Abstract
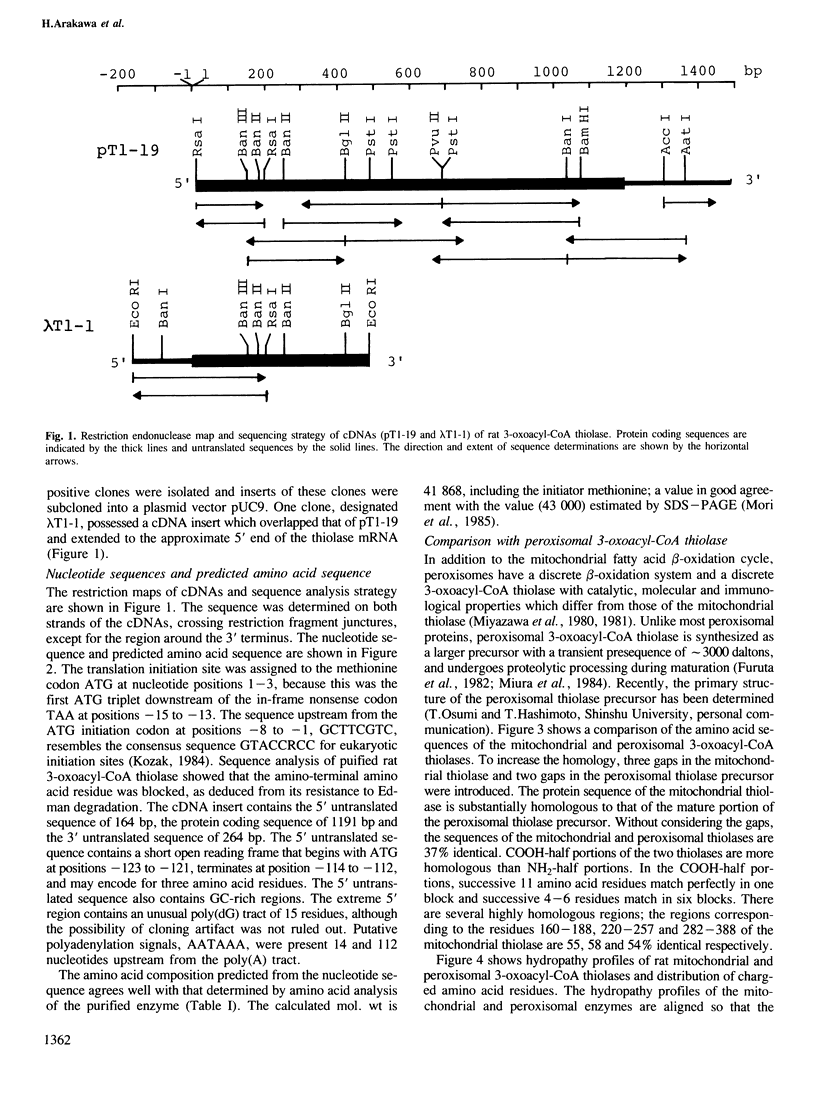
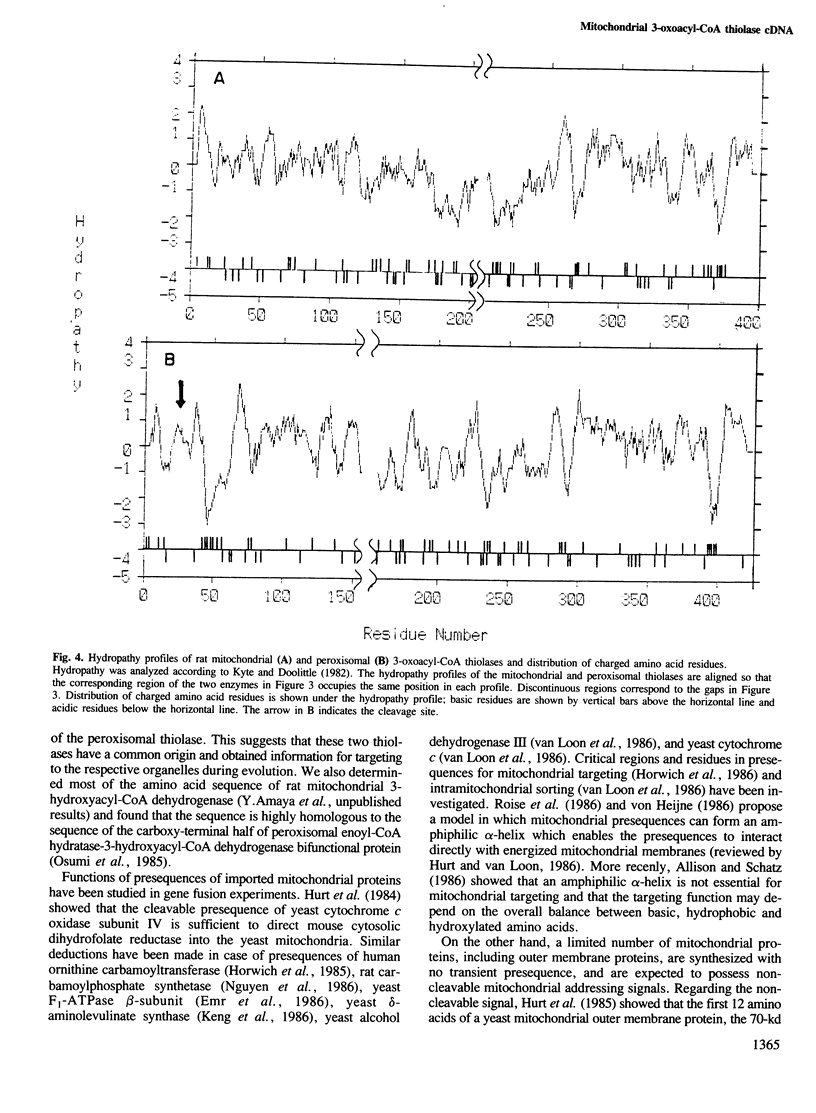
The sorting of homologous proteins between two separate intracellular organelles is a major unsolved problem. 3-Oxoacyl-CoA thiolase is localized in mitochondria and peroxisomes, and provides a good system for the study on the problem. Unlike most mitochondrial matrix proteins, mitochondrial 3-oxoacyl-CoA thiolase in rats is synthesized with no transient presequence and possess information for mitochondrial targeting and import in the mature protein. Two overlapping cDNA clones contained an open reading frame encoding a polypeptide of 397 amino acid residues (predicted Mr = 41,868), a 5' untranslated sequence of 164 bp, a 3' untranslated sequence of 264 bp and a poly(A) tract. The amino acid sequence of the mitochondrial thiolase is 37% identical with that of the mature portion of rat peroxisomal 3-oxoacyl-CoA thiolase precursor. These results suggest that the two thiolases have a common origin and obtained information for targeting to respective organelles during evolution. Two portions in the mitochondrial thiolase that may serve as a mitochondrial targeting signal are presented.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison D. S., Schatz G. Artificial mitochondrial presequences. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9011–9015. doi: 10.1073/pnas.83.23.9011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaya Y., Takiguchi M., Hashimoto T., Mori M. Molecular cloning of cDNA for rat mitochondrial 3-hydroxyacyl-CoA dehydrogenase. Eur J Biochem. 1986 Apr 1;156(1):9–14. doi: 10.1111/j.1432-1033.1986.tb09541.x. [DOI] [PubMed] [Google Scholar]
- Borst P. How proteins get into microbodies (peroxisomes, glyoxysomes, glycosomes). Biochim Biophys Acta. 1986 May 5;866(4):179–203. doi: 10.1016/0167-4781(86)90044-8. [DOI] [PubMed] [Google Scholar]
- Doonan S., Marra E., Passarella S., Saccone C., Quagliariello E. Transport of proteins into mitochondria. Int Rev Cytol. 1984;91:141–186. doi: 10.1016/s0074-7696(08)61316-9. [DOI] [PubMed] [Google Scholar]
- Emr S. D., Vassarotti A., Garrett J., Geller B. L., Takeda M., Douglas M. G. The amino terminus of the yeast F1-ATPase beta-subunit precursor functions as a mitochondrial import signal. J Cell Biol. 1986 Feb;102(2):523–533. doi: 10.1083/jcb.102.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felipo V., Grisolía S. Transport and regulation of polypeptide precursors of mature mitochondrial proteins. Curr Top Cell Regul. 1984;23:217–249. doi: 10.1016/b978-0-12-152823-2.50010-6. [DOI] [PubMed] [Google Scholar]
- Furuta S., Hashimoto T., Miura S., Mori M., Tatibana M. Cell-free synthesis of the enzymes of peroxisomal beta-oxidation. Biochem Biophys Res Commun. 1982 Mar 30;105(2):639–646. doi: 10.1016/0006-291x(82)91482-6. [DOI] [PubMed] [Google Scholar]
- Hay R., Böhni P., Gasser S. How mitochondria import proteins. Biochim Biophys Acta. 1984 Jan 27;779(1):65–87. doi: 10.1016/0304-4157(84)90004-2. [DOI] [PubMed] [Google Scholar]
- Horwich A. L., Kalousek F., Fenton W. A., Pollock R. A., Rosenberg L. E. Targeting of pre-ornithine transcarbamylase to mitochondria: definition of critical regions and residues in the leader peptide. Cell. 1986 Feb 14;44(3):451–459. doi: 10.1016/0092-8674(86)90466-6. [DOI] [PubMed] [Google Scholar]
- Horwich A. L., Kalousek F., Mellman I., Rosenberg L. E. A leader peptide is sufficient to direct mitochondrial import of a chimeric protein. EMBO J. 1985 May;4(5):1129–1135. doi: 10.1002/j.1460-2075.1985.tb03750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurt E. C., Müller U., Schatz G. The first twelve amino acids of a yeast mitochondrial outer membrane protein can direct a nuclear-coded cytochrome oxidase subunit to the mitochondrial inner membrane. EMBO J. 1985 Dec 16;4(13A):3509–3518. doi: 10.1002/j.1460-2075.1985.tb04110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurt E. C., Pesold-Hurt B., Schatz G. The cleavable prepiece of an imported mitochondrial protein is sufficient to direct cytosolic dihydrofolate reductase into the mitochondrial matrix. FEBS Lett. 1984 Dec 10;178(2):306–310. doi: 10.1016/0014-5793(84)80622-5. [DOI] [PubMed] [Google Scholar]
- Keng T., Alani E., Guarente L. The nine amino-terminal residues of delta-aminolevulinate synthase direct beta-galactosidase into the mitochondrial matrix. Mol Cell Biol. 1986 Feb;6(2):355–364. doi: 10.1128/mcb.6.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lazarow P. B., Fujiki Y. Biogenesis of peroxisomes. Annu Rev Cell Biol. 1985;1:489–530. doi: 10.1146/annurev.cb.01.110185.002421. [DOI] [PubMed] [Google Scholar]
- Lazarow P. B. Rat liver peroxisomes catalyze the beta oxidation of fatty acids. J Biol Chem. 1978 Mar 10;253(5):1522–1528. [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton B. The oxoacyl-coenzyme A thiolases of animal tissues. Biochem J. 1973 Apr;132(4):717–730. doi: 10.1042/bj1320717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura S., Mori M., Takiguchi M., Tatibana M., Furuta S., Miyazawa S., Hashimoto T. Biosynthesis and intracellular transport of enzymes of peroxisomal beta-oxidation. J Biol Chem. 1984 May 25;259(10):6397–6402. [PubMed] [Google Scholar]
- Miura S., Takiguchi M., Matsue H., Amaya Y., Tatibana M., Shigesada K., Osumi T., Hashimoto T., Mori M. Molecular cloning of cDNA for rat mitochondrial 3-oxoacyl-CoA thiolase. Eur J Biochem. 1986 Jan 15;154(2):479–484. doi: 10.1111/j.1432-1033.1986.tb09422.x. [DOI] [PubMed] [Google Scholar]
- Miyazawa S., Furuta S., Osumi T., Hashimoto T., Ui N. Properties of peroxisomal 3-ketoacyl-coA thiolase from rat liver. J Biochem. 1981 Aug;90(2):511–519. doi: 10.1093/oxfordjournals.jbchem.a133499. [DOI] [PubMed] [Google Scholar]
- Miyazawa S., Osumi T., Hashimoto T. The presence of a new 3-oxoacyl-CoA thiolase in rat liver peroxisomes. Eur J Biochem. 1980 Feb;103(3):589–596. doi: 10.1111/j.1432-1033.1980.tb05984.x. [DOI] [PubMed] [Google Scholar]
- Mori M., Matsue H., Miura S., Tatibana M., Hashimoto T. Transport of proteins into mitochondrial matrix. Evidence suggesting a common pathway for 3-ketoacyl-CoA thiolase and enzymes having presequences. Eur J Biochem. 1985 May 15;149(1):181–186. doi: 10.1111/j.1432-1033.1985.tb08909.x. [DOI] [PubMed] [Google Scholar]
- Nguyen M., Argan C., Lusty C. J., Shore G. C. Import and processing of hybrid proteins by mammalian mitochondria in vitro. J Biol Chem. 1986 Jan 15;261(2):800–805. [PubMed] [Google Scholar]
- Osumi T., Ishii N., Hijikata M., Kamijo K., Ozasa H., Furuta S., Miyazawa S., Kondo K., Inoue K., Kagamiyama H. Molecular cloning and nucleotide sequence of the cDNA for rat peroxisomal enoyl-CoA: hydratase-3-hydroxyacyl-CoA dehydrogenase bifunctional enzyme. J Biol Chem. 1985 Jul 25;260(15):8905–8910. [PubMed] [Google Scholar]
- Ozasa H., Furuta S., Miyazawa S., Osumi T., Hashimoto T., Mori M., Miura S., Tatibana M. Biosynthesis of enzymes of rat-liver mitochondrial beta-oxidation. Eur J Biochem. 1984 Nov 2;144(3):453–458. doi: 10.1111/j.1432-1033.1984.tb08487.x. [DOI] [PubMed] [Google Scholar]
- Roise D., Horvath S. J., Tomich J. M., Richards J. H., Schatz G. A chemically synthesized pre-sequence of an imported mitochondrial protein can form an amphiphilic helix and perturb natural and artificial phospholipid bilayers. EMBO J. 1986 Jun;5(6):1327–1334. doi: 10.1002/j.1460-2075.1986.tb04363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzbauer J. E., Tamkun J. W., Lemischka I. R., Hynes R. O. Three different fibronectin mRNAs arise by alternative splicing within the coding region. Cell. 1983 Dec;35(2 Pt 1):421–431. doi: 10.1016/0092-8674(83)90175-7. [DOI] [PubMed] [Google Scholar]
- Seubert W., Lamberts I., Kramer R., Ohly B. On the mechanism of malonyl-CoA-independent fatty acid synthesis. I. The mechanism of elongation of long-chain fatty acids by acetyl-CoA. Biochim Biophys Acta. 1968 Dec 18;164(3):498–517. doi: 10.1016/0005-2760(68)90180-x. [DOI] [PubMed] [Google Scholar]
- Staack H., Binstock J. F., Schulz H. Purification and properties of a pig heart thiolase with broad chain length specificity and comparison of thiolases from pig heart and Escherichia coli. J Biol Chem. 1978 Mar 25;253(6):1827–1831. [PubMed] [Google Scholar]
- Takiguchi M., Miura S., Mori M., Tatibana M., Nagata S., Kaziro Y. Molecular cloning and nucleotide sequence of cDNA for rat ornithine carbamoyltransferase precursor. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7412–7416. doi: 10.1073/pnas.81.23.7412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Loon A. P., Brändli A. W., Schatz G. The presequences of two imported mitochondrial proteins contain information for intracellular and intramitochondrial sorting. Cell. 1986 Mar 14;44(5):801–812. doi: 10.1016/0092-8674(86)90846-9. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Mitochondrial targeting sequences may form amphiphilic helices. EMBO J. 1986 Jun;5(6):1335–1342. doi: 10.1002/j.1460-2075.1986.tb04364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]



