stretch and Ca2+ activate BK channels, while depolarization activates Kv channels to hyperpolarize and limit depolarization in a negative-feedback manner (167). The net result, other than contraction, is a dominant Cav1.2-mediated calcium influx which increases expression of smooth muscle-specific genes (also known as differentiation markers) such as smooth muscle specific myosin heavy chain (SMMHC) and smooth muscle alpha actin (SMaA) (32, 167). The transcription factors that drive smooth muscle specific gene expression, myocardin and to a lesser extent MEF, are also increased by pressure-induced Ca influx (32, 167). Pressure/stretch is known to regulate smooth muscle synthesis of connective tissue growth factor (CTGF), collagen, and fibronectin, indicating that pressure can influence vessel wall matrix composition through smooth muscle (30–32, 167) (Figure 2). As highlighted elsewhere, the seminal studies of Folkow and co-workers (74, 75) infer that changes in arterial wall thickness can exacerbate effects on arterial pressure in vivo.
B. Endothelial Shear Stress
In 1933, Schretzenmayr (268) exposed cat femoral arteries to an increase in blood flow via stimulation of the hindleg motor nerves and observed a gradual increase in femoral artery diameter. This may be the first study to demonstrate that conduit arteries are able to react to forces exerted by the circulating blood. The role of endothelial cells in the “detection” of changes in flow, and their production of vasoactive second messengers, was only recognized later.
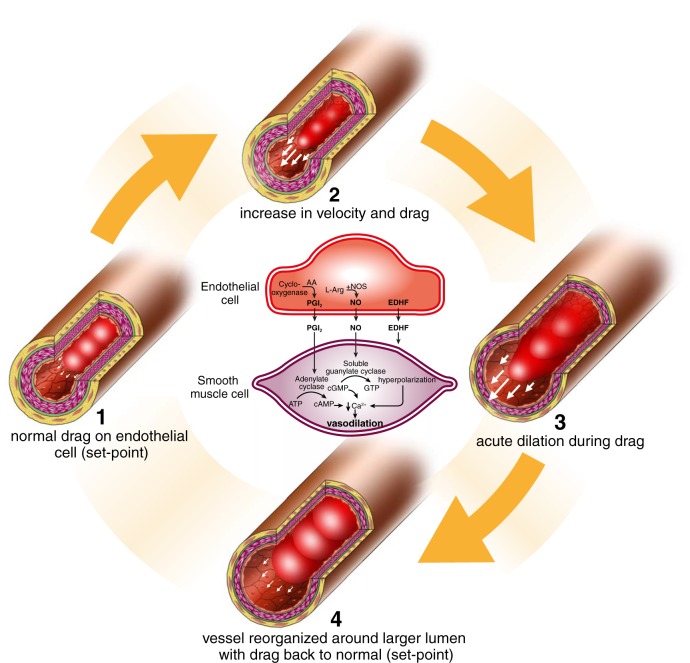
In 1975, Rodbard (256) published a prescient account of endothelial transducer function. He proposed four steps (Figure 3), starting with exposure of the endothelium to an increase in viscous drag (step 1), or “shear stress.” Any increase in flow that increases this drag (step 2) subsequently triggers acute dilation (step 3), a functional change that tends to homeostatically modify the initial increase in shear. When exposed to prolonged periods of change in flow and shear, vessel remodeling can occur, whereby local drag is returned toward the norm by virtue of structural arterial modification (step 4). In 1980, Furchgott (79) published his famous experiment, which provided evidence for the importance of the endothelium in mediating vasodilation in response to acetylcholine through the release of a vasodilator substance, EDRF, later described as NO. This Nature paper was preceded by another, which observed that vasodilator prostaglandins were produced by the endothelium (208), although the role of shear stress in this transduction process was demonstrated somewhat later (160, 263).
FIGURE 3.
Rendering of Rodbard's prediction of “flow-dependent, endothelium-mediated dilation” and remodeling. Exposure of the endothelium to an increase in viscous drag or “shear stress” (step 2) triggers dilation (step 3), a functional change that tends to homeostatically modify the initial increase in shear (see also Figure 9). When exposed to prolonged periods of change in flow and shear, vessel remodeling can occur, whereby drag forces are “structurally” normalized (step 4). These predictions were ultimately verified in both animals (and humans).
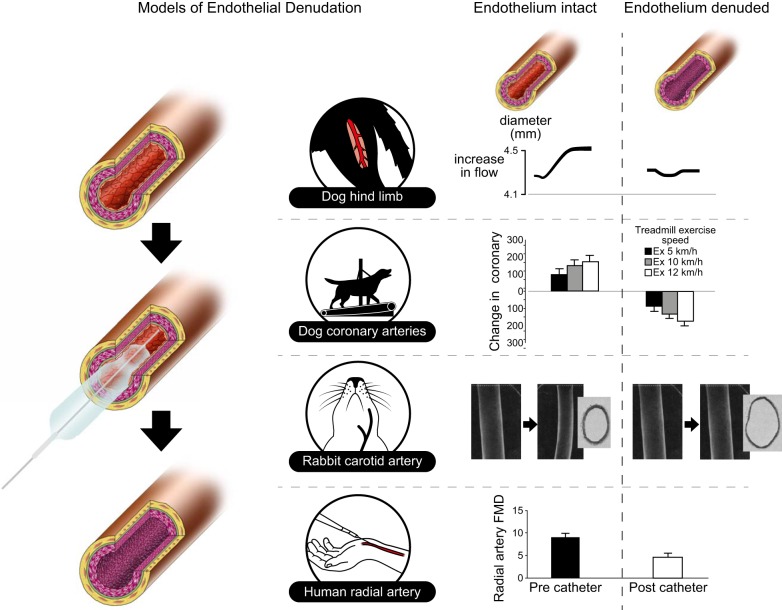
Studies in the early 1980s provided experimental evidence for Rodbard's assumptions that an increase in flow induces artery dilation (134), most likely through the release of a dilator signal from the endothelium (263). Pohl et al. (246) performed a series of experiments in which they demonstrated that in vivo infusion of acetylcholine and augmentation of arterial flow elicited remarkably similar dilation, whereas mechanical removal of the endothelial layer abolished these responses (Figure 4). In the same year, Rubanyi et al. (263) reinforced the ability of increases in flow to induce an endothelium-dependent dilation, and also found that, in addition to prostacyclin, flow triggers the release of another relaxing substance to mediate vasodilation. Subsequently, Berdeaux et al. (21) (Figure 4) showed exercise-intensity-dependent vasodilation of canine epicardial coronary arteries was converted to vasoconstriction after mechanical endothelial denudation. Taken together, these studies provided further evidence for the importance of the functional integrity of the endothelium in the integrated control of arterial diameter, with the endothelium being hypothesized to protect against the vasoconstrictive effects of catecholamines released during exercise (21).
FIGURE 4.
Summary of the outcomes of studies that explored the impact of endothelial denudation on conduit artery functional and structural responses. Endothelial removal has typically been achieved using intra-arterial balloon inflation. In endothelium intact arteries, increases in flow and shear induce dilation in the dog hindlimb [From Pohl et al. (246), with permission from American Heart Association], coronary arteries [From Berdeaux et al. (21), with permission from American Heart Association] and the human radial artery [From Dawson et al. (61)]. All functional and structural adaptive responses are abolished or attenuated in the absence of an intact endothelial layer. Remodeling of rabbit carotid arteries in response to chronic decreases in flow and shear induced using unilateral ligation is also endothelium dependent [From Langille and O'Donnell (167), with permission from American Heart Association]. These studies highlight the importance of the endothelium in the mediation of (acute and chronic) changes in diameter.
In 1989, two human-based studies reported that increases in brachial artery flow induced by distal cuff deflation around the forearm were followed by dose-dependent dilation of the artery (8, 275). These observations prompted introduction of the “flow-mediated dilation (FMD)” approach as an in vivo bioassay of endothelium-dependent vascular relaxation in humans. This noninvasive approach uses a 5-min cuff occlusion of the forearm to induce increased shear stress in the brachial artery and consequent dilation (44), quantified by noninvasive high-resolution ultrasound. Human data support the endothelial and NO dependence of brachial FMD (98), and radial artery FMD is significantly reduced after endothelial denudation in vivo (61) (Figure 4).
The endothelium is also essential in mediating structural arterial adaptation. A decrease in common carotid artery size was observed in response to chronic reductions in blood flow, while such adaptation was abolished when the endothelium was removed (167) (Figure 4). A subsequent study further explored the role of endothelium-derived NO in adaptive changes in diameter (307) (Figure 4). Blood flow through the carotid artery was chronically increased by an arteriovenous fistula, which caused the diameter to increase (causing normalization of shear stress). However, animals in which NO synthesis was pharmacologically blocked showed attenuated adaptation of carotid diameter. These findings suggest that the endothelium, through NO-dependent pathways, plays a role in remodeling of vessel diameters in response to increases in shear stress.
1. Detection of shear stress by the endothelium
Mechanotransduction at the luminal surface of the endothelium is initiated by shear stress detection by ion channels (K+, Ca2+, Na+, Cl−), cell membrane receptors (tyrosine kinase receptors), G proteins, caveolae, and the plasma membrane lipid bilayer (10) (Figure 2). Furthermore, the lumen is lined with glycocalyx, a glycoprotein-polysaccharide structure that is specifically responsible for shear stress-induced NO production (10). There is evidence for primary cilia that are linked to shear stress-mediated production of NO (10), and shear has also been proposed to be detected by the cytoskeleton of the endothelial cells, largely through its connection to integrins (VE-cadherin and integrin) and a mechanosensory complex (platelet endothelial cell adhesion molecule-1). A possible explanation for the involvement of multiple, distinct types of mechanotransduction is that shear stress, in contrast with pressure, is a relatively weak force (10). Therefore, highly sensitive mechanisms seem necessary to sense shear stress (Figure 2), including the detection of complex patterns of shear.
2. Relevance of the pattern of shear
A) CORRELATIONAL STUDIES.
In 1969, Caro et al. (38) studied the incidence of atherosclerosis in low versus high shear regions and branch points in the celiac, mesenteric, and renal arteries. They found that, relative to areas of higher shear (i.e., inner wall of branch points), regions of low shear (i.e., outer wall of daughter vessels) revealed greater burden of atherosclerotic lesions. The authors proposed that local hemodynamics play a fundamental role in the etiology of atherosclerotic disease (38). Of relevance to the present review, Caro et al. (38) were likely the first to articulate that “physical exercise involving increase of cardiac output, and hence increased shear rate, might retard the development of atheroma.” Along these lines, post mortem human carotid artery specimens exhibit the greatest atherosclerotic burden at the outer wall of the vessel, a region subjected to low levels of shear stress and substantial flow reversal (325). Studies in large animals also support the idea that atherosclerosis preferentially develops in susceptible regions of the vasculature. Indeed, low and oscillatory shear regions occur in porcine coronary and peripheral conduit arteries at geometrically irregular sites (i.e., branch points, bifurcations, and curvatures), and these regions are highly atherosclerosis prone (49, 57, 110, 139, 165, 245). Furthermore, it was recently reported in a pig model of familial hypercholesterolemia (251) that the distal portion of the aorta, subjected to disturbed blood flow profiles, presents approximately threefold greater levels of atherosclerotic burden relative to the proximal portion of descending aorta, which is exposed to more unidirectional flow (236).
B) IN VITRO STUDIES.
Intricate mechanotransduction and signaling mechanisms operate in concert to alter endothelial gene expression and function, and adaptation depends on the characteristics of the shear stress stimulus (148). Initial in vitro studies demonstrated that oscillatory shear promotes endothelial inflammation, activation, and abnormal alignment of endothelial cells (58), whereas laminar shear reduces inflammation-related events such as leukocyte-endothelial adhesion (179). Using sophisticated technology, more recent studies showed that expression of pro-atherogenic genes is increased in cultured endothelial cells subjected to shear patterns replicating in vivo patterns in atheroprone regions, in contrast to cells exposed to patterns characteristic of protected regions (35, 126, 228). Pro-atherogenic shear patterns induce production of NADPH oxidase- and mitochondria-derived superoxide radicals, augment production of endothelin-1, and upregulate VCAM-1 and ICAM-1 (51, 54, 142, 143, 228, 284, 318), all critical early events in the development of atherosclerosis.
The role of shear stress patterns in modulating vascular health is also supported by studies in isolated and pressurized arteries. For example, isolated rat soleus feed arteries exposed to high flow and shear stress for 4 h exhibited increased expression of eNOS mRNA and enhanced endothelium-dependent dilation (321). Conversely, exposure of rat carotid arteries to low levels of shear stress for 4 h promoted the upregulation of adhesion molecules such ICAM-1 and VCAM-1 (236). Others have shown that pig carotid arteries subjected to oscillatory shear for 3 days exhibited a marked impairment in endothelium-dependent vasorelaxation (80), an effect that was accompanied by reduced eNOS mRNA and protein expression. Also, evidence exists indicating that flow reversal significantly reduces NO bioavailability in isolated porcine femoral arteries due to increased superoxide production (188), a known molecular mediator of atherogenesis (234). NADPH oxidase appears to be the principal source of retrograde flow-induced ROS generation in isolated arteries (89, 188). Taken together, the data from in vitro cell culture and isolated vessel preparations firmly support the view that disturbed shear stress patterns stimulate a pro-atherogenic endothelial cell phenotype.
C) IN VIVO STUDIES.
Data demonstrating that disturbed shear stress profiles produce detrimental vascular effects are also available from in vivo studies in animals. The partial carotid artery ligation model has provided a valuable experimental model for the in vivo study of disturbed shear stress profiles (162, 221). Due to ligation of all but one of the distal branches, and a consequent increase in downstream vascular resistance, the proximal portion of the carotid artery is chronically exposed to disturbed blood flow. This model leads to substantial wall thickening with leukocyte infiltration and smooth muscle proliferation within 2 wk in normal mice (162) and endothelial dysfunction and advanced atherosclerotic lesions in ApoE−/− mice (221). These functional alterations are preceded by rapid molecular phenotypic changes. In this regard, expression of more than 500 genes is altered within 2 days following ligation (226). This experimental model has provided considerable insights related to the mechanisms underlying disturbed shear stress-induced atherosclerosis. Leukocytes have been reported to promptly accumulate into the arterial wall during initiation and progression of disturbed flow-induced atherosclerosis (3). In addition, eNOS uncoupling (184), NADPH oxidase-derived superoxide radicals (221), fibronectin polymerization (46), interleukin (IL)-17 signaling (189), and adhesion molecules such as PECAM-1 (45) are all involved in the development of atherosclerosis caused by partial carotid ligation in mice. Of note, studies in which the partial ligation model was superimposed onto models of obesity (181) and renovascular hypertension (147) indicate that the pro-atherogenic effects of disturbed shear stress may be more prominent when accompanied by cardiovascular risk factors.
C. Importance of Interaction Between Hemodynamic Forces
Exercise has complex effects on hemodynamics that result in increased blood flow and shear stress, increased frequency of pulsatile changes in pressures and flows, and increased arterial systolic and pulse pressures. These complex hemodynamic effects of exercise can contribute to the expression of pro-atherogenic vascular phenotypes, especially when the hemodynamics are asynchronous. Dancu et al. (55) showed that synchronous pulsatile changes in diameter, flow and blood pressure have different effects on silicon tubes lined by endothelium, compared with the effects of asynchronous changes. Synchronous hemodynamics exist when flow, heart rate and pressures have the same time courses, i.e., peak pressure, peak flow and peak diameter occur at nearly the same time. Synchronous hemodynamics are often seen in the aorta, whereas asynchronous hemodynamics are common in the coronary arteries. Indeed, peak coronary flow is observed in early diastole, when pressure and coronary artery diameter are low. Therefore, asynchronous hemodynamics are normal for coronary arteries (56). When synchronous and asynchronous changes in pressure, tube diameter and gene expression in endothelial cells cultured in silicon tubes are examined in more detail, results demonstrate that asynchronous pulsations in shear stress and circumferential strain result in decreased eNOS expression, but increased ET-1 expression in endothelial cells (55, 56). The fact that coronary arteries are exposed to asynchronous hemodynamics may therefore explain, in part, the propensity of these arteries to develop atherosclerosis. During exercise, however, coronary blood flow becomes pulsatile with positive flow in both systole and diastole. The relatively greater exercise-induced increase in shear stress and blood flow (4- to 6-fold increase), combined with small increase in systolic pressures and nonoscillatory coronary blood flow, results in net anti-atherogenic signals (171, 173).
III. HEMODYNAMIC STIMULI DURING ACUTE EXERCISE IN HUMANS
A. Effects of Exercise on Hemodynamic Forces
1. Shear stress in vascular territories perfusing active areas
At the onset of exercise, blood flow and shear stress markedly increase in active regions in an exercise-intensity-dependent manner, to meet increased metabolic demand (95, 270, 290). For example, handgrip exercise, which causes minor changes in blood pressure and cardiac output, induces large hyperemic responses, suggesting that vasodilation in downstream resistance vessels is the major cause of increased blood flow during handgrip exercise (95). Lower limb exercise, which engages a larger muscle volume and consequently increases blood pressure and cardiac output, is associated with increases in femoral artery blood flow that result from changes in downstream resistance vessel dilation in concert with the increases in central driving pressure. Studies that have examined local vasodilator mechanisms contributing to exercise-hyperemia have generally found a redundancy of vasodilator mechanisms (153), which means that blocking single pathways does not importantly impair exercise-hyperemia. This redundancy ensures that blood flow to exercising muscle is highly protected, even in the absence, or attenuated presence, of key vasodilator pathways. Therefore, local vasodilator mechanisms along with increases in arterial pressure and cardiac output contribute to exercise hyperemia, leading to significant increases in shear stress in the active areas during exercise.
While a large number of studies have explored exercise hyperemic mechanisms, little attention has been paid to the impact of exercise on the pattern of blood flow and shear stress. This likely relates to the technical difficulty and limitations associated with contemporary techniques to validly assess shear stress patterns in physically active limbs, such as in the lower limbs during cycling or running exercise. However, some studies have examined brachial artery shear stress pattern during local handgrip exercise. In agreement with studies adopting techniques to assess bulk blood flow, Green et al. (95) found that brachial artery blood flow and shear rate increased with incremental levels of handgrip exercise in healthy subjects. An intensity-dependent increase in antegrade shear, with negligible levels of retrograde shear rate, was observed during handgrip exercise (95). This study also explored the role of NO during incremental handgrip exercise. Largely in agreement with observations regarding redundancy and blockade effects during exercise-hyperemia (153), blood flow and shear rate patterns of the brachial artery during incremental handgrip exercise were modestly altered in the presence of a NO synthase blocker (95). These data demonstrate that increases in mean shear rate to an active limb are largely mediated through increases in antegrade shear rate, with negligible changes in retrograde shear rate, while NO is not obligatory to mediate these changes in shear pattern.
The increase in antegrade shear induced by handgrip exercise is not stable, but consists of a highly fluctuating pattern of antegrade shear, at least partly induced by muscle contractions (Figure 5). Previous work examined whether such a fluctuating pattern of shear stress (due to handgrip exercise), compared with gradual elevation in shear stress, affects the ability of arteries to dilate (248). It was demonstrated that, when matched for mean shear, comparable conduit artery dilation was achieved during handgrip exercise and heating. This indicates that muscle contractions per se are not obligatory for the impact of shear on conduit artery dilation.
FIGURE 5.
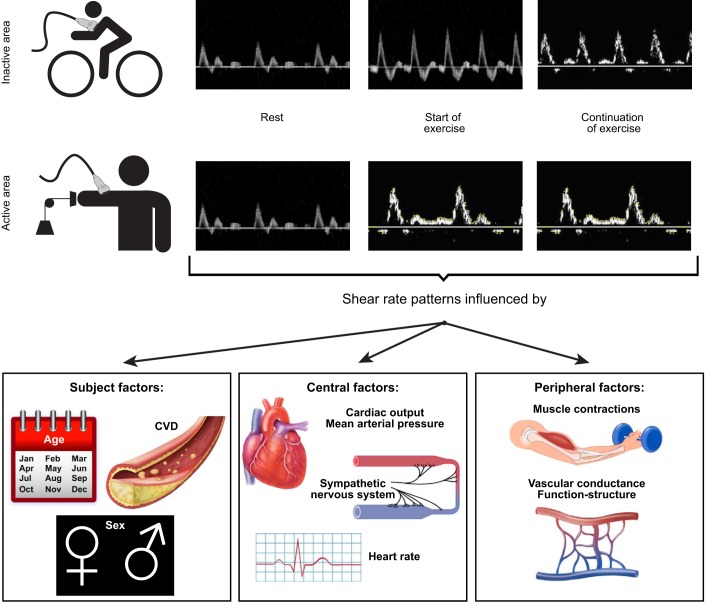
Brachial artery Doppler trace during leg cycle exercise (A; brachial artery representing an inactive region) and during handgrip exercise (B; brachial artery representing an active region) at rest, at the start of exercise, and during continuation of exercise. The (time-dependent) changes in Doppler patterns are influenced by subject as well as central and peripheral factors, summarized in the lower panel.
Despite a significant amount of work exploring exercise hyperemia and its underlying mechanisms, surprisingly little is known about the relative impact of distinct changes in shear stress pattern during different forms of exercise in the active limbs. This knowledge may importantly contribute to our understanding of adaptations in vascular function and structure in physically active regions. It is also important to consider that distinct forms of exercise have impacts on vascular compression and the transduction of forces related to transmural pressure (14). Given evidence that these factors have impacts on arterial adaptation, the differential impact of exercise involving sustained (e.g., rowing) versus cyclical (e.g., rhythmic handgrip) muscular contraction warrants further investigation (261, 262).
2. Shear stress in vascular territories perfusing inactive areas: start of exercise
Early studies indicating that predominant lower limb exercise training induced adaptation in upper limb vascular function (186, 193, 194; previously reviewed in Ref. 104) stimulated interest in the patterns of shear stress occurring in vascular territories other than those feeding active musculature.
Historical evidence suggests that blood flow to the inactive upper limbs decreases during the initial stages of lower limb cycle exercise, and is subsequently restored as exercise continues (25). This study, involving indirect quantification of blood flow using oxygen saturation levels of axillary venous blood, reported a biphasic response in total arm blood flow during the lower limb exercise, characterized by initial decreases from resting values, with subsequent increase in flow. More recently, imaging technologies have confirmed this pattern. Small increases in antegrade flow are accompanied by substantial increases in retrograde flow (92, 93) through the brachial artery during the initial stages of cycle ergometer exercise. The increase in retrograde flow may relate to activation of the sympathetic nervous system (SNS) and an increase in downstream vascular resistance (43, 240). Alternatively, the immediate increase in retrograde shear at the onset of exercise may be caused by an immediate increase in microvascular critical closing pressure (113). This immediate increase in brachial artery retrograde shear at the start of cycle exercise remains relatively stable as exercise work loads increase (92, 93). In contrast, higher cycle exercise intensities are associated with larger increases in antegrade shear, likely due to increases in cardiac output. Consequently, brachial artery mean blood flow shows a biphasic pattern, with a decrease at lower exercise intensities and an increase in blood flow at higher exercise intensity levels (92, 93).
The pattern of shear stress described above depends on the type of exercise performed (290). Cycling and walking, both representing rhythmic lower limb exercise, result in the typical oscillatory shear rate pattern. Blood flow patterns were markedly different during leg kicking, which was linked to a systolic blood pressure-driven increase in antegrade shear rate, without changes in retrograde shear. The different shear patterns suggest that distinct stimuli are responsible for the resulting change in shear stress during exercise. It should be acknowledged that pulsatile pressure and heart rate (and therefore cyclic circumferential strain) also differ markedly between these types of exercise. While leg kicking exercise is associated with small changes in heart rate and is typically sustained for 5–10 min, rhythmic exercise can be sustained for prolonged periods at relatively high heart rates and significant elevation in blood pressure. These differences likely contribute to the distinct shear stress patterns between different types of exercise, which have implications for vascular cell signal transduction and consequent arterial adaptation in humans.
3. What are the mechanisms for changes in shear stress at the start of exercise?
Blocking NO synthase causes a significant drop in brachial artery mean blood flow during cycling, especially at higher work loads (93, 95). These observations suggest that upper limb blood flow during lower limb cycle exercise is, at least partly, mediated through endothelium-mediated release of NO. To better understand the hemodynamic stimuli responsible for NO production under these circumstances, the role of increases in heart rate was explored in the absence of exercise-induced changes in pulse pressure (92). Heart rate was increased in patients with implanted pacemakers to levels similar to those observed during lower limb exercise. In the absence of increases in pulse pressure, isolated increases in pulsatility induced no change in brachial artery blood flow or the contribution of NO to the blood flow response. This suggests that pulse pressure, rather than pulse frequency, may be important for NO production in the upper limb during lower limb cycle exercise in vivo (92).
4. Shear stress in vascular territories perfusing inactive areas: continuation of exercise
During prolonged lower limb exercise, brachial artery blood flow and shear stress patterns in the inactive upper limbs undergo marked changes. In addition to central factors (i.e., cardiac output, arterial pressure, SNS), dilation of resistance arteries and skin microcirculation occur during prolonged exercise, mainly as a thermoregulatory response to facilitate heat exchange. This thermoregulatory dilation leads to decreases in peripheral vascular resistance, which subsequently affect the upstream conduit artery blood flow and shear stress patterns.
Simmons et al. (274) examined the time course of changes in skin perfusion and brachial artery shear stress patterns during prolonged cycle exercise. At the start of cycle exercise, the increase in brachial artery retrograde shear rate was accompanied with a modest decrease in cutaneous vascular resistance. This suggests that forearm cutaneous resistance does not mediate the initial changes in brachial artery blood flow patterns during lower limb exercise. More likely, these changes in shear pattern are mediated through increases in downstream skeletal muscle vascular resistance. Continuation of moderate-intensity cycle exercise decreased vascular resistance, as the cutaneous microcirculation dilated to subserve thermoregulation. Hence, initial retrograde flow patterns, observed at the onset of exercise due to peripheral vasoconstriction under the effects of the SNS, eventually resolve as vascular resistance diminishes as a consequence of thermoregulatory dilation (239, 274). Indeed, forearm cooling at the end of the exercise bout significantly increased forearm and skin vascular resistance and, subsequently, increased retrograde shear (274). These data indicate the importance of integrative changes in human physiological responses to exercise. Changes in blood flow response to exercise per se, along with thermoregulatory modification of systemic blood flow distribution and hemodynamics, both contribute to the ultimate pattern of blood flow and shear stress through human arteries in vivo.
Another relevant question is whether exercise per se is essential to the modulation of arterial diameter in response to changes in shear stress. Carter et al. (39) reported a dose-dependent dilation of the brachial artery in response to stepwise increases in shear stress that were exercise independent. The hypothesis tested was that, if artery dilation during exercise is a consequence of changes in arterial shear stress, then similar changes in shear stress in the absence of exercise should induce a similar magnitude of dilation. It was observed that heating of the forearm (39, 239) or legs (41) at rest caused comparable arterial dilation in response to increases in brachial artery shear stress. More importantly, some of these studies have performed bilateral assessment of the brachial artery, with unilateral cuff inflation to effectively attenuate the heat- or exercise-induced increase in blood flow and shear in one arm, leaving the contralateral arm unaffected. Abolishing the exercise- or heat-induced increase in blood flow and shear stress prevented brachial artery dilation under these experimental conditions. Such within-subject designs involving simultaneously derived measurements, that control for systemic factors and subject variability, strongly imply that shear stress is an important stimulus to acutely dilate conduit arteries in humans. These studies provide insight into the observation that repeated whole body heating (e.g., sauna) may confer clinical benefits in terms of vascular function and health (37, 178).
5. Non-shear stress hemodynamic stimuli mediating artery vasomotion during exercise
Exercise causes marked increases in transmural pressure, a stimulus that reduces arterial diameter in studies using isolated preparations and animals (59, 151). Examining the impact of transmural pressure in humans in vivo is challenging due to the confounding influence of concurrent changes in shear stress which typically accompany alterations in pressure. In a recent study, Atkinson et al. (14) utilized 30-min unilateral handgrip exercise to induce systemic elevation in blood pressure. This approach was not associated with changes in shear rate in the resting contralateral arm, or changes in SNS activation, providing a model to isolate the impact of transmural pressure from that of shear rate in vivo. Unilateral handgrip exercise caused a stepwise decrease in contralateral brachial artery diameter in the resting limb, whereas these decreases in diameter were mitigated in the active limb by exercise-induced elevation in shear stress (14). This work supports the role of transmural pressure in the regulation of vascular tone and suggests active competition in distinct vascular beds between the effects of transmural wall pressure changes and changes in localized shear stress.
Taken together, hemodynamic stimuli, including shear stress and transmural pressure, markedly differ between vessels supplying active and nonactive areas, but also differ between various types of exercise. Functional and structural characteristics of the cardiovascular system also affect the hemodynamic responses to exercise (20). The various factors influencing shear stress patterns in conduit arteries during exercise are summarized (Figure 5), highlighting the complex, integrative nature of the exercise stimulus. Insight into the different hemodynamic stimuli may improve our understanding of the impact of exercise training on adaptations in vascular function and structure and the consequent implications for vascular health (106, 107).
B. Impact of Different Shear Stress Patterns on Artery Function
Studies have demonstrated that acute exercise can lead to an immediate increase in endothelium-mediated dilation (60). To examine the relative importance of shear stress in these functional changes, Tinken et al. (305) examined brachial artery vasodilator function, using the FMD test, before and after 30-min handgrip exercise (i.e., metabolically driven), cycle exercise (i.e., thermoregulatory-driven), and forearm heating (i.e., non-exercise driven) (305). After successfully increasing shear stress levels, FMD significantly improved. Given the marked differences between the three interventions in pulse pressure and pulse frequency, these results highlight the importance of shear stress in mediating acute changes in endothelium-mediated dilation. Indeed, unilaterally attenuating the shear stress stimulus, with preservation of the pulse pressure and frequency, abolished the improvement in FMD (305). This suggests that elevation in shear stress, independent of exercise, directly impacts vascular function in humans.
Given the intensity-dependent relationship between exercise and hyperemia, higher intensity exercise (and therefore larger shear stress) may lead to incremental increases in postexercise vascular function. However, most studies that have explored this relationship have reported a decrease in vascular function immediately after high-intensity cycle exercise (60), which may be followed by a rebound recovery of function one or more hours after the cessation of the bout. For example, cycle exercise at 70–85% impaired FMD post exercise, a response not observed following exercise at 50% of maximal heart rate (22). In addition to increases in shear stress, strenuous exercise also mediates other effects such as the production of ROS and activation of the SNS (90). These potentially detrimental effects may mitigate beneficial shear stress effects of exercise (60). To address these competing impacts, Atkinson et al. (13) examined the effect of incremental levels of handgrip exercise on brachial artery vascular function. Such exercise increases shear stress in the brachial artery, without producing the same degree of reflex sympathetic activation or hormonal change associated with exercise using a larger muscle group, such as lower limb exercise. The dose-dependent increase in brachial artery blood flow and shear stress in response to hand gripping was associated with post-exercise improvement in vascular function following 1 h of recovery from the bout, at the highest exercise only. These data provide further evidence that increases in shear stress, per se, can improve vascular function, possibly in a dose-dependent manner.
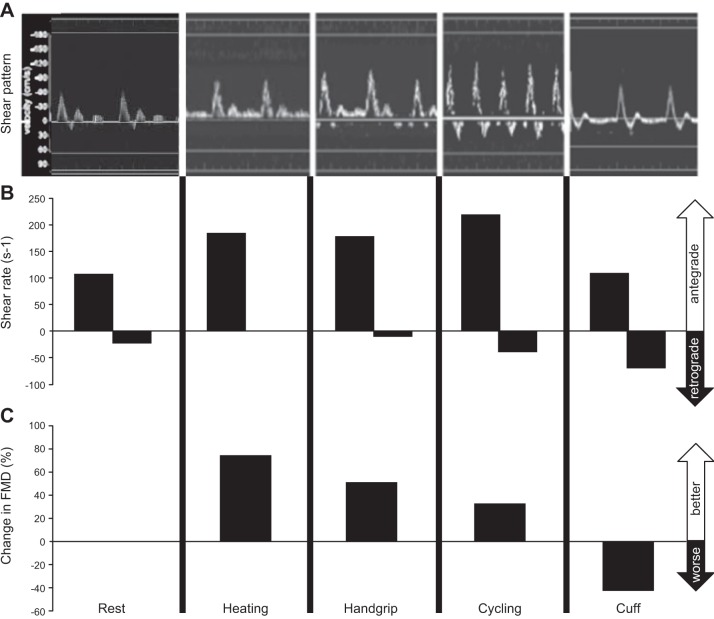
To explore the relevance of the pattern of shear stress, one aforementioned study compared the effects of 30-min forearm heating, handgrip exercise, and leg cycle exercise (305). Mean shear levels under each condition were matched by manipulating the exercise intensities. Comparable improvements in vascular function were observed under each condition. Unilateral forearm cuff inflation reduced mean blood flows in the contralateral arm under each of the experimental conditions, causing distinct shear patterns. Vascular function did not change after heating or handgrip exercise in the cuffed arm, whereas FMD decreased after cycle exercise in the cuffed arm, a condition associated with much greater retrograde flow and shear stress. This study therefore suggested that decreases in FMD occur particularly after exercise that induces a retrograde shear component, such as is evident in the upper limbs during leg cycling. A subsequent study utilized subdiastolic cuff inflation (25, 50, and 75 mmHg) in resting subjects to explore the impact of 30-min exposures to incremental levels of retrograde shear rate, with matched levels of antegrade shear rate (291). Manipulation of the magnitude of retrograde shear in a dose-dependent manner leads to a stepwise decrease in FMD. Taken together, these data are largely in agreement with previous work in animals (see sect. IIB2), and support a role for shear stress in the alteration of endothelial function, with distinct shear patterns potentially leading to different changes (Figure 6).
FIGURE 6.
Doppler trace (A), blood flow and shear rate patterns (B), and consequent acute changes in vascular function (measured as the flow-mediated dilation, FMD; C). Distinct patterns of blood flow and shear in the brachial artery induced by forearm heating, handgrip exercise, cycle exercise, and cuff manipulation have different impacts on the function of the artery, assessed immediately before and after each intervention. Data are derived from Tinken et al. (305) and Thijssen et al. (291). Taken together, these data are largely in agreement with previous work in animals (see sect. IIB2) and support a role for shear stress in the alteration of endothelial function, with distinct shear patterns leading to different changes in function.
If the pattern of shear stress is important, as these studies suggest, the return of brachial artery retrograde shear to baseline values and increase in antegrade shear during prolonged exercise may be advantageous. These changes reflect conversion from a potentially pro-atherogenic stimulus (patterns dominated by a retrograde component) to an anti-atherogenic stimulus. This likely represents the predominant stimulus to which arteries are exposed during prolonged exercise. The differential impacts of exercise intensity on shear patterns may also be a relevant consideration, with lower intensities potentially inducing less detrimental patterns. The protective effects of shear in this regard should be considered in the context of the epidemiological evidence, which indicates that the greatest impact on cardiovascular events occurs from adoption of lower levels of physical activity and that the benefits trail off as the volume of PA increases. The relevance of the pattern of shear stress for adaptation in vascular function and structure to exercise training is further discussed below.
IV. VASCULAR ADAPTATIONS TO EXERCISE TRAINING IN HUMANS: ROLE OF HEMODYNAMIC FACTORS
A. Adaptations in Vascular Function
1. Conduit arteries
Studies in subjects who exhibit impaired endothelial function, such as those possessing CV risk factors (e.g., hypertension, hypercholesterolemia, type 2 diabetes mellitus, obesity) or with established CVD (e.g., heart failure, peripheral artery disease), have typically revealed improvement in conduit artery function (measured as the FMD) following exercise training (85, 104, 193, 194, 244, 296, 315, 316). Indeed, a recent meta-analysis of randomized controlled trials (12) confirmed earlier proposals (104, 196), that exercise training improves FMD, with larger improvements in populations with cardiometabolic disorders. These findings also confirm observations from our group, in which data on 182 subjects who underwent supervised center-based exercise training were pooled. The strong inverse relation between pretraining FMD and improvement in FMD (100) suggested that conduit artery endothelial function is highly amenable to improvement, especially in subjects with the presence of CV disease and/or risk. Similarly, exercise training is able to improve coronary artery diameter, coronary blood flow responses to intracoronary administration of acetylcholine, and coronary blood flow reserve to adenosine infusion (119, 170).
To understand the role of hemodynamic stimuli on vascular adaptation to training, Hambrecht et al. (114) studied the impact of 4 wk of cycle exercise training on the internal mammary artery of CAD patients (114) (Figure 7). Data from the harvested arteries indicated a twofold increase in eNOS expression and fourfold higher eNOS Ser1177 phosphorylation after 4-wk training (114). The upregulation of eNOS Ser1177 is of particular relevance, since phosphorylation of eNOS at position Ser1177 is linked to shear stress transduction. Moreover, a correlation was present between improvement in endothelial function in vivo and shear-dependent eNOS phosphorylation. These data suggest that exercise causes activation of eNOS, through a shear stress-induced/Akt-dependent increase in eNOS phosphorylation on Ser1177, ultimately leading to improvement in endothelial function. These results are supported by reports that exercise training improved endothelium-dependent dilation in peripheral and coronary arteries in humans (114, 115, 117, 193, 194, 315, 316) and a porcine model of early-stage atherosclerotic disease (302, 322, 323).
FIGURE 7.
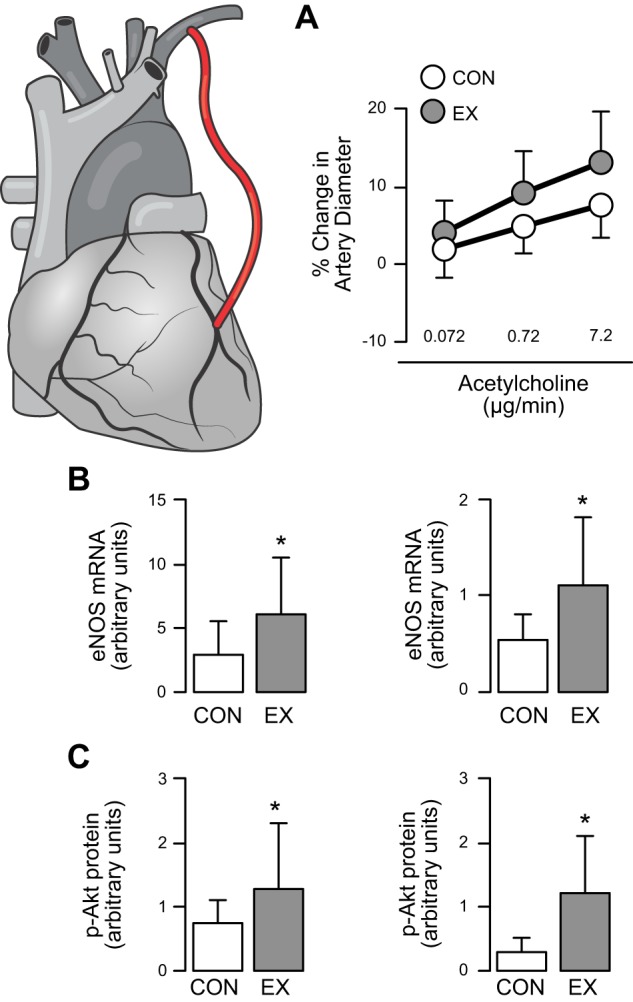
Impact of exercise training in humans with coronary disease. Relative to a nontrained control group (CON), 4 wk of exercise training (EX) increased in vivo acetylcholine-induced vasodilation of the left internal mammary artery (LIMA) (A), increased endothelial nitric oxide synthase (eNOS) mRNA and protein expression in LIMA (B), as well as increased phosphorylation of Akt at Ser473 and eNOS at Ser1177 (C), an effect likely mediated by shear stress. Data are means ± SD. *Statistical significance between groups. [Redrawn from Hambrecht et al. (114), with permission from American Heart Association.]
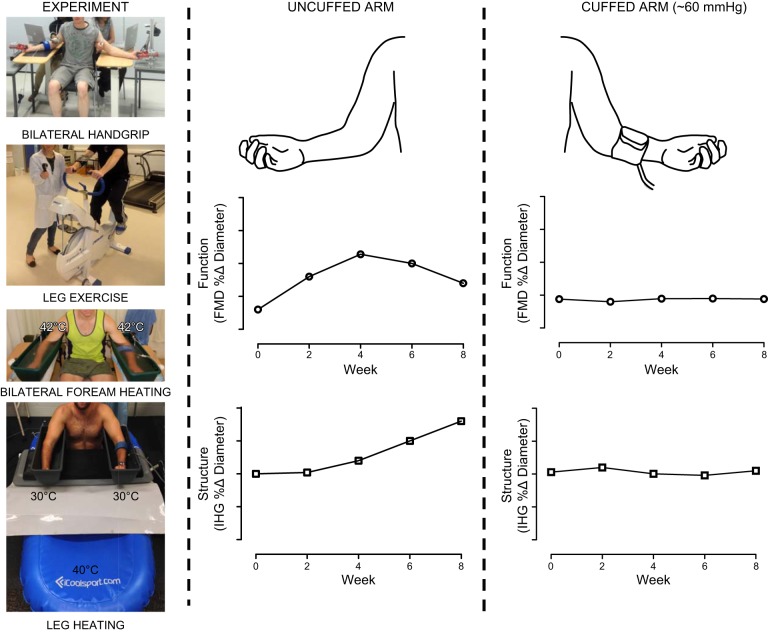
A crucial role for shear stress in mediating vascular adaptation was described in a subsequent series of human in vivo studies, which adopted the model of unilateral, sub-diastolic cuff inflation to attenuate shear stress during exercise (Figure 8). By performing simultaneous bilateral assessments of vascular function and structure, this approach provided a within-subject model to explore the importance of shear stress, especially since both arteries were exposed to similar levels of circulating stimuli, reflex activation, and pressure-related hemodynamics. Adopting this design, 8 wk of bilateral handgrip exercise training (306) and cycle exercise training (22) resulted in significant, time-dependent changes in vasodilator function and structure of the brachial artery in the noncuffed arm. In marked contrast, these exercise training-related adaptations across 8 wk of exercise training were nonexistent in the arm devoid of shear stress due to cuff inflation.
FIGURE 8.
Summary of a series of experiments involving unilateral manipulation of arterial shear stress through the brachial arteries to ascertain the contribution of shear stress to changes in artery function (flow-mediated dilation, FMD) and structure (peak reactive hyperemic dilation induced by ischemic handgrip exercise, IHG). Partial cuff inflation on one arm was used to “clamp” shear at lower levels than the uncuffed and free flowing contralateral limb. lnterventions included bilateral handgrip exercise training, leg exercise training, bilateral forearm heating (42 °C) and leg heating (40 °C) at clamped forearm temperatures (30 °C). Arterial adaptation only occurred in the limb in which shear stress increased; cuff placement substantially decreased blood flow and shear during bouts of exposure, and abolished adaptation in both artery function and structure. Note the biphasic changes in function and the increases in artery size as function resiles (see also Figure 9).
To further evaluate the importance of shear stress, subsequent studies induced repeated episodic increases in brachial artery shear stress using an exercise-independent heating stimulus (41, 222). Exposure to forearm heating increases brachial artery shear stress, whereas inflating a blood pressure cuff around the forearm abolishes such changes (222). Eight weeks of exposure to forearm heating caused a time-dependent improvement in brachial artery vasodilator function and structure, while no adaptation was apparent in the cuffed arm (222). Both arms were directly exposed to heat, which may impact the findings. Therefore, experiments were repeated using 8-wk episodic submersion of the lower limbs in warm water, leading to forearm hyperemia subserving thermoregulation (41). In keeping with previous studies, adaptations in brachial artery function occurred in the uncuffed arm, but not in the cuffed arm in which shear stress was not elevated. These observations support the idea that increases in shear, independent of the method, induce vascular adaptations.
It is important to emphasize that the relative contribution of different vasodilator and constrictor pathways to the improvement in conduit artery function following training remains largely unknown. Specifically, while some previous work has indicated that training-induced improvements in FMD are largely mediated through NO (98), other less well studied mechanisms are nonetheless likely to contribute (136). In addition, while many studies have reported no change in endothelium-independent smooth muscle-mediated dilation following training, most of these adopted a near maximal dose of NO donor, and it is possible that changes in smooth muscle function as a result of training have been overlooked. Indeed, some animal studies have observed changes in artery function that are endothelium-independent as a result of training (170). These research questions require further investigation to fully address the pathways underlying generalized improvement in conduit artery function in response to exercise training in humans.
Another biophysical property of large arteries, arterial wall stiffness, can be reliably measured via pulse wave velocity (PWV), which strongly relates to atherosclerotic disease (314). Measurement of PWV compliments measures of FMD in that PWV captures structural along with functional health of the arterial wall. The increase in risk for CV events is 30% for every 1SD change in PWV (17), a 1-m/s increase in PWV leads to a 7% increase of the hazard for CV events (314) and PWV improves 10-yr risk classification by 13%. PWV is therefore a commonly adopted tool to examine conduit artery stiffness, with studies distinguishing between “central” (femoral-carotid PWV) and “peripheral” stiffness (brachial-ankle PWV). Recent work analyzed all randomized controlled studies of the impact of exercise training on both measures (11). While a generalized effect of exercise training was observed, a somewhat larger effect size was related to longer duration of training and in those with lower a priori levels of arterial stiffness. Furthermore, a larger reduction in PWV after exercise training was observed for brachial-ankle PWV compared with carotid-femoral PWV. This suggests that exercise has a larger effect on “peripheral” conduit artery stiffness compared with aortic stiffness (11). Possibly, more muscular, stiffer peripheral arteries allow for larger adaptations of arterial wall properties in response to exercise training, compared with central, more elastic arteries. In agreement with this hypothesis, one study reported that exercise training improves peripheral artery stiffness (i.e., popliteal artery), in the absence of changes in a central (carotid) artery (249). Few studies have directly examined the importance of distinct hemodynamic factors in mediating changes in the stiffness of arteries in humans.
2. Resistance arteries
Traditionally, the impact of exercise training in resistance arteries has been studied using forearm strain-gauge plethysmography (154). When combined with intrabrachial infusion of agonists or antagonists, this allows for detailed insight into the mechanisms underlying changes with training (154). Adopting a cross-sectional design, Green et al. (101) explored the impact of regular exercise on resistance artery endothelial function and found no differences in endothelial function (infusion of acetylcholine), or contribution of NO to basal resting tone (l-NMMA), between the dominant and nondominant arms of elite tennis players. Similarly, there was no impact on NO-mediated vasodilation as a result of 4 wk on unilateral handgrip exercise training (96). Kingwell et al. (158) observed that 4 wk cycle training did not change forearm resistance artery endothelial function, whereas an improvement in basal NO function was observed. Subsequent studies performed in healthy subjects have reported conflicting results regarding the impact of exercise training on resistance artery endothelial function, with some showing improvement (16, 209), but many reporting no change (195, 233). The majority of longitudinal studies in healthy subjects suggest that exercise training does not “supra-normalize” resistance vessel endothelial function (104).
Studies performed in subjects with impaired resistance artery endothelial function have been more consistent. Exercise training in middle-aged subjects improved forearm resistance vessel endothelial function (65) and NO bioavailability (281). Exercise training also improves endothelial function and/or increases the contribution of NO to basal tone in subjects with hypertension (131), type 2 diabetes mellitus (193, 223), obesity (202), and hypercholesterolemia (183, 316) and in subjects with coronary artery disease (114, 315) and heart failure (115, 116, 192, 194). Nonetheless, not all studies uniformly demonstrate improvement in resistance artery endothelial function (5, 16), and this may relate to the short duration and/or insufficient exercise intensity used in some studies. There are also well-established impacts of sex hormones on the function of arteries (102, 213) and some preliminary evidence that the impact of training may differ (26, 108, 214), although interactions between hormones and shear stress in terms of arterial adaptation have not been directly addressed in humans. Taken together, these findings strongly support the notion that exercise training improves resistance artery vascular function in subjects with CV risk or disease, in whom endothelial function is initially impaired (104).
Recent studies have focused on the potential impact of exercise training on the vasodilator prostacyclin (PGI2). Hellsten et al. (129) found that 8 wk of exercise training in hypertensive participants increased the formation of interstitial adenosine and PGI2, which may contribute to improved vascular responses after exercise training (129). Work from the same group demonstrated that exercise training increased PGI2 muscle protein levels and muscle interstitial concentrations in older men (88), while training-induced increases in PGI2 plasma levels were found in postmenopausal women (227) and hypertensive subjects (123). Others found that exercise training can improve the PGI2 pathway in humans (330), further supporting a role for upregulation of the prostanoid system to improve endothelial function after exercise training.
Vasoconstrictors endothelin (ET)-1 and angiotensin II (ANG II) do not importantly contribute to the regulation of baseline vascular tone in healthy volunteers (111, 294, 312) and aerobic exercise training does not alter these vasoconstrictor pathways in healthy volunteers. In contrast, vasoconstrictor pathways are upregulated in subjects with cardiovascular disease or risk and aerobic training is able to partly reverse the contribution of ET-1 to baseline vascular tone in older humans (297, 310). Exercise training is also associated with decreased plasma and muscle levels of ET-1 (191, 227). With regard to ANG II, training in CAD patients caused a 49% reduction in ANG II-induced vasoconstriction (2). Taken together, exercise training improves vasoconstrictor pathways in individuals who, a priori, demonstrate an increased contribution of vasoconstrictors to vascular tone.
The SNS is a highly relevant vasoactive pathway, particularly in the context of exercise and training effects on resistance vessel function. Heart rate variability, a measure of autonomic balance, improves as a result of exercise training (219, 243), especially in those with autonomic disorders (243). Others have found that plasma norepinephrine decreases following training in heart failure patients. This effect may differ between healthy subjects and those with elevated norepinephrine (34). Consistent with these findings, training decreases age-related impairment in baroreflex function (207). Furthermore, muscle sympathetic nerve activity decreases after a period of exercise training, especially in subjects with elevated SNS activity (42, 232, 258). Finally, exercise training induces cyclic activation of brain stem centers, including the rostral ventrolateral medulla, which may modify central sympathetic output and vasoconstriction (217). Generally, these studies support the notion that exercise training decreases SNS activity level and SNS-mediated vasoconstriction. In contrast, some studies performed in healthy volunteers provide compelling evidence that exercise training does not lower SNS activity (258, 259). In fact, Sugawara et al. (281) demonstrated that aerobic training in healthy volunteers increased basal SNS vasoconstrictor tone (using α-adrenoceptor blockade) (281). This observation concurs with some evidence for elevated sympathetic tone following training in healthy subjects (6). Despite this apparent increase in resting tone, basal blood flows were similar after training, probably as a consequence of compensatory increases in NO-mediated vasodilator function (281). In keeping with this, exercise training can increase NO-mediated vascular tone, despite preserved resting blood flow (158). These lines of evidence support the contention that increased training-induced increases in vasodilator function or arterial remodeling may be counteracted by increased sympathetic tone, with no resultant change in resting blood flow or arterial diameter despite enhanced vasodilator capacity.
3. Microcirculation
Coronary arterioles from exercise-trained pigs exhibit enhanced myogenic constriction compared with arterioles from sedentary pigs (218), and similar results were found in exercise-trained rats (121). This enhanced tone may be due to altered calcium-dependent protein kinase C (PKC) signaling in the coronary smooth muscle cells (163) and increased voltage-gated calcium currents in smooth muscle of large arterioles through L-type calcium channels (31) (Figure 2). The increased constriction in response to stretch (myogenic reactivity) is not accompanied by changes in receptor-mediated vasoconstriction (ET-1, acetylcholine) or to direct stimulation of voltage-gated calcium channel activation with the L-type calcium channel agonist BAY K 8644 or by K+ (172). Exercise training may increase activity of Kv and KCa channels of coronary vascular smooth muscles and/or alter calcium control by sarcoplasmic reticulum (33, 128, 169).
Exercise training increases the maximal adenosine-induced increase in coronary blood flow per gram of myocardium in both dogs and miniature swine in vivo. Although these results demonstrate that coronary blood flow capacity is increased by exercise training, resting blood flow and blood flow during submaximal exercise (same absolute intensities) are equal or slightly lower after exercise training. At similar levels of cardiac work, coronary blood flow is not changed by exercise training, suggesting a minimal effect on the coupling between myocardial metabolism and coronary blood flow (170).
Exercise training has also been reported to increase endothelium-dependent dilation in response to intracoronary serotonin (30) and bradykinin in coronary arterioles (64–157 μm in diameter) isolated from exercise-trained swine (218). The increased bradykinin-induced dilation appeared to be the result of increased NO release from eNOS, because l-NMMA inhibited dilation to a greater extent in arterioles from exercise-trained pigs and eliminated the difference between trained and sedentary groups, suggesting exercise training enhances NO production by NOS (218). Consistent with this interpretation, subsequent work revealed increased endothelial NOS expression in coronary arterioles of exercise-trained swine (176). The observation that cytosolic copper/zinc superoxide dismutase (SOD-1) was upregulated in coronary arterioles of trained pigs (265) suggests that the increased endothelium-dependent vasodilator responses were, at least in part, the result of decreased quenching of NO by superoxide. In both exercise-trained and control arterioles, indomethacin decreased vasodilator responses without altering the exercise effect. Importantly, the sodium nitroprusside response did not differ between sedentary and trained swine (218), implying that exercise increased NO production in the endothelium. Indeed, Laughlin et al. (176) demonstrated increases in NO synthase content in the coronary endothelium of exercise-trained normal pigs.
In coronary arterioles isolated from animal models of vascular disease, exercise training is reported to increase basal myogenic tone and endothelium-dependent dilation (73, 130). Fogarty et al. (73) showed that vascular endothelial growth factor (VEGF165)-mediated vasodilation was enhanced by exercise training via elevated NO bioavailability. So, available evidence indicates that in animal models of coronary artery disease and in patients with coronary disease, exercise training increases endothelium-dependent dilation in coronary arterioles (86, 185, 253).
4. Cutaneous microcirculation: an active vessel bed during exercise
During exercise in humans, when a given threshold is reached, cutaneous vasodilation increases linearly with increases in core temperature until a plateau is achieved. Exercise training modifies this response, causing a leftward shift of the relation between cutaneous vasodilation and core temperature (i.e., vasodilation at a lower threshold) and a higher plateau (i.e., larger blood volume to the skin for heat dissipation) (254). In understanding these adaptations, a previous study linked responses to changes in blood volume (144). Ikegawa et al. (144) trained healthy men for 5 days and reinforced the presence of a leftward shift for the temperature threshold for skin vasodilation, increased plateau, and expansion in plasma volume (∼10%). When these tests were repeated after removal of the increase in plasma volume, the leftward shift in temperature threshold for cutaneous vasodilation and increase in plateau were eliminated. These results suggest that initial training-induced adaptations in cutaneous blood flow importantly depend on expansion of circulating blood or plasma volume (144). While these adaptations seem essential for systemic thermoregulatory purposes, exercise training may also affect intrinsic microvascular function. These intrinsic adaptations may be particularly relevant to the prevention of microvascular disease and its manifestations.
Studies investigating intrinsic cutaneous microvascular function have utilized skin laser-Doppler to assess local skin flux responses to substances such as acetylcholine (administered using iontophoresis or microdialysis), local heating, and/or reactive hyperemia. Local heating is often applied, especially since the plateau phase after sustained local heating is largely NO mediated (204) and can provide an index of NO-mediated microvascular function. A cross-sectional study found that the heating plateau phase was significantly larger in exercise-trained individuals compared with their sedentary peers (255), suggesting that exercise training is associated with improved NOavailability in the skin, a finding that supports observations in the studies that adopted iontophoresis or microdialysis (164, 317). Studies have also explored the effects of exercise training on cutaneous reactive hyperemia. Although the technique and data analysis differ between studies, cross-sectional comparisons report larger skin hyperemia responses in favor of the trained participants (76, 182, 311). Since these skin responses are correlated with nitrite/nitrate concentration (76) and plasma antioxidant capacity (77), larger skin microcirculatory responses to heat or ischemia observed with training may be related to the NO pathway and/or oxidative capacity.
Studies adopting longitudinal, prospective designs confirm these cross-sectional observations, in that regular exercise training improves cutaneous vascular function. These studies indicate that cutaneous responsiveness to both local heating stimuli and acetylcholine microdialysis were enhanced in response to exercise training in young (164, 317) and older humans (27, 132). Black et al. (27) also explored the role of the NO pathway in these adaptations by blocking NO production before and after training. They found that improvement in cutaneous microvascular responsiveness to exercise training was, in large part, due to improvements of the NO pathway (27).
The stimulus responsible for the intrinsic cutaneous vascular function adaptations to exercise training may relate to the hemodynamic impact of repeated exposure to increases in skin blood flow. To test this hypothesis, the impact of repeated episodic elevation in cutaneous blood flow, achieved by directly heating both forearms, was examined (42°C, 8 wk, 3 session/wk), while unilateral manipulation using cuff inflation attenuated cutaneous dilation in one arm (97). After 8 wk of this conditioning, cutaneous vasodilator responses to local heating were enhanced in the uncuffed arm, whereas this adaptation was not observed in the cuffed arm. A similar study found that abolishing forearm cutaneous vasodilation in response to 8 wk of repeated lower limb heating also prevented adaptation in the skin microvascular observed in the arm exposed to repeated increases in flow (40). In these studies, cutaneous vasodilation was accompanied by increases in forearm skin temperature during lower limb heating (40) and direct forearm heating (97). Therefore, heat application per se may represent a stimulus for adaptation, possibly by virtue of interactions between NO and heat shock proteins (81). Indeed, when the increases in skin temperature in response to lower limb heating were “clamped” by submersion of one forearm in thermoneutral water, skin microvascular adaptations differed from those observed in the “unclamped” limb, in which both temperature and blood flow increased (40). These results support an evolving hypothesis that repeated increases in skin blood flow induce intrinsic skin microvascular function, while changes in skin temperature may contribute to the nature of the adaptation. The relationship between these findings and recent elegant observations by Alexander et al. (4) that impaired skin blood flow responses in subjects with cardiovascular risk factors may be dependent on tetrahydrobiopterin coupling of NO synthase, remains to be determined (4).
B. Adaptation in Vascular Structure
1. Conduit arteries
An early observation of enlargement of vessels in response to intense exercise training dates to 1961 and relates to the autopsy of Clarence DeMar who ran 34 marathons (including 7 wins of the Boston Marathon) (53) and in whom “unusually large coronary arteries” were described post mortem. More recently, ultrasound- and MR-based techniques have confirmed the idea that regular exercise training is associated with enlarged coronary artery size (125, 225) and dilator capacity. This type of remodeling is analogous to the concept, originally introduced by Morganroth et al. (215), that regular (endurance) exercise training involves repeated hemodynamic stimuli that remodel the heart. Larger coronary arteries are found after exercise training and may facilitate the increased oxygen demand associated with cardiac hypertrophy and increased cardiac work loads during exercise (170).
Athletes also exhibit increased diameter in large peripheral arteries (i.e., aorta, carotid, subclavian arteries), relative to matched sedentary controls (327), and exercise training studies have revealed remodeling of conduit arteries (66, 224, 279, 293), providing direct evidence that regular exercise increases conduit artery lumen diameter. To better understand the process of remodeling, studies have explored whether remodeling occurs regionally (i.e., related to local processes) or consistently across vascular beds (i.e., related to systemic processes). Huonker and co-workers (140, 141) found that wheelchair athletes (engaged with upper body exercise) possess enhanced dimensions in the aortic arch and subclavian artery, but smaller diameters of the femoral artery, compared with able-bodied controls. In line with these observations, Rowley and co-workers (261, 262) found larger brachial artery diameters in canoeists and kayakers, while within-subject differences were present between the dominant and nondominant brachial arteries of elite squash players. Longitudinal studies involving unilateral leg cycle (206) or unilateral upper arm exercise (329), combined with bilateral assessment of diameter, provided further evidence that exercise training leads to localized adaptation in conduit artery diameter. Taken together, these studies strongly suggest that exercise training induces localized adaptation of conduit artery diameter, typically supplying the physically active limb.
Studies performed in animals demonstrate that experimentally increasing blood flow leads to significant outward remodeling. Inhibition of NO synthesis, either by administration of NO synthesis inhibitors (307) or in eNOS knockout mice (264), results in no changes in conduit artery diameter in response to chronic increases in shear stress (Figure 4). The importance for the NO pathway was confirmed in subsequent work, in which changes in eNOS gene expression strongly correlated with the magnitude of change in shear stress levels (309). Studies in humans also suggest that repeated exposure to shear stress, associated with exercise training, represents a key stimulus for remodeling. When exploring bilateral brachial artery adaptations to handgrip (306) or cycle (22) exercise training, unilateral shear manipulation with subdiastolic cuff inflation prevented increases in peak brachial artery diameter observed in the arm exposed to episodic increases in shear. Hence, data from animals and humans support an important role for shear stress in the mediation of structural outward remodeling of conduit arteries.
A widely adopted hypothesis to explain remodeling of conduit arteries is that changes in diameter represent an attempt to normalize shear stress (156). Unfortunately, most previous studies in humans have not documented shear stress, limiting insight into the concept that shear stress is “regulated” by structural arterial adaptation. In a cross-sectional study, larger femoral artery diameter and blood flow in endurance-trained athletes were reported, compared with controls, while mean and peak shear stress levels were comparable (266). Furthermore, 8 wk cycle exercise training resulted in an increase in the diameter and blood flow of the ascending and abdominal aorta, while blood velocity and shear stress were preserved (205). While studies are not conclusive, this work provides some support for the notion that shear stress is regulated via remodeling (66, 140). It is important to emphasize that most of the interventional exercise training studies have been undertaken over relatively brief time periods (4–12 wk), whereas many of the classic structural adaptation findings relate to cross-sectional comparison of controls to athletes who have trained for decades. Nonetheless, those studies that have involved longer training interventions have generally produced results consistent with the cross-sectional findings (279).
To understand the requirement for artery remodeling, it is important to appreciate the relationship between blood flow and oxygen consumption. For example, one-legged exercise training caused a significant increase in femoral artery diameter that was strongly related to the increase in the one-legged maximal oxygen uptake (r = 0.86) (206). These findings support observations in healthy volunteers that conduit artery diameter is related to lean mass (135, 329). Indeed, the marked differences in femoral artery diameter between subjects with paraplegia and controls disappeared after correcting for differences in thigh lean mass (230). In agreement with these findings, a similar time course of changes in femoral artery diameter and leg muscle volume is reported following a spinal cord injury (62) or during a period of functional electrical stimulation-assisted exercise training in spinal cord-injured individuals (295). Therefore, regional increases in blood flow, tightly coupled with the metabolic demand of the distal muscle, are associated with training-induced arterial remodeling and facilitate the ability to perform aerobic work.
A) CONDUIT ARTERIES (WALL THICKNESS).
When directly comparing endurance-trained and sedentary populations, most studies found no significant differences in carotid wall thickness between trained and untrained cohorts (288). In contrast, Rowley and co-workers (261, 262) found a smaller carotid artery wall thickness in elite athletes compared with sedentary controls. One explanation for these differences in findings is that elite athletes are exposed to a larger volume, duration, and intensity of exercise than recreationally active subjects (212, 247, 285). A recent study reported a significant decrease in carotid artery wall thickness after 6 mo of endurance or resistance exercise training (279). This longitudinal work provides further evidence that carotid artery wall thickness is modifiable, and another recent study showed that 8 wk of exercise training induced comparable changes in wall thickness in the popliteal (i.e., supplying the active lower limbs) and the carotid (i.e., supplying a nonactive area) arteries (108). Therefore, cross-sectional and longitudinal training studies both suggest potent effects of training that can mediate reductions in conduit artery wall thickness supplying the active and nonactive areas (288).
To address the hemodynamic stimuli contributing to adaptation in wall thickness, Rowley et al. (261) compared brachial artery wall thickness between the dominant and nondominant arm of squash players. This model was based on the assumption that the brachial artery in the dominant arm is exposed to higher levels of shear stress, whereas systemic hemodynamic stimuli are similarly present in both arms. No differences in brachial artery wall thickness were found between the arms. In a follow-up study, brachial and femoral artery wall thickness were compared between elite athletes engaged in lower limb versus upper limb exercise, healthy controls, wheelchair controls, and athletes (262). All athletes (able bodied and wheelchair bound) showed a smaller brachial and femoral artery wall thickness compared with their physically inactive peers. These findings suggest that shear stress is not the sole or key stimulus responsible for arterial wall remodeling in humans. A further study provided more direct evidence for this hypothesis by performing bilateral handgrip exercise, with unilateral cuff manipulation to manipulate the shear stress stimulus (292). Despite successfully manipulating the shear stress response between the limbs, brachial artery wall thickness showed a small, but significant gradual decline in both arms as a result of training. These findings suggest that adaptations in wall thickness in response to exercise training may occur largely independently of localized elevations in shear stress.
In interpreting studies that address the impact of exercise training on arterial wall thickness, it should be acknowledged that the arteries are constantly modifying their vasomotor tone. Thijssen et al. (298) demonstrated that reducing vascular tone through sublingual administration of glyceryl trinitrate leads to a generalized and marked acute decrease in wall thickness in both young and older humans. Changes in arterial wall thickness may therefore be mediated, at least in part, by changes in functional tone, along with structural remodeling in the vessel wall. This is particularly relevant in the case of exercise training, since training impacts vascular tone and function.
According to Laplace's Law, an increase in diameter leads to an increase of the circumferential wall stress, typically followed by an increased wall thickness to lower wall stress (given the inverse relation between wall thickness and wall stress). Indeed, such adaptations have been described in the process of atherosclerosis. Regular exercise training also leads to an increase in diameter and, therefore, will lead to an increase in circumferential wall stress. However, as described above, exercise training has been associated with a decrease in wall thickness. Accordingly, one hypothesis is that exercise training leads to remodeling of the arterial wall and tissue organization that result in the ability to sustain higher wall tension in the presence of decreased thickness.
The ability of exercise training to alter arterial wall characteristics may be relevant for atherosclerotic plaque development. Recent animal studies suggest that exercise training is associated with stabilization of atherosclerotic plaque and increased content of collagen and elastin (273). In a retrospective analysis in humans, higher physical fitness levels were associated with high fibrous volume and fibrous cap thickness of coronary plaques (324). Recently, 12 wk of exercise training in patients with CAD decreased the necrotic plaque core (190). These effects of exercise training on conduit artery wall characteristics may have important clinical implications in terms of both plaque stabilization as well as the evolution of plaque volume and rupture.
2. Resistance arteries
A traditional approach to assess resistance artery “structure” involves creating a stimulus that leads to peak blood flow, often through prolonged ischemia (>15 min) or ischemia combined with exercise. An inability to further increase flow suggests that peak blood flow reflects a “structural ceiling” or capacity measure of the vascular bed. Using this technique, Sinoway et al. (276) compared the dominant and nondominant forearms of recreational tennis players to assess the impact of prolonged intensive exercise. They found that the preferred limb exhibited higher peak vasodilator responses than the nonpreferred limbs, while no bilateral differences in forearm peak blood flows were observed in controls (276). Comparable findings were later observed when comparing vascular responses between both arms in elite tennis players (101) and elite squash players (261), but also when comparing leg peak blood flow between elite athletes and controls (277). The presence of a higher peak blood flow after exercise training, either adopting between- or within-subject comparisons, supports the ability of exercise training to cause remodeling of resistance arteries.
Resistance artery remodeling is a localized adaptation. Studies involving cycle exercise training found no changes in forearm peak blood flow (22, 261, 262). These observations suggest that dominant local hemodynamic factors, such as shear stress, contribute to remodeling of resistance arteries (as is the case in conduit arteries, described above). Some support for this was provided by Tinken et al. (306), who found that the increase in brachial artery peak blood flow after 8 wk handgrip exercise training was abolished when exercise-induced increases in blood flow were clamped using a unilateral cuff manipulation. Interestingly, 8 wk repeated elevation in brachial shear stress through cycle exercise training (22) or heat exposure (222) did not alter brachial artery peak blood flow. Therefore, resistance artery remodeling in response to exercise training is tightly coupled to metabolic work performed by the muscle area perfused by the vascular bed. Indeed, lower limb peak blood flow, but not forearm peak blood flow, is coupled with whole body peak oxygen consumption (152, 278). These data suggest that repeated increases in shear stress represent an important stimulus for enlargement of resistance arteries. That is, structural enlargement of skeletal muscle resistance vessels seems to require an increase in metabolic work which leads to the increased skeletal muscle blood flow and shear stress stimulus.
3. Microcirculation
The enhanced intrinsic vasodilator capacity of the muscle microvasculature following training may conceivably result from the increase in capillary density that occurs with training (7). Histochemical analyses of muscle biopsies, often taken from the quadriceps muscle, assess capillary density and the capillary-to-fiber ratio. The majority of studies examining the impact of exercise training, either adopting cross-sectional (athletes vs. controls) or longitudinal design, demonstrate significant and marked increases in the total number of capillaries, capillary-to-fiber ratio, and capillary density in response to different training regimes (mode, intensity, and duration) and across various age groups (133). These structural changes in skeletal muscle microvasculature have been strongly linked to improvements in local and whole body peak oxygen uptake. For example, a previous study found that increases in capillary density represent an early adaptation during exercise training that precedes the improvement in peak oxygen uptake (70). Therefore, growth of capillaries represents an important adaptation to regular exercise training that enables sufficient diffusion capacity, even under highly demanding conditions during which muscle blood flow is profound. However, studies have suggested that an increase in capillary density may not necessarily affect muscle blood flow supplying the skeletal muscle (198). More likely, the increase in capillary density may prolong transit time of red blood cells through the muscle capillaries, leading to an increased time frame for gas exchange within the muscle capillaries (36, 69, 177). These beneficial adaptations are hypothesized to contribute to an improved microvascular milieu that allows for more efficient diffusion of oxygen from the capillaries to (muscle) cells (87), whereas the locus of control of muscle blood flow lies further upstream from these small vessels, in feed arterioles and resistance vessels (271).
Few studies have explored the importance of hemodynamic stimuli in mediating microvascular adaptation, mainly because these hemodynamic stimuli are extremely difficult to quantify and manipulate, particularly in humans. Some studies, however, have provided indirect insight into factors that may contribute to microvascular adaptation to training. Esbjornsson et al. (72) examined whether skeletal muscle capillarization in response to 4 wk one-legged cycle training is different when performed under local ischemia. By performing leg cycle exercise in a sealed, +50 mmHg pressure chamber, local blood flow to the exercising limb was impaired. Consequently, the limb exposed to the impaired blood flow (and ischemia) during exercise showed a larger increase in capillary-to-fiber ratio after training compared with the contralateral limb that underwent the same amount of work under normoxic conditions (72). In agreement with this study, exercise training performed under hypoxia showed a larger improvement in capillary density (83) or capillary-to-fiber ratio (161) compared with exercise training under normoxia. These findings suggest that (localized) hypoxia, possibly mediated by exercise, represents an important modulator for structural adaptation in the skeletal microvasculature (36, 133).
In addition to hypoxia, mechanical forces that are present during muscle activity, such as shear stress and passive stretch, contribute to adaptation in muscle capillary growth. Angiogenic factors involved in mediating these adaptations include vascular endothelial growth factor (VEGF). During muscle contractions, VEGF increases in the muscle and binds to VEGF receptors on the capillary endothelium. As a direct consequence, VEGF triggers an angiogenic process that contributes to remodeling of the capillary vascular bed. Furthermore, exercise-induced release of VEGF-containing vesicles in the circulation leads to rapid replenishment of the VEGF stores. Consequently, this allows for VEGF secretion upon exposure of a subsequent bout of exercise (133). To reiterate, growth in capillaries, while important for gas exchange, may not be a significant determinant of blood pressure regulation in vivo (271).
4. Cutaneous microcirculation: an active vessel bed during exercise
Little is currently known regarding the impact of exercise training on microvascular structural changes in the skin. It is feasible that repeated exercise bouts that stimulate thermoregulatory cutaneous vasodilatation may induce remodeling, either in the form of enlargement of small arterioles or increases in capillary density. Based on an analogy to the training-induced adaptation in skeletal muscle (87), the latter may be associated with prolonged transit time with implications for the efficiency of heat transfer. Until recently, technological limitations have hampered our understanding of cutaneous adaptation, but newer imaging techniques may provide novel insights in the future (Carter HH, Gong P, Kirk RW, Es′haghian S, Atkinson CL, Sampson DD, Green DJ, McLaughlin RA. Optical coherence tomography in the assessment of acute changes in cutaneous vascular function in humans. J Appl Physiol. In press).
C. Changes in Vascular Cell Gene Expression Induced by Exercise Training
A recent series of studies evaluated the effects of regular exercise on the vascular transcriptome in pigs (238) and rats (174, 175, 235). A common finding is that the effects of exercise are heterogeneous across vascular beds. In a recent and comprehensive study (174), next-generation, transcriptome-wide RNA sequencing (RNA-Seq) technology was used to assess the effects of exercise training on transcriptional profiles in skeletal muscle arterioles isolated from the soleus and gastrocnemius muscles of Otsuka Long Evans Tokushima Fatty (OLETF) rats, a model of obesity and type 2 diabetes. In this study rats underwent a 12-wk endurance exercise training program, interval sprint training program, or remained sedentary. Endurance exercise caused the greatest number of changes in gene expression in the soleus and white gastrocnemius 2a arterioles, with little to no changes in the feed arteries. In contrast, interval sprint training produced considerable changes in gene expression in the feed arteries. Ingenuity-pathway analysis revealed 18 pathways with significant changes in gene expression when analyzed across vessels (174). From this comprehensive analysis it was concluded that training-induced changes in arteriolar gene expression patterns differ by muscle fiber type composition and along the arteriolar tree. It should also be noted that the effects of exercise on gene expression may manifest to a greater extent in the arteries perfusing the working muscles; however, effects of exercise beyond the active muscle beds are also apparent (238), providing evidence that the effects of vascular exercise are systemic but not homogeneous. Studies in humans introduced the notion of a systemic impact of training on vascular adaptation and follow-up studies investigated the potential contribution of hemodynamic stimuli to this adaptation (93–95, 290, 305). In addition, reviews summarized the mechanisms related to vascular adaptations beyond active muscle beds (104, 196, 237).
In addition, using RNA sequencing, the extent to which the effects of obesity (i.e., differences between obese OLETF rats and lean-counterparts rats) on aortic endothelial gene expression could be reversed by endurance exercise was recently reported (146, 235). Exercise altered expression of 324 endothelial genes but only partially or totally re stored expression of 8.6% of 396 genes affected by obesity (146, 235). This finding, that only a small fraction of endothelial transcriptional changes produced by obesity can be offset by regular exercise, further supports the notion that exercise exerts direct effects on the artery wall, independent of reductions in obesity and other related co-morbidities (106).
Finally, it is important to emphasize that studies of the myriad factors that impact vasomotor function in humans have emphasized the notion of compensatory redundancy in control (267). Conclusions based on gene expression data should be informed by functional assessment of the relative importance of different pathways to the integrated adaptive response to training, particularly given that few pathways appear to be obligatory in the acute functional response to exercise (187).
V. WHICH FACTORS MODERATE THE ADAPTATION TO TRAINING?
A. Distinct Adaptations to Different Forms of Exercise Training
1. Type (modality) of exercise
Most studies focused on the differential impact of distinct types (modalities) of exercise have compared athletes and controls or fit and unfit individuals, but inter-subject differences introduce significant bias in terms of the true impact of exercise training, limiting the validity of implications regarding the impacts of exercise per se (279). Spence et al. (279) directly compared the impact of endurance versus resistance exercise training within subjects on conduit artery vascular adaptation. Six months of upper limb-dominant resistance training improved brachial, but not femoral, artery resting and peak diameter (indicative of structural remodeling) and vascular function. In contrast, lower limb endurance exercise training increased resting and peak femoral, but not brachial, artery diameter and vascular function (279). These observations of distinct adaptations between resistance and endurance exercise may be linked to site-specific elevation in blood flow (and shear stress) in the active limbs during exercise (i.e., the hemodynamic stimulus), rather than the type of exercise per se. Future studies, adopting direct comparison between different types of exercise within subjects, are required to understand the differences between exercise types, but also the importance of the magnitude of exercise-induced elevation in blood flow and shear rate.
2. Exercise intensity
Few studies have performed direct comparisons between the same mode of exercise training, performed at different intensity levels, on vascular function and structure. Goto et al. (90) performed an elegant study which randomized subjects to perform 12-wk cycle exercise training at mild, moderate, or high intensity. Only moderate-intensity exercise was associated with improvement in NO-mediated endothelial function and a decrease in markers of oxidative stress. The absence of adaptation after high-intensity exercise training was hypothesized to be the result of the induction of significant oxidative stress during each bout of intense exercise, potentially mitigating the effects of exercise on the endothelium. Some evidence for this hypothesis was provided in a follow-up study, where the authors demonstrated that high-intensity, but not mild- or moderate-intensity, exercise caused increases in markers for oxidative stress (91). These observations are largely in line with studies examining the acute effects of exercise on vascular function. While low-to-moderate exercise intensity shows somewhat conflicting results (24, 305), an increase in exercise intensity is typically associated with a (larger) decrease in vascular function (60) immediately post-exercise. Taken together, these studies support the notion that a dose-response relationship exists in terms of functional responses of the vasculature to exercise and training, and that higher intensities of exercise may truncate benefits of training via impacts on inflammation and oxidative stress. It is important to reiterate that the epidemiological evidence suggests that the largest impact on vascular risk occurs from the adoption of lower volumes and intensities of physical activity.
Somewhat in contrast to this paradigm, studies on the effects of high-intensity interval training (HIIT), which is characterized by repeated exposure to short bouts of exercise (1–4 min) performed at near or supramaximal level (84, 320), suggest potentially superior effects compared with traditional endurance exercise. Some studies have compared the impact of HIIT to more conventional endurance training on various outcome measures, including vascular function and structure. Recent work suggested that HIIT leads to superior improvements in vascular function compared with endurance training (250), although selection bias and inconsistencies in the FMD protocol may contribute to these findings. Little work has explored the potential (superior) effects of HIIT on resistance arteries and/or microvessels, while concerns about the potential health risks of HIIT have not yet been ruled out (157). Taken together, this relatively new field on HIIT requires further work to better understand the potential benefits, if any, over more traditional and graduated approaches to intensity prescription, particularly in higher risk populations.
B. Distinct Time Course in Adaptation in Different Vascular Properties
Animal and human data support the existence of different time-course effects of adaptation in artery function and structure as a consequence of exercise training (104). In healthy animals, 1–4 wk of exercise training improved vasodilator function in conduit arteries (200), muscle arterioles (282) and the aorta (64) and was associated with increased eNOS expression in pulmonary arteries (150). In marked contrast, studies adopting 16–20 wk exercise training have not consistently shown augmented endothelial function (199) or changes in eNOS expression (149). Nonetheless, longer training duration is associated with enlargement of arterial diameters. Based on these cross-sectional observations, Laughlin (168) proposed that initial improvements in vascular dilator function act to normalize shear stress during exercise bouts, whereas continuing exercise will result in a more “permanent” normalization of shear stress. As a consequence of structural enlargement, initial improvement in vascular function returns towards baseline (Figure 9).
FIGURE 9.
Time-dependent changes in vascular dilator function (gray line) and structure (red line) across a period of exercise training in healthy volunteers. Laughlin (168) proposed that initial improvements in vascular dilator function contribute to normalize shear stress during exercise bouts, whereas continuing exercise results in more “permanent” normalization of shear stress. Human studies designed to test this proposal confirmed that both brachial and popliteal artery function and structure adapt according to distinct time course across 8 wk of exercise training in healthy volunteers (304) and that such adaptation is shear dependent (23, 306).
A human study designed to test this proposal utilized repeated assessments of the time course of adaptation of vascular function and structure in response to exercise training. Tinken et al. (304) examined both brachial and popliteal artery function and structure across 8 wk of exercise training in healthy volunteers. The results confirm the hypothesis that exercise training leads to an initial improvement in vasodilator function, which returns toward baseline once structural remodeling occurs. Comparable findings of time-dependent adaptation in vasodilator function and structure have been reported in subsequent studies involving cycle exercise (22), handgrip exercise (306), resistance training with blood flow restriction (138), and also local (222) and systemic (41) heating in the absence of an exercise stimulus. Taken together, these data in humans provide strong evidence for time-dependent adaptations in vascular dilator function and structure across a period of exercise training, although the impacts of cardiovascular ageing and/or the presence of endothelial dysfunction may modulate the relative time course.
C. Interaction Between Changes in Function and Structure
Seminal work on relationships between arterial structure and function of arteries was performed by Folkow in the mid-1950s (75). His work focused on the arterial structural adjustments observed in hypertension and explored the impact of a thickened arteriolar wall on vascular resistance and blood pressure. Based on calculations, Folkow (74, 75) revealed that an increased wall-to-lumen ratio would produce exaggerated luminal changes to any vasoactive stimulus. Contraction of smooth muscle also causes increased wall tissue mass that has impact on luminal dimensions. Thickening of the arterial wall, therefore, is accompanied by vascular hyper-reactivity, even in the absence of changes in vasoactive signal transduction. Recently, this hypothesis regarding interaction between structural and functional characteristics was supported in conduit arteries in humans in vivo (300). Shear-dependent and -independent vasodilation was strongly and positively related to wall-to-lumen ratio, with a larger dilation observed in conduit arteries that exhibit a larger wall-to-lumen ratio. Therefore, structural changes of the arterial wall likely impact functional responses and adaptation.
D. Impact of Cardiovascular Disease on Hemodynamic Stimuli and Vascular Adaptation to Exercise
1. Hemodynamic stimuli during exercise
CV risk and disease may alter blood flow responses to exercise. Early studies performed in the 1940s revealed (1, 25) differences between healthy and diseased populations in terms of changes in blood flow to the hands. In agreement with these observations, others reported impaired ability to lower vascular resistance during exercise in subjects with CV disease or risk (29). These distinct vascular responses between healthy subjects and subjects with CV disease potentially contribute to altered shear stress patterns during exercise.
Heart failure (HF) patients exhibit impaired vasodilator responses to passive heat exposure, the mechanism for which was at least partly due to impaired NO-mediated dilator function (105). During exposure to 38°C in a heat chamber, HF patients demonstrated attenuated heating-induced vasodilation of the skin, and controls exhibited elevated NO dilator function. Also during cycle exercise, HF is associated with attenuated skin temperature responses compared with healthy controls (18). Interestingly, a recent study compared cycle exercise-induced brachial artery shear stress between HF patients and healthy age-matched controls (20). It was observed that HF was associated with an exaggerated exercise-induced increase in retrograde shear stress and attenuated increase in antegrade shear stress, which both remained present throughout the 30-min cycle bout. Previous work has linked cutaneous vasodilation to attenuation of retrograde shear during prolonged cycle exercise in healthy volunteers (274). Accordingly, attenuated cutaneous vasodilator responses to passive heat and exercise in HF patients may contribute to the distinct antegrade and retrograde shear stress patterns during exercise in HF. Understanding these interactions is important as they suggest that hemodynamic effects may blunt the beneficial impacts of exercise on vascular structure and function.
Exercise in subjects with CV disease/risk is also associated with exaggerated exercise-induced blood pressure responses (29) which may relate to the presence of endothelial dysfunction, oxidative stress, and/or impaired neurohormonal activation. Subjects with exaggerated exercise-induced blood pressure responses also exhibit transient postexercise hypertension, rather than the hypotension normally present in healthy volunteers after exercise (82). Therefore, subjects with CV disease and/or risk may be exposed to higher pressure responses during and after exercise, potentially impacting subsequent vascular adaptation.
2. Adaptation to exercise
Studies in animals have provided some evidence that CV risk factors impair shear stress mechanotransduction (328) and attenuate NO release upon elevation in shear stress (283). The presence of impaired ability to detect and respond to hemodynamic stimuli, in combination with altered hemodynamics during exercise (see above), may affect vascular adaptation to exercise training. In contrast to this hypothesis, studies in humans have typically found improvement in vascular function after exercise training in populations with CV disease or risk (104). Moreover, a recent study found larger improvement in vascular function in those with a priori impaired function (100). Therefore, despite exposure to unfavorable hemodynamic stimuli, there is sufficient evidence for improvement of vascular function as a result of exercise training in subjects with CV risk or disease.
Some studies suggest that CV risk factors may impair vascular structural adaptation to training. For example, studies in animals have demonstrated that expansion of resistance arteries occurred in young animals after 1–2 wk exposure to high flow, while such adaptation was absent in older animals (68, 78). Interestingly, the capacity for arterial expansion in older animals was restored under co-infusion of drugs directly impacting vasodilator function of these arteries (68, 78). In young and older humans, our laboratories explored vascular changes in response to increases in brachial artery retrograde shear stress (269, 299). We found that 30-min and 2-wk elevation in retrograde shear stress caused a decrease in endothelial function and smaller diameter in young subjects, while such adaptations were not observed in older individuals (299). Furthermore, Hansen et al. (122) found that hypertension is associated with an attenuated exercise-induced release of VEGF, an important angiogenic factor that is linked to capillarization (see above). The attenuated release of VEGF after exercise was also associated with a limited effect on capillary density after training. In another exercise training study, 6 wk of exercise training caused a significant increase in the capillary-to-fiber ratio in healthy controls, while such adaptations were absent in heart transplant recipients (166).
To summarize this work, subjects with CV disease or risk may demonstrate less favorable hemodynamic response patterns during exercise and/or impaired angiogenic or adaptive responses to training. Nonetheless, subjects with CV risk and/or disease remain highly adaptive in the terms of exercise-induced improvement in vascular function, whilst some evidence suggests that structural adaptations are less likely to occur in those with CV risk and/or disease. These data highlight the complexity of the integrative stimuli evoked by exercise and their ultimate impacts on vascular adaptation.
VI. SUMMARY AND IMPLICATIONS FOR EXERCISE SCIENCE AND HEALTH
Exercise is anti-atherogenic, and increasing physical activity has a profound impact on cardiovascular risk. While some of this is due to exercise-mediated modification of traditional cardiovascular risk factors, exercise is a relatively weak poly-pill compared with the impacts of pharmacological agents (303). In contrast, the beneficial impacts of exercise on CV risk exceed that expected from changes in CV risk factors alone, and this risk factor gap (106, 155) may be filled, at least in part, by the direct impacts of exercise on the artery wall. On the evidence presented in this review, it is clear that the hemodynamic impacts of exercise on blood flow and pressure transduce acute changes in vascular function and that repeated exercise leads to arterial adaptation in humans. Exercise can be considered an evolutionary stimulus to maintaining human vascular health. In the same way that exercise is accepted as a stimulus to the maintenance of musculoskeletal function in the face of ageing, frailty, and disease, exercise and associated hemodynamic forces are a direct form of vascular medicine in humans.
It is timely, on the 400th anniversary of the lectures which revolutionized science by revealing the importance of the movement of the heart and blood in animals, to reflect that the introduction to Exercitatio Anatomica de Motu Cordis et Sanguinis in Animalibus states “Very many maintain that all we know is still infinitely less than all that still remains unknown. . . .,” a statement that remains as true in the age of high-resolution noninvasive imaging, as it was in the time of Harvey's anatomical exercises.
GRANTS
D. J. Green is supported by National Health and Medical Research Council Principal Research Fellowship Grant APP1080914. D. H. J. Thijssen is supported by the Netherlands Heart Foundation Grant 2009T064. J. Padilla is supported by the National Institutes of Health Grants K01HL125503 and R21DK105368.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
acknowledgments
Address for reprint requests and other correspondence: D. J. Green, School of Sports Science, Exercise and Health M408, Univ. of Western Australia, Crawley, 6009, Australia (e-mail: danny.green@uwa.edu.au).
REFERENCES
- 1.Abramson DI, Fierst SM, Flachs K. Effect of muscular exercise upon the peripheral circulation in patients with valvular heart disease. J Clin Invest 21: 747–750, 1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams V, Linke A, Krankel N, Erbs S, Gielen S, Mobius-Winkler S, Gummert JF, Mohr FW, Schuler G, Hambrecht R. Impact of regular physical activity on the NAD(P)H oxidase and angiotensin receptor system in patients with coronary artery disease. Circulation 111: 555–562, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Alberts-Grill N, Rezvan A, Son DJ, Qiu H, Kim CW, Kemp ML, Weyand CM, Jo H. Dynamic immune cell accumulation during flow-induced atherogenesis in mouse carotid artery: an expanded flow cytometry method. Arterioscler Thromb Vasc Biol 32: 623–632, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alexander LM, Kutz JL, Kenney WL. Tetrahydrobiopterin increases NO-dependent vasodilation in hypercholesterolemic human skin through eNOS-coupling mechanisms. Am J Physiol Regul Integr Comp Physiol 304: R164–R169, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allemann Y, Vetter C, Kartal N, Eyer S, Stengel SM, Saner H, Hess OM. Effect of mild endurance exercise training and pravastatin on peripheral vasodilatation of forearm resistance vessels in patients with coronary artery disease. Eur J Cardiovasc Prev Rehabil 12: 332–340, 2005. [DOI] [PubMed] [Google Scholar]
- 6.Alvarez GE, Halliwill JR, Ballard TP, Beske SD, Davy KP. Sympathetic neural regulation in endurance-trained humans: fitness vs. fatness. J Appl Physiol 98: 498–502, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Andersen P, Henriksson J. Capillary supply of the quadriceps femoris muscle of man: adaptive response to exercise. J Physiol 270: 677–690, 1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson EA, Mark AL. Flow-mediated and reflex changes in large peripheral artery tone in humans. Circulation 79: 93–100, 1989. [DOI] [PubMed] [Google Scholar]
- 9.Anderson L, Oldridge N, Thompson DR, Zwisler AD, Rees K, Martin N, Taylor RS. Exercise-based cardiac rehabilitation for coronary heart disease: Cochrane systematic review and meta-analysis. J Am Coll Cardiol 67: 1–12, 2016. [DOI] [PubMed] [Google Scholar]
- 10.Ando J, Yamamoto K. Flow detection and calcium signalling in vascular endothelial cells. Cardiovasc Res 99: 260–268, 2013. [DOI] [PubMed] [Google Scholar]
- 11.Ashor AW, Lara J, Siervo M, Celis-Morales C, Mathers JC. Effects of exercise modalities on arterial stiffness and wave reflection: a systematic review and meta-analysis of randomized controlled trials. PLoS One 9: e110034, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ashor AW, Lara J, Siervo M, Celis-Morales C, Oggioni C, Jakovljevic DG, Mathers JC. Exercise modalities and endothelial function: a systematic review and dose-response meta-analysis of randomized controlled trials. Sports Med 45: 279–296, 2015. [DOI] [PubMed] [Google Scholar]
- 13.Atkinson CL, Carter HH, Dawson EA, Naylor LH, Thijssen DH, Green DJ. Impact of handgrip exercise intensity on brachial artery flow-mediated dilation. Eur J Appl Physiol 115: 1705–1713, 2015. [DOI] [PubMed] [Google Scholar]
- 14.Atkinson CL, Carter HH, Naylor LH, Dawson EA, Marusic P, Hering D, Schlaich MP, Thijssen DH, Green DJ. Opposing effects of shear-mediated dilation and myogenic constriction on artery diameter in response to handgrip exercise in humans. J Appl Physiol 119: 858–864, 2015. [DOI] [PubMed] [Google Scholar]
- 16.Bank AJ, Shammas RA, Mullen K, Chuang PP. Effects of short-term forearm exercise training on resistance vessel endothelial function in normal subjects and patients with heart failure. J Card Fail 4: 193–201, 1998. [DOI] [PubMed] [Google Scholar]
- 17.Ben-Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, Boutouyrie P, Cameron J, Chen CH, Cruickshank JK, Hwang SJ, Lakatta EG, Laurent S, Maldonado J, Mitchell GF, Najjar SS, Newman AB, Ohishi M, Pannier B, Pereira T, Vasan RS, Shokawa T, Sutton-Tyrell K, Verbeke F, Wang KL, Webb DJ, Willum Hansen T, Zoungas S, McEniery CM, Cockcroft JR, Wilkinson IB. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol 63: 636–646, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benda N, Eijsvogels T, Van Dijk A, Bellersen L, Thijjssen DH, Hopman MTE. Altered core and skin temperature responses to endurance exercise in heart failure patients and heatlhy controls. Eur J Prev Cardiol. In press. [DOI] [PubMed]
- 20.Benda NM, Seeger JP, van Lier DP, Bellersen L, van Dijk AP, Hopman MT, Thijssen DH. Heart failure patients demonstrate impaired changes in brachial artery blood flow and shear rate pattern during moderate-intensity cycle exercise. Exp Physiol 100: 463–474, 2015. [DOI] [PubMed] [Google Scholar]
- 21.Berdeaux A, Ghaleh B, Dubois-Rande JL, Vigue B, Drieu La Rochelle C, Hittinger L, Giudicelli JF. Role of vascular endothelium in exercise-induced dilation of large epicardial coronary arteries in conscious dogs. Circulation 89: 2799–2808, 1994. [DOI] [PubMed] [Google Scholar]
- 22.Birk GK, Dawson EA, Atkinson C, Haynes A, Cable NT, Thijssen DH, Green DJ. Brachial artery adaptation to lower limb exercise training: role of shear stress. J Appl Physiol 112: 1653–1658, 2012. [DOI] [PubMed] [Google Scholar]
- 24.Birk GK, Dawson EA, Batterham AM, Atkinson G, Cable T, Thijssen DH, Green DJ. Effects of exercise intensity on flow mediated dilation in healthy humans. Int J Sports Med 34: 409–414, 2013. [DOI] [PubMed] [Google Scholar]
- 25.Bishop JM, Donald KW, Taylor SH, Wormald PN. The blood flow in the human arm during supine leg exercise. J Physiol 137: 294–308, 1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Black MA, Cable NT, Thijssen DH, Green DJ. Impact of age, sex, and exercise on brachial artery flow-mediated dilatation. Am J Physiol Heart Circ Physiol 297: H1109–H1116, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Black MA, Green DJ, Cable NT. Exercise prevents age-related decline in nitric-oxide-mediated vasodilator function in cutaneous microvessels. J Physiol 586: 3511–3524, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blair SN, Kohl HW 3rd Barlow CE, Paffenbarger RS Jr, Gibbons LW, Macera CA. Changes in physical fitness and all-cause mortality A prospective study of healthy and unhealthy men. JAMA 273: 1093–1098, 1995. [PubMed] [Google Scholar]
- 29.Bond V Jr, Franks BD, Tearney RJ, Wood B, Melendez MA, Johnson L, Iyriboz Y, Bassett DR Jr. Exercise blood pressure response and skeletal muscle vasodilator capacity in normotensives with positive and negative family history of hypertension. J Hypertens 12: 285–290, 1994. [PubMed] [Google Scholar]
- 30.Bove AA, Dewey JD. Proximal coronary vasomotor reactivity after exercise training in dogs. Circulation 71: 620–625, 1985. [DOI] [PubMed] [Google Scholar]
- 31.Bowles DK, Hu Q, Laughlin MH, Sturek M. Exercise training increases L-type calcium current density in coronary smooth muscle. Am J Physiol Heart Circ Physiol 275: H2159–H2169, 1998. [DOI] [PubMed] [Google Scholar]
- 32.Bowles DK, Laughlin MH. Mechanism of beneficial effects of physical activity on atherosclerosis and coronary heart disease. J Appl Physiol 111: 308–310, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bowles DK, Wamhoff BR. Coronary smooth muscle adaptation to exercise: does it play a role in cardioprotection? Acta Physiol Scand 178: 117–121, 2003. [DOI] [PubMed] [Google Scholar]
- 34.Braith RW, Schofield RS, Hill JA, Casey DP, Pierce GL. Exercise training attenuates progressive decline in brachial artery reactivity in heart transplant recipients. J Heart Lung Transplant 27: 52–59, 2008. [DOI] [PubMed] [Google Scholar]
- 35.Brooks AR, Lelkes PI, Rubanyi GM. Gene expression profiling of human aortic endothelial cells exposed to disturbed flow and steady laminar flow. Physiol Gen 9: 27–41, 2002. [DOI] [PubMed] [Google Scholar]
- 36.Brown MD. Exercise and coronary vascular remodelling in the healthy heart. Exp Physiol 88: 645–658, 2003. [DOI] [PubMed] [Google Scholar]
- 37.Brunt VE, Howard MJ, Francisco MA, Ely BR, Minson CT. Passive heat therapy improves endothelial function, arterial stiffness and blood pressure in sedentary humans. J Physiol 594: 5329–5342, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Caro CG, Fitz-Gerald JM, Schroter RC. Arterial wall shear and distribution of early atheroma in man. Nature 223: 1159–1161, 1969. [DOI] [PubMed] [Google Scholar]
- 39.Carter HH, Dawson EA, Birk GK, Spence AL, Naylor LH, Cable NT, Thijssen DH, Green DJ. Effect of SR manipulation on conduit artery dilation in humans. Hypertension 61: 143–150, 2013. [DOI] [PubMed] [Google Scholar]
- 40.Carter HH, Spence AL, Atkinson CL, Pugh CJ, Cable NT, Thijssen DH, Naylor LH, Green DJ. Distinct effects of blood flow and temperature on cutaneous microvascular adaptation. Med Sci Sports Exercise 46: 2113–2121, 2014. [DOI] [PubMed] [Google Scholar]
- 41.Carter HH, Spence AL, Atkinson CL, Pugh CJ, Naylor LH, Green DJ. Repeated core temperature elevation induces conduit artery adaptation in humans. Eur J Appl Physiol 114: 859–865, 2014. [DOI] [PubMed] [Google Scholar]
- 42.Carter JR, Ray CA. Sympathetic neural adaptations to exercise training in humans. Auton Neurosci 188: 36–43, 2015. [DOI] [PubMed] [Google Scholar]
- 43.Casey DP, Padilla J, Joyner MJ. Alpha-adrenergic vasoconstriction contributes to the age-related increase in conduit artery retrograde and oscillatory shear. Hypertension 60: 1016–1022, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, Lloyd JK, Deanfield JE. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet 340: 1111–1115, 1992. [DOI] [PubMed] [Google Scholar]
- 45.Chen Z, Tzima E. PECAM-1 is necessary for flow-induced vascular remodeling. Arterioscler Thromb Vasc Biol 29: 1067–1073, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chiang HY, Korshunov VA, Serour A, Shi F, Sottile J. Fibronectin is an important regulator of flow-induced vascular remodeling. Arterioscler Thromb Vasc Biol 29: 1074–1079, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cholesterol Treatment Trialists C, Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, Peto R, Barnes EH, Keech A, Simes J, Collins R. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 376: 1670–1681, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chomistek AK, Chiuve SE, Jensen MK, Cook NR, Rimm EB. Vigorous physical activity, mediating biomarkers, and risk of myocardial infarction. Med Sci Sports Exercise 43: 1884–1890, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Civelek M, Manduchi E, Riley RJ, Stoeckert CJ, Davies PF. Coronary artery endothelial transcriptome in vivo/clinical perspective. Circulation Cardiovasc Genet 4: 243–252, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Clausen JP. Effect of physical training on cardiovascular adjustments to exercise in man. Physiol Rev 57: 779–815, 1977. [DOI] [PubMed] [Google Scholar]
- 51.Conway DE, Williams MR, Eskin SG, McIntire LV. Endothelial cell responses to atheroprone flow are driven by two separate flow components, low time-average shear stress and fluid flow reversal. Am J Physiol Heart Circ Physiol 298: H367–H374, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cummins PM, von Offenberg Sweeney N, Killeen MT, Birney YA, Redmond EM, Cahill PA. Cyclic strain-mediated matrix metalloproteinase regulation within the vascular endothelium: a force to be reckoned with. Am J Physiol Heart Circ Physiol 292: H28–H42, 2007. [DOI] [PubMed] [Google Scholar]
- 53.Currens JH, White PD. Half a century of running. Clinical, physiologic and autopsy findings in the case of Clarence DeMar (“Mr Marathon”). N Engl J Med 265: 988–993, 1961. [DOI] [PubMed] [Google Scholar]
- 54.Dai G, Kaazempur-Mofrad MR, Natarajan S, Zhang Y, Vaughn S, Blackman BR, Kamm RD, Garcia-Cardena G, Gimbrone MA. Distinct endothelial phenotypes evoked by arterial waveforms derived from atherosclerosis-susceptible and -resistant regions of human vasculature. Proc Natl Acad Sci USA 101: 14871–14876, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dancu MB, Berardi DE, Vanden Heuvel JP, Tarbell JM. Asynchronous shear stress and circumferential strain reduces endothelial NO synthase and cyclooxygenase-2 but induces endothelin-1 gene expression in endothelial cells. Arterioscler Thromb Vasc Biol 24: 2088–2094, 2004. [DOI] [PubMed] [Google Scholar]
- 56.Dancu MB, Berardi DE, Vanden Heuvel JP, Tarbell JM. Atherogenic endothelial cell eNOS and ET-1 responses to asynchronous hemodynamics are mitigated by conjugated linoleic acid. Ann Biomed Eng 35: 1111–1119, 2007. [DOI] [PubMed] [Google Scholar]
- 57.Davies PF, Civelek M, Fang Y, Guerraty MA, Passerini AG. Endothelial heterogeneity associated with regional athero-susceptibility and adaptation to disturbed blood flow in vivo. Semin Thromb Hemost 36: 265–275, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Davies PF, Remuzzi A, Gordon EJ, Dewey CF Jr, Gimbrone MA Jr. Turbulent fluid shear stress induces vascular endothelial cell turnover in vitro. Proc Natl Acad Sci USA 83: 2114–2117, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Davis MJ, Hill MA. Signaling mechanisms underlying the vascular myogenic response. Physiol Rev 79: 387–423, 1999. [DOI] [PubMed] [Google Scholar]
- 60.Dawson EA, Green DJ, Cable NT, Thijssen DH. Effects of acute exercise on flow mediated dilatation (FMD) in healthy humans. J Appl Physiol 115: 1589–1598, 2013. [DOI] [PubMed] [Google Scholar]
- 61.Dawson EA, Rathore S, Cable NT, Wright DJ, Morris JL, Green DJ. Impact of catheter insertion using the radial approach on vasodilatation in humans. Clin Sci 118: 633–640, 2010. [DOI] [PubMed] [Google Scholar]
- 62.De Groot PC, Bleeker MW, van Kuppevelt DH, van der Woude LH, Hopman MT. Rapid and extensive arterial adaptations after spinal cord injury. Arch Phys Med Rehab 87: 688–696, 2006. [DOI] [PubMed] [Google Scholar]
- 63.DeFina LF, Haskell WL, Willis BL, Barlow CE, Finley CE, Levine BD, Cooper KH. Physical activity versus cardiorespiratory fitness: two (partly) distinct components of cardiovascular health? Prog Cardiovasc Dis 57: 324–329, 2015. [DOI] [PubMed] [Google Scholar]
- 64.Delp MD, Laughlin MH. Time course of enhanced endothelium-mediated dilation in aorta of trained rats. Med Sci Sports Exercise 29: 1454–1461, 1997. [DOI] [PubMed] [Google Scholar]
- 65.DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation 102: 1351–1357, 2000. [DOI] [PubMed] [Google Scholar]
- 66.Dinenno FA, Tanaka H, Monahan KD, Clevenger CM, Eskurza I, DeSouza CA, Seals DR. Regular endurance exercise induces expansive arterial remodelling in the trained limbs of healthy men. J Physiol 534: 287–295, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Draney MT, Herfkens RJ, Hughes TJ, Pelc NJ, Wedding KL, Zarins CK, Taylor CA. Quantification of vessel wall cyclic strain using cine phase contrast magnetic resonance imaging. Ann Biomed Eng 30: 1033–1045, 2002. [DOI] [PubMed] [Google Scholar]
- 68.Dumont O, Pinaud F, Guihot AL, Baufreton C, Loufrani L, Henrion D. Alteration in flow (shear stress)-induced remodelling in rat resistance arteries with aging: improvement by a treatment with hydralazine. Cardiovasc Res 77: 600–608, 2008. [DOI] [PubMed] [Google Scholar]
- 69.Duncker DJ, Bache RJ. Regulation of coronary blood flow during exercise. Physiol Rev 88: 1009–1086, 2008. [DOI] [PubMed] [Google Scholar]
- 70.Duscha BD, Robbins JL, Jones WS, Kraus WE, Lye RJ, Sanders JM, Allen JD, Regensteiner JG, Hiatt WR, Annex BH. Angiogenesis in skeletal muscle precede improvements in peak oxygen uptake in peripheral artery disease patients. Arterioscler Thromb Vasc Biol 31: 2742–2748, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dzau VJ, Gibbons GH, Morishita R, Pratt RE. New perspectives in hypertension research. Potentials of vascular biology. Hypertension 23: 1132–1140, 1994. [DOI] [PubMed] [Google Scholar]
- 72.Esbjornsson M, Jansson E, Sundberg CJ, Sylven C, Eiken O, Nygren A, Kaijser L. Muscle fibre types and enzyme activities after training with local leg ischaemia in man. Acta Physiol Scand 148: 233–241, 1993. [DOI] [PubMed] [Google Scholar]
- 73.Fogarty JA, Muller-Delp JM, Delp MD, Mattox ML, Laughlin MH, Parker JL. Exercise training enhances vasodilation responses to vascular endothelial growth factor in porcine coronary arterioles exposed to chronic coronary occlusion. Circulation 109: 664–670, 2004. [DOI] [PubMed] [Google Scholar]
- 74.Folkow B. Cardiovascular structural adaptation: Its role in the initiation and maintenance of primary hypertension. Clin Sci Mol Med 55 Suppl 4: 3s–22s, 1978. [DOI] [PubMed] [Google Scholar]
- 75.Folkow B, Grimby G, Thulesius O. Adaptive structural changes in the vascular walls in hypertension and their relation to the control of peripheral resistance. Acta Physiol Scand 44: 255, 1958. [DOI] [PubMed] [Google Scholar]
- 76.Franzoni F, Galetta F, Morizzo C, Lubrano V, Palombo C, Santoro G, Ferrannini E, Quinones-Galvan A. Effects of age and physical fitness on microcirculatory function. Clin Sci 106: 329–335, 2004. [DOI] [PubMed] [Google Scholar]
- 77.Franzoni F, Plantinga Y, Femia FR, Bartolomucci F, Gaudio C, Regoli F, Carpi A, Santoro G, Galetta F. Plasma antioxidant activity and cutaneous microvascular endothelial function in athletes and sedentary controls. Biomed Pharmacother 58: 432–436, 2004. [DOI] [PubMed] [Google Scholar]
- 78.Freidja ML, Vessieres E, Clere N, Desquiret V, Guihot AL, Toutain B, Loufrani L, Jardel A, Procaccio V, Faure S, Henrion D. Heme oxygenase-1 induction restores high-blood-flow-dependent remodeling and endothelial function in mesenteric arteries of old rats. J Hypertens 29: 102–112, 2011. [DOI] [PubMed] [Google Scholar]
- 79.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 288: 373–376, 1980. [DOI] [PubMed] [Google Scholar]
- 80.Gambillara V, Chambaz C, Montorzi G, Roy S, Stergiopulos N, Silacci P. Plaque-prone hemodynamics impair endothelial function in pig carotid arteries. Am J Physiol Heart Circ Physiol 290: H2320–H2328, 2006. [DOI] [PubMed] [Google Scholar]
- 81.Garcia-Cardena G, Fan R, Shah V, Sorrentino R, Cirino G, Papapetropoulos A, Sessa WC. Dynamic activation of endothelial nitric oxide synthase by Hsp90. Nature 392: 821–824, 1998. [DOI] [PubMed] [Google Scholar]
- 82.Gaudreault V, Despres JP, Rheaume C, Bergeron J, Almeras N, Tremblay A, Poirier P. Exercise-induced exaggerated blood pressure response in men with the metabolic syndrome: the role of the autonomous nervous system. Blood Press Monit 18: 252–258, 2013. [DOI] [PubMed] [Google Scholar]
- 83.Geiser J, Vogt M, Billeter R, Zuleger C, Belforti F, Hoppeler H. Training high–living low: changes of aerobic performance and muscle structure with training at simulated altitude. Int J Sports Med 22: 579–585, 2001. [DOI] [PubMed] [Google Scholar]
- 84.Gibala MJ, McGee SL. Metabolic adaptations to short-term high-intensity interval training: a little pain for a lot of gain? Exercise Sport Sci Rev 36: 58–63, 2008. [DOI] [PubMed] [Google Scholar]
- 85.Gielen S, Laughlin MH, O'Conner C, Duncker DJ. Exercise training in patients with heart disease: review of beneficial effects and clinical recommendations. Prog Cardiovasc Dis 57: 347–355, 2015. [DOI] [PubMed] [Google Scholar]
- 86.Gielen S, Schuler G, Adams V. Cardiovascular effects of exercise training: molecular mechanisms. Circulation 122: 1221–1238, 2010. [DOI] [PubMed] [Google Scholar]
- 87.Gliemann L, Buess R, Nyberg M, Hoppeler H, Odriozola A, Thaning P, Hellsten Y, Baum O, Mortensen SP. Capillary growth, ultrastructure remodelling and exercise training in skeletal muscle of essential hypertensive patients. Acta Physiol 214: 210–220, 2015. [DOI] [PubMed] [Google Scholar]
- 88.Gliemann L, Schmidt JF, Olesen J, Bienso RS, Peronard SL, Grandjean SU, Mortensen SP, Nyberg M, Bangsbo J, Pilegaard H, Hellsten Y. Resveratrol blunts the positive effects of exercise training on cardiovascular health in aged men. J Physiol 591: 5047–5059, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Godbole AS, Lu X, Guo X, Kassab GS. NADPH oxidase has a directional response to shear stress. Am J Physiol Heart Circ Physiol 296: H152–H158, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Goto C, Higashi Y, Kimura M, Noma K, Hara K, Nakagawa K, Kawamura M, Chayama K, Yoshizumi M, Nara I. Effect of different intensities of exercise on endothelium-dependent vasodilation in humans: role of endothelium-dependent nitric oxide and oxidative stress. Circulation 108: 530–535, 2003. [DOI] [PubMed] [Google Scholar]
- 91.Goto C, Nishioka K, Umemura T, Jitsuiki D, Sakagutchi A, Kawamura M, Chayama K, Yoshizumi M, Higashi Y. Acute moderate-intensity exercise induces vasodilation through an increase in nitric oxide bioavailiability in humans. Am J Hypertens 20: 825–830, 2007. [DOI] [PubMed] [Google Scholar]
- 92.Green D, Cheetham C, Henderson C, Weerasooriya R, O'Driscoll G. Effect of cardiac pacing on forearm vascular responses and nitric oxide function. Am J Physiol Heart Circ Physiol 283: H1354–H1360, 2002. [DOI] [PubMed] [Google Scholar]
- 93.Green D, Cheetham C, Mavaddat L, Watts K, Best M, Taylor R, O'Driscoll G. Effect of lower limb exercise on forearm vascular function: contribution of nitric oxide. Am J Physiol Heart Circ Physiol 283: H899–H907, 2002. [DOI] [PubMed] [Google Scholar]
- 94.Green D, Cheetham C, Reed C, Dembo L, O'Driscoll G. Assessment of brachial artery blood flow across the cardiac cycle: retrograde flows during cycle ergometry. J Appl Physiol 93: 361–368, 2002. [DOI] [PubMed] [Google Scholar]
- 95.Green DJ, Bilsborough W, Naylor LH, Reed C, Wright J, O'Driscoll G, Walsh JH. Comparison of forearm blood flow responses to incremental handgrip and cycle ergometer exercise: relative contribution of nitric oxide. J Physiol 562: 617–628, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Green DJ, Cable NT, Fox C, Rankin JM, Taylor RR. Modification of forearm resistance vessels by exercise training in young men. J Appl Physiol 77: 1829–1833, 1994. [DOI] [PubMed] [Google Scholar]
- 97.Green DJ, Carter HH, Fitzsimons MG, Cable NT, Thijssen DH, Naylor LH. Obligatory role of hyperaemia and shear stress in microvascular adaptation to repeated heating in humans. J Physiol 588: 1571–1577, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Green DJ, Dawson EA, Groenewoud HM, Jones H, Thijssen DH. Is flow-mediated dilation nitric oxide mediated?: a meta-analysis. Hypertension 63: 376–382, 2014. [DOI] [PubMed] [Google Scholar]
- 100.Green DJ, Eijsvogels T, Bouts YM, Maiorana AJ, Naylor LH, Scholten RR, Spaanderman ME, Pugh CJ, Sprung VS, Schreuder T, Jones H, Cable T, Hopman MT, Thijssen DH. Exercise training and artery function in humans: nonresponse and its relationship to cardiovascular risk factors. J Appl Physiol 117: 345–352, 2014. [DOI] [PubMed] [Google Scholar]
- 101.Green DJ, Fowler DT, O'Driscoll JG, Blanksby BA, Taylor RR. Endothelium-derived nitric oxide activity in forearm vessels of tennis players. J Appl Physiol 81: 943–948, 1996. [DOI] [PubMed] [Google Scholar]
- 102.Green DJ, Hopkins ND, Jones H, Thijssen DH, Eijsvogels TM, Yeap BB. Sex differences in vascular endothelial function and health in humans: impacts of exercise. Exp Physiol 101: 230–242, 2016. [DOI] [PubMed] [Google Scholar]
- 103.Green DJ, Jones H, Thijssen D, Cable NT, Atkinson G. Flow-mediated dilation and cardiovascular event prediction: does nitric oxide matter? Hypertension 57: 363–369, 2011. [DOI] [PubMed] [Google Scholar]
- 104.Green DJ, Maiorana A, O'Driscoll G, Taylor R. Effect of exercise training on endothelium-derived nitric oxide function in humans. J Physiol 561: 1–25, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Green DJ, Maiorana AJ, Siong JH, Burke V, Erickson M, Minson CT, Bilsborough W, O'Driscoll G. Impaired skin blood flow response to environmental heating in chronic heart failure. Eur Heart J 27: 338–343, 2006. [DOI] [PubMed] [Google Scholar]
- 106.Green DJ, O'Driscoll G, Joyner MJ, Cable NT. Exercise and cardiovascular risk reduction: time to update the rationale for exercise? J Appl Physiol 105: 766–768, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Green DJ, Spence A, Rowley N, Thijssen DH, Naylor LH. Vascular adaptation in athletes: is there an ‘athlete’s artery'? Exp Physiol 97: 295–304, 2012. [DOI] [PubMed] [Google Scholar]
- 108.Green DJ, Swart A, Exterkate A, Naylor LH, Black MA, Cable NT, Thijssen DH. Impact of age, sex and exercise on brachial and popliteal artery remodelling in humans. Atherosclerosis 210: 525–530, 2010. [DOI] [PubMed] [Google Scholar]
- 109.Green DJ, Walsh JH, Maiorana A, Best MJ, Taylor RR, O'Driscoll JG. Exercise-induced improvement in endothelial dysfunction is not mediated by changes in CV risk factors: pooled analysis of diverse patient populations. Am J Physiol Heart Circ Physiol 285: H2679–H2687, 2003. [DOI] [PubMed] [Google Scholar]
- 110.Greer SA, Hays VW, Speer VC, McCall JT. Effect of dietary fat, protein and cholesterol on atherosclerosis in swine. J Nutr 90: 183–190, 1966. [DOI] [PubMed] [Google Scholar]
- 111.Groothuis JT, Thijssen DH, Rongen GA, Deinum J, Danser AH, Geurts AC, Smits P, Hopman MT. Angiotensin II contributes to the increased baseline leg vascular resistance in spinal cord-injured individuals. J Hypertens 28: 2094–2101, 2010. [DOI] [PubMed] [Google Scholar]
- 112.Hallal PC, Andersen LB, Bull FC, Guthold R, Haskell W, Ekelund U, Lancet Physical Activity Series Working Group. Global physical activity levels: surveillance progress, pitfalls, and prospects. Lancet 380: 247–257, 2012. [DOI] [PubMed] [Google Scholar]
- 113.Halliwill JR, Minson CT. Retrograde shear: backwards into the future? Am J Physiol Heart Circ Physiol 298: H1126–H1127, 2010. [DOI] [PubMed] [Google Scholar]
- 114.Hambrecht R, Adams V, Erbs S, Linke A, Krankel N, Shu Y, Baither Y, Gielen S, Thiele H, Gummert JF, Mohr FW, Schuler G. Regular physical activity improves endothelial function in patients with coronary artery disease by increasing phosphorylation of endothelial nitric oxide synthase. Circulation 107: 3152–3158, 2003. [DOI] [PubMed] [Google Scholar]
- 115.Hambrecht R, Fiehn E, Weigl C, Gielen S, Hamann C, Kaiser R, Yu J, Adams V, Niebauer J, Schuler G. Regular physical exercise corrects endothelial dysfunction and improves exercise capacity in patients with chronic heart failure. Circulation 98: 2709–2715, 1998. [DOI] [PubMed] [Google Scholar]
- 116.Hambrecht R, Gielen S, Linke A, Fiehn E, Yu J, Walther C, Schoene N, Schuler G. Effects of exercise training on left ventricular function and peripheral resistance in patients with chronic heart failure: a randomized trial. JAMA 283: 3095–3101, 2000. [DOI] [PubMed] [Google Scholar]
- 117.Hambrecht R, Hilbrich L, Erbs S, Gielen S, Fiehn E, Schoene N, Schuler G. Correction of endothelial dysfunction in chronic heart failure: additional effects of exercise training and oral l-arginine supplementation. J Am Coll Cardiol 35: 706–713, 2000. [DOI] [PubMed] [Google Scholar]
- 118.Hambrecht R, Walther C, Mobius-Winkler S, Gielen S, Linke A, Conradi K, Erbs S, Kluge R, Kendziorra K, Sabri O, Sick P, Schuler G. Percutaneous coronary angioplasty compared with exercise training in patients with stable coronary artery disease: a randomized trial. Circulation 109: 1371–1378, 2004. [DOI] [PubMed] [Google Scholar]
- 119.Hambrecht R, Wolf A, Gielen S, Linke A, Hofer J, Erbs S, Schoene N, Schuler G. Effect of exercise on coronary endothelial function in patients with coronary artery disease. N Engl J Med 342: 454–460, 2000. [DOI] [PubMed] [Google Scholar]
- 120.Hamer M, Ingle L, Carroll S, Stamatakis E. Physical activity and cardiovascular mortality risk: possible protective mechanisms? Med Sci Sports Exercise 44: 84–88, 2012. [DOI] [PubMed] [Google Scholar]
- 121.Hanna MA, Taylor CR, Chen B, La HS, Maraj JJ, Kilar CR, Behnke BJ, Delp MD, Muller-Delp JM. Structural remodeling of coronary resistance arteries: effects of age and exercise training. J Appl Physiol 117: 616–623, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hansen AH, Nielsen JJ, Saltin B, Hellsten Y. Exercise training n ormalizes skeletal muscle vascular endothelial growth factor levels in patients with essential hypertension. J Hypertens 28: 1176–1185, 2010. [DOI] [PubMed] [Google Scholar]
- 123.Hansen AH, Nyberg M, Bangsbo J, Saltin B, Hellsten Y. Exercise training alters the balance between vasoactive compounds in skeletal muscle of individuals with essential hypertension. Hypertension 58: 943–949, 2011. [DOI] [PubMed] [Google Scholar]
- 124.Harrison DG, Widder J, Grumbach I, Chen W, Weber M, Searles C. Endothelial mechanotransduction, nitric oxide and vascular inflammation. J Internal Med 259: 351–363, 2006. [DOI] [PubMed] [Google Scholar]
- 125.Haskell WL, Sims C, Myll J, Bortz WM, St Goar FG, Alderman EL. Coronary artery size and dilating capacity in ultradistance runners. Circulation 87: 1076–1082, 1993. [DOI] [PubMed] [Google Scholar]
- 126.Hastings NE, Simmers MB, McDonald OG, Wamhoff BR, Blackman BR. Atherosclerosis-prone hemodynamics differentially regulates endothelial and smooth muscle cell phenotypes and promotes pro-inflammatory priming. Am J Physiol Cell Physiol 293: C1824–C1833, 2007. [DOI] [PubMed] [Google Scholar]
- 127.Hawley JA, Hargreaves M, Joyner MJ, Zierath JR. Integrative biology of exercise. Cell 159: 738–749, 2014. [DOI] [PubMed] [Google Scholar]
- 128.Heaps CL, Bowles DK, Sturek M, Laughlin MH, Parker JL. Enhanced L-type Ca2+ channel current density in coronary smooth muscle of exercise-trained pigs is compensated to limit myoplasmic free Ca2+ accumulation. J Physiol 528: 435–445, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hellsten Y, Jensen L, Thaning P, Nyberg M, Mortensen S. Impaired formation of vasodilators in peripheral tissue in essential hypertension is normalized by exercise training: role of adenosine and prostacyclin. J Hypertens 30: 2007–2014, 2012. [DOI] [PubMed] [Google Scholar]
- 130.Henderson KK, Turk JR, Rush JW, Laughlin MH. Endothelial function in coronary arterioles from pigs with early-stage coronary disease induced by high-fat, high-cholesterol diet: effect of exercise. J Appl Physiol 97: 1159–1168, 2004. [DOI] [PubMed] [Google Scholar]
- 131.Higashi Y, Sasaki S, Kurisu S, Yoshimizu A, Sasaki N, Matsuura H, Kajiyama G, Oshima T. Regular aerobic exercise augments endothelium-dependent vascular relaxation in normotensive as well as hypertensive subjects: role of endothelium-derived nitric oxide. Circulation 100: 1194–1202, 1999. [DOI] [PubMed] [Google Scholar]
- 132.Hodges GJ, Sharp L, Stephenson C, Patwala AY, George KP, Goldspink DF, Tim Cable N. The effect of 48 weeks of aerobic exercise training on cutaneous vasodilator function in post-menopausal females. Eur J Appl Physiol 108: 1259–1267, 2010. [DOI] [PubMed] [Google Scholar]
- 133.Hoier B, Hellsten Y. Exercise-induced capillary growth in human skeletal muscle and the dynamics of VEGF. Microcirculation 21: 301–314, 2014. [DOI] [PubMed] [Google Scholar]
- 134.Holtz J, Forstermann U, Pohl U, Giesler M, Bassenge E. Flow-dependent, endothelium-mediated dilation of epicardial coronary arteries in conscious dogs: effects of cyclooxygenase inhibition. J Cardiovasc Pharmacol 6: 1161–1169, 1984. [PubMed] [Google Scholar]
- 135.Hopkins ND, Green DJ, Tinken TM, Sutton L, McWhannell N, Thijssen DH, Cable NT, Stratton G, George K. Does conduit artery diameter vary according to the anthropometric characteristics of children or men? Am J Physiol Heart Circ Physiol 297: H2182–H2187, 2009. [DOI] [PubMed] [Google Scholar]
- 136.Hornig B, Maier V, Drexler H. Physical training improves endothelial function in patients with chronic heart failure. Circulation 93: 210–214, 1996. [DOI] [PubMed] [Google Scholar]
- 137.Hu FB. Globalization of diabetes: the role of diet, lifestyle, and genes. Diabetes Care 34: 1249–1257, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hunt JE, Galea D, Tufft G, Bunce D, Ferguson RA. Time course of regional vascular adaptations to low load resistance training with blood flow restriction. J Appl Physiol 115: 403–411, 2013. [DOI] [PubMed] [Google Scholar]
- 139.Huo Y, Wischgoll T, Kassab GS. Flow patterns in three-dimensional porcine epicardial coronary arterial tree. Am J Physiol Heart Circ Physiol 293: H2959–H2970, 2007. [DOI] [PubMed] [Google Scholar]
- 140.Huonker M, Schmid A, Schmidt-Trucksass A, Grathwohl D, Keul J. Size and blood flow of central and peripheral arteries in highly trained able-bodied and disabled athletes. J Appl Physiol 95: 685–691, 2003. [DOI] [PubMed] [Google Scholar]
- 141.Huonker M, Schmid A, Sorichter S, Schmidt-Trucksab A, Mrosek P, Keul J. Cardiovascular differences between sedentary and wheelchair-trained subjects with paraplegia. Med Sci Sports Exercise 30: 609–613, 1998. [DOI] [PubMed] [Google Scholar]
- 142.Hwang J, Ing MH, Salazar A, Lassegue B, Griendling K, Navab M, Sevanian A, Hsiai TK. Pulsatile versus oscillatory shear stress regulates NADPH oxidase subunit expression: implication for native LDL oxidation. Circ Res 93: 1225–1232, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hwang J, Saha A, Boo YC, Sorescu GP, McNally JS, Holland SM, Dikalov S, Giddens DP, Griendling KK, Harrison DG, Jo H. Oscillatory shear stress stimulates endothelial production of O2− from p47phox-dependent NAD(P)H oxidases, leading to monocyte adhesion. J Biol Chem 278: 47291–47298, 2003. [DOI] [PubMed] [Google Scholar]
- 144.Ikegawa S, Kamijo Y, Okazaki K, Masuki S, Okada Y, Nose H. Effects of hypohydration on thermoregulation during exercise before and after 5-day aerobic training in a warm environment in young men. J Appl Physiol 110: 972–980, 2011. [DOI] [PubMed] [Google Scholar]
- 145.Inaba Y, Chen JA, Bergmann SR. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: a meta-analysis. Int J Cardiovasc Imaging 26: 631–640, 2010. [DOI] [PubMed] [Google Scholar]
- 146.Jenkins NT, Padilla J, Thorne P, Martin J, Rector R, Davis J, Laughlin M. Transcriptome-wide RNA sequencing analysis of rat skeletal muscle feed arteries. I. Impact of obesity. J Appl Physiol 116: 1017–1032, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Jin SX, Shen LH, Nie P, Yuan W, Hu LH, Li DD, Chen XJ, Zhang XK, He B. Endogenous renovascular hypertension combined with low shear stress induces plaque rupture in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol 32: 2372–2379, 2012. [DOI] [PubMed] [Google Scholar]
- 148.Johnson BD, Mather KJ, Wallace JP. Mechanotransduction of shear in the endothelium: basic studies and clinical implications. Vasc Med 16: 365–377, 2011. [DOI] [PubMed] [Google Scholar]
- 149.Johnson LR, Laughlin MH. Chronic exercise training does not alter pulmonary vasorelaxation in normal pigs. J Appl Physiol 88: 2008–2014, 2000. [DOI] [PubMed] [Google Scholar]
- 150.Johnson LR, Rush JW, Turk JR, Price EM, Laughlin MH. Short-term exercise training increases ACh-induced relaxation and eNOS protein in porcine pulmonary arteries. J Appl Physiol 90: 1102–1110, 2001. [DOI] [PubMed] [Google Scholar]
- 151.Johnson PC. The myogenic response. In: Comprehensive Physiology. New York: Wiley, 2011. [Google Scholar]
- 152.Jondeau G, Katz SD, Toussaint JF, Dubourg O, Monrad ES, Bourdarias JP, LeJemtel TH. Regional specificity of peak hyperemic response in patients with congestive heart failure: correlation with peak aerobic capacity. J Am Coll Cardiol 22: 1399–1402, 1993. [DOI] [PubMed] [Google Scholar]
- 153.Joyner MJ, Casey DP. Regulation of increased blood flow (hyperemia) to muscles during exercise: a hierarchy of competing physiological needs. Physiol Rev 95: 549–601, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Joyner MJ, Dietz NM, Shepherd JT. From Belfast to Mayo and beyond: the use and future of plethysmography to study blood flow in human limbs. J Appl Physiol 91: 2431–2441, 2001. [DOI] [PubMed] [Google Scholar]
- 155.Joyner MJ, Green DJ. Exercise protects the cardiovascular system: effects beyond traditional risk factors. J Physiol 587: 5551–5558, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kamiya A, Togawa T. Adaptive regulation of wall shear stress to flow change in the canine carotid artery. Am J Physiol Heart Circ Physiol 239: H14–H21, 1980. [DOI] [PubMed] [Google Scholar]
- 157.Keteyian SJ. Swing and a miss or inside-the-park home run: which fate awaits high-intensity exercise training? Circulation 126: 1431–1433, 2012. [DOI] [PubMed] [Google Scholar]
- 158.Kingwell BA, Sherrard B, Jennings GL, Dart AM. Four weeks of cycle training increases basal production of nitric oxide from the forearm. Am J Physiol Heart Circ Physiol 272: H1070–H1077, 1997. [DOI] [PubMed] [Google Scholar]
- 159.Kodama S, Saito K, Tanaka S, Maki M, Yachi Y, Asumi M, Sugawara A, Totsuka K, Shimano H, Ohashi Y, Yamada N, Sone H. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA 301: 2024–2035, 2009. [DOI] [PubMed] [Google Scholar]
- 160.Koller A, Sun D, Kaley G. Role of shear stress and endothelial prostaglandins in flow- and viscosity-induced dilation of arterioles in vitro. Circ Res 72: 1276–1284, 1993. [DOI] [PubMed] [Google Scholar]
- 161.Kon M, Ohiwa N, Honda A, Matsubayashi T, Ikeda T, Akimoto T, Suzuki Y, Hirano Y, Russell AP. Effects of systemic hypoxia on human muscular adaptations to resistance exercise training. Physiol Rep 2: 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Korshunov VA, Berk BC. Flow-induced vascular remodeling in the mouse: a model for carotid intima-media thickening. Arterioscler Thromb Vasc Biol 23: 2185–2191, 2003. [DOI] [PubMed] [Google Scholar]
- 163.Korzick DH, Laughlin MH, Bowles DK. Alterations in PKC signaling underlie enhanced myogenic tone in exercise-trained porcine coronary resistance arteries. J Appl Physiol 96: 1425–1432, 2004. [DOI] [PubMed] [Google Scholar]
- 164.Kvernmo HD, Stefanovska A, Kirkeboen KA, Osterud B, Kvernebo K. Enhanced endothelium-dependent vasodilatation in human skin vasculature induced by physical conditioning. Eur J Appl Physiol Occup Physiol 79: 30–36, 1998. [DOI] [PubMed] [Google Scholar]
- 165.LaMack JA, Himburg HA, Friedman MH. Distinct profiles of endothelial gene expression in hyperpermeable regions of the porcine aortic arch and thoracic aorta. Atherosclerosis 195: e35–41, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lampert E, Mettauer B, Hoppeler H, Charloux A, Charpentier A, Lonsdorfer J. Skeletal muscle response to short endurance training in heart transplant recipients. J Am Coll Cardiol 32: 420–426, 1998. [DOI] [PubMed] [Google Scholar]
- 167.Langille BL, O'Donnell F. Reductions in arterial diameter produced by chronic decreases in blood flow are endothelium-dependent. Science 231: 405–407, 1986. [DOI] [PubMed] [Google Scholar]
- 168.Laughlin MH. Endothelium-mediated control of coronary vascular tone after chronic exercise training. Med Sci Sports Exercise 27: 1135–1144, 1995. [PubMed] [Google Scholar]
- 169.Laughlin MH, Joseph B. Wolfe Memorial lecture. Physical activity in prevention and treatment of coronary disease: the battle line is in exercise vascular cell biology. Med Sci Sports Exercise 36: 352–362, 2004. [DOI] [PubMed] [Google Scholar]
- 170.Laughlin MH, Bowles DK, Duncker DJ. The coronary circulation in exercise training. Am J Physiol Heart Circ Physiol 302: H10–H23, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Laughlin MH, Korthuis RJ, Duncker DJ, Bache RJ. Control of blood flow to cardiac and skeletal muscle during exercise. In: Handbook of Physiology. Exercise: Regulation and Integration of Multiple Systems. Bethesda, MD: Am. Physiol. Soc, 1996, p. 705–769. [Google Scholar]
- 172.Laughlin MH, Muller JM. Vasoconstrictor responses of coronary resistance arteries in exercise-trained pigs. J Appl Physiol 84: 884–889, 1998. [DOI] [PubMed] [Google Scholar]
- 173.Laughlin MH, Newcomer SC, Bender SB. Importance of hemodynamic forces as signals for exercise-induced changes in endothelial cell phenotype. J Appl Physiol 104: 588–600, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Laughlin MH, Padilla J, Jenkins NT, Thorne PK, Martin JS, Rector RS, Akter S, Davis JW. Exercise-induced differential changes in gene expression among arterioles of skeletal muscles of obese rats. J Appl Physiol 119: 583–603, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Laughlin MH, Padilla J, Jenkins NT, Thorne PK, Martin JS, Rector RS, Akter S, Davis JW. Exercise training causes differential changes in gene expression in diaphragm arteries and 2A arterioles of obese rats. J Appl Physiol 119: 604–616, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Laughlin MH, Pollock JS, Amann JF, Hollis ML, Woodman CR, Price EM. Training induces nonuniform increases in eNOS content along the coronary arterial tree. J Appl Physiol 90: 501–510, 2001. [DOI] [PubMed] [Google Scholar]
- 177.Laughlin MH, Ripperger J. Vascular transport capacity of hindlimb muscles of exercise-trained rats. J Appl Physiol 62: 438–443, 1987. [DOI] [PubMed] [Google Scholar]
- 178.Laukkanen T, Khan H, Zaccardi F, Laukkanen JA. Association between sauna bathing and fatal cardiovascular and all-cause mortality events. JAMA Intern Med 175: 542–548, 2015. [DOI] [PubMed] [Google Scholar]
- 179.Lawrence MB, McIntire LV, Eskin SG. Effect of flow on polymorphonuclear leukocyte/endothelial cell adhesion. Blood 70: 1284–1290, 1987. [PubMed] [Google Scholar]
- 180.Lee DC, Sui X, Artero EG, Lee IM, Church TS, McAuley PA, Stanford FC, Kohl HW 3rd, Blair SN. Long-term effects of changes in cardiorespiratory fitness and body mass index on all-cause and cardiovascular disease mortality in men: the Aerobics Center Longitudinal Study. Circulation 124: 2483–2490, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lee HY, Youn SW, Oh BH, Kim HS. Kruppel-like factor 2 suppression by high glucose as a possible mechanism of diabetic vasculopathy. Korean Circ J 42: 239–245, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Lenasi H, Strucl M. Effect of regular physical training on cutaneous microvascular reactivity. Med Sci Sports Exerc 36: 606–612, 2004. [DOI] [PubMed] [Google Scholar]
- 183.Lewis TV, Dart AM, Chin-Dusting JP, Kingwell BA. Exercise training increases basal nitric oxide production from the forearm in hypercholesterolemic patients. Arterioscler Thromb Vasc Biol 19: 2782–2787, 1999. [DOI] [PubMed] [Google Scholar]
- 184.Li L, Chen W, Rezvan A, Jo H, Harrison DG. Tetrahydrobiopterin deficiency and nitric oxide synthase uncoupling contribute to atherosclerosis induced by disturbed flow. Arterioscler Thromb Vasc Biol 31: 1547–1554, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Linke A, Erbs S, Hambrecht R. Exercise and the coronary circulation-alterations and adaptations in coronary artery disease. Prog Cardiovasc Dis 48: 270–284, 2006. [DOI] [PubMed] [Google Scholar]
- 186.Linke A, Schoene N, Gielen S, Hofer J, Erbs S, Schuler G, Hambrecht R. Endothelial dysfunction in patients with chronic heart failure: systemic effects of lower-limb exercise training. J Am Coll Cardiol 37: 392–397, 2001. [DOI] [PubMed] [Google Scholar]
- 187.Lopez MG, Silva BM, Joyner MJ, Casey DP. Roles of nitric oxide and prostaglandins in the hyperemic response to a maximal metabolic stimulus: redundancy prevails. Eur J Appl Physiol 113: 1449–1456, 2013. [DOI] [PubMed] [Google Scholar]
- 188.Lu X, Kassab GS. Nitric oxide is significantly reduced in ex-vivo porcine arteries during reverse flow because of increased superoxide production. J Physiol 561: 575–582, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Madhur MS, Funt SA, Li L, Vinh A, Chen W, Lob HE, Iwakura Y, Blinder Y, Rahman A, Quyyumi AA, Harrison DG. Role of interleukin 17 in inflammation, atherosclerosis, and vascular function in apolipoprotein e-deficient mice. Arterioscler Thromb Vasc Biol 31: 1565–1572, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Madssen E, Moholdt T, Videm V, Wisloff U, Hegbom K, Wiseth R. Coronary atheroma regression and plaque characteristics assessed by grayscale and radiofrequency intravascular ultrasound after aerobic exercise. Am J Cardiol 114: 1504–1511, 2014. [DOI] [PubMed] [Google Scholar]
- 191.Maeda S, Miyauchi T, Kakiyama T, Sugawara J, Iemitsu M, Irukayama-Tomobe Y, Murakami H, Kumagai Y, Kuno S, Matsuda M. Effects of exercise training of 8 weeks and detraining on plasma levels of endothelium-derived factors, endothelin-1 and nitric oxide, in healthy young humans. Life Sci 69: 1005–1016, 2001. [DOI] [PubMed] [Google Scholar]
- 192.Maiorana A, O'Driscoll G, Cheetham C, Collis J, Goodman C, Rankin S, Taylor R, Green D. Combined aerobic and resistance exercise training improves functional capacity and strength in CHF. J Appl Physiol 88: 1565–1570, 2000. [DOI] [PubMed] [Google Scholar]
- 193.Maiorana A, O'Driscoll G, Cheetham C, Dembo L, Stanton K, Goodman C, Taylor R, Green D. The effect of combined aerobic and resistance exercise training on vascular function in type 2 diabetes. J Am Coll Cardiol 38: 860–866, 2001. [DOI] [PubMed] [Google Scholar]
- 194.Maiorana A, O'Driscoll G, Dembo L, Cheetham C, Goodman C, Taylor R, Green D. Effect of aerobic and resistance exercise training on vascular function in heart failure. Am J Physiol Heart Circ Physiol 279: H1999–H2005, 2000. [DOI] [PubMed] [Google Scholar]
- 195.Maiorana A, O'Driscoll G, Dembo L, Goodman C, Taylor R, Green D. Exercise training, vascular function, and functional capacity in middle-aged subjects. Med Sci Sports Exercise 33: 2022–2028, 2001. [DOI] [PubMed] [Google Scholar]
- 196.Maiorana A, O'Driscoll G, Taylor R, Green D. Exercise and the nitric oxide vasodilator system. Sports Med 33: 1013–1035, 2003. [DOI] [PubMed] [Google Scholar]
- 197.Manson JE, Hu FB, Rich-Edwards JW, Colditz GA, Stampfer MJ, Willett WC, Speizer FE, Hennekens CH. A prospective study of walking as compared with vigorous exercise in the prevention of coronary heart disease in women. N Engl J Med 341: 650–658, 1999. [DOI] [PubMed] [Google Scholar]
- 198.Maxwell LC, White TP, Faulkner JA. Oxidative capacity, blood flow, and capillarity of skeletal muscles. J Appl Physiol 49: 627–633, 1980. [DOI] [PubMed] [Google Scholar]
- 199.McAllister RM, Kimani JK, Webster JL, Parker JL, Laughlin MH. Effects of exercise training o n responses of peripheral and visceral arteries in swine. J Appl Physiol 80: 216–225, 1996. [DOI] [PubMed] [Google Scholar]
- 200.McAllister RM, Laughlin MH. Short-term exercise training alters responses of porcine femoral and brachial arteries. J Appl Physiol 82: 1438–1444, 1997. [DOI] [PubMed] [Google Scholar]
- 201.McLenachan JM, Williams JK, Fish RD, Ganz P, Selwyn AP. Loss of flow-mediated endothelium-dependent dilation occurs early in the development of atherosclerosis. Circulation 84: 1273–1278, 1991. [DOI] [PubMed] [Google Scholar]
- 202.Mestek ML, Westby CM, Van Guilder GP, Greiner JJ, Stauffer BL, DeSouza CA. Regular aerobic exercise, without weight loss, improves endothelium-dependent vasodilation in overweight and obese adults. Obesity 18: 1667–1669, 2010. [DOI] [PubMed] [Google Scholar]
- 203.Mills EJ, Rachlis B, Wu P, Devereaux PJ, Arora P, Perri D. Primary prevention of cardiovascular mortality and events with statin treatments: a network meta-analysis involving more than 65,000 patients. J Am Coll Cardiol 52: 1769–1781, 2008. [DOI] [PubMed] [Google Scholar]
- 204.Minson CT, Berry LT, Joyner MJ. Nitric oxide and neurally mediated regulation of skin blood flow during local heating. J Appl Physiol 91: 1619–1626, 2001. [DOI] [PubMed] [Google Scholar]
- 205.Miyachi M, Iemitsu M, Okutsu M, Onodera S. Effects of endurance training on the size and blood flow of the arterial conductance vessels in humans. Acta Physiol Scand 163: 13–16, 1998. [DOI] [PubMed] [Google Scholar]
- 206.Miyachi M, Tanaka H, Yamamoto K, Yoshioka A, Takahashi K, Onodera S. Effects of one-legged endurance training on femoral arterial and venous size in healthy humans. J Appl Physiol 90: 2439–2444, 2001. [DOI] [PubMed] [Google Scholar]
- 207.Monahan KD, Dinenno FA, Tanaka H, Clevenger CM, DeSouza CA, Seals DR. Regular aerobic exercise modulates age-associated declines in cardiovagal baroreflex sensitivity in healthy men. J Physiol 529: 263–271, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Moncada S, Gryglewski R, Bunting S, Vane JR. An enzyme isolated from arteries transforms prostaglandin endoperoxides to an unstable substance that inhibits platelet aggregation. Nature 263: 663–665, 1976. [DOI] [PubMed] [Google Scholar]
- 209.Montero D, Walther G, Diaz-Canestro C, Pyke KE, Padilla J. Microvascular dilator function in athletes: a systematic review and meta-analysis. Med Sci Sports Exercise 47: 1485–1494, 2015. [DOI] [PubMed] [Google Scholar]
- 210.Moore SC, Patel AV, Matthews CE, Berrington de Gonzalez A, Park Y, Katki HA, Linet MS, Weiderpass E, Visvanathan K, Helzlsouer KJ, Thun M, Gapstur SM, Hartge P, Lee IM. Leisure time physical activity of moderate to vigorous intensity and mortality: a large pooled cohort analysis. PLoS Med 9: e1001335, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Mora S, Cook N, Buring JE, Ridker PM, Lee IM. Physical activity and reduced risk of cardiovascular events: potential mediating mechanisms. Circulation 116: 2110–2118, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Moreau KL, Donato AJ, Seals DR, Dinenno FA, Blackett SD, Hoetzer GL, Desouza CA, Tanaka H. Arterial intima-media thickness: site-specific associations with HRT and habitual exercise. Am J Physiol Heart Circ Physiol 283: H1409–H1417, 2002. [DOI] [PubMed] [Google Scholar]
- 213.Moreau KL, Hildreth KL, Meditz AL, Deane KD, Kohrt WM. Endothelial function is impaired across the stages of the menopause transition in healthy women. J Clin Endocrinol Metab 97: 4692–4700, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Moreau KL, Stauffer BL, Kohrt WM, Seals DR. Essential role of estrogen for improvements in vascular endothelial function with endurance exercise in postmenopausal women. J Clin Endocrinol Metab 98: 4507–4515, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Morganroth J, Maron BJ, Henry WL, Epstein SE. Comparative left ventricular dimensions in trained athletes. Ann Internal Med 82: 521–524, 1975. [DOI] [PubMed] [Google Scholar]
- 216.Morris JN. Forward. In: Epidemiologic Methods in Physical Activity Studies, edited by Lee IM, Blair SN, Manson JE. York: NYU Press, 2009, p. 3–12. [Google Scholar]
- 217.Mueller PJ. Physical (in)activity-dependent alterations at the rostral ventrolateral medulla: influence on sympathetic nervous system regulation. Am J Physiol Regul Integr Comp Physiol 298: R1468–R1474, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Muller JM, Myers PR, Laughlin MH. Vasodilator responses of coronary resistance arteries of exercise-trained pigs. Circulation 89: 2308–2314, 1994. [DOI] [PubMed] [Google Scholar]
- 219.Munk K, Andersen NH, Schmidt MR, Nielsen SS, Terkelsen CJ, Sloth E, Botker HE, Nielsen TT, Poulsen SH. Remote ischemic conditioning in patients with myocardial infarction treated with primary angioplasty: impact on left ventricular function assessed by comprehensive echocardiography and gated single-photon emission CT. Circ Cardiovasc Imaging 3: 656–662, 2010. [DOI] [PubMed] [Google Scholar]
- 220.Naci H, Ioannidis JP. Comparative effectiveness of exercise and drug interventions on mortality outcomes: metaepidemiological study. BMJ 347: f5577, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Nam D, Ni CW, Rezvan A, Suo J, Budzyn K, Llanos A, Harrison D, Giddens D, Jo H. Partial carotid ligation is a model of acutely induced disturbed flow, leading to rapid endothelial dysfunction and atherosclerosis. Am J Physiol Heart Circ Physiol 297: H1535–H1543, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Naylor LH, Carter H, FitzSimons MG, Cable NT, Thijssen DH, Green DJ. Repeated increases in blood flow, independent of exercise, enhance conduit artery vasodilator function in humans. Am J Physiol Heart Circ Physiol 300: H664–H669, 2011. [DOI] [PubMed] [Google Scholar]
- 223.Naylor LH, Davis EA, Kalic RJ, Paramalingam N, Abraham MB, Jones TW, Green DJ. Exercise training improves vascular function in adolescents with type 2 diabetes. Physiol Rep 4: 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Naylor LH, O'Driscoll G, Fitzsimons M, Arnolda LF, Green DJ. Effects of training resumption on conduit arterial diameter in elite rowers. Med Sci Sports Exercise 38: 86–92, 2006. [DOI] [PubMed] [Google Scholar]
- 225.Nguyen PK, Terashima M, Fair JM, Varady A, Taylor-Piliae RE, Iribarren C, Go AS, Haskell WL, Hlatky MA, Fortmann SP, McConnell MV. Physical activity in older subjects is associated with increased coronary vasodilation: the ADVANCE study. JACC Cardiovasc Imaging 4: 622–629, 2011. [DOI] [PubMed] [Google Scholar]
- 226.Ni CW, Qiu H, Rezvan A, Kwon K, Nam D, Son DJ, Visvader JE, Jo H. Discovery of novel mechanosensitive genes in vivo using mouse carotid artery endothelium exposed to disturbed flow. Blood 116: e66–73, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Nyberg M, Seidelin K, Andersen TR, Overby NN, Hellsten Y, Bangsbo J. Biomarkers of vascular function in premenopausal and recent postmenopausal women of similar age: effect of exercise training. Am J Physiol Regul Integr Comp Physiol 306: R510–R517, 2014. [DOI] [PubMed] [Google Scholar]
- 228.O'Keeffe LM, Muir G, Piterina AV, McGloughlin T. Vascular cell adhesion molecule-1 expression in endothelial cells exposed to physiological coronary wall shear stresses. J Biomech Eng 131: 081003, 2009. [DOI] [PubMed] [Google Scholar]
- 229.Odden MC, Coxson PG, Moran A, Lightwood JM, Goldman L, Bibbins-Domingo K. The impact of the aging population on coronary heart disease in the United States. Am J Med 124: 827–833, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Olive JL, Dudley GA, McCully KK. Vascular remodeling after spinal cord injury. Med Sci Sports Exercise 35: 901–907, 2003. [DOI] [PubMed] [Google Scholar]
- 231.Olshansky SJ, Passaro DJ, Hershow RC, Layden J, Carnes BA, Brody J, Hayflick L, Butler RN, Allison DB, Ludwig DS. A potential decline in life expectancy in the United States in the 21st century. N Engl J Med 352: 1138–1145, 2005. [DOI] [PubMed] [Google Scholar]
- 232.Oneda B, Cardoso CG Jr, Forjaz CL, Araujo TG, Bernardo FR, de Gusmao JL, Pinto LG, Labes E, Abrahao SB, Mion D Jr, Fonseca AM, Tinucci T. Effects of estrogen therapy and aerobic training on sympathetic activity and hemodynamics in healthy postmenopausal women: a double-blind randomized trial. Menopause 21: 369–375, 2014. [DOI] [PubMed] [Google Scholar]
- 233.Oudegeest-Sander MH, Olde Rikkert MG, Smits P, Thijssen DH, van Dijk AP, Levine BD, Hopman MT. The effect of an advanced glycation end-product crosslink breaker and exercise training on vascular function in older individuals: a randomized factorial design trial. Exp Gerontol 48: 1509–1517, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Pacher Pl Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev 87: 315–424, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Padilla J, Jenkins N, Thorne P, Martin J, Rector R, Davis J, Laughlin M. Transcriptome-wide RNA sequencing analysis of rat skeletal muscle feed arteries. II. Impact of exercise training in obesity. J Appl Physiol 116: 1033–1047, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Padilla J, Jenkins NT, Laughlin MH, Fadel PJ. Blood pressure regulation VIII: resistance vessel tone and implications for a pro-atherogenic conduit artery endothelial cell phenotype. Eur J Appl Physiol 114: 531–544, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Padilla J, Simmons GH, Bender SB, Arce-Esquivel AA, Whyte JJ, Laughlin MH. Vascular effects of exercise: endothelial adaptations beyond active muscle beds. Physiology 26: 132–145, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Padilla J, Simmons GH, Davis JW, Whyte JJ, Zderic TW, Hamilton MT, Bowles DK, Laughlin MH. Impact of exercise training on endothelial transcriptional profiles in healthy swine: a genome-wide microarray analysis. Am J Physiol Heart Circ Physiol 301: H555–H564, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Padilla J, Simmons GH, Vianna LC, Davis MJ, Laughlin MH, Fadel PJ. Brachial artery vasodilatation during prolonged lower limb exercise: role of shear rate. Exp Physiol 96: 1019–1027, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Padilla J, Young CN, Simmons GH, Deo SH, Newcomer SC, Sullivan JP, Laughlin MH, Fadel PJ. Increased muscle sympathetic nerve activity acutely alters conduit artery shear rate patterns. Am J Physiol Heart Circ Physiol 298: H1128–H1135, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Paffenbarger RS Jr, Hyde RT, Wing AL, Hsieh CC. Physical activity, all-cause mortality, and longevity of college alumni. N Engl J Med 314: 605–613, 1986. [DOI] [PubMed] [Google Scholar]
- 242.Paffenbarger RS Jr, Kampert JB, Lee IM, Hyde RT, Leung RW, Wing AL. Changes in physical activity and other lifeway patterns influencing longevity. Med Sci Sports Exerc 26: 857–865, 1994. [PubMed] [Google Scholar]
- 243.Pagkalos M, Koutlianos N, Kouidi E, Pagkalos E, Mandroukas K, Deligiannis A. Heart rate variability modifications following exercise training in type 2 diabetic patients with definite cardiac autonomic neuropathy. Br J Sports Med 42: 47–54, 2008. [DOI] [PubMed] [Google Scholar]
- 244.Parmenter BJ, Dieberg G, Smart NA. Exercise training for management of peripheral arterial disease: a systematic review and meta-analysis. Sports Med 45: 231–244, 2015. [DOI] [PubMed] [Google Scholar]
- 245.Passerini AG, Polacek DC, Shi C, Francesco NM, Manduchi E, Grant GR, Pritchard WF, Powell S, Chang GY, Stoeckert CJ Jr, Davies PF. Coexisting proinflammatory and antioxidative endothelial transcription profiles in a disturbed flow region of the adult porcine aorta. Proc Natl Acad Sci USA 101: 2482–2487, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Pohl U, Holtz J, Busse R, Bassenge E. Crucial role of endothelium in the vasodilator response to increased flow in vivo. Hypertension 8: 37–44, 1986. [DOI] [PubMed] [Google Scholar]
- 247.Popovic M, Puchner S, Endler G, Foraschik C, Minar E, Bucek RA. The effects of endurance and recreational exercise on subclinical evidence of atherosclerosis in young adults. Am J Med Sci 339: 332–336, 2010. [DOI] [PubMed] [Google Scholar]
- 248.Pyke KE, Poitras V, Tschakovsky ME. Brachial artery flow-mediated dilation during handgrip exercise: evidence for endothelial transduction of the mean shear stimulus. Am J Physiol Heart Circ Physiol 294: H2669–H2679, 2008. [DOI] [PubMed] [Google Scholar]
- 249.Rakobowchuk M, Tanguay S, Burgomaster KA, Howarth KR, Gibala MJ, MacDonald MJ. Sprint interval and traditional endurance training induce similar improvements in peripheral arterial stiffness and flow-mediated dilation in healthy humans. Am J Physiol Regul Integr Comp Physiol 295: R236–R242, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Ramos JS, Dalleck LC, Tjonna AE, Beetham KS, Coombes JS. The impact of high-intensity interval training versus moderate-intensity continuous training on vascular function: a systematic review and meta-analysis. Sports Med 45: 679–692, 2015. [DOI] [PubMed] [Google Scholar]
- 251.Rapacz J, Hasler-Rapacz J, Taylor KM, Checovich WJ, Attie AD. Lipoprotein mutations in pigs are associated with elevated plasma cholesterol and atherosclerosis. Science 234: 1573–1577, 1986. [DOI] [PubMed] [Google Scholar]
- 252.Ras RT, Streppel MT, Draijer R, Zock PL. Flow-mediated dilation and cardiovascular risk prediction: A systematic review with meta-analysis. Int J Cardiol 168: 344–351, 2013. [DOI] [PubMed] [Google Scholar]
- 253.Ribeiro F, Alves AJ, Duarte JA, Oliveira J. Is exercise training an effective therapy targeting endothelial dysfunction and vascular wall inflammation? Int J Cardiol 141: 214–221, 2010. [DOI] [PubMed] [Google Scholar]
- 254.Roberts MF, Wenger CB, Stolwijk JA, Nadel ER. Skin blood flow and sweating changes following exercise training and heat acclimation. J Appl Physiol 43: 133–137, 1977. [DOI] [PubMed] [Google Scholar]
- 255.Roche DM, Rowland TW, Garrard M, Marwood S, Unnithan VB. Skin microvascular reactivity in trained adolescents. Eur J Appl Physiol 108: 1201–1208, 2010. [DOI] [PubMed] [Google Scholar]
- 256.Rodbard S. Vascular caliber. Cardiology 60: 4–49, 1975. [DOI] [PubMed] [Google Scholar]
- 257.Ross R, Wight TN, Strandness E, Thiele B. Human atherosclerosis. I. Cell constitution and characteristics of advanced lesions of the superficial femoral artery. Am J Pathol 114: 79–93, 1984. [PMC free article] [PubMed] [Google Scholar]
- 258.Roveda F, Middlekauff HR, Rondon MU, Reis SF, Souza M, Nastari L, Barretto AC, Krieger EM, Negrao CE. The effects of exercise training on sympathetic neural activation in advanced heart failure: a randomized controlled trial. J Am Coll Cardiol 42: 854–860, 2003. [DOI] [PubMed] [Google Scholar]
- 259.Rowell LB. Human Cardiovascular Control. New York: Oxford Univ. Press, 1993, p. 162–241. [Google Scholar]
- 260.Rowell LB, Brengelmann GL, Blackmon JR, Bruce RA, Murray JA. Disparities between aortic and peripheral pulse pressures induced by upright exercise and vasomotor changes in man. Circulation 37: 954–964, 1968. [DOI] [PubMed] [Google Scholar]
- 261.Rowley NJ, Dawson EA, Birk GK, Cable NT, George K, Whyte G, Thijssen DH, Green DJ. Exercise and arterial adaptation in humans: uncoupling localized and systemic effects. J Appl Physiol 110: 1190–1195, 2011. [DOI] [PubMed] [Google Scholar]
- 262.Rowley NJ, Dawson EA, Hopman MT, George K, Whyte GP, Thijssen DH, Green DJ. Conduit diameter and wall remodelling in elite athletes and spinal cord injury. Med Sci Sports Exercise 44: 844–849, 2012. [DOI] [PubMed] [Google Scholar]
- 263.Rubanyi GM, Romero JC, Vanhoutte PM. Flow-induced release of endothelium-derived relaxing factor. Am J Physiol Heart Circ Physiol 250: H1145–H1149, 1986. [DOI] [PubMed] [Google Scholar]
- 264.Rudic RD, Shesely EG, Maeda N, Smithies O, Segal SS, Sessa WC. Direct evidence for the importance of endothelium-derived nitric oxide in vascular remodeling. J Clin Invest 101: 731–736, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Rush JW, Laughlin MH, Woodman CR, Price EM. SOD-1 expression in pig coronary arterioles is increased by exercise training. Am J Physiol Heart Circ Physiol 279: H2068–H2076, 2000. [DOI] [PubMed] [Google Scholar]
- 266.Schmidt-Trucksass A, Schmid A, Brunner C, Scherer N, Zach G, Keul J, Huonker M. Arterial properties of the carotid and femoral artery in endurance-trained and paraplegic subjects. J Appl Physiol 89: 1956–1963, 2000. [DOI] [PubMed] [Google Scholar]
- 267.Schrage WG, Joyner MJ, Dinenno FA. Local inhibition of nitric oxide and prostaglandins independently reduces forearm exercise hyperaemia in humans. J Physiol 557: 599–611, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Schretzenmayr A. Uber kreislaufregulatorische vorgange an den grossen arterien bei der muskelarbeit. Pflügers Arch 232: 743–748, 1933. [Google Scholar]
- 269.Schreuder TH, Green DJ, Hopman MT, Thijssen DH. Impact of retrograde shear rate on brachial and superficial femoral artery flow-mediated dilation in older subjects. Atherosclerosis 241: 199–204, 2015. [DOI] [PubMed] [Google Scholar]
- 270.Schreuder TH, van Lotringen JH, Hopman MT, Thijssen DH. Impact of endothelin blockade on acute exercise-induced changes in blood flow and endothelial function in type 2 diabetes mellitus. Exp Physiol 99: 1253–1264, 2014. [DOI] [PubMed] [Google Scholar]
- 271.Segal SS. Cell-to-cell communication coordinates blood flow control. Hypertension 23: 1113–1120, 1994. [DOI] [PubMed] [Google Scholar]
- 272.Shephard RJ. A short history of occupational fitness and health promotion. Preventive Med 20: 436–445, 1991. [DOI] [PubMed] [Google Scholar]
- 273.Shimada K, Mikami Y, Murayama T, Yokode M, Fujita M, Kita T, Kishimoto C. Atherosclerotic plaques induced by marble-burying behavior are stabilized by exercise training in experimental atheroscleros is. Int J Cardiol 151: 284–289, 2011. [DOI] [PubMed] [Google Scholar]
- 274.Simmons GH, Padilla J, Young CN, Wong BJ, Lang JA, Davis MJ, Laughlin MH, Fadel PJ. Increased brachial artery retrograde shear rate at exercise onset is abolished during prolonged cycling: role of thermoregulatory vasodilation. J Appl Physiol 110: 389–397, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Sinoway LI, Hendrickson C, Davidson WR Jr, Prophet S, Zelis R. Characteristics of flow-mediated brachial artery vasodilation in human subjects. Circ Res 64: 32–42, 1989. [DOI] [PubMed] [Google Scholar]
- 276.Sinoway LI, Musch TI, Minotti JR, Zelis R. Enhanced maximal metabolic vasodilatation in the dominant forearms of tennis players. J Appl Physiol 61: 673–678, 1986. [DOI] [PubMed] [Google Scholar]
- 277.Snell PG, Martin WH, Buckey JC, Blomqvist CG. Maximal vascular leg conductance in trained and untrained men. J Appl Physiol 62: 606–610, 1987. [DOI] [PubMed] [Google Scholar]
- 278.Snell PG, Martin WH, Buckey JC, Blomqvist CG. Maximal vascular leg conductance in trained and untrained men. J Appl Physiol 62: 606–610, 1987. [DOI] [PubMed] [Google Scholar]
- 279.Spence AL, Carter HH, Naylor LH, Green DJ. A prospective randomized longitudinal study involving 6 months of endurance or resistance exercise. Conduit artery adaptation in humans. J Physiol 591: 1265–1275, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Sugawara J, Komine H, Hayashi K, Yoshizawa M, Otsuki T, Shimojo N, Miyauchi T, Yokoi T, Maeda S, Tanaka H. Systemic alpha-adrenergic and nitric oxide inhibition on basal limb blood flow: effects of endurance training in middle-aged and older adults. Am J Physiol Heart Circ Physiol 293: H1466–H1472, 2007. [DOI] [PubMed] [Google Scholar]
- 282.Sun D, Huang A, Koller A, Kaley G. Short-term daily exercise activity enhances endothelial NO synthesis in skeletal muscle arterioles of rats. J Appl Physiol 76: 2241–2247, 1994. [DOI] [PubMed] [Google Scholar]
- 283.Sun D, Huang A, Yan EH, Wu Z, Yan C, Kaminski PM, Oury TD, Wolin MS, Kaley G. Reduced release of nitric oxide to shear stress in mesenteric arteries of aged rats. Am J Physiol Heart Circ Physiol 286: H2249–H2256, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Takabe W, Jen N, Ai L, Hamilton R, Wang S, Holmes K, Dharbandi F, Khalsa B, Bressler S, Barr ML, Li R, Hsiai TK. Oscillatory shear stress induces mitochondrial superoxide production: implication of NADPH oxidase and c-Jun NH2-terminal kinase signaling. Antioxid Redox Signal 15: 1379–1388, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Tanaka H, Seals DR, Monahan KD, Clevenger CM, DeSouza CA, Dinenno FA. Regular aerobic exercise and the age-related increase in carotid artery intima-media thickness in healthy men. J Appl Physiol 92: 1458–1464, 2002. [DOI] [PubMed] [Google Scholar]
- 286.Taylor RS, Unal B, Critchley JA, Capewell S. Mortality reductions in patients receiving exercise-based cardiac rehabilitation: how much can be attributed to cardiovascular risk factor improvements? Eur J Cardiovasc Prev Rehabil 13: 369–374, 2006. [DOI] [PubMed] [Google Scholar]
- 287.Thijssen DH, Black MA, Pyke KE, Padilla J, Atkinson G, Harris RA, Parker B, Widlansky ME, Tschakovsky ME, Green DJ. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol 300: H2–H12, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Thijssen DH, Cable NT, Green DJ. Impact of exercise training on arterial wall thickness in humans. Clin Sci 122: 311–322, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Thijssen DH, Dawson EA, Black MA, Hopman MT, Cable NT, Green DJ. Brachial artery blood flow responses to different modalities of lower limb exercise. Med Sci Sports Exercise 41: 1072–1079, 2009. [DOI] [PubMed] [Google Scholar]
- 291.Thijssen DH, Dawson EA, Tinken TM, Cable NT, Green DJ. Retrograde flow and shear rate acutely impair endothelial function in humans. Hypertension 53: 986–992, 2009. [DOI] [PubMed] [Google Scholar]
- 292.Thijssen DH, Dawson EA, van den Munckhof IC, Tinken TM, den Drijver E, Hopkins N, Cable NT, Green DJ. Exercise-mediated changes in conduit artery wall thickness in humans: role of shear stress. Am J Physiol Heart Circ Physiol 301: H241–H246, 2011. [DOI] [PubMed] [Google Scholar]
- 293.Thijssen DH, de Groot PC, Smits P, Hopman MT. Vascular adaptations to 8-week cycling training in older men. Acta Physiol 190: 221–228, 2007. [DOI] [PubMed] [Google Scholar]
- 294.Thijssen DH, Ellenkamp R, Kooijman M, Pickkers P, Rongen GA, Hopman MT, Smits P. A causal role for endothelin-1 in the vascular adaptation to skeletal muscle deconditioning in spinal cord injury. Arterioscler Thromb Vasc Biol 27: 325–331, 2007. [DOI] [PubMed] [Google Scholar]
- 295.Thijssen DH, Ellenkamp R, Smits P, Hopman MT. Rapid vascular adaptations to training and detraining in persons with spinal cord injury. Arch Physical Med Rehab 87: 474–481, 2006. [DOI] [PubMed] [Google Scholar]
- 296.Thijssen DH, Maiorana AJ, O'Driscoll G, Cable NT, Hopman MT, Green DJ. Impact of inactivity and exercise on the vasculature in humans. Eur J Appl Physiol 108: 845–875, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Thijssen DH, Rongen GA, van Dijk A, Smits P, Hopman MT. Enhanced endothelin-1-mediated leg vascular tone in healthy older subjects. J Appl Physiol 103: 852–857, 2007. [DOI] [PubMed] [Google Scholar]
- 298.Thijssen DH, Scholten RR, van den Munckhof IC, Benda N, Green DJ, Hopman MT. Acute change in vascular tone alters intima-media thickness. Hypertension 58: 240–246, 2011. [DOI] [PubMed] [Google Scholar]
- 299.Thijssen DH, Schreuder TH, Newcomer SW, Laughlin MH, Hopman MT, Green DJ. Impact of 2-weeks continuous increase in retrograde shear stress on brachial artery vasomotor function in young and older men. J Am Heart Assoc 4: 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Thijssen DH, Willems L, van den Munckhof I, Scholten R, Hopman MT, Dawson EA, Atkinson G, Cable NT, Green DJ. Impact of wall thickness on conduit artery function in humans: is there a “Folkow” effect? Atherosclerosis 217: 415–419, 2011. [DOI] [PubMed] [Google Scholar]
- 301.Thoma R. Untersuchungen uber die histogenese und histomechanik des gefassystems. Stuttgart, Germany: Ferdinand Enke Verlag, 1893. [Google Scholar]
- 302.Thompson MA, Henderson KK, Woodman CR, Turk JR, Rush JW, Price E, Laughlin MH. Exercise preserves endothelium-dependent relaxation in coronary arteries of hypercholesterolemic male pigs. J Appl Physiol 96: 1114–1126, 2004. [DOI] [PubMed] [Google Scholar]
- 303.Thompson PD, Buchner D, Pina IL, Balady GJ, Williams MA, Marcus BH, Berra K, Blair SN, Costa F, Franklin B, Fletcher GF, Gordon NF, Pate RR, Rodriguez BL, Yancey AK, Wenger NK, American Heart Association Council on Clinical Cardiology Subcommittee on Exercise, Rehabilitation, and Prevention, American Heart Association Council on Nutrition, Physical Activity, and Metabolism, Subcommittee on Physical Activity. Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease: a statement from the Council on Clinical Cardiology (Subcommittee on Exercise, Rehabilitation, and Prevention) and the Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity). Circulation 107: 3109–3116, 2003. [DOI] [PubMed] [Google Scholar]
- 304.Tinken TM, Thijssen DH, Black MA, Cable NT, Green DJ. Time course of change in vasodilator function and capacity in response to exercise training in humans. J Physiol 586: 5003–5012, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Tinken TM, Thijssen DH, Hopkins N, Black MA, Dawson EA, Minson CT, Newcomer SC, Laughlin MH, Cable NT, Green DJ. Impact of shear rate modulation on vascular function in humans. Hypertension 54: 278–285, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Tinken TM, Thijssen DH, Hopkins N, Dawson EA, Cable NT, Green DJ. Shear stress mediates endothelial adaptations to exercise training in humans. Hypertension 55: 312–318, 2010. [DOI] [PubMed] [Google Scholar]
- 307.Tronc F, Wassef M, Esposito B, Henrion D, Glagov S, Tedgui A. Role of NO in flow-induced remodeling of the rabbit common carotid artery. Arterioscler Thromb Vasc Biol 16: 1256–1262, 1996. [DOI] [PubMed] [Google Scholar]
- 308.Turnbull F. Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. Lancet 362: 1527–1535, 2003. [DOI] [PubMed] [Google Scholar]
- 309.Tuttle JL, Nachreiner RD, Bhuller AS, Condict KW, Connors BA, Herring BP, Dalsing MC, Unthank JL. Shear level influences resistance artery remodeling: wall dimensions, cell density, and eNOS expression. Am J Physiol Heart Circ Physiol 281: H1380–H1389, 2001. [DOI] [PubMed] [Google Scholar]
- 310.Van Guilder GP, Westby CM, Greiner JJ, Stauffer BL, DeSouza CA. Endothelin-1 vasoconstrictor tone increases with age in healthy men but can be reduced by regular aerobic exercise. Hypertension 50: 403–409, 2007. [DOI] [PubMed] [Google Scholar]
- 311.Vassalle C, Lubrano V, Domenici C, L'Abbate A. Influence of chronic aerobic exercise on microcirculatory flow and nitric oxide in humans. Int J Sports Med 24: 30–35, 2003. [DOI] [PubMed] [Google Scholar]
- 312.Verhaar MC, Strachan FE, Newby DE, Cruden NL, Koomans HA, Rabelink TJ, Webb DJ. Endothelin-A receptor antagonist-mediated vasodilatation is attenuated by inhibition of nitric oxide synthesis and by endothelin-B receptor blockade. Circulation 97: 752–756, 1998. [DOI] [PubMed] [Google Scholar]
- 314.Vlachopoulos C, Aznaouridis K, Stefanadis C. Aortic stiffness for cardiovascular risk prediction: just measure it, just do it! J Am Coll Cardiol 63: 647–649, 2014. [DOI] [PubMed] [Google Scholar]
- 315.Walsh JH, Bilsborough W, Maiorana A, Best M, O'Driscoll GJ, Taylor RR, Green DJ. Exercise training improves conduit vessel function in patients with coronary artery disease. J Appl Physiol 95: 20–25, 2003. [DOI] [PubMed] [Google Scholar]
- 316.Walsh JH, Yong G, Cheetham C, Watts GF, O'Driscoll GJ, Taylor RR, Green DJ. Effects of exercise training on conduit and resistance vessel function in treated and untreated hypercholesterolaemic subjects. Eur Heart J 24: 1681–1689, 2003. [DOI] [PubMed] [Google Scholar]
- 317.Wang JS. Effects of exercise training and detraining on cutaneous microvascular function in man: the regulatory role of endothelium-dependent dilation in skin vasculature. Eur J Appl Physiol 93: 429–434, 2005. [DOI] [PubMed] [Google Scholar]
- 318.Willett NJ, Kundu K, Knight SF, Dikalov S, Murthy N, Taylor WR. Redox signaling in an in vivo murine model of low magnitude oscillatory wall shear stress. Antioxid Redox Signal 15: 1369–1378, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Williams PT, Thompson PD. Walking versus running for hypertension, cholesterol, and diabetes mellitus risk reduction. Arterioscler Thromb Vasc Biol 33: 1085–1091, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Wisloff U, Stoylen A, Loennechen JP, Bruvold M, Rognmo O, Haram PM, Tjonna AE, Helgerud J, Slordahl SA, Lee SJ, Videm V, Bye A, Smith GL, Najjar SM, Ellingsen O, Skjaerpe T. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: a randomized study. Circulation 115: 3086–3094, 2007. [DOI] [PubMed] [Google Scholar]
- 321.Woodman CR, Price EM, Laughlin MH. Shear stress induces eNOS mRNA expression and improves endothelium-dependent dilation in senescent soleus muscle feed arteries. J Appl Physiol 98: 940–946, 2005. [DOI] [PubMed] [Google Scholar]
- 322.Woodman CR, Turk JR, Rush JW, Laughlin MH. Exercise attenuates the effects of hypercholesterolemia on endothelium-dependent relaxation in coronary arteries from adult female pigs. J Appl Physiol 96: 1105–1113, 2004. [DOI] [PubMed] [Google Scholar]
- 323.Woodman CR, Turk JR, Williams DP, Laughlin MH. Exercise training preserves endothelium-dependent relaxation in brachial arteries from hyperlipidemic pigs. J Appl Physiol 94: 2017–2026, 2003. [DOI] [PubMed] [Google Scholar]
- 324.Yoshikawa D, Ishii H, Kurebayashi N, Sato B, Hayakawa S, Ando H, Hayashi M, Isobe S, Okumura T, Hirashiki A, Takeshita K, Amano T, Uetani T, Yamada S, Murohara T. Association of cardiorespiratory fitness with characteristics of coronary plaque: assessment using integrated backscatter intravascular ultrasound and optical coherence tomography. Int J Cardiol 162: 123–128, 2013. [DOI] [PubMed] [Google Scholar]
- 325.Zarins CK, Giddens DP, Bharadvaj BK, Sottiurai VS, Mabon RF, Glagov S. Carotid bifurcation atherosclerosis. Quantitative correlation of plaque localization with flow velocity profiles and wall shear stress. Circ Res 53: 502–514, 1983. [DOI] [PubMed] [Google Scholar]
- 326.Zeiher AM, Drexler H, Wollschlaeger H, Saurbier B, Just H. Coronary vasomotion in response to sympathetic stimulation in humans: importance of the functional integrity of the endothelium. J Am Coll Cardiol 14: 1181–1190, 1989. [DOI] [PubMed] [Google Scholar]
- 327.Zeppilli P, Vannicelli R, Santini C, Dello Russo A, Picani C, Palmieri V, Cameli S, Corsetti R, Pietrangeli L. Echocardiographic size of conductance vessels in athletes and sedentary people. Int J Sports Med 16: 38–44, 1995. [DOI] [PubMed] [Google Scholar]
- 328.Zhang X, Cheng R, Rowe D, Sethu P, Daugherty A, Yu G, Shin HY. Shear-sensitive regulation of neutrophil flow behavior and its potential impact on microvascular blood flow dysregulation in hypercholesterolemia. Arterioscler Thromb Vasc Biol 34: 587–593, 2014. [DOI] [PubMed] [Google Scholar]
- 329.Zoeller RF, Angelopoulos TJ, Thompson BC, Wenta MR, Price TB, Thompson PD, Moyna NM, Seip RL, Clarkson PM, Gordon PM, Pescatello LS, Devaney JM, Gordish-Dressman H, Hoffman EP, Visich PS. Vascular remodeling in response to 12 wk of upper arm unilateral resistance training. Med Sci Sports Exercise 41: 2003–2008, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Zoladz JA, Majerczak J, Duda K, Chlopicki S. Endurance training increases exercise-induced prostacyclin release in young, healthy men–relationship with VO2max. Pharmacol Rep 62: 494–502, 2010. [DOI] [PubMed] [Google Scholar]