We assessed limb position sense during movement in patients with cerebellar damage and found deficits in proprioceptive acuity during both passive and active movement. The effect of cerebellar damage was most apparent when individuals relied on both timing and spatial information during active movement. Thus proprioceptive acuity during active movements may be reliant on the motor system’s ability to predict motor output.
Keywords: proprioception, temporal, spatial, cerebellum, ataxia
Abstract
Proprioception, the sense of limb position and motion, is essential for generating accurate movements. Limb position sense has typically been studied under static conditions (i.e., the fixed position of a limb in space), with less known about dynamic position sense (i.e., limb position during movement). Here we investigated how a person’s estimate of hand position varies when using spatial or temporal information to judge the unseen hand’s location during reaching. We assessed the acuity of dynamic position sense in two directions, orthogonal to hand movement, which only requires spatial information, and in line with hand movement, which has both spatial and temporal components. Our results showed that people have better proprioceptive acuity in the orthogonal condition where only spatial information is used. We then assessed whether cerebellar damage impairs proprioceptive acuity in both tasks during passive and active movement. Cerebellar patients showed reduced acuity in both tasks and in both movement conditions relative to age-matched controls. However, patients’ deficits were most apparent when judgments of active movement relied on temporal information. Furthermore, both cerebellar patient and control performance correlated with the trial-to-trial variability of their active movements: subjects are worse at the proprioceptive tasks when movements are variable. Our results suggest that, during active movements, proprioceptive acuity may be reliant on the motor system’s ability to predict motor output. Therefore, the resultant proprioceptive deficits occurring after cerebellar damage may be related to a more general impairment in movement prediction.
NEW & NOTEWORTHY We assessed limb position sense during movement in patients with cerebellar damage and found deficits in proprioceptive acuity during both passive and active movement. The effect of cerebellar damage was most apparent when individuals relied on both timing and spatial information during active movement. Thus proprioceptive acuity during active movements may be reliant on the motor system’s ability to predict motor output.
proprioception is the perception of body position and movement in the absence of vision. We know that it is vital for making accurate reaching movements because without proprioception large errors in movement occur (Ghez et al. 1995; Gordon et al. 1995). Although it was once considered a single sense, many studies have shown that proprioception can be divided into two distinct senses: limb position and limb motion (Proske and Gandevia 2009, 2012; Sittig et al. 1985). Evidence for the classification of proprioception into two senses includes the capacity to alter the perception of position without changing the perception of motion (Allen and Proske 2006; Brown et al. 2003). In fact, if a limb is moved slowly enough, instead of feeling that their limb is being moved with a certain velocity, subjects will simply report a discrete change in position (McCloskey 1973). In line with this, Kurtzer et al. (2005) showed that distinct populations of cortical neurons fire in response to changes in limb position and motion.
Another distinction often made between the two senses is that limb position is a static sense, whereas limb motion is a dynamic sense. Accordingly, the perception of limb position is most often assessed in static situations, where subjects are asked to estimate the fixed position of a limb in space (see studies reviewed in Proske and Gandevia 2009). However, recent work suggests that the sense of limb position should also be thought of in dynamic situations (Goble and Brown 2009; Yousif et al. 2015). This is because different movements about the shoulder and elbow joints elicit changes in muscle spindle activity that can alter proprioceptive localization ability (Proske and Gandevia 2009; van Beers et al. 1998, 2002; Wilson et al. 2010). One example of a dynamic proprioception task would be to ask a subject to assess where they think their limb was during a movement (e.g., were you to the left or right of a fixed target?). In this type of task, only spatial information about limb position is relevant, despite the fact that the limb is moving. However, in most natural situations, it is necessary to estimate the limb position “on the fly” during a movement. For example, a baseball batter needs to continually assess arm position during the swing to properly align bat and ball. This is clearly a complicated estimate, which necessarily relies on sensing both the position and velocity of the limbs. In other words, this type of dynamic proprioceptive estimate requires both spatial and temporal information.
There are no studies that directly compare proprioceptive ability in tasks with spatial vs. temporal-spatial information. Work in the visual domain suggests that these two information sources are processed via different neural circuits. Tasks with a temporal component seem to rely on cerebellar processing, whereas those with only a spatial component do not (Ivry and Diener 1991; O’Reilly et al. 2008; Roth et al. 2013). Grill et al. (1994) found that individuals with cerebellar damage show proprioceptive deficits when passively discriminating movement velocity, but not when discriminating movement amplitude. However, Bhanpuri et al. (2013) found that discrimination of movement amplitude was impaired by cerebellar damage when movements were actively generated. Importantly, estimating movement velocity requires integrating limb position over time, whereas amplitude discrimination requires assessing differences between the spatial positions of different movement end points. Therefore, while passive proprioceptive judgments relying on temporal information may be cerebellum dependent, this distinction between temporal and spatial information in dynamic proprioception becomes less clear during active movement.
Here we examined the sense of limb position in a dynamic proprioceptive task. First, we sought to quantify proprioceptive acuity using passive movements to control for variable motor output between subjects. We tested participants’ ability to judge the position of a visual cursor relative to their unseen hand while it was moved either along the x- or y-axis of the task space. The cursor was shifted either orthogonal to movement direction (i.e., where only spatial information is relevant) or in line with movement (i.e., when both spatial and temporal information are important). Two movement directions were tested to assess the influence of different movements at the shoulder and elbow joints. Second, we sought to assess dynamic proprioceptive acuity in our task during both active movement and passive movement, as well as assay the effects of cerebellar damage. We found that proprioceptive acuity is better when only spatial information is relevant compared with when both spatial and temporal information must be used, and that this was not differentially affected by movement direction. Additionally, we found that cerebellar damage impairs dynamic proprioceptive acuity during both active and passive movement, and this is most apparent when proprioceptive judgments rely on temporal information about limb movement.
MATERIALS AND METHODS
Subjects
All subjects gave written, informed consent, and protocols were approved by the Johns Hopkins Institutional Review Board. For experiment 1, we recruited a total of 25 right-handed subjects, ranging in age between 18 and 33 yr. These subjects were divided into two groups, each performing different experimental tasks. Young control (YC) group 1 performed four tasks (8 women, 4 men, mean age ± SD: 25.8 ± 3.9 yr). YC group 2 performed two tasks (7 women, 6 men, mean age ± SD: 25.1 ± 4.5 yr). For experiment 2, we recruited 11 patients with cerebellar degeneration and 13 healthy older controls (OC) matched for age and sex (patients: 3 women, 8 men, mean age ± SD: 62.0 ± 10.5 yr; controls: 3 women, 10 men, mean age ± SD: 62.5 ± 9.6 yr). All subjects in experiment 2 had cutaneous mechanoreception assessed with the monofilament test, a standard clinical exam done on the index fingertips to assess peripheral sensory loss (Campbell 2005). All cerebellar patients and OC subjects were within the normal range for cutaneous mechanoreception (≤0.40 g) (Thornbury and Mistretta 1981). Clinical tests of passive proprioception were also normal for all study participants. Cerebellar patient movement impairments were assessed using the International Cooperative Ataxia Rating Scale (Trouillas et al. 1997). Further details about patient characteristics and other participants are presented in Table 1.
Table 1.
Subject characteristics
| ICARS |
|||||||
|---|---|---|---|---|---|---|---|
| Subject | Sex | Age, yr | DH | Hand Fine Touch, g | Diagnosis | Total (/100) | Kinetic (/52) |
| CB1 | M | 61 | R | 0.16 | SCA 6/8 | 66 | 25 |
| CB2 | F | 66 | R | 0.07 | ADCA III | 54 | 18 |
| CB3 | M | 63 | R | 0.40 | ADCA III | 19 | 6 |
| CB4 | M | 80 | R | 0.16 | ADCA III | 45 | 23 |
| CB5 | M | 76 | R | 0.16 | Sporadic | 34 | 8 |
| CB6 | M | 43 | L | 0.04 | SCA 8 | 59 | 22 |
| CB7 | M | 53 | R | 0.40 | SCA 7 | 49 | 15 |
| CB8 | F | 64 | R | 0.16 | SCA 6 | 39 | 19 |
| CB9 | M | 63 | L | 0.16 | SCA 6 | 13 | 4 |
| CB group | F = 2/9 | 63.2 ± 11.0 | L = 2 | 0.19 ± 0.13 | 42.0 ± 17.7 | 15.6 ± 7.8 | |
| OC group | F = 3/9 | 59.8 ± 9.6 | L = 0 | 0.23 ± 0.13 | |||
| YC group 1 | F = 6/10 | 25.9 ± 4.3 | L = 0 | ||||
| YC group 2 | F = 6/10 | 25.4 ± 5.0 | L = 0 | ||||
Group data are means ± SD. CB, cerebellar patient; OC, older control subject; YC, younger control subject; M, male; F, female; DH, dominant hand; R, right; L, left; g, grams; ADCA, autosomal dominant cerebellar ataxia type III; SCA, spinocerebellar ataxia types 6, 7, and 8; Sporadic, sporadic adult-onset cerebellar ataxia; ICARS, International Cooperative Ataxia Rating Scale.
Apparatus and Task Considerations
All experimental tasks were performed using the KINARM exoskeleton robot system (BKIN Technologies, Kingston, Canada; Scott 1999). The subject’s arm was supported by trays, with the index finger resting on a Velcro square. The shoulder and elbow joints of the subject’s arm were aligned with the corresponding joints on the robot. Arm movements were made in the horizontal plane below a screen which blocked vision of the arm. The projection of the visual feedback onto the screen was calibrated to appear in the same plane as the subject’s arm. Furthermore, the visual display’s inherent lag (compared with the movement of the arm/exoskeleton) was compensated for by shifting the visual feedback during movement forward in the direction of movement. Given that all movements were constrained to straight-line trajectories and visual feedback was only shifted while movements were occurring, this resulted in exact alignment of visual feedback and fingertip position.
To assess the necessary range of perceptual shifts, both a large movement length and long movement duration were needed. Consequently, movement velocity needed to be slower than during a typical reaching movement. We specified both movement length and duration during the passive and active tasks, training subjects in the active task to make slow, continuous movements of the correct duration. During active movements at slow speeds, velocity profiles are multipeaked rather than bell-shaped, resulting in extended time spent near peak velocity. Given this, a constant velocity profile was used for the passive movement rather than a bell-shaped velocity profile.
Procedure
Experiment 1.
To assess the differences in proprioceptive acuity due to available proprioceptive information and movement direction, subjects in YC group 1 performed two tests of proprioceptive acuity during passive robot-controlled movement. One test required estimating limb position in the direction of movement (the temporal inline task), and the other required estimating limb position orthogonal to movement direction (the spatial orthogonal task). Subjects performed the temporal inline and spatial orthogonal tasks twice over 2 days to assess different movement directions. In one session participants were moved parallel to the x-axis from left to right (Fig. 1A). In the other session they were moved parallel to the y-axis from the bottom of the task space to the top. Task order and movement direction order were counterbalanced across subjects.
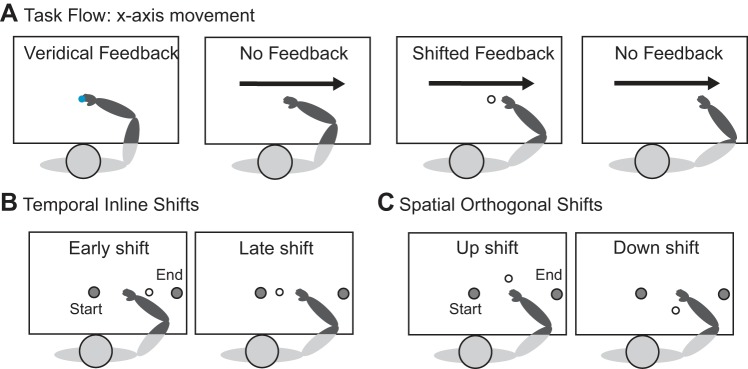
Fig. 1.
Schematic of the task flow and feedback shifts for the x-axis movements. A: passive tasks. Subjects are moved to the start position. A blue dot appears on the screen for 3 s to show the actual index fingertip position. Their arm is then passively moved along the x-axis in the rightward direction. During this movement, subjects are shown shifted feedback of their fingertip position (white dot) for 600 ms. Active tasks: Subjects are first passively moved to the start position. Then they must hold their fingertip in the start position for 3 s, during which a blue dot appears on the screen to show the actual fingertip position. The blue dot then disappears, cueing subjects to actively move their arm along the x-axis (the movement is constrained to the x-axis using a force channel). During their movement, shifted feedback of their fingertip position (white dot) appears for 600 ms. They continue their movement until they hit the unseen end target and are stopped by the robot. In both tasks, judgments about the position of the shifted feedback relative to the fingertip are reported after movement completion. B: schematic of the feedback shifts used in the temporal inline task (shown for the x-axis movements): During the rightward movement, the white visual dot is shifted early or late in time compared with the actual index fingertip trajectory. C: schematic of the feedback shifts used in the spatial orthogonal task (shown for the x-axis movements): During the rightward movement, the white visual dot is shifted either up or down from the actual index fingertip position.
A trial began with the robot passively moving a subject’s right index finger to the start position. A visual cursor representing the position of the index fingertip (0.5 cm radius blue circle) was then shown for 3 s to ensure that the subject’s estimate of arm position did not drift over time. After this the visual cursor was extinguished for the remainder of the trial. The robot then passively moved the subject’s hand at a constant velocity of 9 cm/s for 3 s (preceded by a 500-ms ramp-up and followed by a 500-ms ramp-down). After constant velocity was reached, a visual dot (0.5-cm radius white circle) appeared on the screen for 600 ms, moving at the same velocity as the subject’s fingertip. The precise timing of this appearance during the constant velocity movement was randomized from trial to trial to prevent the subject from anticipating the dot’s presentation. Subjects were told that the dot represented their index fingertip, but that its position had been shifted relative to their actual fingertip position. They were asked to compare the movement of the dot to the perceived movement of their fingertip.
In the temporal inline task the visual dot’s movement was spatially in line with the subject’s fingertip movement, but its position was shifted in time to appear either too early (i.e., ahead of the fingertip) or too late (i.e., behind the fingertip) (Fig. 1B). Nine different temporal shifts were tested in a randomized order (−800, −600, −400, −200, 0, 200, 400, 600, and 800 ms relative to the fingertip). On each trial, subjects were instructed to verbally report whether they felt the dot was shifted “early” or “late” relative to their fingertip. To implement the early time shifts, each time shift was multiplied by the constant velocity to calculate the corresponding position shift. For consistency, the same procedure was subsequently used for the late time shifts.
In the spatial orthogonal task, the visual dot was presented temporally in line with the fingertip movement, but its position was shifted in space orthogonal to the fingertip movement direction. Nine spatial shifts were tested in a randomized order (−30, −20, −13, −7, 0, 7, 13, 20, and 30 mm). Here, subjects were instructed to verbally report whether they felt the dot was shifted “up” or “down” (x-axis movement, Fig. 1C) or “left” or “right” (y-axis movement) relative to their fingertip.
Both the temporal inline and spatial orthogonal tasks used a two-alternative forced choice design with the method of constant stimuli. Subjects completed 12 trials at each shift for a total of 108 trials per task. Each task was split into 6 blocks of 18 trials, and subjects received breaks between each block. Before starting each task, subjects were given a practice block with larger shift values than in the experimental blocks (temporal shifts of ±1,000 ms, spatial shifts of ±40 mm), and completed trials until they understood the task and were correctly completing it.
The temporal inline and spatial orthogonal tasks differed in two important ways: first, in the direction of visual cursor shift, and, second, in the information to which subjects were cued to focus their attention when assessing the cursor position relative to their fingertip. To assess the influence of informational cueing, a second group of subjects (YC group 2) completed both the spatial orthogonal and temporal inline tasks with movement in the x-axis, as described above. However, for this group of subjects, the temporal inline task directions were changed. Here, subjects were told that the dot had been shifted to the right or left, instead of early or late, relative to the index fingertip. This served to cue them to spatial signals, rather than temporal signals, to estimate limb movement. The data from these subjects were compared with the x-axis movement data from YC group 1.
Experiment 2.
The objectives of this experiment were twofold. First, we sought to assess the influence of active movement on performance of the two dynamic proprioception tasks studied in experiment 1. Second, we sought to study the contribution of the cerebellum to the dynamic proprioception tasks by comparing the performance of individuals with cerebellar damage and healthy controls matched for age and sex. In two sessions, on separate days, subjects completed the temporal inline and spatial orthogonal tasks during passive, robot-controlled movement, as well as during active, self-generated movement. Movement condition (passive vs. active) and task order (temporal inline vs. spatial orthogonal) were counterbalanced across subjects. Results from experiment 1 revealed no effect of movement direction (x-axis movement vs. y-axis movement); therefore, subjects in experiment 2 performed movements along the x-axis only.
The passive movement condition for both the temporal inline and spatial orthogonal tasks was completed as indicated for experiment 1 with two additional temporal shifts and spatial shifts in each task, respectively. This resulted in 11 temporal shifts (0, ±200, ±400, ±600, ±800, and ±1,000 ms relative to the fingertip) and 11 spatial shifts (0, ±7, ±13, ±20, ±30, ±40 mm relative to the fingertip position) that were tested in a randomized order within each task. Each task was split into 6 blocks of 22 trials for a total of 132 trials per task. Prior to beginning each task, participants were given a practice block with larger shift values than those in the experimental blocks (temporal shifts of ±1,200 ms, spatial shifts of ±50 mm).
The active movement condition differed from the passive movement condition in that subjects now actively generated the movement of the fingertip along the x-axis. To ensure that all subjects were able to perform the active movement at the desired movement speed, subjects performed 50 trials of the movement only before beginning the perceptual tasks and were given feedback about their movement duration.
A trial in the active movement condition began with the robot passively moving a subject’s right index finger to the start position (shown as a red circle with a 1.5 cm radius). The subject was then required to actively hold his or her finger in the start position for 3 s, during which time a visual cursor representing the position of the index finger was shown (0.5 cm radius blue circle). After 3 s, both the visual cursor and the start position were extinguished, which cued the subject to begin the active movement along the x-axis. During this movement, an unseen force channel was put in place to prevent any movement of the fingertip that was not in the direction of the x-axis. On each trial, subjects were instructed to move slowly and continuously to the right until they hit an unseen wall (i.e., were stopped by the robot). While subjects were actively moving, a shifted visual dot (0.5 cm radius white circle) appeared on the screen and moved at the same velocity as the subject’s fingertip for 600 ms, disappearing before the movement ended.
The active movement condition of the spatial orthogonal task employed the same spatial shifts as in the passive movement condition. The active condition of the temporal inline task employed temporal shifts of 0, ±200, ±400, ±600, and ±800 ms, with temporal shifts of ±1,000 ms used for the practice block. As in the passive condition, each time shift was multiplied by the velocity to calculate the corresponding position shift. To maintain consistency across subjects, regardless of their movement velocity, the constant velocity from the passive task was used.
In all tasks, trials where subjects did not see the visual dot, or moved inappropriately during the active tasks (e.g., stopped during the movement or moved too fast, causing the dot to cut off early), were repeated.
EMG Recordings
Electromyographic (EMG) signals were recorded with the Bagnoli EMG system (Delsys, Boston, MA). Surface electrodes were positioned on five muscles in the right arm: brachioradialis, biceps brachii, triceps brachii, pectoralis major, and posterior deltoid. An amplifier gain of 10,000 was applied, and the data were sampled at 1 kHz. During the passive practice trials, EMG signals were monitored online, and subjects were told to relax their arm more if resisting the robot. EMG signals continued to be monitored online throughout all passive tasks to ensure passive behavior.
Analysis
Subjects who could not complete the experiment (e.g., judgments of visual dot position were not different from chance for all shifts) or who had persistent EMG activity during the passive movement were excluded from analysis. This affected data for three subjects in experiment 1 (1 from YC group 1, and 2 from YC group 2), and two control subjects (from the OC group), and one cerebellar patient in experiment 2. Data from one OC subject in experiment 2 were excluded because tests of passive mechanoreception revealed sensory loss in the upper extremities. Data from one additional cerebellar patient were excluded from analysis due to the presence of extracerebellar signs in the clinical exam.
The spatial orthogonal task used position shifts, whereas the temporal inline task used time shifts. To compare orthogonal and inline tasks, all shift values had to be represented in the spatial domain. To do this, the temporal shifts were multiplied by the movement velocity (9 cm/s). The proportion of trials where a subject reported the visual dot to be “early” (in the temporal inline task with temporal cues), “up” (in the spatial orthogonal task with movement along the x-axis), or “right” (in the temporal inline task with spatial cues and spatial orthogonal task with movement along the y-axis) relative to the finger was determined for each shift value. The data were then fit with a psychometric (logistic) function (Wichmann and Hill 2001). Individual subject fitting was done for each task and movement direction. If a subject had even one poor data fit, their data were discarded from analysis (experiment 1: 1 subject each from YC groups 1 and 2; experiment 2: 1 control subject from the OC group discarded after day 1; for poor fit criteria see Wichmann and Hill 2001). Overall, less than 2.1% of all fits were poor.
From the fitted psychometric function, we calculated two measures to quantify a subject’s sensory predictions: point of subjective equality and just noticeable difference (JND). The point of subjective equality, or bias, is where a subject perceives the visual dot to be aligned with his or her fingertip. In other words, the bias is the point on the psychometric function equal to a 50% probability (e.g., subjects respond “early” for one-half the trials and “late” for the other one-half). To assess bias in both experiments, a one-sample t-test was run on each task’s average group bias to determine whether it was significantly different from zero. In addition, in experiment 2, a mixed-model ANOVA was used to compare bias in cerebellar patients and controls separately in the passive tasks and active tasks, with a between-subjects factor of group (cerebellar patients, controls) and a within-subjects factor of task (spatial orthogonal, temporal inline).
JND, a measure of proprioceptive acuity, quantifies the steepness of the psychometric function. It is calculated as the difference between the 50 and 75% probabilities on the psychometric function. A smaller JND indicates better acuity. To compare JNDs in experiment 1, a repeated-measures ANOVA was used for group 1, with factors of task (spatial orthogonal, temporal inline) and movement direction (x-axis, y-axis). A mixed-model ANOVA was used for group 2, with a between-subjects factor of group (group 1, group 2) and a within-subjects factor of task (spatial orthogonal, temporal or spatial inline). To compare JNDs between cerebellar patients and control participants in experiment 2, mixed-model ANOVAs were used in the passive and active movement conditions, with a between-subjects factor of group (cerebellar patients, OCs) and a within-subjects factor of task (spatial orthogonal, temporal inline). Post hoc analysis of interactions was done using tests of simple effects. In experiment 2, we did not compare subject performance between the passive and active movement conditions. This was because the active condition had dual requirements: subjects had to precisely control movement speed while simultaneously performing the perceptual task. The passive condition only required subjects to perform the perceptual task. As such, we felt it most appropriate to compare cerebellar subjects to their matched controls in each movement condition separately.
To examine the reliability of motor output in the active movement condition, we computed the variability of each participant’s movement speed across trials. Additionally, correlations were performed to examine whether relationships existed between the reliability of motor output and proprioceptive acuity in that task. Correlational analysis was also performed to test whether proprioceptive acuity was related to ataxia symptomology in the cerebellar patient group.
All statistical analysis was completed using Statistica (StatSoft, Tulsa, OK) and SPSS (IBM, Armonk, NY) software, with significance set at 0.05.
RESULTS
Experiment 1
Here we assessed proprioceptive acuity in two tasks requiring dynamic estimates of limb position. In both tasks, a robot passively moved participants’ hands along a path that paralleled either the x- or y-axis of the task space. The temporal inline task required participants to report the position of a visual dot that was shifted in time along the movement path relative to their index finger. The spatial orthogonal task required participants to report the position of a visual dot that was shifted in space, orthogonal to the movement path, relative to their index finger. A range of temporal and spatial shifts were tested, and the reported positions of the visual dot were used to generate psychometric functions. From these functions, we determined each subject’s proprioceptive bias and JND for each movement direction in each task.
Figure 2A shows individual fitted psychometric functions for an example subject. Note that in all four tasks there are multiple points at or near both zero and one, indicating that this subject was consistently able to correctly identify the largest temporal and spatial shifts. The 50% probability point for this subject’s psychometric function is close to zero, which means that they did not have any substantial bias in their perception. However, the psychometric functions are sharper for the spatial orthogonal task compared with the temporal inline task, demonstrating better acuity. The direction of movement did not seem to make a difference (i.e., compare x-axis and y-axis movements for each task).
Fig. 2.
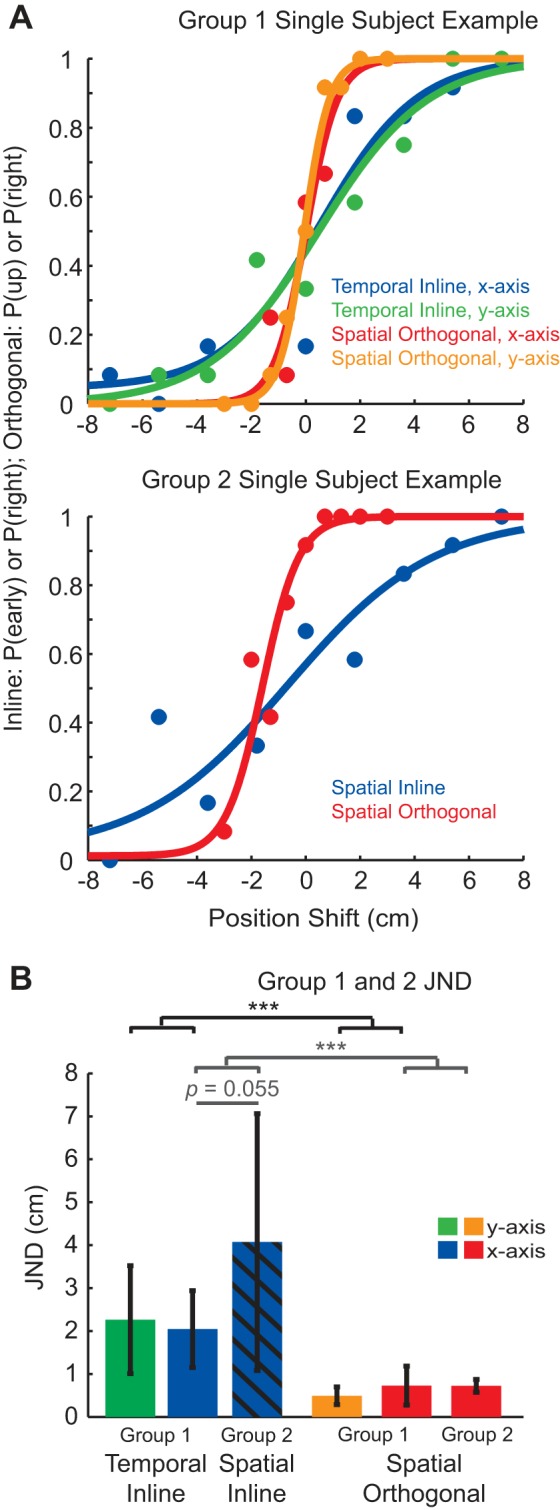
Proprioceptive acuity in young controls during passive movement. A: single-subject examples for the four tasks performed by group 1 (top) and the two tasks performed by group 2 (bottom). The proportion of responses at every shift was calculated separately for individual subjects in each task. A psychometric function was then fit to the data. B: the average JND for groups 1 and 2. In group 1, JND was smaller (better acuity) for the spatial orthogonal task than the temporal inline task, regardless of movement direction (black significance bars). Group 2 performed the temporal inline task with spatial cues. Here, subjects indicated whether the dot was shifted to the right or left (instead of early or late). Cueing subjects to the spatial information in group 2 resulted in larger JNDs, but these were not significantly different than the JNDs found for the temporal inline task in group 1 (gray significance bars). Both groups ran subjects on the same spatial orthogonal task with no difference in JND. Values are means ± SD. ***P < 0.001.
At the group level, subjects had minimal proprioceptive bias when they were moved along the y-axis. One-sample t-tests showed that these biases were not significantly different from zero for either the temporal inline [t(9) = −0.73, P = 0.48] or the spatial orthogonal [t(9) = 0.50, P = 0.63] tasks. However, when subjects were moved along the x-axis, they did show a significant nonzero bias in both tasks. This proprioceptive bias was positive in the temporal inline task [mean ± SD 1.22 ± 0.66 cm; t(9) = 5.83, P < 0.001] and negative in the spatial orthogonal task [−0.73 ± 0.48 cm; t(9) = −4.78, P < 0.001].
We focused our analysis on the JND (75% probability minus 50% probability on the psychometric function), which represents proprioceptive acuity. Repeated-measures ANOVA showed a significant main effect of task [F(1,9) = 36.11, P < 0.001] driven by a smaller JND for the spatial orthogonal task compared with the temporal inline task (Fig. 2B). No main effect of movement direction [F(1,9) = 0.0035, P = 0.95], or interaction between the task and movement direction was found [F(1,9) = 3.23, P = 0.11]. Therefore, proprioceptive acuity was better in the spatial orthogonal task and worse in the temporal inline task, regardless of movement direction.
Given the improvement in proprioceptive acuity in the spatial orthogonal task, we asked whether cueing subjects to spatial signals would change their acuity in the temporal inline task. A second group of subjects was tested on the temporal inline task, but were told that the dot was shifted to the right or left (spatial) of the index finger instead of early or late (temporal). We compared the mean JND from the group who performed the temporal inline task with spatial cues to that from the first group who performed the temporal inline task with temporal cues. (Fig. 2B, compare blue striped bar to solid bar). Mixed-model ANOVA showed a significant main effect of task, where the JND for the spatial orthogonal task was significantly smaller than that for the temporal inline task [F(1,18) = 24.37, P < 0.001]. The interaction between factors group and task was also significant [F(1,18) = 4.62, P = 0.045]. Post hoc means comparisons showed that the interaction was driven by significantly greater JND within group 2 for the temporal inline task with spatial cueing compared with the spatial orthogonal task (P < 0.001). The comparison between the temporal inline task with temporal cueing (group 1) and the temporal inline task with spatial cueing (group 2) was not significant (P = 0.055). Taken together, these results showed that emphasizing spatial cues in the temporal inline task did not differentially affect performance.
Experiment 2
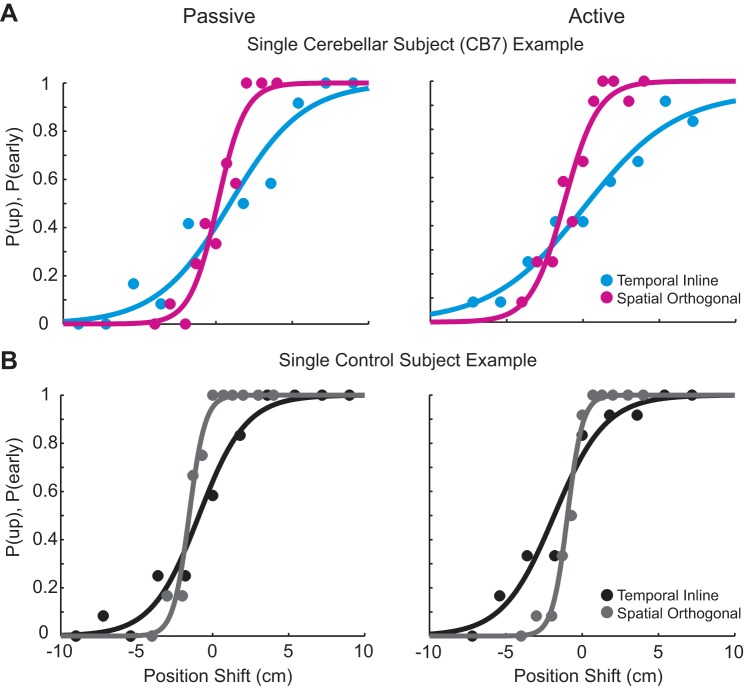
In this experiment, we compared dynamic proprioception during both passive and active movement between patients with cerebellar damage and controls matched for age and sex. Figure 3 shows example data for a cerebellar patient (Fig. 3A) and an OC (Fig. 3B). Both subjects have data points that span the tested shifts, with points near zero and one for each task. In addition, the psychometric functions match well with the data points. This indicates that we were able to capture proprioceptive ability in all tasks for both groups.
Fig. 3.
Example cerebellar patient and older control data during the passive and active movement conditions. Single cerebellar subject (A) and control subject (B) examples for the temporal inline and spatial orthogonal tasks during passive movement (left) and active movement (right) are shown. Both subjects spanned the breadth of behavior, with points near zero and one in each task. Each subject’s data were subsequently fit with a psychometric function.
In the example psychometric functions shown in Fig. 3, the proprioceptive bias varies slightly between tasks, but remains quite small. This is consistent with the group averages for bias, which were all <2 cm (see Table 2). Mixed-model ANOVAs comparing proprioceptive bias across the two groups and tasks showed no significant effects and no interaction for both the passive and active movement conditions separately. These results indicate that, although participants exhibited proprioceptive biases in some experimental conditions, they were not systematic.
Table 2.
Group biases for experiment 2
| Task | CB Group | OC Group |
|---|---|---|
| Passive spatial orthogonal | −0.27 ± 1.13 | −1.19 ± 0.65 |
| Passive temporal inline | −0.63 ± 3.08 | −1.37 ± 2.41 |
| Active spatial orthogonal | −0.91 ± 1.09 | −0.75 ± 0.98 |
| Active temporal inline | 0.73 ± 3.43 | −1.87 ± 2.06 |
Values are means ± SD in centimeters (cm). CB, cerebellar patient; OC, older control subject.
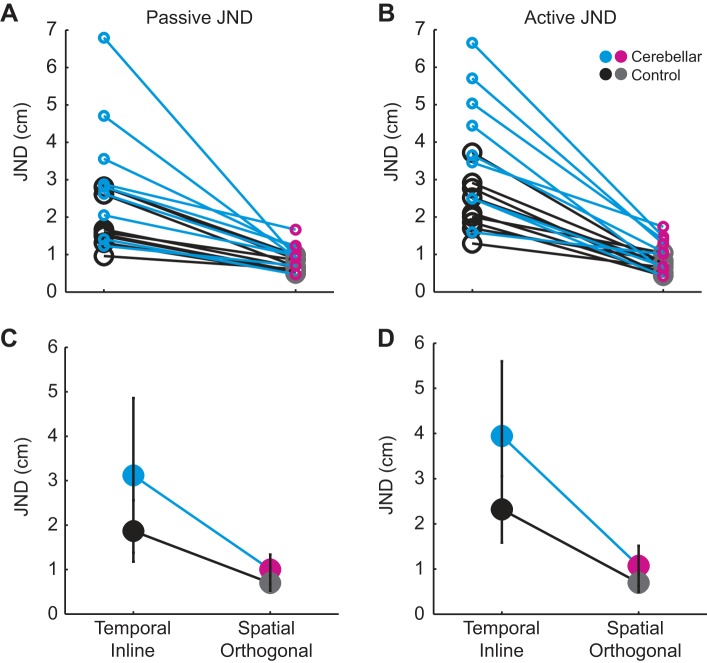
To measure proprioceptive acuity, we computed the JND. Figure 4, A and B, shows individual subject JNDs for each task within the passive and active movement conditions, respectively. All subjects performed worse in the temporal inline task compared with the spatial orthogonal task.
Fig. 4.
Comparison of cerebellar patient and older control experimental results during passive and active movement. All individual subject JNDs for the passive movement condition (A) and active movement condition (B) are shown. For each movement condition, a subject’s JND in the temporal inline and spatial orthogonal tasks is connected by a line (cyan for cerebellar patients and black for older controls). C: group average JND during the passive movement condition. There was a significant main effect of group (P = 0.034), indicating that cerebellar patients had worse acuity than controls. There was also a significant main effect of task (P < 0.001), indicating that for both groups acuity was better in the spatial orthogonal task than the temporal inline task. D: group average JND during the active movement condition. There were significant main effects of both group (P = 0.010) and task (P < 0.001). There was also a significant interaction (P = 0.039), which was driven by worse acuity in the cerebellar patients compared with controls for the temporal inline task. Values are means ± SD.
Figure 4, C and D, shows group data. Note that the general pattern of results looks similar across movement conditions: the cerebellar group showed worse acuity in both the temporal and spatial tasks. Also, both groups showed worse acuity in the temporal inline task compared with the spatial orthogonal task. Statistical analysis of the passive movement condition using mixed-model ANOVA showed significant main effects of group [F(1,16) = 5.379, P = 0.034] and task [F(1,16) = 30.077, P < 0.001], but the interaction was not significant [F(1,16) = 2.495, P = 0.134; Fig. 4C]. In the active movement condition, mixed-model ANOVA for JND also resulted in significant main effects of group [F(1,16) = 8.470, P = 0.010] and task [F(1,16) = 65.579, P < 0.001]. Here however, the interaction between group and task was significant [F(1,16) = 5.058, P = 0.039; Fig. 4D]. Post hoc means comparisons showed that the interaction was driven by JNDs in the temporal inline task, where cerebellar patients had significantly worse acuity than healthy controls (P = 0.016). In other words, in the active movement condition, the cerebellar group’s acuity worsened in the temporal inline task to a greater extent than controls.
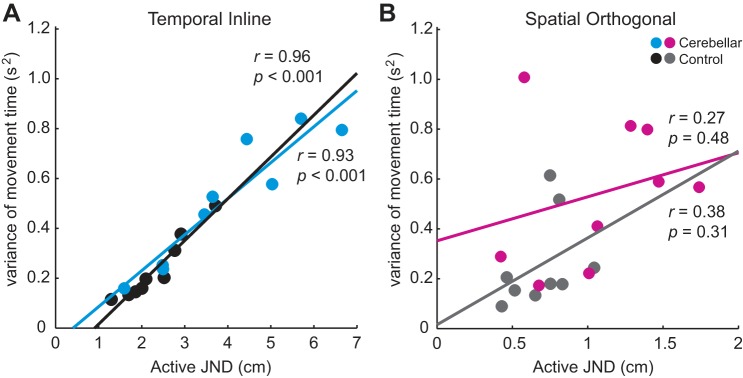
Our finding of reduced proprioceptive acuity in cerebellar patients led us to compare the active movement dynamics of this group with controls. Subjects were supposed to complete their active movements in 3,500–4,500 ms. Both groups had an average movement time falling within that time window, with no difference between groups or tasks in movement time (mean ± SD; spatial orthogonal task: control = 3,987 ± 219 ms, cerebellar = 4,005 ± 237 ms; temporal inline task: control = 4,065 ± 284 ms, cerebellar = 4,000 ± 341 ms). However, when we considered each subject’s trial-to-trial variance of movement time, a pattern emerged. In the temporal inline task, we found strong, positive correlations between JND in the active movement condition and within subject movement time variance for both cerebellar patients and controls (cerebellar: r = 0.93, P < 0.001; control: r = 0.96, P < 0.001; Fig. 5A). In contrast, within-subject movement time variance was not significantly correlated with active JND in the spatial orthogonal task (cerebellar: r = 0.27, P = 0.48; control: r = 0.38, P = 0.31; Fig. 5B).
Fig. 5.
Proprioceptive acuity during active movement correlates with movement time variance during the temporal inline task but not the spatial orthogonal task. Plots show correlations between JND in the active movement condition and within-subject movement time variance separately for cerebellar patients and older control subjects. A: temporal inline task. B: spatial orthogonal task.
There was also a significant correlation between cerebellar patients’ JND in the active movement condition of the spatial orthogonal task and their total International Cooperative Ataxia Rating Scale (ICARS) score (r = 0.70, P = 0.035). However, no other correlations between task performance and ICARS score were found for the patient group (all P > 0.06).
DISCUSSION
In two experiments, we sought to assess dynamic proprioceptive acuity during passive, robot-controlled movement and active self-movement in healthy control participants and individuals with cerebellar damage. In experiment 1, we found that healthy individuals show better proprioceptive acuity when asked to compare the position of a visual cursor to the position of their hand when the cursor was shifted orthogonal to movement direction (i.e., when only spatial information was relevant) vs. in line with movement direction (i.e., when temporal and spatial information were relevant). When the cursor was shifted in line with movement, cueing participants to spatial information (rather than temporal information) did not improve performance. This suggested that differences in informational cueing were not the primary reason for differences in proprioceptive acuity between tasks. Additionally, acuity did not change with different movement directions (i.e., along the x- vs. y-axes of the task space). In experiment 2, we found that patients with cerebellar damage had reduced dynamic proprioceptive acuity compared with age-matched, healthy controls in both passive and active movement conditions. Within each movement condition, both groups showed worse acuity in the temporal inline task, compared with the spatial orthogonal task. Notably, the cerebellar group’s acuity worsened with the temporal inline task to a greater extent than did controls in the active movement condition (indicated by the significant interaction between group and task), whereas this was not the case in the passive movement condition. This suggests that the effect of cerebellar damage on dynamic proprioceptive acuity was most apparent when hand position estimates relied on a combination of temporal and spatial information during active movement.
Overall, our results align with the hypothesis that the cerebellum is important for sensory perception during movement. Previous work has found that cerebellar damage impairs proprioception in tasks that require the estimation of movement (Bhanpuri et al. 2013; Grill et al. 1994), but does not impair proprioception in some baseline tasks that require estimating end-point limb position (i.e., localization; Izawa et al. 2012). This is in line with the cerebellum having an integral role in state estimation during movement. Specifically, the cerebellum is thought to generate predictions of limb states that will result from motor commands (Miall et al. 2007; Shadmehr and Krakauer 2008). These predictions are necessary during movement to compensate for delays in the relaying and processing of afferent information (Wolpert at al. 1998). Importantly, state estimation involves the integration of these predictions with incoming sensory signals (Körding and Wolpert 2006). Thus hand position sense in our study may have been based on a combination of sensory and predictive signals. Motor commands in active movement would make predictive input even more important, and this may underlie why cerebellar patients seemed to show greater differences from controls in this condition of our task.
We found that both cerebellar patients and controls showed reduced proprioceptive acuity when limb position estimates relied on the processing of temporal information compared with when they required spatial information alone. One explanation for these results may be that, in the temporal inline task, the cursor appeared along the path of the finger, whereas, in the spatial orthogonal task, it did not. This means that, in the temporal inline task, subjects had to make continual instantaneous estimates of their moving fingertip position to compare it to the position of the cursor. Due to time delays in the processing of proprioceptive feedback, sensory information would be less reliable when generating these inline state estimates. Thus state estimates in the temporal inline task would rely more heavily on predictive signals, and this could have made subjects more uncertain about their limb position. In contrast, estimates of fingertip position in the spatial orthogonal task were not continuously changing along the axis in which the comparison was made, because participants were not moving in the direction of the cursor shift. This may have made it easier for participants to discriminate whether the displayed cursor was shifted in the spatial orthogonal task.
In support of a role for predictive signals in participants’ hand position sense, we found that proprioceptive acuity for both cerebellar patients and healthy controls was related to the reliability of their movement. We found that proprioceptive acuity in the active movement condition of the temporal inline task correlated with movement time variance in both groups, such that individuals with higher variance from one movement to the next had worse proprioceptive acuity overall. We also found that some proprioceptive impairments in cerebellar patients were related to their deficits in motor control. Patients’ proprioceptive acuity in the active movement condition of the spatial orthogonal task correlated with their total score on the ICARS, such that patients with worse acuity tended to have higher scores (higher scores denote more severely impaired movement). The motor deficits that result from cerebellar damage are thought to reflect impaired formation of predictive internal models of limb states (Bastian 2006). Previous work has shown that the specific proprioceptive deficits seen during single-joint, active movements by cerebellar patients can be induced in healthy controls by unpredictably perturbing their movement (Bhanpuri et al. 2013). This suggests that, during movement, proprioceptive acuity may critically depend on the ability of the motor system to predict motor output, and the proprioceptive deficits seen following cerebellar damage could be related to a more general impairment in movement prediction.
In experiment 1, proprioceptive acuity was not differentially affected by the direction of movement. This was surprising. Previous work studying proprioceptive localization in the upper extremity has found differences in acuity with different movement directions (van Beers et al. 1998, 2002; Wilson et al. 2010), which potentially resulted from differences in muscle spindle activity for different changes in shoulder and elbow joint angles (Goodwin et al. 1972; Proske and Gandevia 2009). In our task, although the shoulder moved through roughly the same angular change for both movement directions, the elbow moved through an angular change that was approximately six times greater in the y-axis movement compared with the x-axis movement. So why were our subjects unable to take advantage of the increased afferent information during the y-axis movement? A possible reason for the discrepancy between previous results and ours might lie in the time at which proprioceptive estimates were made. Previous work showing changes in proprioceptive acuity with different movement directions involved proprioceptive estimates that were made after movement completion. In our task, estimates were generated during movement execution. It is possible that the brain is unable to make use of extra afferent information from larger changes in joint angle until after a movement is complete. Therefore, the increased change in elbow angle between the two movement directions tested in experiment 1 could not influence proprioceptive acuity online. It would be interesting for future work to directly compare differences in proprioceptive acuity during movement, and after its completion to further our understanding of sensory processing in these two limb states.
In both experiments, we found that participants tended to show baseline biases in estimates of fingertip position. In general, these biases were positive in the temporal inline task and negative in the spatial orthogonal task. A positive bias in the temporal inline task meant that subjects thought their hand was further rightward than it actually was. A negative bias in the spatial orthogonal task indicated that subjects thought their hand was closer to the body than it actually was. These group level biases align with the proprioceptive biases reported in other studies (Crowe et al. 1987; Wilson et al. 2010). Importantly, biases were very small and showed no systematic differences between groups.
In summary, we have found that there is a clear difference in proprioceptive acuity when assessing hand position orthogonal to (spatial) vs. in line with (temporal and spatial) the direction of hand movement. Furthermore, individuals with cerebellar damage showed deficits in proprioceptive acuity compared with controls, which were most apparent when hand position was assessed during active movement in line with the direction of movement. We interpret these results as evidence that the inline task requires the brain to use temporal and spatial information to make predictions about the hand while it is in flight, and that the cerebellum might play an essential role in this ability. Cerebellar circuits have long been known to be a critical part of a predictive mechanism for motor control. Our results add to this notion by showing that the cerebellar processing may also be part of a predictive mechanism for proprioceptive sense.
GRANTS
This research was supported by National Institutes of Health Grants R01 HD-040289 to A. J. Bastian and F31 NS-086399 to H. M. Weeks.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
H.M.W. and A.J.B. conceived and designed research; H.M.W. performed experiments; H.M.W., A.S.T., and A.J.B. analyzed data; H.M.W., A.S.T., and A.J.B. interpreted results of experiments; H.M.W. prepared figures; H.M.W. and A.S.T. drafted manuscript; H.M.W., A.S.T., and A.J.B. edited and revised manuscript; H.M.W., A.S.T., and A.J.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the members of the Center for Movement Studies at the Kennedy Krieger Institute for helping to coordinate experiments, and the patients for volunteering time to participate in these studies.
REFERENCES
- Allen TJ, Proske U. Effect of muscle fatigue on the sense of limb position and movement. Exp Brain Res 170: 30–38, 2006. doi: 10.1007/s00221-005-0174-z. [DOI] [PubMed] [Google Scholar]
- Bastian AJ. Learning to predict the future: the cerebellum adapts feedforward movement control. Curr Opin Neurobiol 16: 645–649, 2006. doi: 10.1016/j.conb.2006.08.016. [DOI] [PubMed] [Google Scholar]
- Bhanpuri NH, Okamura AM, Bastian AJ. Predictive modeling by the cerebellum improves proprioception. J Neurosci 33: 14301–14306, 2013. doi: 10.1523/JNEUROSCI.0784-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LE, Rosenbaum DA, Sainburg RL. Limb position drift: implications for control of posture and movement. J Neurophysiol 90: 3105–3118, 2003. doi: 10.1152/jn.00013.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell W. DeJong’s The Neurologic Examination. Baltimore, MD: Lippincott Williams and Wilkins, 2005. [Google Scholar]
- Crowe A, Keessen W, Kuus W, van Vliet R, Zegeling A. Proprioceptive accuracy in two dimensions. Percept Mot Skills 64: 831–846, 1987. doi: 10.2466/pms.1987.64.3.831. [DOI] [PubMed] [Google Scholar]
- Ghez C, Gordon J, Ghilardi MF. Impairments of reaching movements in patients without proprioception. II. Effects of visual information on accuracy. J Neurophysiol 73: 361–372, 1995. [DOI] [PubMed] [Google Scholar]
- Goble DJ, Brown SH. Dynamic proprioceptive target matching behavior in the upper limb: effects of speed, task difficulty and arm/hemisphere asymmetries. Behav Brain Res 200: 7–14, 2009. doi: 10.1016/j.bbr.2008.11.034. [DOI] [PubMed] [Google Scholar]
- Goodwin GM, McCloskey DI, Matthews PB. The persistence of appreciable kinesthesia after paralysing joint afferents but preserving muscle afferents. Brain Res 37: 326–329, 1972. doi: 10.1016/0006-8993(72)90679-8. [DOI] [PubMed] [Google Scholar]
- Gordon J, Ghilardi MF, Ghez C. Impairments of reaching movements in patients without proprioception. I. Spatial errors. J Neurophysiol 73: 347–360, 1995. [DOI] [PubMed] [Google Scholar]
- Grill SE, Hallett M, Marcus C, McShane L. Disturbances of kinaesthesia in patients with cerebellar disorders. Brain 117: 1433–1447, 1994. doi: 10.1093/brain/117.6.1433. [DOI] [PubMed] [Google Scholar]
- Ivry RB, Diener HC. Impaired velocity perception in patients with lesions of the cerebellum. J Cogn Neurosci 3: 355–366, 1991. doi: 10.1162/jocn.1991.3.4.355. [DOI] [PubMed] [Google Scholar]
- Izawa J, Criscimagna-Hemminger SE, Shadmehr R. Cerebellar contributions to reach adaptation and learning sensory consequences of action. J Neurosci 32: 4230–4239, 2012. doi: 10.1523/JNEUROSCI.6353-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Körding KP, Wolpert DM. Bayesian decision theory in sensorimotor control. Trends Cogn Sci 10: 319–326, 2006. doi: 10.1016/j.tics.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Kurtzer I, Herter TM, Scott SH. Random change in cortical load representation suggests distinct control of posture and movement. Nat Neurosci 8: 498–504, 2005. doi: 10.1038/nn1420. [DOI] [PubMed] [Google Scholar]
- McCloskey DI. Differences between the senses of movement and position shown by the effects of loading and vibration of muscles in man. Brain Res 61: 119–131, 1973. doi: 10.1016/0006-8993(73)90521-0. [DOI] [PubMed] [Google Scholar]
- Miall RC, Christensen LOD, Cain O, Stanley J. Disruption of state estimation in the human lateral cerebellum. PLoS Biol 5: e316, 2007. doi: 10.1371/journal.pbio.0050316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly JX, Mesulam MM, Nobre AC. The cerebellum predicts the timing of perceptual events. J Neurosci 28: 2252–2260, 2008. doi: 10.1523/JNEUROSCI.2742-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proske U, Gandevia SC. The kinaesthetic senses. J Physiol 587: 4139–4146, 2009. doi: 10.1113/jphysiol.2009.175372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proske U, Gandevia SC. The proprioceptive senses: their roles in signaling body shape, body position and movement, and muscle force. Physiol Rev 92: 1651–1697, 2012. doi: 10.1152/physrev.00048.2011. [DOI] [PubMed] [Google Scholar]
- Roth MJ, Synofzik M, Lindner A. The cerebellum optimizes perceptual predictions about external sensory events. Curr Biol 23: 930–935, 2013. doi: 10.1016/j.cub.2013.04.027. [DOI] [PubMed] [Google Scholar]
- Scott SH. Apparatus for measuring and perturbing shoulder and elbow joint positions and torques during reaching. J Neurosci Methods 89: 119–127, 1999. doi: 10.1016/S0165-0270(99)00053-9. [DOI] [PubMed] [Google Scholar]
- Shadmehr R, Krakauer JW. A computational neuroanatomy for motor control. Exp Brain Res 185: 359–381, 2008. doi: 10.1007/s00221-008-1280-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sittig AC, Denier van der Gon JJ, Gielen CC. Separate control of arm position and velocity demonstrated by vibration of muscle tendon in man. Exp Brain Res 60: 445–453, 1985. doi: 10.1007/BF00236930. [DOI] [PubMed] [Google Scholar]
- Thornbury JM, Mistretta CM. Tactile sensitivity as a function of age. J Gerontol 36: 34–39, 1981. doi: 10.1093/geronj/36.1.34. [DOI] [PubMed] [Google Scholar]
- Trouillas P, Takayanagi T, Hallett M, Currier RD, Subramony SH, Wessel K, Bryer A, Diener HC, Massaquoi S, Gomez CM, Coutinho P, Ben Hamida M, Campanella G, Filla A, Schut L, Timann D, Honnorat J, Nighoghossian N, Manyam B; The Ataxia Neuropharmacology Committee of the World Federation of Neurology . International Cooperative Ataxia Rating Scale for pharmacological assessment of the cerebellar syndrome. J Neurol Sci 145: 205–211, 1997. doi: 10.1016/S0022-510X(96)00231-6. [DOI] [PubMed] [Google Scholar]
- van Beers RJ, Sittig AC, Denier van der Gon JJ. The precision of proprioceptive position sense. Exp Brain Res 122: 367–377, 1998. doi: 10.1007/s002210050525. [DOI] [PubMed] [Google Scholar]
- van Beers RJ, Wolpert DM, Haggard P. When feeling is more important than seeing in sensorimotor adaptation. Curr Biol 12: 834–837, 2002. doi: 10.1016/S0960-9822(02)00836-9. [DOI] [PubMed] [Google Scholar]
- Wichmann FA, Hill NJ. The psychometric function: I. Fitting, sampling, and goodness of fit. Percept Psychophys 63: 1293–1313, 2001. doi: 10.3758/BF03194544. [DOI] [PubMed] [Google Scholar]
- Wilson ET, Wong J, Gribble PL. Mapping proprioception across a 2D horizontal workspace. PLoS One 5: e11851, 2010. [Erratum. PLoS One 5: 9, 2010.] doi: 10.1371/journal.pone.0011851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpert DM, Miall RC, Kawato M. Internal models in the cerebellum. Trends Cogn Sci 2: 338–347, 1998. doi: 10.1016/S1364-6613(98)01221-2. [DOI] [PubMed] [Google Scholar]
- Yousif N, Cole J, Rothwell J, Diedrichsen J. Proprioception in motor learning: lessons from a deafferented subject. Exp Brain Res 233: 2449–2459, 2015. doi: 10.1007/s00221-015-4315-8. [DOI] [PubMed] [Google Scholar]