In a study of feline locomotor behavior we found that the preferred strategy to avoid a small obstacle is circumvention. During circumvention, stride direction changes but length and temporal structure are preserved. Another strategy, stepping over the obstacle, is used in narrow walkways. During overstepping, two strides adjust. A stride preceding the obstacle decreases in length and duration. The following stride negotiating the obstacle increases in height while retaining normal temporal structure and nearly normal length.
Keywords: locomotion, motor control, obstacle avoidance, spatial navigation
Abstract
Avoiding obstacles is essential for successful navigation through complex environments. This study aimed to clarify what strategies are used by a typical quadruped, the cat, to avoid obstacles during walking. Four cats walked along a corridor 2.5 m long and 25 or 15 cm wide. Obstacles, small round objects 2.5 cm in diameter and 1 cm in height, were placed on the floor in various locations. Movements of the paw were recorded with a motion capture and analysis system (Visualeyez, PTI). During walking in the wide corridor, cats’ preferred strategy for avoiding a single obstacle was circumvention, during which the stride direction changed while stride duration and swing-to-stride duration ratio were preserved. Another strategy, stepping over the obstacle, was used during walking in the narrow corridor, when lateral deviations of walking trajectory were restricted. Stepping over the obstacle involved changes in two consecutive strides. The stride preceding the obstacle was shortened, and swing-to-stride ratio was reduced. The obstacle was negotiated in the next stride of increased height and normal duration and swing-to-stride ratio. During walking on a surface with multiple obstacles, both strategies were used. To avoid contact with the obstacle, cats placed the paw away from the object at a distance roughly equal to the diameter of the paw. During obstacle avoidance cats prefer to alter muscle activities without altering the locomotor rhythm. We hypothesize that a choice of the strategy for obstacle avoidance is determined by minimizing the complexity of neuro-motor processes required to achieve the behavioral goal.
NEW & NOTEWORTHY In a study of feline locomotor behavior we found that the preferred strategy to avoid a small obstacle is circumvention. During circumvention, stride direction changes but length and temporal structure are preserved. Another strategy, stepping over the obstacle, is used in narrow walkways. During overstepping, two strides adjust. A stride preceding the obstacle decreases in length and duration. The following stride negotiating the obstacle increases in height while retaining normal temporal structure and nearly normal length.
avoiding obstacles during locomotion is a normal motor behavior of animals. It involves visual detection of an object obstructing walking in the desired direction, selection of a strategy to avoid contact with the object, and appropriate adaptation of locomotor movements. Cognitive and kinematic aspects of obstacle avoidance have been studied in great detail primarily in humans, which is quite understandable given the ultimate interest in human motor behavior and the relative simplicity of conducting such experiments in human subjects.
In numerous studies a large variety of configurations of obstacles, their locations, and timings of their presentation during walking on walkways and treadmill belts have been used to analyze how humans avoid obstacles (Bauby and Kuo 2000; Berard and Vallis 2006; Chen et al. 1991, 1994; Chou et al. 2001; Mohagheghi et al. 2004; Moraes et al. 2004, 2007; Owings and Grabiner 2004; Patla et al. 1989, 1999; Patla and Rietdyk 1993; Santos et al. 2010; Warren et al. 1986; Weerdesteyn et al. 2005; Zijlstra et al. 1995). These studies showed that humans adapt their strategies for avoiding obstacles according to the context of the experimental task. When subjects walked along a walkway with an arbitrarily placed object, they adjusted the lengths of a few strides to place a foot in a convenient location in front of the object (Chen et al. 1991, 1994; Patla et al. 1991; Patla and Greig 2006). The length of the following stride, which negotiated the obstacle, did not change in any definite fashion (Berard and Vallis 2006; Chou et al. 2001; Schulz 2012). When the obstacle was specifically positioned close to the area of normal foot placement during unobstructed walking and time to react to the obstacle was ample, subjects consistently increased the length of the stride that negotiated the obstacle (Chen et al. 1991, 1994; Moraes et al. 2004, 2007; Patla et al. 1989, 1999; Weerdesteyn et al. 2005). When the size of the object was comparable to the size of the foot and occupied only a small fraction of the walking pathway, subjects could alter the direction of the walking trajectory. Depending on features of the experimental space, the subjects could place the targeted foot either medial or lateral to the object while continuing progressive walking (Moraes et al. 2007; Patla et al. 1999). When avoiding the obstacle, human subjects attempted to minimize the displacement of the foot from its landing spot during unobstructed walking (Moraes et al. 2004, 2007).
To understand motor behavior for obstacle avoidance during locomotion of terrestrial animals, studies in both biped and quadruped species are essential. Extrapolating knowledge of strategies for obstacle avoidance gained from studies of bipedal humans to quadrupedal species is productive, but not entirely accurate. Biomechanics and neural control of locomotor movements in bipedal and quadrupedal species are fundamentally similar but not identical (Alexander 1989; Biewener 2006; D’Août et al. 2004; Vilensky 1987). Even among bipedal species, locomotor mechanics are not the same; for example, patterns of the force exerted on the ground during walking differs among humans, apes, and birds (Alexander 2004). Until now, obstacle avoidance in quadrupeds has been studied only fragmentarily, focusing mostly on the kinematics of limbs while stepping over obstacles (Aoki et al. 2012; Drew 1993; Krouchev and Drew 2013; Lavoie et al. 1995; Lajoie and Drew 2007; McFadyen et al. 1999; Sato et al. 2012). The aim of this study was to elucidate the strategies for obstacle avoidance used by quadrupeds during walking, the spatial and temporal characteristics of strides associated with these strategies, and what determines a choice between available strategies.
As subjects of the study we selected domestic cats, whose locomotor kinematics and neural control of walking have been studied in great detail. In cats, as in other quadrupeds, the forelimbs not only lead the way but also bear a greater portion of the weight of the body compared with the hindlimbs and considerably contribute to propulsive movement of the body during walking (Alexander 1980; Manter 1938; Pandy et al. 1988; Prilutsky et al. 2005). Thus we focused on the movements of the forelimbs in cats avoiding obstacles during walking. We analyzed three spatial characteristics of strides: length, direction, and height. For temporal characteristics, we analyzed the duration of the step cycle and the swing-to-stride duration ratio.
We found that to avoid a single small obstacle cats preferred to walk around it rather than step over it. The temporal structure of the strides did not change during circumvention of the obstacle. If environmental constraints required cats to step over the obstacle, they shortened the stride preceding the obstacle by reducing the duration of the swing phase. Cats negotiated the obstacle with a following stride of increased elevation of the paw but normal temporal structure. During walking along a pathway cluttered with multiple obstacles, cats avoided obstacles by implementing a complex adaptation of both spatial and temporal parameters of strides.
Preliminary results of this study have been published in abstract form (Marlinski et al. 2014).
METHODS
Experiments were conducted with four adult cats: one female, SF (2.9 kg), and three males, FM (3.7 kg), MI (3.5 kg), and TI (3.8 kg). The experimental protocol was in compliance with the National Institutes of Health guidelines for the care and use of animals in research and was approved by the Barrow Neurological Institute Animal Care and Use Committee.
Locomotor tasks.
The cats were habituated to the experimental environment and engaged in locomotor behavior with food reinforcement. The cats were trained to walk in a chamber that consisted of three connected corridors, each 250 cm long, 25 cm wide, and 40 cm high (Fig. 1B). An external wall in one of the corridors was made of a transparent plastic material, which permitted visual monitoring and recording of the walking cat. We refer to this part of the chamber as the test corridor. For some experiments, the width of the test corridor was reduced to 15 cm. The floor of the chamber was covered with a flat black rubberized material. All experiments were conducted under normal laboratory illumination.
Fig. 1.
Experimental design. A: photograph of a cat walking along the corridor cluttered with multiple small obstacles. B: diagram of the walking chamber and location of the motion capture system. C: reconstruction of the placement of each of the 4 paws—right forepaw (black circles), left forepaw (white circles), right hindpaw (black squares), and left hindpaw (white squares)—during unobstructed walking in the wide corridor. Locations of the symbols are averages of 6 placements.
We studied the locomotor behavior of cats during both unobstructed and obstructed walking. The obstructions to walking were created by placing small obstacles on the floor of the test corridor. The obstacle we used was a white round wooden object 2.5 cm in diameter and 1 cm in height (Fig. 1A). The size of the object was close to the size of the cat’s paw, which is ~3 cm in diameter. Objects were mounted on selected sites on the floor with double-sided adhesive tape. The white color of the object and the black color of the walkway’s surface contrasted sharply, allowing cats to easily identify the obstacle. The object was not harmful to the cat in any manner. Three different obstacle arrangements were used: 1) a single obstacle in the wide (25 cm) corridor, 2) a single obstacle in the narrow (15 cm) corridor, and 3) multiple obstacles in the wide corridor. During walking in the chamber, cats avoided touching walls. In the wide corridor, which was significantly wider than the width of the cat’s body, cats could change walking trajectory without touching walls (Fig. 1A). In the narrow corridor, which was only slightly wider than the cat’s body, cats could not deviate from straightforward walking without touching the walls.
To study obstacle avoidance during walking along both wide and narrow walkways obstructed with a single obstacle (experiments 1 and 2, respectively), we placed the object on a site of one of the right forepaw placements observed during unobstructed walking. We chose 2nd, 3rd, or 4th placements, which were in the middle of the test corridor and located at different distances from the entrance of the corridor. At the beginning of a recording session, a cat walked 12–24 times along the unobstructed wide or narrow walkway and typical longitudinal and transverse coordinates of right forepaw placements were noted. The object was then placed on the site of one of these placements, and the cat continued walking along the obstructed walkway for 20–24 more passages.
Experiment 1—single obstacle in the wide corridor—consisted of two parts. In the first part, the obstacle was positioned with respect to the walking cat: on a site of one of the right forepaw placements typical for unobstructed walking. In the second part, a position for the obstacle was chosen taking into account both the walking cat and the environment. The obstacle was placed on the midline of the walkway with the longitudinal coordinate of one of the right forepaw placements typically observed during unobstructed walking.
In experiment 2—single obstacle in the narrow corridor—the object was initially placed on a site of a right forepaw placement typical for unobstructed walking and then displaced sequentially in increments of 2.5 cm toward the entrance of the corridor. The purpose of displacing the object was to determine how cats modify their walking when the distance between the paw and the obstacle is shorter than the normal length of the stride.
In experiment 3—multiple obstacles in the wide corridor—we studied obstacle avoidance during walking along the walkway obstructed with 59 objects placed haphazardly on the floor of the corridor. The objects occupied 4% of the surface of the walkway, leaving the great part of the walkway available for safe stepping but challenging the cat to make accurate foot placements. To position the objects on the walkway in different layouts, we used templates made of cardboards perforated in different fashions. Figure 1A shows one such layout. To prevent cats from memorizing object locations, a new layout was presented to the cat in each experimental session. During recording sessions, the order of the walking tasks was randomized. During each recording session, cats walked 20–40 times along the obstructed walkway and 12–24 times along the walkway without obstacles.
Recording procedure.
A computerized, active-marker three-dimensional real-time motion capture and analysis system, Visualeyez (VZ-4000, PTI), was used to record the cat’s movements while the cat passed through the test corridor. The cameras of the Visualeyez were placed 2.7 m away from the transparent external wall of the corridor (Fig. 1B). To monitor limb movement, light-emitting diodes (LEDs) were attached with an adhesive tape to the cat’s skin covering the metacarpal joint of both forelimbs and the metatarsal joint of both hindlimbs. Light, flexible 32-gauge wires (Cooner Wire) connected the LEDs to a tracker control module of the Visualeyez (dimensions 6 × 4 × 1.5 cm, weight 31.4 g), which was attached to the cat’s body with an adhesive tape. The module was connected to an acquisition system via a flexible cable coupled with a slip-ring assembly. Cats were habituated to wear the module, LEDs, and wires. After training, they did not show any signs of discomfort when wearing them and appeared to walk normally. After completion of each recording session, a separate LED was placed on sites where the obstacle was mounted during the session. Five additional LEDs were mounted on the external side of the test corridor at the level of the chamber’s floor and served as reference points during data analysis. Signals from all LEDs were sampled at a frequency of 200 Hz.
Data processing.
Three-dimensional coordinates of LEDs were exported for further processing in MATLAB (MathWorks, Lowell, MA) and Excel (Microsoft, Redmond, WA). The Cartesian coordinates of paw placements on the floor were determined at the time intervals when the vertical coordinate of the LED was at a local minimum. Figure 1C shows a representative reconstruction of the placements of all four paws during unobstructed walking in the wide corridor. In this study, analysis was focused on the stepping pattern and placements of the right forepaw.
For quantitative analysis, we selected passages through the test corridor that met two criteria. First, we included only passages in which the cat began walking in the test corridor with the same forelimb during unobstructed and obstructed walking. Second, we accepted only passages in which locations of the first placement of the right forepaw were uniform, restricted to an area of ~6 cm in diameter, which corresponds approximately to 2 diameters of a cat’s paw. As a result, for each locomotor task completed by a cat during each recording session we obtained a sample of 6–32 passages. The number of discarded passages varied between 2 and 10 for each session.
We took the center of the paw as the location of the forepaw placement. To estimate coordinates of the center of the paw, we approximated the area of the paw as a circle. The LED mounted on the skin covering the metacarpal joint was located between the most lateral and the most posterior points of the circle. We assumed that angular distances between the LED and these points were 45°. Thus the coordinates of paw placement were estimated as
where A and B are longitudinal and transverse coordinates, respectively. The subscript i indicates the center of the paw, and the subscript L indicates the LED. RP is a radius of the forepaw (1.7 cm in cats FM and TI, 1.6 cm in cat MI, and 1.5 cm in cat SF).
To characterize spatial parameters of strides we estimated their length, direction, and height. The stride length (L) was calculated as
where A and B are longitudinal and transverse coordinates of two consecutive (i and i+1) placements of the right forepaw. The direction of the stride was defined as an angular deviation away from the longitudinal axis of the walkway. The stride direction in degrees (φ) was calculated as
Deviations to the left were taken as positive and deviations to the right as negative values. The elevation of the paw during the swing phase of the stride was calculated as a difference between the local maximum and minimum in vertical coordinates of the paw during the stride.
To characterize the spatial relationship between placement of the paw and the obstacle we calculated the distance (D) between the forepaw and the nearest object:
where Ai and AO are longitudinal coordinates of the paw placement and of the object, respectively; Bi and BO are the respective transverse coordinates. RO is the radius of the object (1.3 cm). To normalize this distance to the size of the cat’s body, we calculated a ratio of D to the paw diameter in each cat.
We defined the stride as a sequence of the stance phase followed by the swing phase of the right forelimb. A part of the stride when the longitudinal velocity of the LED was equal or below a threshold was classified as a stance, while a part when this velocity was above the threshold was classified as a swing. The threshold of 0.15 m/s was defined empirically in accordance with the sampling noise. We chose the right forepaw because movements of the attached LED were visible to recording cameras at all times, while movements of the LED on the left forepaw were partially obstructed by the right forelimb. We evaluated the proportion of swing duration in the stride as a ratio (%): swing duration/stride duration × 100. This ratio characterized the temporal relationship of stride phases during different locomotor tasks and was complementary to the commonly used stride duty factor (%; stance duration/stride duration × 100). We also calculated the velocity of the right paw during swing as a stride length divided by swing duration.
For statistical analysis of spatial and temporal parameters of strides we grouped the data for the 1st, 2nd, 3rd, 4th, and 5th strides from accepted passages in each recording session. Descriptive statistics (means ± SD) of samples of variables were calculated for each group of strides (the group of 1st strides, the group of 2nd strides, etc.). Parameters of corresponding strides during unobstructed and obstructed walking were compared with t-test for two related samples (the group of 1st strides of unobstructed walking was compared with the group of 1st strides of obstructed walking, etc.). To determine statistical significance of changes in the duration, direction, length, and height of strides during obstructed walking we applied a one-sided binomial test. To complete the test, we divided samples of each of these variables into two categories. The first category included samples that were significantly different from control samples recorded during unobstructed walking. The second category included samples that were similar to control samples. The confidence level for all statistical tests was taken to be 95%.
RESULTS
Experiment 1: single obstacle in wide corridor.
In experiment 1, we studied how cats avoided a single obstacle while walking in the wide corridor. Representative reconstructions of right paw placements during unobstructed and obstructed walking are shown in Figs. 2–5. Figures illustrate different features of walking trajectory: changes in the direction of one or two consecutive strides during avoidance of the obstacle placed on a typical site of right paw placement (Fig. 2 and Fig. 3), changes in the direction of strides during avoidance of the obstacle placed in the midline of the test corridor (Fig. 4), and concurrent changes in the direction and length of strides during avoidance of the obstacle (Fig. 5). In these figures, the gray and black shapes represent paw placements during unobstructed and obstructed walking, respectively. Strides are depicted as arrows. Average lengths and directions of strides are indicated with numbers above and below arrows, respectively.
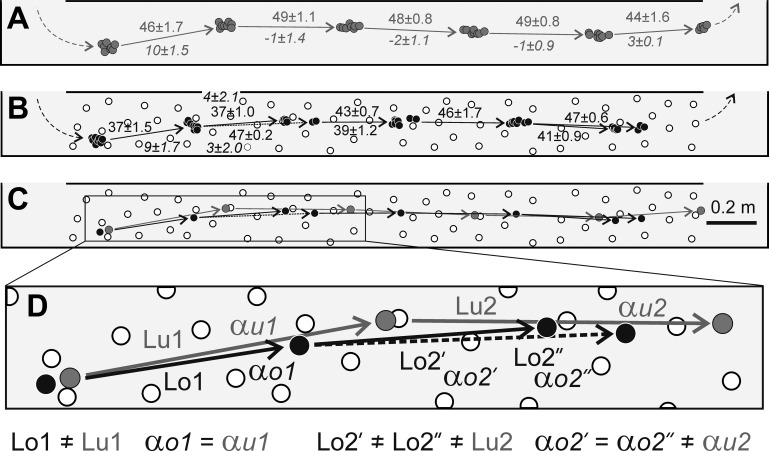
Fig. 2.
A change in the direction of the stride to circumvent an obstacle during walking in the wide corridor (experiment 1, part 1; cat SF). A: right forelimb placements during unobstructed walking along the corridor. Each placement is depicted by a gray circle. Average length (cm, numbers above arrows) and direction (°, numbers below arrows) are indicated for each stride. B: paw placements during walking along the corridor obstructed with a single object. Each placement is depicted by a black circle. The object is depicted by a white circle. C: average paw placements during unobstructed and obstructed walking superimposed. The last stride before the obstacle and a corresponding unobstructed stride are highlighted with a box. D: magnification of the highlighted fragment in C. Lo, length of obstructed stride; Lu, length of unobstructed stride; αo, direction of obstructed stride; αu, direction of unobstructed stride.
Fig. 5.
Adaptations of both length and direction of strides during obstacle circumvention (experiment 1, part 2; cat SF). A: right forelimb placements during unobstructed walking. B: right forelimb placements during walking along the corridor obstructed with an object. C: average placements during unobstructed and obstructed walking superimposed. A fragment showing 2 strides preceding the obstacle and corresponding unobstructed stride is highlighted with a box. D: magnification of the highlighted fragment in C. Designations as in Fig. 4.
Fig. 3.
Changes in the direction of 2 consecutive strides to circumvent an obstacle during walking in the wide corridor (experiment 1, part 1; cat FM). A: right forelimb placements during unobstructed walking. B: right forelimb placements during walking along the corridor obstructed with an object. C: average paw placements during unobstructed and obstructed walking superimposed. The last 2 strides before the obstacle and corresponding unobstructed strides are highlighted with a box. D: magnification of the highlighted fragment in C. Lo1 and Lo2, lengths of obstructed strides; Lu1 and Lu2, lengths of unobstructed strides; αo1 and αo2, directions of obstructed strides; αu1 and αu2, directions of unobstructed strides. Designations as in Fig. 2.
Fig. 4.
Changes in the direction of the strides to circumvent an obstacle while approaching it from either side (experiment 1, part 2; cat SF). A: right forelimb placements during unobstructed walking. B: right forelimb placements during 9 rounds of walking along the corridor obstructed with an object. Black circles represent paw placements during passages on the left of the obstacle; black diamonds show those of the passages on the right of the obstacle. C: average paw placements during unobstructed and obstructed walking superimposed. A fragment showing 2 strides preceding the obstacle and corresponding unobstructed stride is highlighted with a box. D: magnification of the highlighted fragment in C. Designations as in Fig. 2.
The width of the corridor allowed for substantial lateral deviations of the walking trajectory. In fact, typical trajectories of unobstructed walking were curvilinear (Fig. 2A, Fig. 3A, Fig. 4A, and Fig. 5A). Cats walked in the chamber in the counterclockwise direction. To enter the test corridor, they made a left turn and moved close to the right side of the corridor, making the turn as wide as possible. Then, cats usually progressed closer to the midline of the walkway. Further on, they walked straight ahead until the turn into the next corridor. The last step before the turn often deviated to the left side of the corridor. Cats made between four and six strides in the test corridor. The stride length varied among cats and also varied in each cat among experimental sessions. On average, during unobstructed walking the stride length was 47 ± 3, 49 ± 2, 45 ± 2, and 50 ± 1 cm in cats FM, MI, SF, and TI, respectively.
Our first question was whether a single object placed on the floor of the corridor would be recognized by the cats as an obstacle that affects their walking. We found that the object was indeed perceived by all cats as a peculiar thing, and they consistently avoided stepping on it.
In all recording sessions of experiment 1(13/13; Table 1 and Table 2), cats changed the direction of walking to avoid the obstacle in the corridor. Cats took the same number of strides while walking in the obstructed corridor as during unobstructed walking.
Table 1.
The wide walkway (experiment 1, part 1): a stride negotiating the obstacle positioned on a site of paw placement
| Stride Length, cm |
Stride Direction, ° |
Stride Duration, s |
Swing Duration, s |
Swing Proportion in Stride, % |
Swing Velocity, m/s |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cat | Unobst | Obst | Unobst | Obst | Unobst | Obst | Unobst | Obst | Unobst | Obst | Unobst | Obst | Paw-to-Obstacle Distance, cm |
| FM | |||||||||||||
| 1 | 48 ± 1.3 | 47 ± 1.3 | 3 ± 1.1 | 5 ± 0.9 | 0.72 ± 0.01 | 0.69 ± 0.04 | 0.31 ± 0.02 | 0.30 ± 0.01 | 43 ± 2 | 43 ± 2 | 1.5 ± 0.1 | 1.6 ± 0.1 | 3.7 ± 1.1 |
| 2 | 45 ± 2.4 | 46 ± 3.0 | 0 ± 1.1 | 6 ± 2.0 | 0.58 ± 0.08 | 0.54 ± 0.03 | 0.29 ± 0.02 | 0.27 ± 0.01 | 50 ± 9 | 50 ± 2 | 1.6 ± 0.2 | 1.7 ± 0.7 | 5.0 ± 2.2 |
| 3 | 50 ± 0.7 | 50 ± 0.6 | −1 ± 1.0 | 5 ± 1.8 | 0.58 ± 0.02 | 0.55 ± 0.03 | 0.29 ± 0.02 | 0.28 ± 0.01 | 51 ± 4 | 51 ± 2 | 1.7 ± 0.1 | 1.8 ± 0.1 | 4.8 ± 0.9 |
| MI | |||||||||||||
| 1 | 51 ± 2.0 | 51 ± 0.9 | 3 ± 1.5 | 8 ± 1.8 | 0.60 ± 0.04 | 0.61 ± 0.03 | 0.31 ± 0.02 | 0.31 ± 0.02 | 52 ± 1 | 51 ± 1 | 1.7 ± 0.1 | 1.7 ± 0.1 | 4.0 ± 1.8 |
| 2 | 48 ± 1.3 | 47 ± 3.4 | 3 ± 1.9 | 6 ± 1.3 | 0.58 ± 0.03 | 0.57 ± 0.04 | 0.27 ± 0.01 | 0.28 ± 0.03 | 47 ± 2 | 49 ± 3 | 1.8 ± 0.1 | 1.7 ± 0.1 | 3.7 ± 1.4 |
| 3 | 50 ± 2.5 | 50 ± 4.3 | 1 ± 2.8 | 4 ± 3.2 | 0.56 ± 0.04 | 0.58 ± 0.05 | 0.29 ± 0.01 | 0.29 ± 0.02 | 53 ± 2 | 53 ± 2 | 1.7 ± 0.1 | 1.7 ± 0.1 | 4.2 ± 1.8 |
| SF | |||||||||||||
| 1 | 43 ± 1.6 | 41 ± 0.6 | 4 ± 2.5 | 13 ± 1.3 | 0.73 ± 0.02 | 0.71 ± 0.02 | 0.33 ± 0.01 | 0.30 ± 0.01 | 44 ± 1 | 44 ± 1 | 1.4 ± 0.1 | 1.4 ± 0.1 | 5.5 ± 0.9 |
| TI | |||||||||||||
| 1 | 46 ± 6.0 | 45 ± 0.2 | −3 ± 1.1 | 0 ± 2.1 | 0.68 ± 0.04 | 0.61 ± 0.04 | 0.29 ± 0.02 | 0.25 ± 0.01 | 43 ± 1 | 41 ± 2 | 1.6 ± 0.3 | 1.8 ± 0.1 | 3.0 ± 0.2 |
| 2 | 51 ± 0.8 | 48 ± 1.0 | −1 ± 2.0 | 2 ± 1.2 | 0.69 ± 0.02 | 0.64 ± 0.03 | 0.30 ± 0.01 | 0.27 ± 0.01 | 43 ± 1 | 43 ± 1 | 1.7 ± 0.1 | 1.8 ± 0.1 | 6.3 ± 1.6 |
Values are means ± SD for each cat for numbered sessions as indicated. Obst, a stride preceding the obstacle; Unobst, a corresponding stride of unobstructed walking. Pairs of categories that were different (P < 0.05, t-test) are in bold.
Table 2.
The wide walkway: strides negotiating the obstacle positioned in the midline of the walkway
| Stride Length, cm |
Stride Direction, ° |
Stride Duration, s |
Swing Proportion in Stride, % |
Swing Velocity, m/s |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cat | Unobst | Obst | Unobst | Obst | Unobst | Obst | Unobst | Obst | Unobst | Obst | Paw-to-Obstacle Distance, cm |
| MI | |||||||||||
| 1 | 48 ± 1.3 | 49 ± 2.2 | 3 ± 1.9 | 1 ± 1.0 | 0.58 ± 0.03 | 0.59 ± 0.02 | 47 ± 2 | 45 ± 1 | 1.8 ± 0.1 | 1.8 ± 0.1 | 5.0 ± 0.4 |
| SF | |||||||||||
| 1 | 44 ± 1.4 | 47 ± 0.9 | 4 ± 0.7 | 16 ± 2.5 | 0.74 ± 0.08 | 0.74 ± 0.02 | 44 ± 2 | 45 ± 1 | 1.3 ± 0.1 | 1.4 ± 0.1 | 3.4 ± 0.8 |
| 43 ± 1.0 | 1 ± 0.8 | 0.80 ± 0.07 | 44 ± 2 | 42 ± 2 | 1.3 ± 0.1 | 1.3 ± 0.1 | 6.6 ± 0.9 | ||||
| 2 | 46 ± 0.5 | 39 ± 1.3 | 2 ± 0.4 | 10 ± 1.5 | 0.70 ± 0.05 | 0.77 ± 0.07 | 45 ± 3 | 44 ± 2 | 1.5 ± 0.1 | 1.2 ± 0.1 | 15.7 ± 4.1 |
| 42 ± 0.2 | −3 ± 2.4 | 0.82 ± 0.05 | 42 ± 2 | 1.2 ± 0.1 | 9.0 ± 1.4 | ||||||
| TI | |||||||||||
| 1 | 50 ± 1.0 | 55 ± 1.4 | 0 ± 1.6 | 3 ± 0.9 | 0.68 ± 0.02 | 0.70 ± 0.01 | 43 ± 1 | 42 ± 1 | 1.5 ± 0.1 | 1.9 ± 0.1 | 4.6 ± 0.8 |
| 43 ± 0 | 3 ± 0 | 42 ± 1 | 1.4 ± 0.1 | 5.0 ± 1.0 | |||||||
Values are means ± SD for each cat for numbered sessions as indicated. Obst, a stride preceding the obstacle; Unobst, a corresponding stride of unobstructed walking. Pairs of categories that were different (P < 0.05, t-test) are in bold.
The first part of experiment 1, in which the object was placed on a site of a typical right forepaw placement observed during unobstructed walking, included nine recording sessions: three each with cat FM and cat MI, two with cat TI, and one with cat SF (Table 1). In all these sessions (9/9), during avoidance of the obstacle the cat’s walking trajectories consistently deviated to the left; thus the right forepaw was placed on the left of the object (Fig. 2, Fig. 3; Table 1). Typically, changes in the direction were seen in the last stride preceding the object, as illustrated in Fig. 2. Occasionally, changes in the walking trajectory occurred in the two consecutive strides preceding the object, as shown in Fig. 3. In these cases, an angular deviation of the walking trajectory away from the longitudinal axis of the walkway was similar in both strides. During obstacle avoidance, the length of strides, their duration, the swing-to-stride duration ratio, and paw velocity during the swing did not change (Table 1). The elevation of the forepaw during the swing was also similar during both unobstructed and obstructed walking: 2.7 ± 0.4 and 2.7 ± 0.4 cm in cat FM, 3.1 ± 0.5 and 3.5 ± 1.3 cm in cat MI, 4.7 ± 1.7 and 3.7 ± 0.3 cm in cat SF, and 2.4 ± 0.3 and 2.6 ± 0.4 cm in cat TI (P > 0.05, t-test). After completion of the stride obstructed with the obstacle, the distance between the paw and the object varied between 3 and 6 cm (Table 1). This distance, when calculated as a ratio to the diameter of the paw, was similar in three cats and was close to 1.5 diameters of the paw: 1.3 (cat FM), 1.2 (cat MI), and 1.4 (cat TI). This distance was about 2 diameters of the paw in cat SF: 1.8.
The second part of experiment 1, in which the obstacle was placed on the midline of the walkway, included four recording sessions: one each with cats MI and TI and two with cat SF. During these sessions, the direction of walking trajectory during obstacle avoidance varied. To avoid the obstacle, cat MI consistently placed the right forepaw further to the right compared with typical placement during unobstructed walking (Table 2) and walked on the right of the obstacle. Cats SF and TI avoided the obstacle by walking either on the left or on the right of the object. In both cases, the directions of strides preceding the obstacle were significantly different from the directions of corresponding strides during unobstructed walking (Fig. 4; Table 2). In these two cats, changes in the stride direction were accompanied by changes in the stride length. Strides that deviated to the left lengthened, while strides that deviated to right shortened (Fig. 4; Table 2). The swing-to-stride duration ratio, however, did not change in any of those strides, nor did the stride duration in most of them (Table 2).
In one of the recording sessions (session 2), cat SF walked around the obstacle in a conspicuous manner, modifying both the length and direction of strides (Fig. 5; Table 2). When approaching the obstacle on either the left or right side, the cat shortened the lengths of two strides preceding the object and changed the direction of the last stride. The durations of these strides increased without a change in the swing-to-stride duration ratio, and the forepaw swing velocity decreased. As a result of these advanced adaptations, the forepaw was placed far away from the obstacle, at a distance that corresponded to 3–5 diameters of the paw (Table 2). With the exception of this case, the cats placed the right forepaw away from the object at distances that equaled ~1.5 diameters of the paw (Table 2).
Experiment 2: single obstacle in narrow corridor.
In experiment 2, we studied how cats avoided a single obstacle while walking in the narrow corridor, which restricted lateral deviations. Dimensions of the corridor allowed simple straightforward unobstructed walking but forced the cats to step over the object to avoid it during obstructed walking (Fig. 6). The cats took the same number of strides during both unobstructed and obstructed walking in the narrow corridor as they did in the wide corridor.
Fig. 6.
Stepping over an obstacle during walking in the narrow corridor (experiment 2; cat TI). A: right forelimb placements during unobstructed walking. B: placements during walking along the corridor obstructed with an object positioned on a site of a typical 2nd unobstructed paw placement. C: placements during walking along the corridor obstructed with an object positioned slightly before (2.5 cm) the site of the typical 2nd unobstructed paw placement. D: placements during walking along the corridor obstructed with an object positioned at a greater distance (5 cm) from the site of the 2nd unobstructed paw placement. E: placements during walking along the corridor obstructed with an object positioned at the site of the 3rd unobstructed paw placement. F: placements during walking along the corridor obstructed with an object positioned 2.5 cm before the site of the 3rd unobstructed paw placement. G: placements during walking along the corridor obstructed with an object positioned at a greater distance (5 cm) before the site of the 3rd unobstructed paw placement. H: placements during walking along the corridor obstructed with an object positioned at the site of the 4th unobstructed paw placement. I: placements during walking along the corridor obstructed with an object positioned 2.5 cm before the site of the 4th unobstructed paw placement. J: placements during walking along the corridor obstructed with an object positioned 5 cm before the site of the 4th unobstructed paw placement. In C, F, and I, gray symbols and numbers represent paw placements during passages when the stride was lengthened during stepping over the object. Other designations as in Fig. 2.
Experiment 2 included nine recording sessions: three each with cats SF and TI, two with cat MI, and one with cat FM. In all these sessions, the cats placed the right forepaw before the obstacle occupying a site of a normal paw placement. The strides that preceded the obstacle were shortened in all sessions (9/9; Table 3). Duration of the stride was reduced in only three of nine (33%) sessions. At the same time, in all sessions (9/9) the duration of the swing phase decreased. In the majority of sessions (7/9, 78%, P = 0.09, 1-sided binomial test), a decrease in swing duration was accompanied by a decrease in the swing-to-stride duration ratio (Table 3). The velocity and elevation of the forepaw during the swing phase of these strides did not change (Table 3). After completion of the strides that preceded the obstacle, the distance between the forepaw and the object varied between 3 and 7 cm (Table 3). In three cats (FM, SF, and TI), this distance was close to 1.5 diameters of the paw: 1.4. It was ∼2 diameters of the paw in one cat (MI) only: 1.8.
Table 3.
The narrow walkway (experiment 2): a stride shortened before the obstacle
| Stride Length, cm |
Stride Height, cm |
Stride Duration, s |
Swing Duration, s |
Swing Proportion in Stride, % |
Swing Velocity, m/s |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cat | Unobst | Obst | Unobst | Obst | Unobst | Obst | Unobst | Obst | Unobst | Obst | Unobst | Obst | Paw-to-Obstacle Distance, cm |
| FM | |||||||||||||
| 1 | 47 ± 1.2 | 42 ± 0.5 | 4.3 ± 0.3 | 4.2 ± 0.2 | 0.62 ± 0.03 | 0.63 ± 0.06 | 0.31 ± 0.02 | 0.28 ± 0.02 | 50 ± 1 | 44 ± 1 | 1.6 ± 0.1 | 1.5 ± 0.1 | 4.9 ± 1.4 |
| MI | |||||||||||||
| 1 | 53 ± 1.5 | 46 ± 1.7 | 3.4 ± 0.4 | 3.4 ± 0.2 | 0.62 ± 0.03 | 0.58 ± 0.02 | 0.31 ± 0.02 | 0.27 ± 0.01 | 50 ± 1 | 47 ± 1 | 1.7 ± 0.1 | 1.7 ± 0.1 | 7.2 ± 1.0 |
| 2 | 50 ± 1.7 | 45 ± 1.6 | 3.5 ± 0.2 | 3.5 ± 0.1 | 0.62 ± 0.03 | 0.60 ± 0.01 | 0.31 ± 0.02 | 0.29 ± 0.01 | 50 ± 3 | 49 ± 1 | 1.6 ± 0.1 | 1.6 ± 0.1 | 4.5 ± 1.7 |
| SF | |||||||||||||
| 1 | 40 ± 2.3 | 36 ± 0.3 | 3.8 ± 0.5 | 3.4 ± 0.1 | 0.85 ± 0.04 | 0.88 ± 0.05 | 0.35 ± 0.02 | 0.33 ± 0.01 | 42 ± 2 | 38 ± 2 | 1.1 ± 0.1 | 1.1 ± 0.5 | 3.4 ± 1.0 |
| 2 | 39 ± 2.9 | 34 ± 3.2 | 3.6 ± 0.3 | 3.9 ± 0.7 | 0.85 ± 0.06 | 0.81 ± 0.05 | 0.37 ± 0.02 | 0.32 ± 0.03 | 43 ± 2 | 39 ± 1 | 1.1 ± 0.1 | 1.1 ± 0.1 | 4.3 ± 1.5 |
| 3 | 45 ± 1.3 | 41 ± 1.3 | 2.5 ± 0.3 | 2.3 ± 0.7 | 0.72 ± 0.04 | 0.66 ± 0.01 | 0.31 ± 0.01 | 0.26 ± 0.01 | 43 ± 1 | 40 ± 1 | 1.5 ± 0.1 | 1.6 ± 0.1 | 4.7 ± 1.5 |
| TI | |||||||||||||
| 1 | 51 ± 1.4 | 44 ± 0.3 | 2.0 ± 0.6 | 1.6 ± 0.2 | 0.71 ± 0.05 | 0.68 ± 0.02 | 0.32 ± 0.02 | 0.29 ± 0.04 | 45 ± 1 | 42 ± 1 | 1.6 ± 0.1 | 1.5 ± 0.1 | 4.3 ± 0.7 |
| 2 | 52 ± 1.2 | 43 ± 0.8 | 2.7 ± 0.4 | 3.1 ± 0.3 | 0.70 ± 0.03 | 0.60 ± 0.06 | 0.30 ± 0.01 | 0.25 ± 0.02 | 42 ± 2 | 41 ± 2 | 1.8 ± 0.1 | 1.8 ± 0.1 | 3.2 ± 0.8 |
| 3 | 51 ± 1.2 | 47 ± 2.3 | 2.7 ± 0.2 | 2.6 ± 0.2 | 0.71 ± 0.03 | 0.68 ± 0.02 | 0.31 ± 0.01 | 0.28 ± 0.01 | 43 ± 1 | 41 ± 1 | 1.7 ± 0.1 | 1.7 ± 0.1 | 6.3 ± 1.6 |
Values are means ± SD for each cat for numbered sessions as indicated. Obst, a stride preceding the obstacle negotiation; Unobst, a stride of unobstructed walking. Pairs of categories that were different (P < 0.05, t-test) are in bold.
The strides that negotiated the obstacle were somewhat longer than preceding strides. However, the strides negotiating the obstacle tended to be shorter than normal strides during unobstructed walking, as was seen in seven of nine sessions (78%, P = 0.09, 1-sided binomial test; Fig. 6, B, C, E, F, H, and I; Table 4). The elevation of the paw during obstacle negotiation was always higher than that of normal strides in all sessions (9/9): by 1.1 ± 0.4, 1.5 ± 0.7, 1.2 ± 0.2, and 0.9 ± 0.3 cm in cats FM, MI, SF, and TI, respectively (P < 0.01, t-test). The duration of the strides that negotiated the obstacle was similar to that of unobstructed walking (Table 4). The swing-to-stride duration ratio and the velocity of the forepaw during the swing of these strides usually were also similar (Table 4).
Table 4.
The narrow walkway (experiment 2): a stride negotiating the obstacle after a short preceding stride
| Stride Length, cm |
Stride Height, cm |
Stride Duration, s |
Swing Duration, s |
Swing Proportion in Stride, % |
Swing Velocity, m/s |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cat | Unobst | Obst | Unobst | Obst | Unobst | Obst | Unobst | Obst | Unobst | Obst | Unobst | Obst |
| FM | ||||||||||||
| 1 | 47 ± 1.2 | 44 ± 0.7 | 4.2 ± 0.4 | 5.3 ± 0.1 | 0.62 ± 0.06 | 0.65 ± 0.02 | 0.31 ± 0.02 | 0.31 ± 0.01 | 50 ± 5 | 48 ± 1 | 1.5 ± 0.1 | 1.4 ± 0.1 |
| MI | ||||||||||||
| 1 | 53 ± 1.6 | 47 ± 0.6 | 3.8 ± 0.4 | 5.7 ± 0.7 | 0.62 ± 0.03 | 0.59 ± 0.02 | 0.31 ± 0.02 | 0.29 ± 0.01 | 50 ± 2 | 49 ± 1 | 1.7 ± 0.1 | 1.6 ± 0.1 |
| 2 | 50 ± 1.6 | 49 ± 1.5 | 3.5 ± 0.3 | 4.5 ± 0.3 | 0.64 ± 0.03 | 0.62 ± 0.01 | 0.32 ± 0.02 | 0.31 ± 0.01 | 49 ± 3 | 50 ± 3 | 1.6 ± 0.1 | 1.6 ± 0.1 |
| SF | ||||||||||||
| 1 | 41 ± 1.8 | 42 ± 0.4 | 3.6 ± 0.2 | 4.6 ± 0.5 | 0.84 ± 0.08 | 0.90 ± 0.05 | 0.33 ± 0.02 | 0.36 ± 0.01 | 40 ± 3 | 40 ± 1 | 1.3 ± 0.1 | 1.2 ± 0.1 |
| 2 | 41 ± 2.2 | 39 ± 1.1 | 3.6 ± 0.2 | 4.8 ± 0.5 | 0.84 ± 0.04 | 0.82 ± 0.03 | 0.35 ± 0.03 | 0.33 ± 0.01 | 42 ± 3 | 41 ± 2 | 1.2 ± 0.1 | 1.2 ± 0.1 |
| 3 | 45 ± 3.1 | 41 ± 0.1 | 2.1 ± 0.3 | 3.4 ± 0.1 | 0.72 ± 0.02 | 0.71 ± 0.01 | 0.31 ± 0.02 | 0.33 ± 0.01 | 42 ± 3 | 46 ± 1 | 1.5 ± 0.1 | 1.3 ± 0.1 |
| TI | ||||||||||||
| 1 | 52 ± 1.2 | 46 ± 0.7 | 1.9 ± 0.4 | 2.5 ± 0.2 | 0.72 ± 0.05 | 0.70 ± 0.04 | 0.30 ± 0.02 | 0.30 ± 0.01 | 41 ± 2 | 43 ± 1 | 1.8 ± 0.1 | 1.5 ± 0.1 |
| 2 | 51 ± 1.1 | 46 ± 0.4 | 2.0 ± 0.4 | 3.1 ± 0.3 | 0.74 ± 0.07 | 0.71 ± 0.03 | 0.31 ± 0.02 | 0.32 ± 0.01 | 42 ± 1 | 45 ± 1 | 1.6 ± 0.1 | 1.4 ± 0.1 |
| 3 | 51 ± 1.6 | 46 ± 0.7 | 2.7 ± 0.3 | 3.6 ± 0.2 | 0.70 ± 0.06 | 0.68 ± 0.01 | 0.30 ± 0.02 | 0.29 ± 0.01 | 43 ± 2 | 44 ± 1 | 1.7 ± 0.1 | 1.6 ± 0.1 |
Values are means ± SD for each cat for numbered sessions as indicated. Obst, a stride that negotiated the obstacle; Unobst, corresponding stride of unobstructed walking. Pairs of categories that were different (P < 0.05, t-test) are in bold.
When the object was placed closer to the entrance of the test corridor, at a distance equal to ~90% of the normal stride length, stride adjustments became variable. Cats still preferred to shorten the stride before the object, and negotiated the object with the next stride. Yet, occasionally, cats lengthened the stride and negotiated the object by stepping over it. This is illustrated in representative recordings from cat TI (Fig. 6, C, F, and I). When the object was placed even closer to the entrance of the corridor, at a distance that corresponded to ~80% of the normal stride length, cats always stepped over the object. The length of strides that negotiated the obstacle always increased (Fig. 6, D, G, and J). The elevation of the paw during these strides increased as well: by 2.3 ± 0.3, 1.1 ± 0.3, and 1.8 ± 0.3 cm in cats MI, SF, and TI, respectively. This increase tended to be larger than that in strides that negotiated the obstacle after shortening of the previous stride (P < 0.05 for cat MI and P < 0.02 for cat TI, t-test). An increase in the stride length was accompanied by an increase in the swing-to-stride duration ratio in one cat only (Table 5). The duration and the forepaw swing velocity during these strides did not change (Table 5). After completion of the strides, the distance between the right forepaw and the object varied substantially between 2 and 8 cm (Table 5). In two cats, this distance was close to the diameter of the paw: 0.8 (SF) and 1.2 (MI). In cat TI it was 2 diameters of the paw.
Table 5.
The narrow walkway (experiment 2): a stride lengthened during negotiation of the obstacle
| Stride Length, cm |
Stride Height, cm |
Stride Duration, s |
Swing Duration, s |
Swing Proportion in Stride, % |
Swing Velocity, m/s |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cat | Unobst | Obst | Unobst | Obst | Unobst | Obst | Unobst | Obst | Unobst | Obst | Unobst | Obst | Paw-to-Obstacle Distance, cm |
| MI | |||||||||||||
| 1 | 50 ± 1.7 | 54 ± 0.3 | 3.5 ± 0.2 | 5.6 ± 0.1 | 0.65 ± 0.03 | 0.66 ± 0.02 | 0.31 ± 0.02 | 0.33 ± 0.01 | 50 ± 3 | 50 ± 1 | 1.6 ± 0.1 | 1.7 ± 0.1 | 2.6 ± 0.1 |
| 2 | 46 ± 2.6 | 52 ± 1.3 | 3.6 ± 0.3 | 6.1 ± 0.5 | 0.65 ± 0.03 | 0.73 ± 0.06 | 0.29 ± 0.02 | 0.35 ± 0.03 | 45 ± 2 | 47 ± 2 | 1.6 ± 0.1 | 1.5 ± 0.1 | 5.3 ± 2.1 |
| SF | |||||||||||||
| 1 | 44 ± 1.1 | 48 ± 1.0 | 4.0 ± 0.5 | 5.3 ± 0.8 | 0.74 ± 0.04 | 0.72 ± 0.06 | 0.31 ± 0.02 | 0.31 ± 0.02 | 43 ± 2 | 44 ± 2 | 1.4 ± 0.1 | 1.5 ± 0.1 | 2.3 ± 0.3 |
| 2 | 38 ± 3.6 | 42 ± 2.3 | 2.1 ± 0.2 | 3.0 ± 0.6 | 0.85 ± 0.04 | 0.80 ± 0.06 | 0.35 ± 0.02 | 0.33 ± 0.01 | 40 ± 3 | 40 ± 2 | 1.1 ± 0.1 | 1.2 ± 0.2 | 2.6 ± 0.3 |
| TI | |||||||||||||
| 1 | 50 ± 1.4 | 55 ± 1.5 | 2.7 ± 0.3 | 4.5 ± 0.1 | 0.71 ± 0.02 | 0.67 ± 0.03 | 0.32 ± 0.01 | 0.32 ± 0.02 | 45 ± 1 | 48 ± 1 | 1.6 ± 0.1 | 1.7 ± 0.1 | 7.7 ± 0.6 |
| 2 | 50 ± 1.7 | 56 ± 1.6 | 3.4 ± 0.3 | 5.0 ± 0.1 | 0.72 ± 0.02 | 0.73 ± 0.02 | 0.33 ± 0.01 | 0.35 ± 0.02 | 46 ± 1 | 48 ± 1 | 1.5 ± 0.1 | 1.6 ± 0.1 | 6.1 ± 0.8 |
Values are means ± SD for each cat for numbered sessions as indicated. Obst, a stride that negotiated the obstacle; Unobst, a corresponding stride of unobstructed walking. Pairs of categories that were different (P < 0.05, t-test) are in bold.
While approaching the obstacle, cats adjusted the length of the stride that immediately preceded the obstacle. This was true for strides that preceded the object positioned on a site of any paw placement observed during unobstructed walking. There were no distinct changes in the length of earlier strides, even in tests where the object was located at the end of the corridor and the cats approached it with several strides.
Experiment 3: multiple obstacles in wide corridor.
In experiment 3, we studied how cats avoided obstacles while walking along the wide corridor cluttered with multiple small objects. The data were collected in six recording sessions with cat FM using four different layouts of obstacles, two of which were used twice; in four sessions with cat MI using different layouts; in seven sessions with cat SF using six different layouts, one of which was used twice; and in eight sessions with cat TI using six different layouts, two of which were used twice. Repetitions of walking across the same layouts were done to clarify whether cats did or did not reproduce the pattern of obstacle avoidance during recording sessions held on different days. We found that patterns of forepaw placements during walking along the same layouts of obstacles during different recording sessions were not identical.
All cats avoided the obstacles by implementing a complex adaptation of stride trajectories that included both circumvention and stepping over the objects, as well as an increase in the elevation of the paw. Venn diagrams in Fig. 7 illustrate how often the paw elevation, the length, and the direction of strides changed in each cat during obstructed walking. To obtain these estimates we compared parameters between all 1st, 2nd, 3rd, and 4th strides of unobstructed walking and corresponding 1st, 2nd, 3rd, and 4th strides of obstructed walking during the same recording session. The results were pooled together in each cat, yielding groups of 27, 16, 34, and 33 comparisons between corresponding strides in cats FM, MI, SF, and TI, respectively. To compare results among cats, the results were normalized in a percentage form.
Fig. 7.
Proportions of strides, in which the length (L, areas at top left of each panel), the direction (α, areas at top right of each panel), and the paw elevation during swing (H, areas at bottom of each panel) were altered during walking in the corridor obstructed with multiple objects. Strides with and without changes in the duration and/or the swing-to-stride ratio are combined. Obstructed and unobstructed walking conditions are indicated with indices “o” and “u,” respectively. The percentage of strides, in which neither parameter changed, is indicated at bottom left of each panel. The number of comparisons between groups of obstructed and unobstructed strides is indicated at bottom right of each panel.
During obstructed walking, the most frequent change was a change in the elevation of the paw. It increased in 78%, 100%, 59%, and 61% of strides in cats FM, MI, SF, and TI, respectively (Fig. 7). Paw elevations during obstructed and unobstructed walking were 3.4 ± 0.5 and 2.4 ± 0.3 cm in cat FM, 4.6 ± 0.5 and 3.4 ± 0.3 cm in cat MI, 3.2 ± 0.5 and 2.6 ± 0.7 cm in cat SF, and 4.4 ± 0.5 and 3.8 ± 0.4 cm in cat TI (all were significantly different, P < 0.00001, t-test). In all cats, this increase was ~1 cm, which was equal to the height of the obstacles.
A change in the stride direction during obstructed walking was found in 48%, 63%, 26%, and 33% of strides in cats FM, MI, SF, and TI, respectively (Fig. 7). The second stride shown in Fig. 8, B–D, presents an example of such change. The direction of this stride was different from the direction of the corresponding stride during unobstructed walking (0 ± 1.5° and 6 ± 1.9°, respectively; P < 0.00001, t-test). However, other parameters of this pair of corresponding strides were similar.
Fig. 8.
Selective changes in direction and length of strides during walking in the corridor cluttered with multiple objects (experiment 3; cat SF). A: right forelimb placements during unobstructed walking. B: right forelimb placements during walking along the corridor obstructed with objects. C: average placements during both unobstructed and obstructed walking. Fragments showing strides avoiding obstacles using 2 different strategies and corresponding unobstructed strides are highlighted with 2 boxes. D: magnification of the fragment in C highlighting the change in stride direction. E: magnification of the fragment in C highlighting the change in stride length. Designations as in Fig. 2.
A change in the stride length was found in 41%, 69%, 56%, and 91% of strides in cats FM, MI, SF, and TI, respectively (Fig. 7). The strides were usually shorter during obstructed than unobstructed walking. In three cats (FM, MI, and SF), the strides shortened by ~2 cm. The lengths of these strides were 45.6 ± 0.8 and 47.2 ± 1.9 cm in cat FM (P = 0.002, t-test), 47.4 ± 1.2 and 50.4 ± 2.2 cm in cat MI (P < 0.00001, t-test), and 40.9 ± 1.6 and 43.7 ± 2.0 cm in cat SF (P < 0.00001, t-test), respectively. The third stride shown in Fig. 8, B, C, and E, presents an example of such change. The lengths of the corresponding strides during obstructed and unobstructed walking were 38 ± 1 and 43 ± 1 cm (P < 0.00001, t-test). The swing-to-stride duration ratios of these strides were different as well (36 ± 2% and 39 ± 2%, respectively; P = 0.014, t-test). At the same time, other parameters of this pair of corresponding strides were similar.
Changes in the stride length during obstructed walking found in cat TI were more complex (Fig. 9). This cat had the longest strides during unobstructed walking. After entering the test corridor cluttered with obstacles, this cat significantly reduced the length of all strides from 49.0 ± 0.9 to 42.2 ± 0.5 cm (P < 0.0001, t-test). This general shortening of the strides was complemented with an increase in variations in their length. This variability is seen in second strides shown in Fig. 9, B–D. To avoid the obstacle during these strides, the cat placed the paw either before or after the object. Accordingly, the lengths of strides during these different paw placements differed significantly. However, all these strides were shorter than corresponding strides during unobstructed walking.
Fig. 9.
A gross decrease in the stride length during walking in the wide corridor cluttered with multiple objects (experiment 3; cat TI). A: right forelimb placements during unobstructed walking. B: right forelimb placements during walking along the corridor obstructed with objects. C: average placements during unobstructed and obstructed walking superimposed. A fragment illustrating changes in stride length to avoid the obstacle and corresponding unobstructed stride is highlighted with a box. D: magnification of the highlighted fragment in C. Second obstructed strides of different length are designated with solid and dashed arrows. Lo2′ and Lo2ʺ, 2nd obstructed strides of different length; αo1′ and αo2ʺ, directions of 2nd obstructed strides of different length. Other designations as in Figs. 2 and 3.
Changes in the spatial characteristics of the stride were often accompanied with changes in its temporal characteristics: 56% (15/27), 75% (12/16), 65% (22/34), and 58% (19/33) of the comparisons between corresponding strides in cats FM, MI, SF, and TI, respectively. The duration of the strides changed in half of these comparisons: 48% (13/27), 50% (8/16), 50% (17/34), and 42% (14/33) in cats FM, MI, SF, and TI, respectively. Mostly, the stride duration decreased. A decrease was seen in all altered strides in cats FM and MI (27/27 and 16/16, respectively), in 38% (13/34) of comparisons between strides in cat SF, and in 39% (14/33) of comparisons between strides in cat TI. The swing-to-stride duration ratio changed in a third of comparisons between strides: 29% (8/27), 31% (5/16), 39% (13/34), and 33% (11/33) of comparisons between strides in cats FM, MI, SF, and TI, respectively. No preferences were seen in the type of the change in this ratio. It decreased in 11% (3/27) and increased in 19% (5/27) of comparisons between strides in cat FM, decreased in 12% (2/16) and increased in 19% (3/16) of comparisons between strides in cat MI, and decreased in 18% (6/34) and increased in 20% (7/34) of comparisons between strides in cat SF. Only cat TI typically reduced the swing-to-stride duration ratio [in 30% (10/33) of comparisons between strides] and only rarely increased it [in 3% (1/33) of comparisons between strides]. In approximately one-fifth of strides the duration and the swing-to-stride duration ratio changed concurrently: in 22% (6/27), 24% (8/34), and 18% (6/33) of comparisons between strides in cats FM, SF, and TI, respectively, and in 6% (1/16) of comparisons between strides in cat MI.
Regardless of specific adjustments of the strides during walking along the test corridor obstructed with multiple obstacles, three cats placed the right forepaw closer to the object as compared with walking in the corridor obstructed with just one obstacle. In these cats, the distance between the forepaw and the nearest object was similar: 2.4 ± 0.9 cm (cat FM), 2.3 ± 1.2 cm (cat SF), and 1.9 ± 0.9 cm (cat TI), which corresponded to 0.7, 0.8, and 0.6 diameters of the paw, respectively. These distances were shorter than those during avoidance of a single obstacle in the wide corridor (P < 0.001, t-test). In one cat (MI) the distance between the paw and the obstacle was larger: 3.4 ± 1.9 cm, which corresponded to the diameter of the paw. This distance did not differ from that during avoidance of a single obstacle in the wide corridor (P = 0.17, t-test).
DISCUSSION
Avoidance of obstacles during walking is a common behavioral task for animals. In this study we investigated how the cat—a typical quadruped animal—solves this task when confronted with small objects while walking in environments with different spatial constraints.
We found that cats used two strategies to avoid the obstacle. One strategy was to circumvent the obstacle by changing the direction of strides (Fig. 10D). No other parameters of the strides were altered while implementing this strategy (Fig. 10, E and F). Another strategy was to step over the obstacle while maintaining the direction of walking (Fig. 10G). Features of this strategy included a decrease in the length, the duration, and the swing-to-stride duration ratio of the stride preceding the obstacle and an increase in the elevation of the paw during the stride negotiating the obstacle (Fig. 10, H and I).
Fig. 10.
Strategies for avoiding small obstacles during walking. A: trajectories of 2 consecutive strides (1 and 2) during unobstructed walking. B: length and height of 2 strides during unobstructed walking. C: duration of the stance (St) and swing (Sw) phases of 2 strides during unobstructed walking. D: in an unrestricting environment, obstacle avoidance is achieved by circumvention. The preferred placement of the right paw is to the left of the obstacle at a distance of about the diameter of the paw. E: during circumvention, the length and height of both strides 1 and 2 do not change. F: duration and swing-to-stride duration ratio of strides 1 and 2 do not change. G: in a restricting environment, obstacle avoidance is achieved by stepping over the obstacle. The paw is placed before the obstacle at a distance of the diameter of the paw. The obstacle is negotiated with the next stride. H: the length of the stride preceding the obstacle (1) decreases, but its height does not change. The stride negotiating the obstacle (2) increases in height. This stride (2) is longer than the preceding stride, albeit shorter than normal. I: the duration of the stride preceding the obstacle (1) reduces because of a decrease in the duration of the swing. Temporal characteristics of the stride negotiating the obstacle (2) are similar to those of the normal stride. In A, D, and G, the paw is depicted with a black pictogram. In D and G, the obstacle is depicted with a gray circle. In E, F, H, and I, the gray circle indicates the location of the obstacle. In B, E, and H, black areas show the length and height of strides. In C, F, and I, white and gray areas depict the duration of the stance (St) and swing (Sw) phases of strides. Indices “1” and “2” indicate 2 consecutive strides.
Cats consistently selected the first strategy to avoid a single obstacle in a wide corridor that allowed lateral deviations of the walking trajectory (experiment 1). Cats circumvented the obstacle, which occupied a typical site of the right forepaw placement and specifically confronted movements of the right forelimb (Fig. 2, Fig. 3). During circumvention, the walking trajectory deviated to the left, thus minimizing lateral displacements of the body during the obstacle negotiation. This finding shows that during walking in an open environment a modification of the activities of abductor and adductor muscles is sufficient to avoid a small obstacle while the rhythm of locomotor movements is preserved. Cats also circumvented the obstacle when it was placed on the midline of the corridor and obstructed progressive movement of the whole body of the cat, rather than movement of one forelimb only. In this case, circumventions of the obstacle were either rightward or leftward. Similar behavior was reported in humans who had to bypass an obstacle while walking toward a goal in a virtual environment (Fajen and Warren 2003). When positions of the subject, the object, and the goal were aligned, and thus the length and curvature of rightward or leftward circumventions necessary to avoid the obstacle were similar, the subjects equally chose either a leftward or a rightward trajectory of walking. When the obstacle was directly in the cats’ path, cats walked around it by making a larger or smaller stride to the side of it, thus altering two spatial parameters of the stride (Fig. 4, Fig. 5). However, cats tended to preserve the temporal characteristics of the stride, maintaining its swing-to-stride duration ratio and, in most cases, the duration of strides. This indicates that for obstacle avoidance a modification of the activities of several muscle groups is preferred over modifications of the locomotor rhythm.
It should be noted that the cats never straddled the obstacle. It is very likely that the cats ignored this modification of strides because walking with wide foot separation compromises the cat’s stability during walking. Indeed, it was recently found that, in contrast to standing, an increased lateral interpaw distance during walking dramatically decreases the cat’s body dynamic stability during double support phases of the stride and causes the cat to spend more time in three-legged support phases (Farrell et al. 2014).
While walking along the narrow corridor (experiment 2), cats were forced to step over the obstacle. To do this, cats had to choose how to modify their strides. They could either increase the paw elevation and stride length and negotiate the obstacle with a single stride or decrease the length of the stride before the obstacle and negotiate it with the next stride of increased paw elevation. We have found that cats always used the second approach.
A decrease in the length of the stride before the obstacle was accompanied by a decrease in swing-to-stride duration ratio (Table 3). This decrease was due to a change in the duration of the swing phase of the stride. The observation suggests that descending commands that are based on visual localization of the obstacle target the flexor part of the locomotor central pattern generator. It is consistent with the notion that descending commands originating in the motor cortex are directed primarily to the flexion-related neuronal networks of the spinal cord and are capable of affecting the rhythm of locomotion (Orlovsky 1972; Rho et al. 1999). This is different from changes in the structure of the step cycle during a change in walking velocity when the duration of the stance phase mainly alters, not the duration of the swing phase (Arshavsky et al. 1965; Goslow et al. 1973; Murray 1967). It was suggested that during changes in the walking velocity sensory feedback affects the stance phase more than the swing phase (Yakovenko et al. 2005).
Notably, we found that, despite variability in swing-to-stance duration ratio in the stride preceding the obstacle, the stride duration was usually preserved (Table 3). Thus the duration of stride phases could be modified independently from the overall duration of the stride. Apparently, consistency in the stride duration benefits uninterrupted walking, since changes in the duration of strides of one limb negotiating the obstacle would affect coordination between locomotor movements of all limbs.
We found that the stride that negotiated the obstacle with an increase in paw elevation was usually longer than the preceding stride but still shorter than a normal stride (Table 3, Table 4). A small shortening of the stride negotiating the obstacle may serve to prevent an increase in length of the paw trajectory during swing, which would occur in the stride of normal length but of an increased paw elevation. Similar modification of the length of strides during obstacle avoidance was also observed in another quadruped, the rat. In rats, the length of the stride that negotiated the obstacle was found to be significantly shorter than that of the preceding stride and also shorter than the length of an average stride during unobstructed walking (Sato et al. 2012). We found that, despite alterations in two spatial characteristics of the stride negotiating the obstacle, its overall duration and swing-to-stride duration ratio were preserved (Table 4).
Notably, during stepping over the obstacle, cats divided spatial adjustments of strides in two parts. First, cats adjusted the length of the stride preceding obstacle negotiation to place the paw before the obstacle. This adjustment in the stride length was achieved by a decrease in the duration of the swing phase of this stride. Second, cats adjusted the elevation of the paw during the swing phase of the stride negotiating the obstacle. An increase of the paw elevation was accompanied by attenuation of the stride length, which prevented an increase in the length of the paw trajectory. The strides negotiating the obstacle had temporal characteristics similar to normal stride (Table 5). It appears that a separation of adjustments of the stride length and paw elevation in two successive strides simplified control of limb movements during negotiation of the obstacle.
Adjustments in the length of the stride preceding the obstacle during overstepping had limitations. When the distance between the obstacle and the preceding placement of the forepaw was reduced to ~80% of the normal stride length, cats did not shorten the stride further but lengthened it, and negotiated the object with a single stride. Probably, cats avoided a large shortening of the stride before the obstacle to prevent decline in the longitudinal stability of the body during walking. The shortening of the stride would decrease the already small area of support when only two diagonal limbs support the body and the cat is dynamically unstable in the forward direction (Farrell et al. 2014).
Interestingly, in contrast to quadruped cats, when confronted with an obstacle occupying a site of normal foot placement biped humans prefer to negotiate the obstacle with a single stride of an increased length (Chen et al. 1991; Moraes et al. 2004, 2007; Patla et al. 1999; Weerdesteyn et al. 2005). This difference can be explained by considering the projection of the center of mass of the body on the base of support area in humans and cats. In both humans and cats, there are periods during the stride when the center of mass of the body accelerates forward and downward and the body is dynamically unstable in the sagittal plane (Farrell et al. 2014; Winter 1995; Zijlstra and Hof 2003). This phase of sagittal instability apparently helps to propel the body forward by exploiting the body’s inertia. In humans, the projection of the center of mass, when moving forward, rapidly departs from the base of support area, thus making lengthening of the stride easier to achieve than shortening. In contrast, in the quadruped cat, the center of mass of the body is behind the forelimbs and within the base of support area for the majority of the stride cycle (Alexander 1980; Farrell et al. 2014; Manter 1938; Pandy et al. 1988). Thus, for a cat, shortening of the stride is easier to achieve than lengthening.
When approaching a single obstacle in the wide or narrow walkway, the cats were free to choose between a large-scale adaptation of the last stride preceding the object and a gradual adaptation of several strides before the object. We have found that adaptations typically occurred in the last stride preceding the obstacle. In a similar way, it was reported that the earliest changes in the firing activity of motor cortex neurons in a cat stepping over an obstacle mounted on a treadmill belt occur immediately before stepping over the obstacle, without any consistent changes during preceding steps (Drew 1993). In some of our experiments, however, gradual adaptations, which included changes in the direction of two consecutive strides, were seen as well (Fig. 3). These adaptations were very similar to a human’s strategy in approaching the obstacle. Usually, humans adjust a limited number of strides before negotiating an obstacle. It was reported that young adults preferentially adjust the length of the final stride to avoid the obstacle, while older adults prefer gradual adaptations in walking and usually adjust the length of two strides before the obstacle (Chen et al. 1994). When subjects approached a large, human-scaled obstacle on an initial path deviation occurred about six steps from the obstacle, but final large-scale adjustments of the walking trajectory occurred in the last two steps preceding the obstacle (Gérin-Lajoie et al. 2005). Analogous adaptations of strides were found in skilled long jumpers during the run-up. During acceleration, athletes maintained a stereotypical stride pattern up to the last few strides, with lengths varying considerably to secure accurate placement of the leading foot on the narrow takeoff board (Hay and Koh 1988; Lee et al. 1982).
Dissimilarities in the strategies for obstacle avoidance imply considerable differences in motor commands controlling adjustments of locomotor movements. Execution of the circumvention strategy suggests that the motor commands were forwarded toward the motoneuronal pools controlling muscles but bypassed the neural network of the spinal locomotor rhythm generator (McCrea and Rybak 2008; Rybak et al. 2006). This suggestion is supported by the findings that during avoidance of a single obstacle located on the wide walkway, the direction and, occasionally, the length of the strides changed but their duration and swing-to-stride duration ratio, which reflect the temporal structure of the stride, were preserved. In contrast, the strategy for stepping over the obstacle suggests that distinct descending motor commands were directed toward the locomotor rhythm generator. This suggestion is supported by the fact that the duration and swing-to-stride ratio, along with the length of the stride preceding the obstacle, changed, which indicated alterations in the timer of the generator.
The strategies for obstacle avoidance are not exclusive; they complement each other during walking in complex environments. During walking along the walkway cluttered with multiple obstacles (experiment 3), cats often modified temporal characteristics of the strides, altering the rhythm of the strides and/or the swing-to-stride duration ratio, both of which could happen either concurrently or independently. This suggests the existence of distinct motor commands that control the duration of the whole locomotor cycle and the duration of elements of the cycle. Frequently, changes in the temporal characteristics of strides were accompanied by changes in their spatial characteristics. Besides, in about a third of strides, cats changed only spatial characteristics of the strides, maintaining their temporal structure. We hypothesize that this flexibility in using different strategies for modifying walking was achieved because of concurrent processing of parallel motor commands that originated in distinct brain areas and targeted different groups of spinal neurons.
The ultimate goal of adaptations of locomotor movements during obstructed walking is to step at a safe distance away from the obstacle. When the environment allowed changes in the walking trajectory, as in the wide corridor obstructed with a single object, this distance was 1–1.5 diameters of the cat’s paw. Typically, this distance did not further increase, even though room for a larger deviation from the obstacle was available. In constrained environments, as in the narrow corridor, cats did not have the freedom to deviate far from the obstacle, but they were still able to place the paw at a distance of about a diameter of the paw away from the obstacle. While walking along the corridor cluttered with multiple obstacles, the distance between the paw and the obstacle decreased but was still about half of the diameter of the paw. This behavior is somewhat different from that of humans, whose limb movements during avoidance of small obstacles to a great extent are determined by a desire to minimize foot displacement (Moraes et al. 2004, 2007; Patla et al. 1999). Our data suggest that cats have an estimate of the secure distance between the paw and the obstacle. This distance is larger than the smallest possible displacement of the foot from the obstacle and is approximately equal to the diameter of the paw. Thus the preferred secure distance to the obstacle is scaled accordingly to the dimensions of the body. This is similar to the human strategy for avoidance of large, human-shaped obstructions. It was reported that during walking humans kept the same distance of approximately one-third of their step length away from both stationary and moving obstructions (Gérin-Lajoie et al. 2005). During circumvention of a large obstacle located at the midline, and left or right to the midline of the walkway, the distance between a subject and the obstacle was found to be ~0.2 m (Kolarik et al. 2016a, 2016b). The estimate of body-scaled distance between a subject and an obstacle during circumvention of a large obstacle appears to develop with age: in children this distance was reported to be 0.4 m, which is twice larger than in adults (Hackney et al. 2014). The distance between a subject and an obstacle varies with the visual perception of the environment. It was found that humans walking under conditions that impede orientation because of visual field limitations enlarge the step width to avoid collision with the obstacle (Jansen et al. 2011). It should be noted that during avoidance of the obstacle, cats were very accurate in not only defining a secure distance to the obstacle in the horizontal plane but in the vertical plane as well. When cats stepped over the obstacle they increased the paw elevation according to the height of the obstacle.
We have to acknowledge a few limitations of the study. First, during experiments our cats wore devices necessary for recording limb movements. To minimize the disruptive effect of these devices, the cats were habituated to wearing them. During recording sessions, cats appeared to walk normally. However, we cannot completely rule out the possibility that their behavior was affected by the garment. Still, we want to note that the cats wore the same garment during both unobstructed and obstructed walking, and thus the garment could not be the major reason for differences between the tasks. Second, in our study we did not address paw dominance, though there is evidence that cats can be either right or left pawed (Fabre-Thorpe et al. 1993; Tan and Kutlu 1991). It was reported that in cats the preferred paw performed reaching movements better than the nonpreferred paw (Lorincz and Fabre-Thorpe 1996). However, we did not assess how this difference affected accuracy in placing the paw away from the obstacle during walking.
In summary, the goal of obstacle avoidance during locomotion is to place the foot at a safe distance away from the obstacle. In cats, a preferred secure distance between the foot and the obstacle appears to be close to the size of the paw. The placement of the paw away from the obstacle can be accomplished with two main strategies: circumvention and stepping over the obstacle. During circumvention, only the stride direction and occasionally the length are changed, while the stride duration and swing-to-stride ratio are preserved. Stepping over the obstacle typically involves changes in two consecutive strides. The stride preceding the obstacle is shortened, and its duration and swing-to-stride ratio are reduced. The obstacle is negotiated with the next stride of increased height and normal duration and swing-to-stride ratio. The results suggest that stride adaptations for circumvention are achieved mainly because of motor commands directed toward neuronal circuits controlling musculature. Adaptations necessary for stepping over the obstacle are the result of motor commands to both neuronal circuits controlling muscles and networks of the spinal locomotor rhythm generator. The strategies for obstacle avoidance are not exclusive; rather, they complement each other during walking in complex environments. We suggest that obstacle avoidance can be considered as a task that includes choice between available neuro-motor strategies. Selection of the strategy depends on the environmental context and locomotor behavior preceding the negotiation of the obstacle and might be determined by minimizing the complexity of neuro-motor processes required to achieve the behavioral goal.
GRANTS
This study was supported by the Barrow Neurological Foundation and National Institute of Neurological Disorders and Stroke Grant R01 NS-058659 to I. N. Beloozerova.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
K.M.C., S.H.S., and V.M. performed experiments; K.M.C., S.H.S., and V.M. analyzed data; I.N.B. and V.M. edited and revised manuscript; V.M. conceived and designed research; V.M. interpreted results of experiments; V.M. prepared figures; V.M. drafted manuscript; V.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We are grateful to Michelle Heusser, Aakanksha Saxena, Anagha Deshpande, Trevor Kross, and Hai Tran for their assistance in collecting and analyzing the data.
REFERENCES
- Alexander RM. Optimum walking techniques for quadrupeds and bipeds. J Zool 192: 97–117, 1980. doi: 10.1111/j.1469-7998.1980.tb04222.x. [DOI] [Google Scholar]
- Alexander RM. Optimization and gaits in the locomotion of vertebrates. Physiol Rev 69: 1199–1227, 1989. [DOI] [PubMed] [Google Scholar]
- Alexander RM. Bipedal animals, and their differences from humans. J Anat 204: 321–330, 2004. doi: 10.1111/j.0021-8782.2004.00289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki S, Sato Y, Yanagihara D. Characteristics of leading forelimb movements for obstacle avoidance during locomotion in rats. Neurosci Res 74: 129–137, 2012. doi: 10.1016/j.neures.2012.07.007. [DOI] [PubMed] [Google Scholar]
- Arshavsky YI, Kots YM, Orlovsky GN, Rodionov IM, Shik ML. Investigation of the biomechanics of running by the dog. Biophysics (Oxf) 10: 737–746, 1965. [Google Scholar]
- Bauby CE, Kuo AD. Active control of lateral balance in human walking. J Biomech 33: 1433–1440, 2000. doi: 10.1016/S0021-9290(00)00101-9. [DOI] [PubMed] [Google Scholar]
- Berard JR, Vallis LA. Characteristics of single and double obstacle avoidance strategies: a comparison between adults and children. Exp Brain Res 175: 21–31, 2006. doi: 10.1007/s00221-006-0529-0. [DOI] [PubMed] [Google Scholar]
- Biewener AA. Patterns of mechanical energy change in tetrapod gait: pendula, springs and work. J Exp Zool A Comp Exp Biol 305: 899–911, 2006. doi: 10.1002/jez.a.334. [DOI] [PubMed] [Google Scholar]
- Chen HC, Ashton-Miller JA, Alexander NB, Schultz AB. Stepping over obstacles: gait patterns of healthy young and old adults. J Gerontol 46: M196–M203, 1991. doi: 10.1093/geronj/46.6.M196. [DOI] [PubMed] [Google Scholar]
- Chen HC, Ashton-Miller JA, Alexander NB, Schultz AB. Age effects on strategies used to avoid obstacles. Gait Posture 2: 139–146, 1994. doi: 10.1016/0966-6362(94)90001-9. [DOI] [Google Scholar]
- Chou LS, Kaufman KR, Brey RH, Draganich LF. Motion of the whole body’s center of mass when stepping over obstacles of different heights. Gait Posture 13: 17–26, 2001. doi: 10.1016/S0966-6362(00)00087-4. [DOI] [PubMed] [Google Scholar]
- D’Août K, Vereecke E, Schoonaert K, De Clercq D, Van Elsacker L, Aerts P. Locomotion in bonobos (Pan paniscus): differences and similarities between bipedal and quadrupedal terrestrial walking, and a comparison with other locomotor modes. J Anat 204: 353–361, 2004. doi: 10.1111/j.0021-8782.2004.00292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew T. Motor cortical activity during voluntary gait modifications in the cat. I. Cells related to the forelimbs. J Neurophysiol 70: 179–199, 1993. [DOI] [PubMed] [Google Scholar]
- Fabre-Thorpe M, Fagot J, Lorincz E, Levesque F, Vauclair J. Laterality in cats: paw preference and performance in a visuomotor activity. Cortex 29: 15–24, 1993. doi: 10.1016/S0010-9452(13)80208-0. [DOI] [PubMed] [Google Scholar]
- Fajen BR, Warren WH. Behavioral dynamics of steering, obstacle avoidance, and route selection. J Exp Psychol Hum Percept Perform 29: 343–362, 2003. doi: 10.1037/0096-1523.29.2.343. [DOI] [PubMed] [Google Scholar]
- Farrell BJ, Bulgakova MA, Beloozerova IN, Sirota MG, Prilutsky BI. Body stability and muscle and motor cortex activity during walking with wide stance. J Neurophysiol 112: 504–524, 2014. doi: 10.1152/jn.00064.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gérin-Lajoie M, Richards CL, McFadyen BJ. The negotiation of stationary and moving obstructions during walking: anticipatory locomotor adaptations and preservation of personal space. Mot Contr 9: 242–269, 2005. doi: 10.1123/mcj.9.3.242. [DOI] [PubMed] [Google Scholar]
- Goslow GE Jr, Reinking RM, Stuart DG. The cat step cycle: hind limb joint angles and muscle lengths during unrestrained locomotion. J Morphol 141: 1–41, 1973. doi: 10.1002/jmor.1051410102. [DOI] [PubMed] [Google Scholar]
- Hackney AL, Van Ruymbeke N, Bryden PJ, Cinelli ME. Direction of single obstacle circumvention in middle-aged children. Gait Posture 40: 113–117, 2014. doi: 10.1016/j.gaitpost.2014.03.005. [DOI] [PubMed] [Google Scholar]
- Hay JG, Koh TJ. Evaluating the approach in the horizontal jumps. Int J Sport Biomech 4: 372–392, 1988. doi: 10.1123/ijsb.4.4.372. [DOI] [Google Scholar]
- Jansen SE, Toet A, Werkhoven PJ. Human locomotion through a multiple obstacle environment: strategy changes as a result of visual field limitation. Exp Brain Res 212: 449–456, 2011. doi: 10.1007/s00221-011-2757-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolarik AJ, Scarfe AC, Moore BC, Pardhan S. An assessment of auditory-guided locomotion in an obstacle circumvention task. Exp Brain Res 234: 1725–1735, 2016a. doi: 10.1007/s00221-016-4567-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolarik AJ, Scarfe AC, Moore BC, Pardhan S. Echoic sensory substitution information in a single obstacle circumvention task. PLoS One 11: e0160872, 2016b. doi: 10.1371/journal.pone.0160872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krouchev N, Drew T. Motor cortical regulation of sparse synergies provides a framework for the flexible control of precision walking. Front Comput Neurosci 7: 83, 2013. doi: 10.3389/fncom.2013.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lajoie K, Drew T. Lesions of area 5 of the posterior parietal cortex in the cat produce errors in the accuracy of paw placement during visually guided locomotion. J Neurophysiol 97: 2339–2354, 2007. doi: 10.1152/jn.01196.2006. [DOI] [PubMed] [Google Scholar]
- Lavoie S, McFadyen B, Drew T. A kinematic and kinetic analysis of locomotion during voluntary gait modification in the cat. Exp Brain Res 106: 39–56, 1995. doi: 10.1007/BF00241355. [DOI] [PubMed] [Google Scholar]
- Lee DN, Lishman JR, Thomson JA. Regulation of gait in long jumping. J Exp Psychol Hum Percept Perform 8: 448–459, 1982. doi: 10.1037/0096-1523.8.3.448. [DOI] [Google Scholar]
- Lorincz E, Fabre-Thorpe M. Shift of laterality and compared analysis of paw performances in cats during practice of a visuomotor task. J Comp Psychol 110: 307–315, 1996. doi: 10.1037/0735-7036.110.3.307. [DOI] [PubMed] [Google Scholar]
- Manter JT. The dynamics of quadrupedal walking. J Exp Biol 15: 522–540, 1938. [DOI] [PubMed] [Google Scholar]
- Marlinski V, Chu KM, Seto SH, Foe MD, Tran HJ, Izady MH, Beloozerova IN. . Strategies for avoidance of small obstacles on the walking path (Abstract). Neuroscience Meeting Planner 2014: 831.01, 2014. [Google Scholar]
- McCrea DA, Rybak IA. Organization of mammalian locomotor rhythm and pattern generation. Brain Res Brain Res Rev 57: 134–146, 2008. doi: 10.1016/j.brainresrev.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadyen BJ, Lavoie S, Drew T. Kinetic and energetic patterns for hindlimb obstacle avoidance during cat locomotion. Exp Brain Res 125: 502–510, 1999. doi: 10.1007/s002210050708. [DOI] [PubMed] [Google Scholar]
- Mohagheghi AA, Moraes R, Patla AE. The effects of distant and on-line visual information on the control of approach phase and step over an obstacle during locomotion. Exp Brain Res 155: 459–468, 2004. doi: 10.1007/s00221-003-1751-7. [DOI] [PubMed] [Google Scholar]
- Moraes R, Allard F, Patla AE. Validating determinants for an alternate foot placement selection algorithm during human locomotion in cluttered terrain. J Neurophysiol 98: 1928–1940, 2007. doi: 10.1152/jn.00044.2006. [DOI] [PubMed] [Google Scholar]
- Moraes R, Lewis MA, Patla AE. Strategies and determinants for selection of alternate foot placement during human locomotion: influence of spatial and temporal constraints. Exp Brain Res 159: 1–13, 2004. doi: 10.1007/s00221-004-1888-z. [DOI] [PubMed] [Google Scholar]
- Murray MP. Gait as a total pattern of movement. Am J Phys Med 46: 290–333, 1967. [PubMed] [Google Scholar]
- Orlovsky GN. The effect of different descending systems on flexor and extensor activity during locomotion. Brain Res 40: 359–371, 1972. doi: 10.1016/0006-8993(72)90139-4. [DOI] [PubMed] [Google Scholar]
- Owings TM, Grabiner MD. Step width variability, but not step length variability or step time variability, discriminates gait of healthy young and older adults during treadmill locomotion. J Biomech 37: 935–938, 2004. doi: 10.1016/j.jbiomech.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Pandy MG, Kumar V, Berme N, Waldron KJ. The dynamics of quadrupedal locomotion. J Biomech Eng 110: 230–237, 1988. doi: 10.1115/1.3108436. [DOI] [PubMed] [Google Scholar]
- Patla AE, Greig M. Any way you look at it, successful obstacle negotiation needs visually guided on-line foot placement regulation during the approach phase. Neurosci Lett 397: 110–114, 2006. doi: 10.1016/j.neulet.2005.12.016. [DOI] [PubMed] [Google Scholar]
- Patla AE, Prentice SD, Rietdyk S, Allard F, Martin C. What guides the selection of alternate foot placement during locomotion in humans. Exp Brain Res 128: 441–450, 1999. doi: 10.1007/s002210050867. [DOI] [PubMed] [Google Scholar]
- Patla AE, Prentice SD, Robinson C, Neufeld J. Visual control of locomotion: strategies for changing direction and for going over obstacles. J Exp Psychol Hum Percept Perform 17: 603–634, 1991. doi: 10.1037/0096-1523.17.3.603. [DOI] [PubMed] [Google Scholar]
- Patla AE, Rietdyk S. Visual control of limb trajectory over obstacles during locomotion: effect of obstacle height and width. Gait Posture 1: 45–60, 1993. doi: 10.1016/0966-6362(93)90042-Y. [DOI] [Google Scholar]
- Patla AE, Robinson C, Samways M, Armstrong CJ. Visual control of step length during overground locomotion: task-specific modulation of the locomotor synergy. J Exp Psychol Hum Percept Perform 15: 603–617, 1989. doi: 10.1037/0096-1523.15.3.603. [DOI] [Google Scholar]
- Prilutsky BI, Sirota MG, Gregor RJ, Beloozerova IN. Quantification of motor cortex activity and full-body biomechanics during unconstrained locomotion. J Neurophysiol 94: 2959–2969, 2005. doi: 10.1152/jn.00704.2004. [DOI] [PubMed] [Google Scholar]
- Rho MJ, Lavoie S, Drew T. Effects of red nucleus microstimulation on the locomotor pattern and timing in the intact cat: a comparison with the motor cortex. J Neurophysiol 81: 2297–2315, 1999. [DOI] [PubMed] [Google Scholar]
- Rybak IA, Shevtsova NA, Lafreniere-Roula M, McCrea DA. Modelling spinal circuitry involved in locomotor pattern generation: insights from deletions during fictive locomotion. J Physiol 577: 617–639, 2006. doi: 10.1113/jphysiol.2006.118703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos LC, Moraes R, Patla AE. Visual feedforward control in human locomotion during avoidance of obstacles that change size. Mot Contr 14: 424–439, 2010. doi: 10.1123/mcj.14.4.424. [DOI] [PubMed] [Google Scholar]
- Sato Y, Aoki S, Yanagihara D. Gait modification during approach phase when stepping over an obstacle in rats. Neurosci Res 72: 263–269, 2012. doi: 10.1016/j.neures.2011.11.008. [DOI] [PubMed] [Google Scholar]
- Schulz BW. Healthy younger and older adults control foot placement to avoid small obstacles during gait primarily by modulating step width. J Neuroeng Rehabil 9: 69, 2012. doi: 10.1186/1743-0003-9-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan U, Kutlu N. The distribution of paw preference in right-, left-, and mixed pawed male and female cats: the role of a female right-shift factor in handedness. Int J Neurosci 59: 219–229, 1991. doi: 10.3109/00207459108985976. [DOI] [PubMed] [Google Scholar]
- Vilensky JA. Locomotor behavior and control in human and non-human primates: comparisons with cats and dogs. Neurosci Biobehav Rev 11: 263–274, 1987. doi: 10.1016/S0149-7634(87)80013-1. [DOI] [PubMed] [Google Scholar]
- Warren WH Jr, Young DS, Lee DN. Visual control of step length during running over irregular terrain. J Exp Psychol Hum Percept Perform 12: 259–266, 1986. doi: 10.1037/0096-1523.12.3.259. [DOI] [PubMed] [Google Scholar]
- Weerdesteyn V, Nienhuis B, Mulder T, Duysens J. Older women strongly prefer stride lengthening to shortening in avoiding obstacles. Exp Brain Res 161: 39–46, 2005. doi: 10.1007/s00221-004-2043-6. [DOI] [PubMed] [Google Scholar]
- Winter DA. Human balance and posture control during standing and walking. Gait Posture 3: 193–214, 1995. doi: 10.1016/0966-6362(96)82849-9. [DOI] [Google Scholar]
- Yakovenko S, McCrea DA, Stecina K, Prochazka A. Control of locomotor cycle durations. J Neurophysiol 94: 1057–1065, 2005. doi: 10.1152/jn.00991.2004. [DOI] [PubMed] [Google Scholar]
- Zijlstra W, Hof AL. Assessment of spatio-temporal gait parameters from trunk accelerations during human walking. Gait Posture 18: 1–10, 2003. doi: 10.1016/S0966-6362(02)00190-X. [DOI] [PubMed] [Google Scholar]
- Zijlstra W, Rutgers AW, Hof AL, Van Weerden TW. Voluntary and involuntary adaptation of walking to temporal and spatial constraints. Gait Posture 3: 13–18, 1995. doi: 10.1016/0966-6362(95)90804-2. [DOI] [Google Scholar]