Feedback from central pattern generator (CPG) circuits patterns activity of their projection neuron inputs. However, whether the intraburst firing rate between rhythmic feedback inhibition is also impacted by CPG feedback was not known. I establish that CPG feedback can alter the projection neuron intraburst firing rate through interactions with projection neuron intrinsic properties. The contribution of feedback to projection neuron activity level is specific to the modulatory condition, demonstrating a state dependence for this novel role of circuit feedback.
Keywords: central pattern generator, mechanosensory, modulation, postinhibitory rebound
Abstract
Central pattern generator (CPG) motor circuits underlying rhythmic behaviors provide feedback to the projection neuron inputs that drive these circuits. This feedback elicits projection neuron bursting linked to CPG rhythms. The brief periodic interruptions in projection neuron activity in turn influence CPG output, gate sensory input, and enable coordination of multiple target CPGs. However, despite the importance of the projection neuron activity level for circuit output, it remains unknown whether feedback also regulates projection neuron intraburst firing rates. I addressed this issue using identified neurons in the stomatogastric nervous system of the crab, Cancer borealis, a small motor system controlling chewing and filtering of food. Mechanosensory input triggers long-lasting activation of two projection neurons to elicit a chewing rhythm, during which their activity is patterned by circuit feedback. Here I show that feedback increases the intraburst firing rate of only one of the two projection neurons (commissural projection neuron 2: CPN2). Furthermore, this is not a fixed property because the CPN2 intraburst firing rate is decreased instead of increased by feedback when a chewing rhythm is activated by a different modulatory input. I establish that a feedback pathway that does not impact the CPN2 activity level in the control state inhibits CPN2 sufficiently to trigger postinhibitory rebound following mechanosensory stimulation. The rebound increases the CPN2 intraburst firing rate above the rate due only to mechanosensory activation of CPN2. Thus in addition to patterning projection neuron activity, circuit feedback can adjust the intraburst firing rate, demonstrating a novel functional role for circuit feedback to central projection neurons.
NEW & NOTEWORTHY Feedback from central pattern generator (CPG) circuits patterns activity of their projection neuron inputs. However, whether the intraburst firing rate between rhythmic feedback inhibition is also impacted by CPG feedback was not known. I establish that CPG feedback can alter the projection neuron intraburst firing rate through interactions with projection neuron intrinsic properties. The contribution of feedback to projection neuron activity level is specific to the modulatory condition, demonstrating a state dependence for this novel role of circuit feedback.
neural circuits, including central pattern generator (CPG) circuits underlying the generation of rhythmic behaviors, require functional flexibility to meet changing demands (Briggman and Kristan 2008; Marder and Bucher 2007). Different circuit outputs are elicited by a variety of sources, including circuit inputs projecting from higher order ganglia or brain regions to CPG circuits, such as brainstem neurons targeting spinal cord locomotor circuits (Berridge and Waterhouse 2003; Blitz et al. 1999; Nusbaum 2009; Ryczko and Dubuc 2013; Sharples et al. 2014). These central inputs, which are referred to as “descending projections” or “projection neurons” in rhythmic motor systems, are activated by sensory and other modulatory inputs (Briggman and Kristan 2008; Daghfous et al. 2016; Jordan et al. 2008; Marder 2012; Nusbaum 2009; Sharples et al. 2014; Stein 2009). Projection neurons alter circuit output via fast chemical and electrical synapses and modulatory effects on intrinsic and synaptic properties, all of which can be activity dependent (Briggman and Kristan 2008; Cazalis et al. 1985; Garcia et al. 2011; Marder 2012; Nadim and Bucher 2014; Nusbaum 2002; O’Brien 2014; Peng and Horn 1991; Vilim et al. 1996; Whim and Lloyd 1989). These projection neuron effects collectively activate and/or modulate CPG circuits. For instance, individual identified projection neurons activate swimming, crawling, and feeding-related circuits in leeches, crabs, and Aplysia and populations of brainstem neurons activate spinal locomotor circuits in zebrafish, lamprey, and rats (Brodfuehrer et al. 2008; Coleman and Nusbaum 1994; Dubuc et al. 2008; Hurwitz et al. 2005; Kyriakatos et al. 2011; Liu and Jordan 2005). Thus control of projection neuron activity is a key aspect in generating appropriate circuit output.
One regulator of projection neuron activity is CPG feedback. Across species, feedback from CPG circuits elicits projection neuron bursting linked to ongoing motor patterns (Arshavsky et al. 1988; Blitz and Nusbaum 2008; Buchanan and Einum 2008; Dubuc and Grillner 1989; Ezure and Tanaka 1997; Frost and Katz 1996). However, it is only more recently that the functional consequences of periodic interruptions in projection neuron activity, due to circuit feedback, have been identified. These functions include regulating intercircuit coordination, gating sensory signals, and contributing to projection neuron impact on circuit output (Antri et al. 2009; Blitz and Nusbaum 2008, 2012; Kozlov et al. 2014; Spencer and Blitz 2016; Wood et al. 2004). For example, locomotor circuit feedback phasically suppresses the influence of turning signals on lamprey reticulopsinal neurons, ensuring that turns only occur during behaviorally appropriate phases of locomotion (Kozlov et al. 2014). The projection neuron activity level, i.e., the intraburst firing rates between periodic interruptions due to feedback, also has important implications for circuit output. Specifically, intraburst firing rates can regulate the cycle period of rhythmic motor patterns, duration of a particular phase of a motor pattern, and motor neuron firing rates and burst durations (Bartos et al. 1999; Blitz et al. 2004; Jing and Weiss 2005; Severi et al. 2014; Spencer and Blitz 2016). It is unknown whether CPG feedback can regulate the projection neuron intraburst firing rate in addition to linking projection neuron activity to motor output.
The stomatogastric nervous system (STNS) is a preeminent model system for determining cellular mechanisms of neural circuit plasticity and provides unique advantages to studying circuit feedback (Marder and Bucher 2007; Stein 2009). For instance, CPG circuits for generating the pyloric (filtering of chewed food, ~1-s cycle period) and the gastric mill (chewing, ~10-s cycle period) rhythms are located within the stomatogastric ganglion (STG) and consist of a small number of identified neurons with known synaptic connections (Daur et al. 2016; Marder and Bucher 2007; Stein 2009). This includes two feedback neurons, the anterior burster (AB) and interneuron 1 (Int1) neurons, which each occur as a single copy (Fig. 1). AB and Int1 project from the STG through connecting nerves (stn and son) to the commissural ganglia (CoGs) to provide feedback to projection neurons in their ganglia of origin (Fig. 1). Descending projection neurons including modulatory commissural neuron 1 (MCN1) and commissural projection neuron 2 (CPN2) project to the STG to activate and modulate the pyloric and gastric mill motor patterns (Fig. 1) (Coleman and Nusbaum 1994; Norris et al. 1994). MCN1 and CPN2 are activated by multiple pathways including a population of mechanosensory neurons, the ventral cardiac neurons (VCNs) (Fig. 1A) (Beenhakker and Nusbaum 2004; Blitz et al. 2004, 2008; Hedrich et al. 2009). In vitro and in vivo, the VCNs are activated by pressure applied to the cardiac gutter, a structure located at the boundary between the storage and chewing compartments of the foregut (Beenhakker et al. 2004; Diehl et al. 2013). It is hypothesized that distention of the foregut storage compartment due to food intake activates the VCN neurons that in turn trigger chewing of food that will persist when food subsequently moves to the chewing compartment (Beenhakker et al. 2004). Brief VCN stimulation triggers a long-lasting (tens of minutes) activation of MCN1 and CPN2 (Beenhakker et al. 2004; Beenhakker and Nusbaum 2004; Beenhakker et al. 2007; Blitz and Nusbaum 2008). An increase in inward current in MCN1 and CPN2 parallels the long-lasting increased firing rate of these projection neurons after VCN stimulation (Blitz and Nusbaum 2007a). The VCN-triggered activation of MCN1 and CPN2 is necessary and sufficient for driving a long-lasting gastric mill rhythm, which is different from other gastric mill rhythm versions (Beenhakker et al. 2004; Beenhakker and Nusbaum 2004; Blitz et al. 2004, 2008; Diehl et al. 2013; White and Nusbaum 2011) (Fig. 1).
Fig. 1.
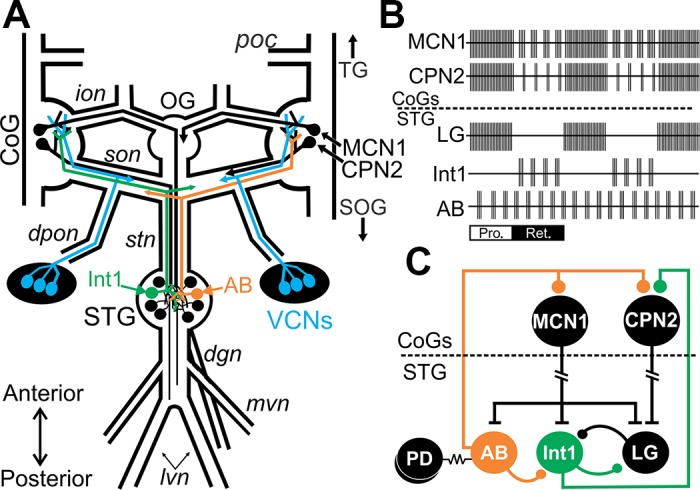
The mechanosensory VCN neurons trigger a gastric mill rhythm through activation of the projection neurons MCN1 and CPN2. A: schematic of the isolated stomatogastric nervous system showing somata locations and projection pathways of CPG interneurons (AB and Int1), projection neurons (MCN1 and CPN2), and the VCN sensory neurons. MCN1, CPN2, and VCN neurons occur bilaterally, but for clarity the full bilateral projection pathways are not drawn. B: schematic represents activity patterns of projection neurons, feedback neurons, and gastric mill circuit neuron LG during the VCN version of the gastric mill rhythm. AB feedback inhibition elicits pauses in MCN1/CPN2 activity during the retraction phase of VCN-gastric mill rhythms. During the protraction phase, MCN1 and CPN2 fire tonically due to presynaptic inhibition of AB within the CoGs (Beenhakker and Nusbaum 2004; Blitz and Nusbaum 2008). C: schematic partial circuit diagram illustrating connectivity among projection neurons, feedback neurons, and circuit neurons. AB and Int1 project from the STG to the CoGs where AB inhibits MCN1 and CPN2 (Blitz and Nusbaum 2008; Coleman and Nusbaum 1994) and Int1 inhibits CPN2 (Norris et al. 1994). The PD neurons are electrically coupled to the pyloric feedback neuron AB (Marder and Eisen 1984). LG inhibits the gastric mill feedback neuron Int1 (Bartos et al. 1999). T bars represent excitatory synapses, ball and stick represent inhibitory synapses, and resistor symbols represent electrical synapses. Break in MCN1 and CPN2 axons indicates additional distance between their somata and their STG terminals. Ganglia: CoG, commissural ganglion; OG, esophageal ganglion; SOG, supraesophageal ganglion; STG, stomatogastric ganglion; TG, thoracic ganglion. Neurons: AB, anterior burster; CPN2, commissural projection neuron 2; Int1, interneuron 1; LG, lateral gastric neuron; MCN1, modulatory commissural neuron 1; PD, pyloric dilator neuron; VCN, ventral cardiac neuron. Nerves: dgn, dorsal gastric nerve; dpon, dorsal posterior esophageal nerve; ion, inferior esophageal nerve; lvn, lateral ventricular nerve; mvn, medial ventricular nerve; poc, postesophageal commissure; son, superior esophageal nerve; stn, stomatogastric nerve. Other: Pro, protraction phase of the gastric mill rhythm; Ret, retraction phase.
In this study, activity in the two feedback pathways in this system was blocked to determine whether each pathway altered the MCN1 and CPN2 activity level during triggered gastric mill rhythms. Feedback selectively increased the CPN2 intraburst firing rate after stimulation of the mechanosensory VCN neurons but decreased the CPN2 intraburst firing rate after stimulation of a different modulatory pathway. The Int1 feedback pathway was ineffective in control conditions. However, after VCN stimulation, Int1 inhibited CPN2 sufficiently to trigger rebound firing and increase CPN2 intraburst activity. In contrast, hyperpolarzing current injection triggered rebound firing both in control conditions and after VCN stimulation. Thus in addition to feedback linking projection neuron activity to motor output, CPG feedback can determine the intraburst firing rate, an important component of projection neuron influence on CPG neurons.
MATERIALS AND METHODS
Animals.
Male Cancer borealis crabs were obtained from commercial suppliers (Fresh Lobster, Gloucester, MA; Ocean Resources, Sedgwick, ME). Crabs were maintained in commercial tanks containing recirculating, filtered, and aerated artificial seawater (10–12°C). Crabs were fed thawed squid pieces twice weekly. Before dissection, crabs were cold anesthetized by packing in ice (30–40 min). The STNS was dissected as described previously (Blitz et al. 2008; Gutierrez and Grashow 2009). Briefly, the foregut was removed from the animal, bisected, and pinned interior-side down in a Sylgard 170 (Fisher Scientific)-coated glass bowl in chilled C. borealis saline. The STNS, including all four ganglia plus their connecting and peripheral nerves (Fig. 1), was then freed from surrounding tissue, removed from the surface of the foregut, and pinned down in a Sylgard 184 (Fisher Scientific)-coated Petri dish. The foregut and nervous system were maintained in chilled (9–12°C) saline throughout the dissection and subsequent experiment.
Solutions.
C. borealis saline included the following (in mM): 440 NaCl, 26 MgCl2, 13 CaCl2, 11 KCl, 10 Trizma base, and 5 maleic acid (pH 7.4–7.6). High-divalent cation saline (HiDi saline) consisted of (in mM): 205 NaCl, 130 MgCl2, 65 CaCl2, 11 KCl, 10 Trizma base, and 5 maleic acid (pH 7.4–7.6). Lowered calcium saline with compensatory manganese to maintain divalent cation concentration (Low Ca2+ saline) consisted of the following (in mM): 440 NaCl, 26 MgCl2, 1.3 CaCl2, 11.7 MnCl2, 11 KCl, 10 Trizma base, and 5 maleic acid (pH 7.4–7.6). Picrotoxin (10−5 M; Sigma) was dissolved directly in saline before use.
Electrophysiology.
Extracellular recordings were made by placing one of a pair of stainless steel wires alongside a nerve and isolating a small region of the nerve with a petroleum jelly well (Vaseline) and placing the other wire in the main bath compartment. Extracellular nerve recordings were amplified using Model 1700 AC Amplifiers (AM Systems).
Intracellular microelectrodes were made from borosilicate glass filled with 0.6 M K2SO4 plus 10 mM KCl or 4 M KAc plus 10 mM KCl (20–25 MΩ). Intracellular signals were amplified using Axoclamp 2B and 900A amplifiers (Molecular Devices) in bridge mode, discontinuous current-clamp mode (3–10 kHz), or discontinuous single electrode voltage-clamp mode (10–15 kHz) and digitized at ~5 kHz using a Micro 1401 data acquisition interface and Spike2 software (Cambridge Electronic Design, Cambridge, UK).
To facilitate intracellular recordings, ganglia were desheathed and viewed with light transmitted through a darkfield condenser (Nikon Instruments). STG and CoG neurons were identified based on their activity patterns, synaptic connectivity, and axonal projection patterns (Beenhakker and Nusbaum 2004; Coleman and Nusbaum 1994; Norris et al. 1994; Weimann et al. 1991). CPN2 was recorded via intra-somatic recordings in the CoG or intra-axonal recordings at the entrance to the STG (CPN2stn) and was identified based on time-locked excitatory postsynaptic potentials (EPSPs) in a gastric mill motor neuron (GM) (Blitz and Nusbaum 1999; Blitz et al. 2004; Norris et al. 1994). CPN2 is the sole source of discrete EPSPs in GM (Norris et al., 1994). The VCN gastric mill rhythm was triggered by trains of extracellular stimulation of the dorsal posterior esophageal nerve (dpon) (Fig. 1; 10 trains of intraburst frequency: 15 Hz; interburst frequency: 0.06 Hz; burst duration: 6 s; and stimulus duration: 1 ms) (Beenhakker et al. 2004; Beenhakker and Nusbaum 2004; Blitz and Nusbaum 2008). The postesophageal commissure (POC) gastric mill rhythm was triggered by extracellular stimulation of the POC (Fig. 1; intraburst frequency: 15 Hz; duration: 30 s) (Blitz et al. 2008; Blitz and Nusbaum 2008, 2012).
To nonselectively block all circuit feedback HiDi saline or low Ca2+ saline was applied selectively to the STG. A Vaseline wall was used to isolate the STG from the anterior ganglia. HiDi saline raises action potential threshold, thereby suppressing spontaneous neuronal activity (Blitz and Nusbaum 1999). Low Ca2+ saline blocks transmitter release, removing modulatory input to the STG and decreasing activity of STG neurons (Beenhakker et al. 2007; Kirby and Nusbaum 2007). CPN2 firing rates measured after VCN stimulation with either low Ca2+ saline (n = 4) or HiDi saline (n = 6) applied to the STG were not different (t8 = 0.787, P = 0.454, t-test). Thus these data sets were combined. All other experiments blocking CPG feedback with altered saline used only HiDi saline.
To selectively eliminate the two circuit feedback pathways independently, intracellular current injections were used. To selectively eliminate feedback from the AB neuron, hyperpolarizing current was injected into the two pyloric dilator (PD) neurons, which are strongly electrically coupled to AB (Marder and Eisen 1984) (Fig. 1C). This is a routine method for eliminating AB activity (Bartos et al. 1999; Blitz and Nusbaum 2012; White and Nusbaum 2011). To control Int1 activity, hyperpolarizing or depolarizing current was injected into the lateral gastric (LG) neuron. Int1 is rhythmically active during ongoing pyloric rhythms and tonically active in the absence of pyloric rhythms (AB hyperpolarized) (Bartos et al. 1999). LG activity effectively silences the Int1 neuron (Bartos et al. 1999; DeLong et al. 2009). Thus maintaining LG activity eliminates Int1 activity and silencing LG allows Int1 activity to occur.
For measuring Int1 synaptic responses (current clamp) and synaptic currents (voltage clamp) in CPN2, PD neurons were hyperpolarized to eliminate AB feedback. In current-clamp experiments, CPN2 membrane potential post-VCN stimulation was matched to the resting potential prestimulation, while CPN2 was maintained at the same holding voltage pre- and post-VCN stimulation (−65 to −75 mV) in voltage-clamp experiments. In all cases, Int1 activity was controlled through manipulations of LG. In other experiments, steps of hyperpolarizing current (−0.5 nA, 6-s duration) were used to assay CPN2 responses to hyperpolarization independent of the Int1 synapse.
Data analysis.
Data analysis was performed using Spike2 (CED) software. Within each gastric mill cycle, the projection neuron intraburst firing rate was measured separately for protraction (LG active) and retraction (LG inactive) phases. LG action potentials with intervals of <2 s were considered to be part of a burst (Diehl et al. 2013). With feedback intact, the intraburst projection neuron firing rates were measured across 10 gastric mill cycles. For 4 out of the 42 gastric mill rhythms analyzed, 5–9 cycles were analyzed due to other manipulations occurring before a full set of 10 cycles. Projection neuron activity consisted of pyloric-timed bursts during VCN-retraction, POC-protraction, and POC-retraction phases. During VCN-protraction, MCN1 and CPN2 lose their pyloric timing and fire continuously throughout the protraction phase (Beenhakker and Nusbaum 2004; Blitz and Nusbaum 2008, 2012). Thus there was a single MCN1/CPN2 burst during VCN-protraction but multiple pyloric-timed bursts during VCN-retraction, POC-protraction, and POC-retraction phases. Projection neuron intraburst frequency was measured as the number of action potentials in a burst minus one divided by the duration of that MCN1 or CPN2 burst (Beenhakker and Nusbaum 2004; Blitz and Nusbaum 2008, 2012). Bursts with only one action potential were not included in this analysis. With feedback blocked, there was no gastric mill rhythm and thus the average firing rate was quantified across a duration (40–100 s) similar to the duration of 10 gastric mill cycles. Analysis of feedback intact intraburst firing rate and no-feedback firing rate was performed with custom written scripts for Spike 2 (freely available: http://stg.rutgers.edu/Resources.html). The average intraburst MCN1 or CPN2 firing rate during VCN and POC gastric mill rhythms, or their average rate in the absence of feedback from each preparation, was then averaged across preparations. The firing rate of only one MCN1 or CPN2 was measured in each preparation.
In some experiments, the CPN2 firing rate was determined by measuring EPSPs in a GM neuron as noted. For these experiments, one CoG was cut away (one ion and one son transected; Fig. 1A) leaving only one CPN2 intact. CPN2 is the only source of discrete EPSPs in the GM neurons. Thus with one CoG intact, EPSPs in GM are a reliable measure of CPN2 activity (Blitz and Nusbaum 1999; Blitz et al. 2008; Norris et al. 1994). To facilitate threshold detection and viewing of CPN2 elicited EPSPs in GM, recordings were filtered in Spike2 software to remove the slow component of the GM waveform.
To examine the decay of the feedback effects on the CPN2 firing rate, feedback from AB, Int1, or both was eliminated for 60 s. During elimination of AB feedback, a gastric mill rhythm continued and thus the CPN2 intraburst firing rate was measured during the first four seconds of the protraction phase closest to the indicated time points. For instance, the control CPN2 intraburst firing rate was measured during the last protraction phase before a manipulation, the protraction phase closest to 10 s after AB feedback was eliminated, and the protraction phase closest to 20 s after AB feedback was eliminated and so on. The onset of protraction occurred within a few seconds of the indicated time point. Elimination of Int1 feedback eliminated the gastric mill rhythm because Int1 is part of the half-center rhythm generator circuit (Marder and Bucher 2007; White and Nusbaum 2011). In this condition, the average CPN2 firing rate was measured in 4-s epochs beginning at each time point, i.e., at 10 s after elimination of Int1 feedback, and the average CPN2 firing rate was measured across a 4-s window, again at 20 s, and so on.
To measure Int1 synaptic current in CPN2 or the effects of Int1 on CPN2 membrane potential, AB feedback was eliminated and Int1 active and Int1 inactive epochs of 6 and 4 s were alternated to mimic Int1 timing during gastric mill rhythms. The CPN2 firing rate was measured in 4-s time blocks before, during, and after Int1 activation. The average voltage or current during Int1 active epochs was subtracted from the average voltage or current during the preceding Int1 inactive epoch to quantify the change in membrane potential or the Int1 synaptic current. For each preparation, the response to three repetitions of a manipulation were averaged. To determine if there were discrete spike-elicited synaptic currents, Int1 action potential timing was determined by the timing of Int1-elicited inhibitory postsynaptic potentials (IPSPs) in the LG neuron. Int1 is the sole source of IPSPs in LG (Bartos et al. 1999). Inhibitory postsynaptic currents (IPSCs) in CPN2 were detected by overlaying multiple sweeps (>25 per recording) aligned to the timing of Int1-elicited action potentials.
To determine sensitivity of the Int1 to CPN2 synapse to CPN2 membrane potential, the average membrane potential in current-clamp recordings, or the average holding current in voltage-clamp recordings was measured during Int1 off and Int1 on epochs after VCN stimulation. In each experiment, the difference between Int1 off and Int1 on was normalized to the membrane potential or holding current during Int1 off.
To quantify the response of CPN2 to hyperpolarizing current injection, the average firing rate for the 4 s before and after the step was measured (number of action potentials minus one divided by 4 s). To compare the extent to which CPN2 displayed rebound after a current step in control vs. post-VCN stimulation, the postinhibitory rebound area (“PIR area”) was measured (Angstadt et al. 2005; Angstadt and Simone 2014). PIR area was measured as the area under the curve (CPN2 membrane potential) with the average precurrent step CPN2 membrane potential (4-s precurrent step) set as the baseline. The area was measured beginning at 200 ms after the end of the step to allow the membrane potential to recover back to the prestep membrane potential (Angstadt and Simone 2014) through to 4-s postcurrent step. Figures were made using Spike2, CorelDraw (vX6; Corel), and SigmaPlot (v13; Systat). Statistical significance was assessed with SigmaPlot or SAS for Windows (v9.4). Each data set was tested for normality (Shapiro-Wilk test) to determine whether a parametric or nonparametric test was to be used. One-way ANOVA, one-way repeated-measures ANOVA test, one-way ANOVA on ranks, two-way within-subject ANOVA (PROC MIXED in SAS), Mann-Whitney rank sum test, t-test, or paired t-tests were performed as indicated. Reported n values are the number of neurons, which coincides with the number of preparations. Significance was considered to be P < 0.05. Data are expressed as means ± SE.
RESULTS
CPG feedback patterns MCN1 and CPN2 activity.
During a VCN-triggered gastric mill rhythm, projection neuron activity is patterned by circuit feedback with distinctions between the protraction (during LG burst) and retraction (LG interburst) phases (Beenhakker and Nusbaum 2004; Blitz and Nusbaum 2008). Specifically, the pyloric interneuron AB projects to the CoGs where it inhibits MCN1 and CPN2. This results in pyloric-timed (~1 s cycle period) interruptions in their activity during the retraction phase of the rhythm (Fig. 1B) (Beenhakker and Nusbaum 2004; Blitz and Nusbaum 2008; Coleman and Nusbaum 1994). However, during the protraction phase, presynaptic inhibition of AB eliminates AB feedback (Blitz and Nusbaum 2008). The gastric mill interneuron Int1 has activity linked to both the pyloric and gastric mill rhythms. In the presence of an applied neuromodulator, Int1 inhibits CPN2 during each retraction phase (Fig. 1, B and C) (Norris et al. 1994), but little is known about its effects during gastric mill rhythms triggered by sensory inputs including the VCNs. There is no evidence of rhythmic Int1 actions on MCN1 (Coleman MJ, Nusbaum MP, Blitz DM, unpublished observations).
Regulation of intraburst firing rate.
The question to be addressed here was whether CPG feedback regulates the projection neuron intraburst firing rate in addition to eliciting rhythmic interruptions in projection neuron activity. To address this question, I compared the VCN-triggered intraburst firing rate of MCN1 and CPN2 in control to their VCN-triggered firing rate after blocking rhythmic AB and Int1 feedback. Feedback was blocked by selectively superfusing the STG with high-divalent cation saline (HiDi) or low Ca2+ saline (see materials and methods) (Beenhakker et al. 2007; Blitz and Nusbaum 1999; Kirby and Nusbaum 2007).
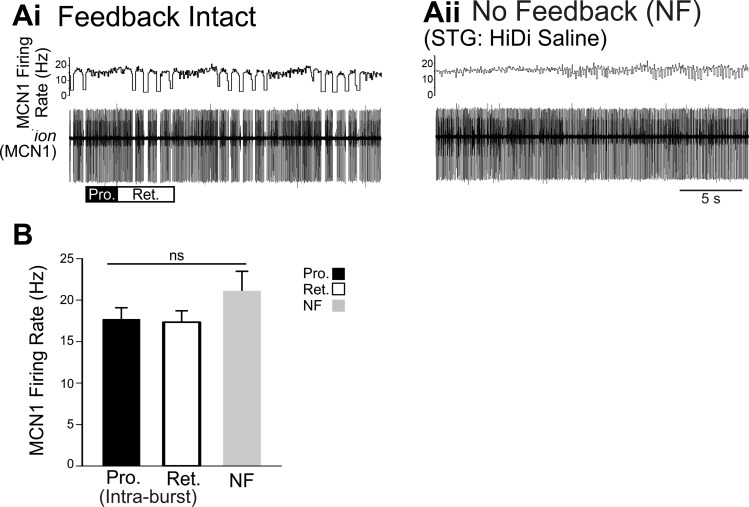
Brief stimulation of the mechanosensory VCN neurons (ten 15-Hz 6-s bursts at 0.06 Hz) triggered tonic MCN1 activity during VCN-protraction but pyloric-timed bursts of activity during VCN-retraction (Beenhakker and Nusbaum 2004; Blitz and Nusbaum 2008) (Fig. 2). In the same preparation when feedback was blocked with HiDi saline applied selectively to the STG, VCN stimulation again triggered activation of MCN1. MCN1 firing pattern was impacted by eliminating CPG feedback [No-Feedback (NF)] (Blitz et al. 2008; White and Nusbaum 2011), whereas the MCN1 firing rate was not altered. In the experiment shown, the MCN1 intraburst firing rate was 16 Hz during protraction and 16 Hz during retraction (Fig. 2Ai). In the absence of feedback, MCN1 fired at 16 Hz (Fig. 2Aii). Across preparations, there was no difference between the average MCN1 intraburst firing rate during VCN-protraction, VCN-retraction, or its firing rate in the absence of feedback (Fig. 2B) (Pro/Ret: n = 9; NF: n = 8; F2,21 = 1.341, P = 0.281, one-way ANOVA). Thus eliminating CPG feedback eliminated the patterning of MCN1 firing but the MCN1 firing rate in the absence of feedback did not differ from the MCN1 intraburst firing rate with feedback intact.
Fig. 2.
When activated by the mechanosensory VCNs, MCN1 intraburst firing rate is not regulated by CPG feedback. A: in an example experiment after VCN stimulation with feedback intact (Ai), MCN1 fired tonically during VCN protraction (Pro., black bar) at ~16 Hz and with a pyloric-timed intraburst rate of ~16 Hz during retraction (Ret., white bar). MCN1 activity was recorded in an extracellular recording of a connecting nerve that it projects through (ion). The instantaneous MCN1 firing rate is plotted above. After VCN stimulation in the absence of feedback (Aii) the average MCN1 firing rate was ~16 Hz. B: across preparations, the average MCN1 intraburst firing rate during protraction (Pro., black bar, n = 9) and retraction (Ret., white bar, n = 9) was not different from the average firing rate in the no-feedback condition (NF, gray bar, n = 8). The retraction intraburst firing rate does not include the pyloric-timed interruptions in firing. nsP > 0.05.
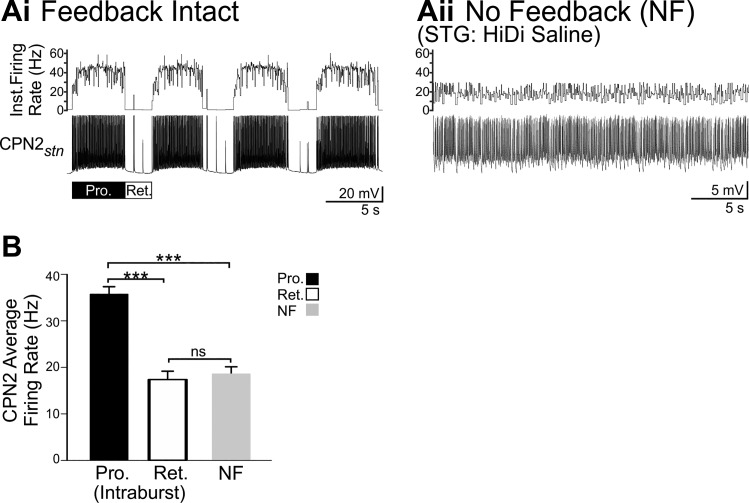
Similar to MCN1, VCN stimulation triggered tonic CPN2 firing during the protraction phase of the VCN-gastric mill rhythm (Beenhakker and Nusbaum 2004; Blitz and Nusbaum 2008) at a peak rate of ~38 Hz in the example shown (Fig. 3Ai). During VCN-retraction, there was weak pyloric-timed CPN2 activity (Fig. 3Ai) (Beenhakker and Nusbaum 2004; Blitz and Nusbaum 2008). VCN stimulation also triggered activation of CPN2 when feedback was blocked with HiDi saline. However, when CPG feedback was blocked, the gastro-pyloric patterning of CPN2 firing was not present after VCN stimulation, and notably, the CPN2 firing rate was lower (average ~16 Hz in an example recording) (Fig. 3Aii) compared with the intraburst firing rate occurring during VCN-protraction with feedback intact. The variability in instantaneous firing rate likely resulted from other synaptic input to CPN2 that remains intact in the absence of feedback. For instance, the anterior gastric receptor (AGR) sensory neuron has multiple centrally located spike initiation zones that are active in vitro (Smarandache et al. 2008; Städele and Stein 2016). AGR elicits electrical excitatory postsynaptic potentials in CPN2, which can elicit CPN2 action potentials (Hedrich et al. 2009; Norris et al. 1994).
The average CPN2 firing rate in the absence of feedback was lower than the intraburst CPN2 firing rate during the protraction phase of VCN gastric mill rhythms (Pro: n = 13; NF: n = 10; F2,31 = 36.967, P < 0.001, one-way ANOVA, Holm-Sidak post hoc test). However, the average intraburst firing rate during VCN-retraction and the average firing rate in the absence of feedback were similar (Ret: n = 11; NF: n = 10; F2,31 = 36.967, P = 0.637; Fig. 3B). This was the case despite fewer action potentials during VCN retraction compared with VCN-no feedback (VCN-ret.: 238 ± 47 spikes, n = 13; VCN-NF: 1,361 ± 166 spikes; n = 10; T10,13 = 185.000, P < 0.001, Mann-Whitney rank sum test). This indicates that when action potentials did occur during VCN-retraction, they occurred with a similar interspike interval to those during the no-feedback condition. As indicated qualitatively in previous studies, the average CPN2 intraburst firing rate was higher during the protraction phase of VCN gastric mill rhythms compared with retraction (Ret: n = 11; NF: n = 10, F2,31 = 36.967, P < 0.001, one-way ANOVA, Holm-Sidak post hoc test; Fig. 3B) (Beenhakker et al. 2004; Beenhakker and Nusbaum 2004). Thus the CPN2 intraburst firing rate when triggered by VCN stimulation, specifically during VCN-protraction, was influenced by CPG feedback.
Fig. 3.
When activated by the mechanosensory VCNs, CPN2 intraburst firing rate is altered by CPG feedback. A: in an example experiment after VCN stimulation with feedback intact (Ai), CPN2 fired at ~38 Hz during protraction (Pro., black bar) and fired only a few action potentials during retraction (Ret., white bar). CPN2 was recorded via intra-axonal impalement in the nerve (stn) it projects through just before entering the STG. CPN2 instantaneous firing rate is plotted above the traces. After VCN stimulation in the absence of feedback (Aii), CPN2 fired at an average frequency of ~16 Hz. The variability in instantaneous firing rate likely resulted from other synaptic input to CPN2 including the AGR sensory neuron which remained spontaneously active in the absence of feedback (Hedrich et al. 2009; Norris et al. 1994; Smarandache et al. 2008; Städele and Stein 2016). B: across preparations, the average CPN2 intraburst firing rate was higher during VCN protraction (Pro., black bar, n = 13) than VCN retraction (Ret., white bar, n = 11) and higher during protraction than its average firing rate in the no-feedback condition (NF, gray bar, n = 10). The retraction intraburst firing rate does not include the pyloric-timed interruptions in firing. ***P < 0.01; nsnonsignificant.
It was possible that the selective increase of the CPN2 intraburst firing rate by CPG feedback was a fixed occurrence in response to any physiological activation of CPN2, which elicits a gastric mill rhythm. Alternatively, regulation of CPN2 activity level by feedback might be a flexible component that differs between motor patterns. To distinguish between these possibilities, I tested whether CPG feedback regulates the CPN2 intraburst firing rate during production of a different gastric mill rhythm triggered by a population of peptidergic neurons, the POC neurons (Blitz et al. 2008).
Brief POC stimulation also triggers a long-lasting increase in MCN1 and CPN2 activity which drives a gastric mill rhythm (Blitz et al. 2008; Blitz and Nusbaum 2008, 2012). Similar to VCN stimulation, the POC-triggered increase in MCN1 and CPN2 activity is paralleled by a long-lasting increase in inward current (Blitz and Nusbaum 2007a). The MCN1 and CPN2 activity patterns, the gastric mill motor pattern, and the resulting muscle and tooth movements all differ between the POC- and VCN-triggered gastric mill rhythms (Beenhakker and Nusbaum 2004; Blitz et al. 2008; Blitz and Nusbaum 2008; Diehl et al. 2013; White and Nusbaum 2011). These unique features of the two rhythms arise from differences in the regulation of CPG feedback to the projection neurons. Specifically, the presynaptic inhibition of AB occurring during VCN-protraction does not occur during POC-protraction (Blitz and Nusbaum 2008). This results in MCN1 and CPN2 activity being interrupted in pyloric time by AB feedback during both retraction and protraction phases of the POC gastric mill rhythm (Figs. 4 and 5) (Blitz et al. 2008; Blitz and Nusbaum 2008, 2012).
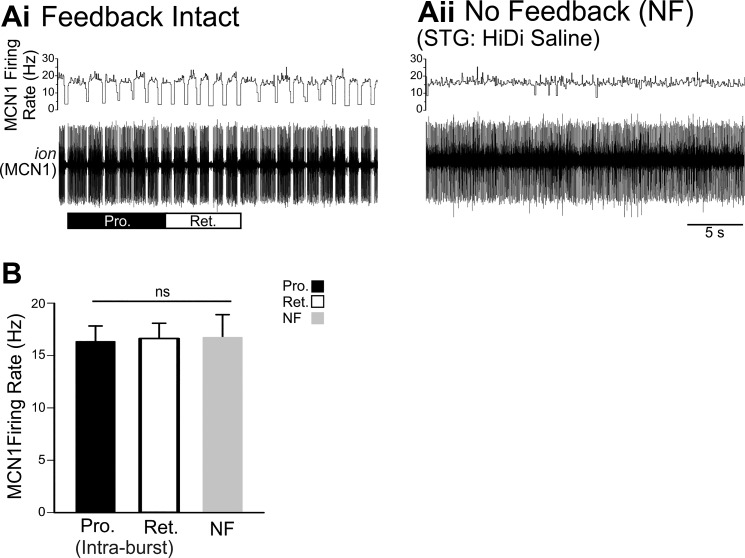
Fig. 4.
When activated by POC stimulation, MCN1 intraburst firing rate is not regulated by circuit feedback. Ai: when triggered by POC stimulation, MCN1 activity is regulated by pyloric-timed AB inhibition during both the protraction and retraction phases. MCN1 was recorded extracellularly in the ion. Aii: in the absence of circuit feedback, pyloric-timed interruptions in MCN1 firing were eliminated. B: across preparations, there was no difference between the MCN1 intraburst firing rate during the protraction and retraction phases of POC-triggered gastric mill rhythms (n = 8) or its average firing rate in the absence of circuit feedback (n = 6). Pyloric-timed interruptions in firing during protraction and retraction were not included in the intraburst firing rates. nsP > 0.05.
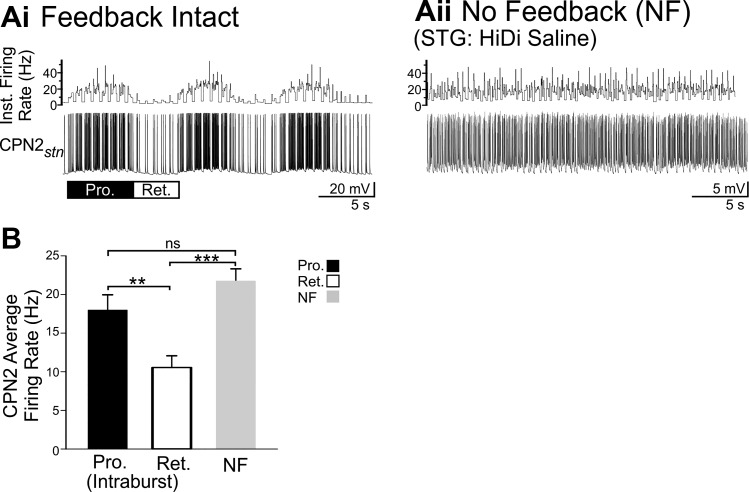
Fig. 5.
When activated by POC stimulation, CPN2 intraburst firing rate is regulated by circuit feedback but differently than when activated by VCN. Ai: when triggered by POC stimulation, CPN2 activity is rhythmically interrupted by pyloric-timed AB inhibition during both the protraction and retraction phases. CPN2 was recorded via intra-axonal impalement in the nerve (stn) it projects through just before entering the STG. Aii: in the absence of circuit feedback, CPN2 fired at an average rate of ~21 Hz, similar to the CPN2 intraburst firing rate during POC protraction. Similar to Fig. 3Aii, CPN2 did not fire at a steady rate in the absence of feedback, likely due to synaptic input including from the AGR sensory neuron (Hedrich et al. 2009; Norris et al. 1994). B: across preparations, the CPN2 intraburst firing rate was higher during protraction than retraction phases of POC-triggered gastric mill rhythms (n = 12) and the average firing rate in the absence of circuit feedback (n = 7) was higher than the intraburst retraction firing rate. Pyloric-timed interruptions in firing during protraction and retraction were not included in the intraburst firing rates. **P < 0.01, ***P < 0.001, nsP > 0.05.
Similar to VCN stimulation, after POC stimulation, the MCN1 intraburst firing rate was not affected by CPG feedback. Pyloric-timed interruptions in MCN1 firing, present during both POC-protraction and POC-retraction (Fig. 4Ai), were eliminated in the no-feedback condition (Fig. 4Aii). However, there was no difference between the MCN1 intraburst firing rate during POC gastric mill rhythms compared with the firing rate in the no-feedback condition (POC-Pro/Ret: n = 8, POC-NF: n = 6, F2,19 = 20.154, P = 0.985, one-way ANOVA; Fig. 4B).
Feedback increased the CPN2 intraburst firing rate after VCN stimulation, but this was not the case after POC stimulation. CPN2 was activated in a pyloric-timed pattern during both protraction and retraction after POC stimulation (15 Hz, 30 s; Blitz et al. 2008; Blitz and Nusbaum 2008, 2012), with a higher intraburst firing rate during protraction than retraction (Fig. 5; POC-Pro/Ret: n = 12; F2,28 = 9.571, P = 0.007, one-way ANOVA, Holm-Sidak post hoc). In the absence of feedback, POC stimulation triggered CPN2 firing without the rhythmic pyloric-timed interruptions (Fig. 5Aii). There were periodic brief increases in the CPN2 firing rate in the absence of feedback. These coincided with spontaneous action potentials in the AGR sensory neuron. In contrast to CPN2 activity after VCN stimulation, across preparations the CPN2 POC-protraction intraburst firing rate was not different from its firing rate in the absence of feedback (POC-Pro: n = 12; POC-NF: n = 7; F2,28 = 9.571, P = 0.180, one-way ANOVA, Holm-Sidak post hoc; Fig. 5B). However, the firing rate in the absence of feedback was higher than during POC-retraction (POC-Ret: n = 12; POC-NF: n = 7; F2,28 = 9.571, P < 0.001, one-way ANOVA, Holm-Sidak post hoc). Thus the CPN2 intraburst firing rate is not regulated by circuit feedback in the same manner simply upon activation of the gastric mill CPG. Instead there is a state dependence, such that during long-lasting activation of a VCN gastric mill rhythm, but not a POC gastric mill rhythm, the CPN2 protraction intraburst firing rate is increased by the presence of CPG feedback. During POC gastric mill rhythms, the CPN2 intraburst firing rate during the retraction phase is lower with feedback intact than without feedback. This indicates that the manner in which CPG feedback regulates projection neuron activity level is specific to the modulatory state of a motor system. The regulation of the intraburst firing rate described here is independent of feedback rhythmically interrupting projection neuron activity. Thus I aimed to determine what underlies selective state-dependent regulation of the intraburst firing rate of a projection neuron by CPG feedback. Specifically in this study, I investigated the mechanism by which CPG feedback increased the intraburst firing rate in CPN2 during a VCN-triggered gastric mill rhythm.
Int1 feedback inhibition.
Int1 selectively inhibiting CPN2 and not MCN1 (Fig. 1C) (Norris et al. 1994) could explain the sensitivity of the VCN-triggered CPN2 but not MCN1 intraburst firing rate to removal of feedback. To test this, AB and Int1 feedback were independently eliminated. Specifically, AB activity was eliminated through hyperpolarization of the electrically coupled PD neurons, and Int1 activity was eliminated by depolarization of the LG neuron, which inhibits Int1 (Fig. 1C) (see materials and methods). For this data set, the CPN2 firing rate was measured by recording CPN2-elicited EPSPs in a GM neuron (see materials and methods).
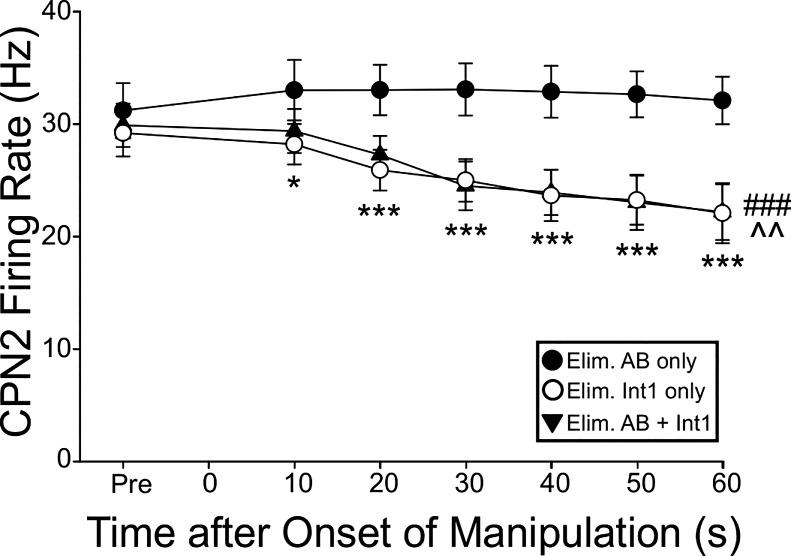
Hyperpolarizing the PD neurons to eliminate AB activity did not alter the CPN2 firing rate. Relative to the last protraction phase before eliminating AB activity, there was no difference in the CPN2 protraction intraburst firing rate, assayed every 10 s up to 60 s (n = 6, F6,100 = 0.28, P = 0.945, Two-way within-subject ANOVA; Fig. 6). Conversely, when LG was depolarized to eliminate Int1 activity, which consequently eliminated the gastric mill rhythm, the CPN2 firing rate decreased (n = 6; F6,100 = 4.47, P = 0.0011, two-way within-subject ANOVA; Fig. 6). Eliminating both AB and Int1 feedback similarly decreased the CPN2 firing rate (n = 6, F6,100 = 4.47, P < 0.0001). The CPN2 firing rate was impacted from 10 s through 60 s after feedback was eliminated (n = 6, F2,100 = 3.369–19.47, P = <0.0001 to 0.029; Fig. 6). Thus the CPN2 firing rate is specifically regulated by feedback from Int1.
Fig. 6.
When activated by VCN stimulation, CPN2 firing rate is regulated only by Int1 feedback. CPN2 firing rate is plotted against time after only AB feedback was eliminated (filled circles, n = 6), only Int1 feedback was eliminated (open circles, n = 6), or both AB and Int1 feedback were eliminated (filled triangles, n = 6). Eliminating Int1 alone, or Int1 plus AB feedback decreased CPN2 firing rate to the same extent. Eliminating only AB feedback did not alter CPN2 firing rate. ^^P < 0.01, Int1 feedback eliminated; ###P < 0.001, Int1 + AB feedback eliminated; *P < 0.05, ***P < 0.001, feedback eliminated at indicated time after onset.
Int1 is reciprocally active with LG and thus can only act on CPN2 during each retraction phase (Fig. 1C). In the presence of a muscarinic acetylcholine receptor agonist, Int1 inhibits CPN2 (Norris et al. 1994). However, it was anecdotally noted that effects of Int1 on CPN2 were rarely observed in normal saline suggesting that Int1 inhibition of CPN2 only occurs during specific modulatory conditions. Thus I examined the influence of Int1 on CPN2 under control conditions and after VCN stimulation.
VCN modulation of Int1 feedback.
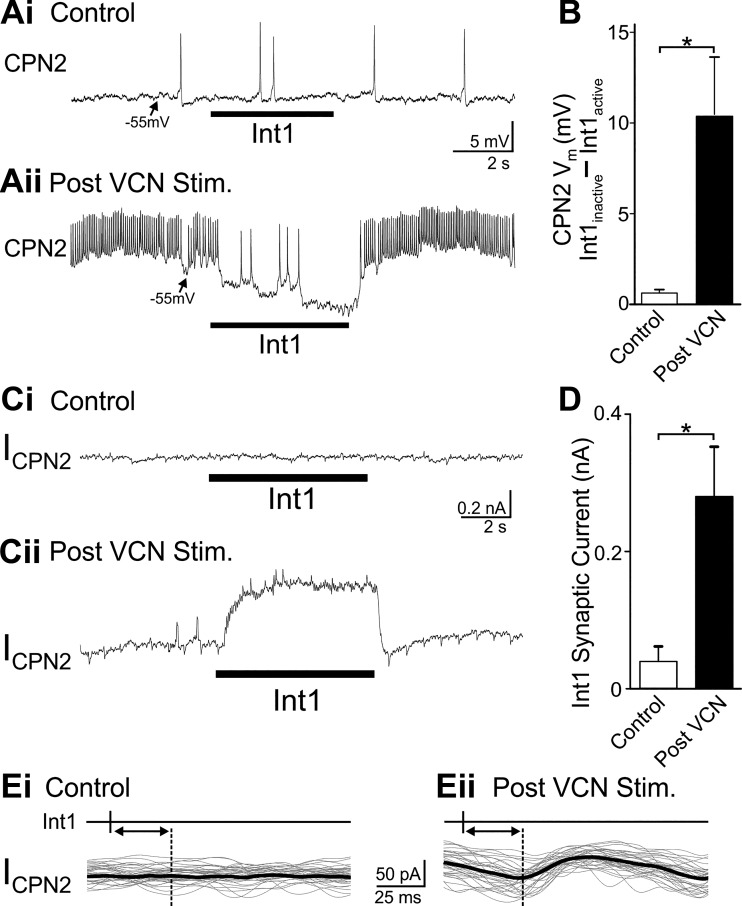
VCN stimulation altered the influence of Int1 feedback on CPN2. To examine Int1 feedback in isolation, AB feedback was eliminated by hyperpolarizing the two PD neurons. In the absence of a pyloric rhythm, Int1 is spontaneously tonically active (Bartos et al. 1999). Rhythmic LG depolarizations (4 s; Int1 inactive) and hyperpolarizations (6 s; Int1 active) were used to mimic gastric mill timing of Int1. In an example current-clamp recording, there was no evident Int1 inhibition of CPN2 in control conditions (Fig. 7Ai). However after VCN stimulation, Int1 activity hyperpolarized CPN2 relative to when Int1 was inactive (Fig. 7Aii). The AB feedback neuron elicits one-for-one spike-mediated IPSPs in CPN2 (Blitz and Nusbaum 2008). However, discrete one-for-one Int1 IPSPs are typically not evident in CPN2 (Norris et al. 1994). Transmission at this synapse appears to occur primarily through graded transmitter release as occurs at synapses in the STG and other systems (Alle and Geiger 2008; Graubard et al. 1980; Raper 1979; Shu et al. 2006). Thus, to compare Int1 synaptic strength in the two conditions, the average membrane potential during Int1 active and inactive time blocks was quantified. In control conditions, there was a 0.6 ± 0.2 mV change in CPN2 membrane potential between when Int1 was active and inactive (n = 6, t5 = −3.288, P = 0.021, paired t-test). After VCN stimulation, the average CPN2 membrane potential was more hyperpolarized during Int1 active periods relative to Int1 inactive (n = 6, t5 = −3.092, P = 0.027). The larger difference in CPN2 membrane potential between Int1 active and inactive time blocks after VCN stimulation was indicative of strengthened Int1 to CPN2 inhibition (n = 6, t5 = −3.053, P = 0.028, paired t-test; Fig. 7B).
Fig. 7.
Int1 inhibition of CPN2 is strengthened after VCN stimulation. Ai: there was no evident change in CPN2 membrane potential elicited by Int1 activity in control conditions. However, after VCN stimulation (Aii) Int1 elicited a hyperpolarization in CPN2 and decreased its spiking activity. B: across preparations, the difference in CPN2 membrane potential between Int1 inactive and Int1 active periods was larger after VCN stimulation (n = 6). Ci: in voltage-clamp recordings there was no evident Int1-elicited synaptic current in CPN2 in control. Cii: after VCN stimulation, Int1 elicited an outward current. D: the average Int1-elicited synaptic current was greater after VCN stimulation compared with control conditions (n = 6). Ei and Eii: sets of 33 overlaid traces with the thick black line indicating the average current in each condition. Traces were triggered by the timing of an Int1 action potential indicated above the traces. In control conditions there was no evident inhibitory postsynaptic current (IPSC) recorded after Int1 action potentials (Ei). After VCN stimulation, there was a reliably occurring IPSC following each Int1 action potential (Eii). The dotted line and double arrow indicates the delay between Int1 action potential and onset of the IPSC in Eii and the same delay in Ei. *P < 0.05.
It does not appear that the enhanced influence of Int1 on CPN2 after VCN stimulation is due to modulation of Int1 activity. The number of Int1 action potentials per pyloric-timed burst was not statistically different before vs. after VCN stimulation (Pre-VCN, 7.8 ± 0.9, Post-VCN, 11.1 ± 0.4, n = 6; F3,14 = 7.469, P = 0.059, one-way ANOVA, Holm-Sidak post hoc), but there was an increase in the Int1 intraburst firing rate elicited by VCN stimulation (Pre-VCN, 12.5 ± 1.0 Hz, Post-VCN, 19.6 ± 0.9 Hz, n = 6; H3 = 12.456, P = 0.048, ANOVA on ranks, Dunn’s post hoc). However, after POC stimulation when Int1 feedback did not increase the CPN2 protraction intraburst firing rate (Fig. 5), Int1 activity was not different from its activity after VCN stimulation [post-VCN (n = 6) vs. post-POC (n = 3): number of spikes/pyloric cycle: 11.1 ± 0.4 vs. 10.4 ± 1.3, F3,14 = 7.469, P = 0.628, one-way ANOVA, Holm-Sidak post hoc; firing rate: 19.6 ± 0.9 vs. 16.8 ± 1.9 Hz, H3 = 12.456, P = 1.00, ANOVA on ranks, Dunn’s post hoc]. This indicates that the level of Int1 activity after VCN stimulation is not sufficient to explain the stronger inhibition of CPN2. I thus next determined whether the Int1 synaptic current was altered by VCN stimulation.
In an example experiment, in control conditions there was no evident difference in holding current before vs. during Int1 activity (Fig. 7Ci). After VCN stimulation, at the same holding voltage (−70 mV), there was an outward current when Int1 was active (Fig. 7Cii). There may have been small discrete IPSCs, but the synaptic current was dominated by an apparently graded component. Thus the difference in average current during Int1 active vs. inactive periods was used as the measure of Int1 synaptic current. Consistently across preparations, there was no difference in holding current between when Int1 was active and inactive in control conditions (n = 7, t6 = −1.368, P = 0.22, paired t-test; Fig. 7D). However, after VCN stimulation, there was an Int1 synaptic current evident in the difference in holding current (n = 7, t6 = −3.901, P = 0.008, paired t-test; Fig. 7D). Thus across preparations VCN stimulation triggered an increase in the amplitude of Int1 synaptic current defined as the difference in holding current between Int1 inactive and Int1 active time blocks (n = 7, t6 = −3.609, P = 0.011, paired t-test; Fig. 7D).
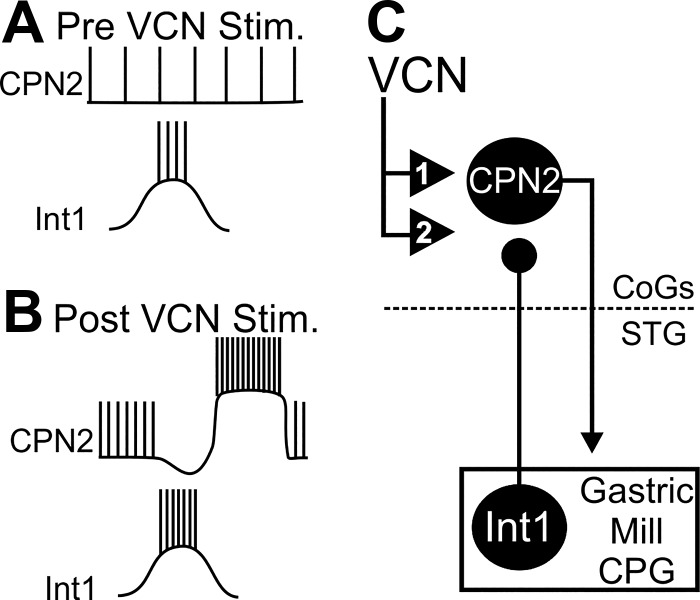
The inhibitory nature of the Int1 to CPN2 synapse previously reported (Norris et al. 1994) and demonstrated in current- and voltage-clamp recordings above conflicts with the cessation of Int1 activity causing decreased CPN2 activity (Fig. 6). To further confirm that the Int1 to CPN2 synapse is a direct inhibitory synapse, I examined whether there were one-for-one spike-mediated IPSCs from Int1 to CPN2. Discrete one-for-one Int1 elicited IPSPs or IPSCs were not readily apparent in the response to a 6-s epoch of Int1 activity (Fig. 7, A and C). In a subset of experiments in which it was possible to monitor Int1 action potentials with simultaneous CPN2 voltage-clamp recordings, in control conditions there were no evident IPSCs in CPN2 time locked to Int1 action potentials (Fig. 7Ei). However, in the same preparations, small (45.2 ± 8.1 pA, n = 2) one-for-one time-locked IPSCs were evident after VCN stimulation (n = 2; Fig. 7Eii). In addition, Int1-timed inhibition of CPN2 was blocked by 10−5 M picrotoxin (n = 3), which blocks ionotropic glutamatergic synapses in the STNS (Golowasch and Marder 1992; Marder and Paupardin-Tritsch 1978; Swensen et al. 2000). Thus far the only known transmitter in Int1 is glutamate (Marder 1987) and Int1 is not GABAergic (Marder and Paupardin-Tritsch 1978; Swensen et al. 2000). Finally, hyperpolarizing current injections (n = 4) or more negative holding potentials (n = 2) decreased the amplitude of Int1-elicited changes in CPN2 membrane potential or holding current, although it was difficult to maintain the CPN2 membrane potential at sufficiently hyperpolarized potentials to reverse the sign of the synapse. The difference in membrane potential or holding current between Int1 off and Int1 on was normalized to the potential/current with Int1 off and compared between a depolarized membrane potential (−52 ± 6.5 mV) and a more hyperpolarized membrane potential (−86 ± 5.7 mV). There was a larger difference at more depolarized membrane potentials compared with hyperpolarized potentials (t5 = 2.831, P = 0.0366, n = 6, paired t-test), indicating that the reversal potential is closer to −86 than to −52 mV, which indicates an inhibitory synapse. Overall, previous findings (Norris et al. 1994) and those reported in this study indicate that the Int1 to CPN2 synapse is a direct inhibitory synapse. The inhibitory nature of the Int1 to CPN2 synapse therefore needed to be reconciled with removal of Int1 activity causing a decrease in VCN-triggered CPN2 activity.
Int1 is only active during retraction, but the CPN2 intraburst firing rate is higher during VCN-protraction compared with its firing rate during the no-feedback condition. I hypothesized that Int1 inhibition, occurring during VCN-retraction, triggers PIR in CPN2 during VCN-protraction. Int1-triggered PIR would then elicit a higher intraburst firing rate during VCN-protraction than that due solely to VCN modulation of CPN2. In response to repeated bouts of Int1 inhibition occurring during a VCN gastric mill rhythm, a sustained PIR could then take tens of seconds to decay, explaining the fall in CPN2 activity upon sustained removal of Int1 activity. PIR with a long decay time constant in response to rhythmic inhibitory input occurs, for instance, in the STG motor neuron LP (Goaillard et al. 2010). In the presence of a muscarinic acetylcholine receptor agonist, Int1 inhibition of CPN2 triggers a rebound when Int1 activity is suppressed. Similar to Int1 inhibition of CPN2, Int1-triggered CPN2 rebound activity was also rarely observed in normal saline (Norris et al. 1994). This suggested that modulation of the Int1 synapse, such as that occurring after VCN stimulation, might enable Int1 to trigger PIR. Thus I next tested the functional impact of Int1 inhibition on CPN2 activity before vs. after VCN stimulation.
Functional impact of strengthened Int1 inhibition of CPN2.
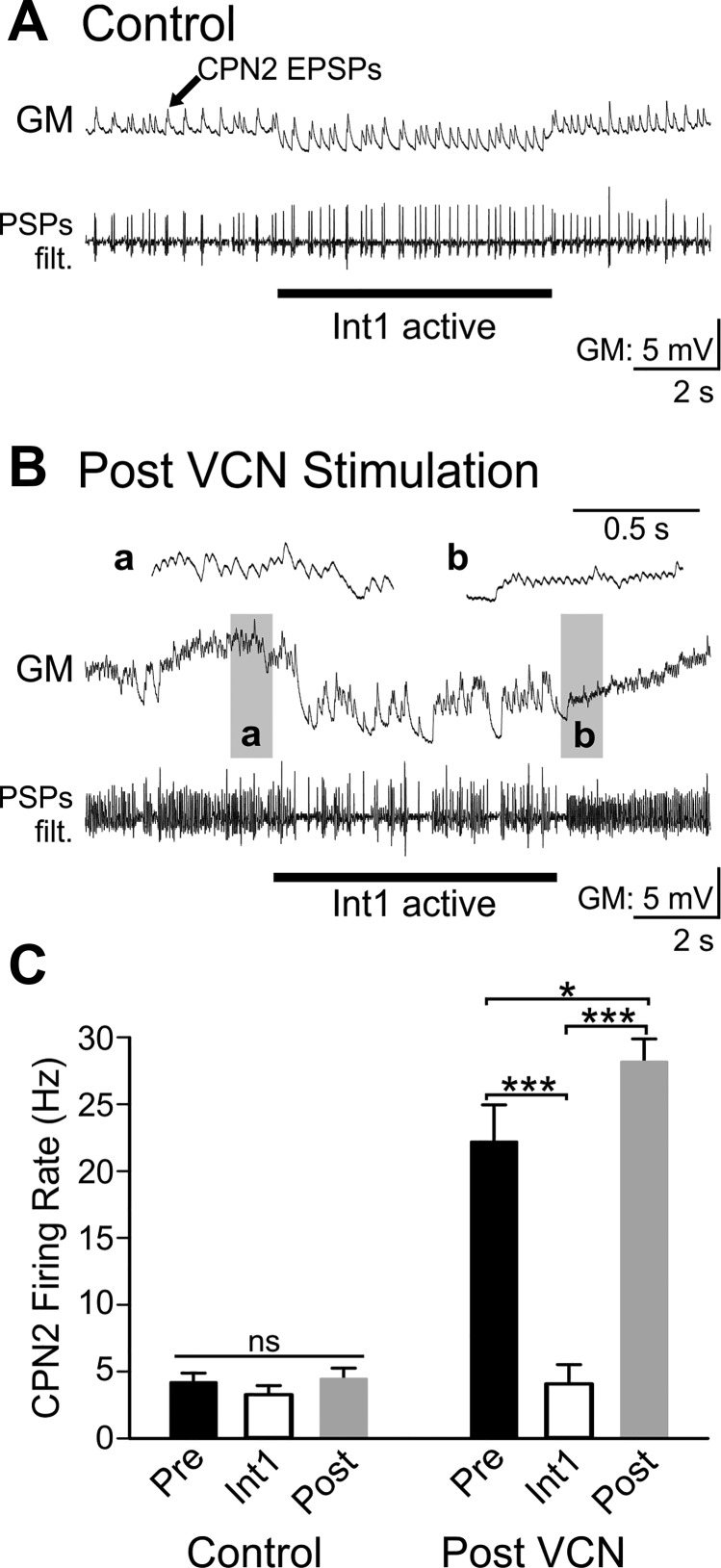
To determine the functional consequences of VCN stimulation strengthening the Int1 to CPN2 synapse, the CPN2 firing rate was measured before, during, and after Int1 active periods. Given the long decay of feedback effects on the CPN2 firing rate (Fig. 6), Int1 activity was suppressed for at least 60 s and Int1 was then activated briefly (6 s). EPSPs in GM were again used to measure CPN2 activity. In control conditions, Int1 activity had no effect on the CPN2 firing rate in the absence of feedback. Additionally, there was no difference in the CPN2 firing rate before vs. after Int1 activation (Fig. 8; n = 7, F2,12 = 2.116, P = 0.163, one-way repeated-measures ANOVA). After VCN stimulation, CPN2 firing rate was higher in the absence of Int1 feedback, yet Int1 decreased the CPN2 firing rate (Fig. 8; F2,10 = 49.399, n = 6, P < 0.001, one-way repeated measures ANOVA, Holm-Sidak post hoc). Furthermore, after VCN stimulation, the CPN2 firing rate was greater after release from Int1 inhibition compared with before Int1 activation (F2,10 = 49.399, n = 6, P = 0.039; Fig. 8). This indicates that the strengthened Int1 feedback after VCN stimulation triggered PIR in CPN2.
Fig. 8.
Int1 inhibits CPN2 and triggers post inhibitory rebound after VCN stimulation, but not in control conditions. A: in control conditions there was weak CPN2 activity recorded as excitatory postsynaptic potentials (EPSPs) in the GM neuron. A filtered version of the GM recording (PSPs filt.) is shown below the original trace. The Int1 active epoch did not alter the CPN2 firing rate, and there was no postinhibitory rebound (PIR) firing after Int1 activity was terminated. Int1 activity was controlled with depolarizing and hyperpolarizing current injections into LG due to its inhibition of Int1 (see materials and methods). GM is electrically coupled to LG and thus hyperpolarized with LG during the Int1 active period. B: post-VCN stimulation, CPN2 firing rate was higher but was decreased during the Int1 active epoch. After the Int1 active epoch CPN2 fired at a higher rate than before activation of Int1. a And b: gray boxed areas at an expanded time scale. C: the average CPN2 firing rate was not altered by Int1 in control conditions (Control Pre vs. Int1) nor was there a rebound in firing rate elicited after a period of Int1 activity (Control Pre vs. Post, n = 7). VCN stimulation triggered increased CPN2 activity. Under this condition, Int1 inhibited CPN2 activity and triggered a rebound in CPN2 firing rate (n = 6). Black bars: Pre-Int1 activity; white bars: Int1 active; gray bars: Post-Int1 activity during control conditions (left) and after VCN stimulation (right). nsP > 0.05, *P < 0.05, ***P < 0.001.
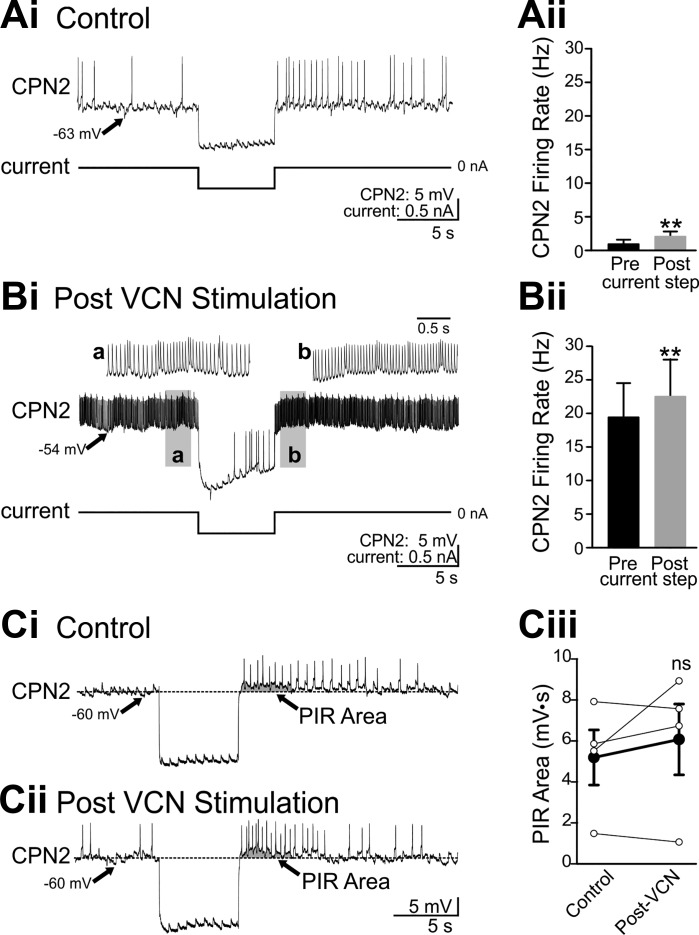
To investigate whether VCN stimulation enables PIR, or PIR is a baseline property of CPN2, CPN2 responses to steps of hyperpolarizing current injection were measured in the absence of feedback. In control conditions, CPN2 was weakly active (Fig. 9A). Current steps of the same duration as Int1 activity (6 s) were used to hyperpolarize CPN2 by a similar amount as Int1 inhibition after VCN stimulation (Int1: 10.4 ± 3.3 mV, n = 5; −0.5-nA current injection: 10.3 ± 2.0 mV, n = 6; t10 = −0.000927, P = 0.999, t-test). In control conditions, in which Int1 did not elicit PIR, hyperpolarizing current steps triggered an increased CPN2 firing rate after the current step compared with preinjection (t5 = −4.483, P = 0.006, paired t-test; Fig. 9A). Similarly, after VCN stimulation, the same amplitude current injection elicited an increased CPN2 firing rate after the end of the current injection (t4 = −4.924, P = 0.008, paired t-test; Fig. 9B). Thus the inability of Int1 to elicit PIR in control conditions is not due to the absence of PIR in CPN2.
Fig. 9.
CPN2 expresses similar post inhibitory rebound properties in control conditions and after VCN stimulation. Hyperpolarizing current injection into CPN2 in control conditions elicited rebound firing after the end of the current step in an example recording (Ai) and across preparations (Aii) (n = 6). After VCN stimulation, the same amplitude current step decreased CPN2 firing and triggered rebound firing after the end of the current step in the example recording (Bi) and across preparations (Bii) (n = 5). a And b: gray boxed areas at an expanded time scale. A step current injection elicited rebound firing in CPN2 in a different preparation than above (A and B) in control conditions (Ci) and after VCN stimulation (Cii), when steady current injection was used to maintain CPN2 at the same membrane potential as in control. To compare PIR in control and post-VCN conditions at the same CPN2 membrane potential, “PIR area” (arrow, gray shading) was measured (see materials and methods). Ciii: there was no consistent change in PIR area (n = 4). EPSPs evident in CPN2 in A–C are due to spontaneous action potentials in the AGR sensory neuron. **P < 0.01, nsP > 0.05.
PIR is expressed in CPN2 both in control conditions and after VCN stimulation; however, it was possible that VCN stimulation modulated PIR in addition to modulating the strength of the Int1 feedback synapse. To compare PIR in the two conditions, I quantified PIR with CPN2 at the same membrane potential before and after VCN stimulation. Constant hyperpolarizing current was injected into CPN2 after VCN stimulation to maintain CPN2 at the same membrane potential as in the control condition. At the same membrane potential and before a hyperpolarizing current step, the CPN2 firing rate was 0.1 ± 0.1 Hz in control conditions and 4.3 ± 1.7 Hz after VCN stimulation (n = 4). Therefore, instead of using the change in firing rate to compare PIR in the two conditions, the PIR area was quantified before and after VCN stimulation (Angstadt et al. 2005; Angstadt and Simone 2014). The PIR area is defined as the area under the CPN2 membrane potential after the end of the current step, with the prestep membrane potential as the baseline (see materials and methods). As above, in control conditions a hyperpolarizing current step elicited rebound firing (Fig. 9Ci). The gray shading indicates the area quantified as PIR area. After VCN stimulation, constant hyperpolarizing current was injected to maintain CPN2 at the pre-VCN membrane potential. In this condition, the same amplitude (−0.5 nA) current injection elicited rebound firing and a similar PIR area (Fig. 9Cii). Across four preparations, there was no consistent difference in PIR area before vs. after VCN stimulation (t3 = −0.983, P = 0.398, paired t-test; Fig. 9Ciii). Overall, VCN stimulation does not appear to elicit a substantial change in PIR properties in CPN2. This indicates that the critical aspect of VCN modulatory actions that enable Int1 to trigger PIR after VCN stimulation but not in control is the enhanced strength of the Int1 to CPN2 synapse.
DISCUSSION
Across invertebrate and vertebrate species, CPG circuits send feedback to their projection neuron inputs, eliciting patterned projection neuron activity linked to motor output (Arshavsky et al. 1988; Blitz and Nusbaum 2008; Ezure and Tanaka 1997; Frost and Katz 1996). The projection neuron intraburst firing rate is an important determinant of circuit output (Bartos et al. 1999; Blitz et al. 2004; Jing and Weiss 2005; Nakamura and Katakura 1995; Severi et al. 2014; Spencer and Blitz 2016), yet it was unknown whether CPG feedback also regulates this aspect of projection neuron activity. During a chewing motor pattern, I found that CPG feedback regulates the projection neuron intraburst firing rate by interacting with intrinsic properties of the projection neuron. Increasing the complexity of this regulation, it is selective for only one of the projection neurons driving the chewing pattern, does not occur in control conditions, and is different during a different chewing motor pattern. Specifically, the modulatory effects of the mechanosensory VCN neurons trigger a long-lasting increased activity in the projection neuron CPN2 (Fig. 10) (Beenhakker et al. 2004; Blitz and Nusbaum 2007a). In this study, I find that VCN stimulation additionally triggers a strengthening of the Int1 to CPN2 feedback synapse. The enhanced Int1 feedback elicits postinhibitory rebound in CPN2, increasing its intraburst firing rate above that due solely to the modulatory sensory actions (Fig. 10).
Fig. 10.
Summary of the VCN modulatory actions on CPN2 and Int1 feedback. A: in control conditions, CPN2 is weakly active and is not inhibited by Int1. B–C: modulatory VCN actions trigger a long-lasting increased CPN2 firing rate relative to control (1) (Beenhakker and Nusbaum 2004; Blitz and Nusbaum 2007a). Additionally, after VCN stimulation, the Int1 inhibition of CPN2 is strengthened (2). The enhanced Int1 inhibition of CPN2 triggers postinhibitory rebound in CPN2, which increases CPN2 intraburst firing rate above that due to VCN modulatory actions on CPN2. Ball and stick represent an inhibitory synapse. Numbered triangles indicate modulatory actions.
Projection neurons select different circuit outputs through classical and modulatory chemical synapses as well as electrical synapses with circuit neurons (Briggman and Kristan 2008; Daghfous et al. 2016; Kyriakatos et al. 2011; Marder 2012; Nusbaum 2002; Sharples et al. 2014). As all of these synaptic connections can be sensitive to presynaptic activity (Cazalis et al. 1985; Fioravante and Regehr 2011; Nadim and Bucher 2014; Nusbaum 2002; O’Brien 2014; Peng and Horn 1991; Vilim et al. 1996; Whim and Lloyd 1989), it follows that the activity patterns and intraburst firing rates of projection neurons would be important in determining circuit output.
Many parameters of motor circuit output such as cycle period, motor neuron activity levels, and phase relationships do in fact vary with projection neuron intraburst firing rates (Bartos et al. 1999; Jing and Weiss 2005; Kyriakatos et al. 2011; Nakamura and Katakura 1995; Severi et al. 2014; Spencer and Blitz 2016). Physiologically, projection neuron firing rates are regulated by the strength of sensory or central inputs and by behavioral states (Blitz et al. 2004; Daghfous et al. 2016; Di Prisco et al. 1997; Jacobs et al. 2002; Rossignol et al. 2006; Velázquez-Ulloa et al. 2003). However, to my knowledge this study provides the first demonstration that the projection neuron intraburst firing rate is also regulated by circuit feedback.
The projection neuron intraburst firing rate may regulate CPG circuit output due to multiple factors. For instance, the amounts and complements of released cotransmitters, the relative efficacy of degradation mechanisms, and the response properties of circuit neurons and synapses all express activity dependence (Marder 2012; Nadim and Bucher 2014; Nusbaum 2002; Vilim et al. 1996; Whim and Lloyd 1989). CPN2 uses primarily fast chemical and electrical synaptic communication to act on circuit neurons (Norris et al. 1994). This is similar to the ionotropic effects of reticulospinal neurons on spinal locomotor circuit neurons (Daghfous et al. 2016; Rossignol et al. 2006). The importance of a higher CPN2 intraburst firing rate is indicated by differences in burst durations and phase relationships of many gastric mill neurons during the VCN vs. the POC gastric mill rhythms (White and Nusbaum 2011). This is relevant to the feedback-elicited increase in CPN2 activity because the higher CPN2 firing rate during VCN-protraction compared with the no-feedback condition is similar to the difference in the CPN2 intraburst firing rate between VCN-protraction and POC-protraction (Figs. 3 and 5). The MCN1 firing rate is similar between POC and VCN stimulations (Figs. 2 and 4). MCN1 and CPN2 are the two projection neurons that elicit the VCN and the POC gastric mill rhythms, and these rhythms are produced by the same core CPG neurons (Beenhakker and Nusbaum 2004; Blitz et al. 2008; Blitz and Nusbaum 2008; White and Nusbaum 2011). Thus the different regulation of the CPN2 firing rate by feedback during VCN vs. POC gastric mill rhythms appears to be a significant contributor to differences in the motor patterns and therefore the resulting muscle and tooth movements (Diehl et al. 2013; White and Nusbaum 2011). Specifically, eliminating the weaker CPN2 activity during POC gastric mill rhythms, which occurs at a similar firing rate as in the VCN-no feedback condition, has a minor effect on the motor pattern (Blitz and Nusbaum 2012). In fact, when MCN1 activity is eliminated during POC gastric mill rhythms, the low CPN2 firing rate, similar to that occurring in the VCN-no feedback condition, is unable to maintain a rhythm (Blitz and Nusbaum 2012). However, eliminating the higher firing rate CPN2 activity during VCN gastric mill rhythms has more dramatic effects on network output, and the VCN-protraction level of activity is sufficient to maintain gastric mill rhythms in the absence of MCN1 (Beenhakker and Nusbaum 2004). Thus a higher CPN2 firing rate is an important contributor to gastric mill network output. In addition to STG level effects, CPN2 has an arborization within the CoGs (Norris et al. 1994) and it is possible that the higher firing rate during VCN gastric mill rhythms is relevant to how it interacts with esophageal (swallowing) network neurons and other neurons originating in, or projecting to, the CoGs.
In addition to direct circuit level effects, a change in the projection neuron intraburst firing rate can also impact circuit output indirectly through sensory feedback loops (Blitz and Nusbaum 2007b; Pearson 2004; Stein 2014). For instance, increased CPN2 intraburst firing rate increases the firing rate of gastric mill motor neurons innervating gastric mill muscles, which have proprioceptors and a tendon organ receptor associated with them (Beenhakker and Nusbaum 2004; Combes et al. 1995; Hedrich et al. 2009; Katz et al. 1989; Norris et al. 1994). These sensory neurons alter the gastric mill motor pattern through both short-loop feedback, acting on gastric mill network neurons, and long-loop feedback, acting on MCN1 and CPN2 projection neurons (Beenhakker et al. 2005; Blitz et al. 2004; Daur et al. 2012; Hedrich et al. 2009; Norris et al. 1994). An additional potential indirect consequence of feedback-elicited changes in the projection neuron intraburst firing rate is interfering with the influence of other inputs acting on a motor pathway through projection neurons. Multiple sensory modalities converge onto the same projection neurons to impact motor pathways (Rossignol et al. 2006; Swallie et al. 2015; Velázquez-Ulloa et al. 2003). In particular, CPN2 is targeted by multiple proprioceptors, chemosensory neurons, and a neuroendocrine input (Beenhakker and Nusbaum 2004; Blitz et al. 2004, 2008; Christie et al. 2004; Hedrich et al. 2009; Norris et al. 1994). After VCN stimulation, CPN2 is no longer sensitive to input from a set of stretch receptors, which alters the impact of this long-loop feedback on motor pattern generation (Beenhakker et al. 2007). The mechanism underlying this gating remains to be determined, but it highlights the flexibility in responsiveness of projection neurons to convergent inputs. Thus regulating projection neuron intraburst firing rate has the potential to alter the output of a motor pathway in both direct and indirect ways.
To generate appropriate movements, modulation of motor systems occurs at multiple levels. This includes changes in projection neuron activity, circuit neuron intrinsic and synaptic properties, sensory processing from transduction through to central actions, and neuromuscular junctions and muscles (Birmingham et al. 2003; Blitz and Nusbaum 2007b; Brezina 2010; Doi and Ramirez 2008; Fort et al. 2004; Nusbaum and Blitz 2012; Nadim and Bucher 2014). CPG feedback was long thought to be a static component of motor systems; however, this study along with our earlier study (Blitz and Nusbaum 2012) reveals that CPG feedback is yet another site of plasticity within motor pathways. In this study we did not distinguish between pre- and postsynaptic modulation of the Int1 to CPN2 feedback synapse, POC neurons presynaptically modulate AB feedback inhibition of MCN1 and CPN2 (Blitz and Nusbaum 2012). Additionally, in hippocampal and corticothalamic circuit modulation of circuit feedback occurs presynaptically (Kyuyoung and Huguenard 2014; Winterer et al. 2011). However, postsynaptic modulatory actions could also contribute to, or entirely account for, the strengthened Int1 synaptic current in CPN2.
Regardless of the locus of modulation, VCN strengthening of Int1 feedback inhibition is critical for feedback to regulate the CPN2 intraburst firing rate. CPN2 expresses PIR properties in control conditions, but the Int1 to CPN2 synapse is ineffective and thus cannot trigger PIR. However, after VCN stimulation, Int1 inhibition of CPN2 is enhanced and able to trigger PIR in CPN2. This increases the CPN2 intraburst firing rate during the protraction phase above that due only to VCN modulatory actions, which is important in determining motor network output as discussed above. CPG feedback also interacts with intrinsic properties in reticulospinal projection neurons in the lamprey locomotor system. In this case, CPG feedback increases the duration of reticulospinal activity triggered by sensory input, prolonging bouts of swimming (Antri et al. 2009). Interactions of CPG feedback with projection neuron intrinsic properties could serve to increase flexibility. For instance, this provides a means by which the patterning effects of CPG feedback could be modulated independently from other effects such as those on intraburst firing rate or response durations.
Modulation of CPG feedback synapses may serve yet additional functions as well. For instance, biological and computational studies indicate that the Int1 to CPN2 feedback synapse is part of a different rhythm generator circuit for the VCN gastric mill rhythm compared with other gastric mill rhythm versions (Akay et al. 2004; Kintos and Nadim 2014). The addition of the Int1 to CPN2 synapse shifts the locus of rhythm generation (Akay et al. 2004; Kintos and Nadim 2014). I find here that the control Int1 feedback synapse is too weak to affect CPN2, and thus modulation of a feedback synapse theoretically enables a particular type of oscillatory network to be activated. Shifting the locus of rhythm generation could have important implications for sensorimotor integration. For instance, shifting rhythm generation from entirely within the network, to partially outside the network, could increase or decrease the impact of sensory feedback depending on whether it acts at the projection neuron level or the network level (Beenhakker et al. 2005, 2007; Hedrich et al. 2009; Rossignol et al. 2006; Stein 2014).
The list of functions of feedback in oscillatory networks has only recently begun to expand, aided in large part by utilizing small CPG circuits (Antri et al. 2009; Blitz and Nusbaum 2012; Kintos and Nadim 2014; Kozlov et al. 2014; Spencer and Blitz 2016; Wood et al. 2004). Previously identified functions include contributing to interactions between related circuits (Wood et al. 2004), constraining the phase in which sensory inputs to projection neurons are effective (Kozlov et al. 2014), and contributing to network neuron activity levels and patterns (Blitz and Nusbaum 2012; Spencer and Blitz 2016). In this study, the ability of CPG feedback to regulate the projection neuron intraburst firing rate has been identified as a novel role for circuit feedback to central projection neurons. However, the ability of CPG feedback to alter the projection neuron intraburst firing rate relies on modulation of feedback. Furthermore, the manner in which feedback regulates projection neuron activity level differs when the projection neurons are activated by different pathways. Thus in future studies it will be important to determine the extent to which the modulatory status of central feedback loops may contribute to state dependence of neural circuits, particularly those that are part of rhythmic motor systems.
GRANTS
This work was supported by National Science Foundation Grant IOS-1153417.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author.
AUTHOR CONTRIBUTIONS
D.M.B. conceived and designed research; D.M.B. performed experiments; D.M.B. analyzed data; D.M.B. interpreted results of experiments; D.M.B. prepared figures; D.M.B. drafted manuscript; D.M.B. edited and revised manuscript; D.M.B. approved final version of manuscript.
ACKNOWLEDGMENTS
I thank M. Beenhakker, M. Kirby, and R. White for contributing a subset of the experiments analyzed in Figs. 2–4, N. Daur for assistance with experiments for Fig. 9, and D. Bucher for Spike2 analysis scripts.
REFERENCES
- Akay T, Wood DE, Nusbaum. MP Reciprocal Inhibition is not necessary for generation of all gastric mill rhythms. Neurosci Meet Plan Wash DC Soc Neurosci Online Program No. 657.2, 2004.
- Alle H, Geiger JR. Analog signalling in mammalian cortical axons. Curr Opin Neurobiol 18: 314–320, 2008. doi: 10.1016/j.conb.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Angstadt JD, Grassmann JL, Theriault KM, Levasseur SM. Mechanisms of postinhibitory rebound and its modulation by serotonin in excitatory swim motor neurons of the medicinal leech. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 191: 715–732, 2005. doi: 10.1007/s00359-005-0628-6. [DOI] [PubMed] [Google Scholar]
- Angstadt JD, Simone AM. Riluzole suppresses postinhibitory rebound in an excitatory motor neuron of the medicinal leech. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 200: 759–775, 2014. doi: 10.1007/s00359-014-0919-x. [DOI] [PubMed] [Google Scholar]
- Antri M, Fénelon K, Dubuc R. The contribution of synaptic inputs to sustained depolarizations in reticulospinal neurons. J Neurosci 29: 1140–1151, 2009. doi: 10.1523/JNEUROSCI.3073-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arshavsky YI, Orlovsky GN, Perret C. Activity of rubrospinal neurons during locomotion and scratching in the cat. Behav Brain Res 28: 193–199, 1988. doi: 10.1016/0166-4328(88)90096-4. [DOI] [PubMed] [Google Scholar]
- Bartos M, Manor Y, Nadim F, Marder E, Nusbaum MP. Coordination of fast and slow rhythmic neuronal circuits. J Neurosci 19: 6650–6660, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beenhakker MP, Blitz DM, Nusbaum MP. Long-lasting activation of rhythmic neuronal activity by a novel mechanosensory system in the crustacean stomatogastric nervous system. J Neurophysiol 91: 78–91, 2004. doi: 10.1152/jn.00741.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beenhakker MP, DeLong ND, Saideman SR, Nadim F, Nusbaum MP. Proprioceptor regulation of motor circuit activity by presynaptic inhibition of a modulatory projection neuron. J Neurosci 25: 8794–8806, 2005. doi: 10.1523/JNEUROSCI.2663-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beenhakker MP, Kirby MS, Nusbaum MP. Mechanosensory gating of proprioceptor input to modulatory projection neurons. J Neurosci 27: 14308–14316, 2007. doi: 10.1523/JNEUROSCI.4404-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beenhakker MP, Nusbaum MP. Mechanosensory activation of a motor circuit by coactivation of two projection neurons. J Neurosci 24: 6741–6750, 2004. doi: 10.1523/JNEUROSCI.1682-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Brain Res Rev 42: 33–84, 2003. doi: 10.1016/S0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- Birmingham JT, Billimoria CP, DeKlotz TR, Stewart RA, Marder E. Differential and history-dependent modulation of a stretch receptor in the stomatogastric system of the crab, Cancer borealis. J Neurophysiol 90: 3608–3616, 2003. doi: 10.1152/jn.00397.2003. [DOI] [PubMed] [Google Scholar]
- Blitz DM, Nusbaum MP. Extrinsic inputs modulate intrinsic currents and circuit feedback to shape projection neuron activity. Neurosci Meet Plan San Diego CA Soc Neurosci Online Program No. 924.21, 2007a.
- Blitz DM, Beenhakker MP, Nusbaum MP. Different sensory systems share projection neurons but elicit distinct motor patterns. J Neurosci 24: 11381–11390, 2004. doi: 10.1523/JNEUROSCI.3219-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitz DM, Christie AE, Coleman MJ, Norris BJ, Marder E, Nusbaum MP. Different proctolin neurons elicit distinct motor patterns from a multifunctional neuronal network. J Neurosci 19: 5449–5463, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitz DM, Nusbaum MP. Distinct functions for cotransmitters mediating motor pattern selection. J Neurosci 19: 6774–6783, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitz DM, Nusbaum MP. Mechanosensory regulation of invertebrate motor systems. In: Invertebrate Neurobiology (Greenspan RJ, North G, eds.). Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press, 2007b. [Google Scholar]
- Blitz DM, Nusbaum MP. State-dependent presynaptic inhibition regulates central pattern generator feedback to descending inputs. J Neurosci 28: 9564–9574, 2008. doi: 10.1523/JNEUROSCI.3011-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitz DM, Nusbaum MP. Modulation of circuit feedback specifies motor circuit output. J Neurosci 32: 9182–9193, 2012. doi: 10.1523/JNEUROSCI.1461-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitz DM, White RS, Saideman SR, Cook A, Christie AE, Nadim F, Nusbaum MP. A newly identified extrinsic input triggers a distinct gastric mill rhythm via activation of modulatory projection neurons. J Exp Biol 211: 1000–1011, 2008. doi: 10.1242/jeb.015222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brezina V. Beyond the wiring diagram: signalling through complex neuromodulator networks. Philos Trans R Soc Lond B Biol Sci 365: 2363–2374, 2010. doi: 10.1098/rstb.2010.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggman KL, Kristan WB Jr. Multifunctional pattern-generating circuits. Annu Rev Neurosci 31: 271–294, 2008. doi: 10.1146/annurev.neuro.31.060407.125552. [DOI] [PubMed] [Google Scholar]
- Brodfuehrer PD, McCormick K, Tapyrik L, Albano AM, Graybeal C. Activation of two forms of locomotion by a previously identified trigger interneuron for swimming in the medicinal leech. Invert Neurosci 8: 31–39, 2008. doi: 10.1007/s10158-007-0064-0. [DOI] [PubMed] [Google Scholar]
- Buchanan JT, Einum JF. The spinobulbar system in lamprey. Brain Res Brain Res Rev 57: 37–45, 2008. doi: 10.1016/j.brainresrev.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazalis M, Dayanithi G, Nordmann JJ. The role of patterned burst and interburst interval on the excitation-coupling mechanism in the isolated rat neural lobe. J Physiol 369: 45–60, 1985. doi: 10.1113/jphysiol.1985.sp015887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie AE, Stein W, Quinlan JE, Beenhakker MP, Marder E, Nusbaum MP. Actions of a histaminergic/peptidergic projection neuron on rhythmic motor patterns in the stomatogastric nervous system of the crab Cancer borealis. J Comp Neurol 469: 153–169, 2004. doi: 10.1002/cne.11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman MJ, Nusbaum MP. Functional consequences of compartmentalization of synaptic input. J Neurosci 14: 6544–6552, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combes D, Simmers J, Moulins M. Structural and functional characterization of a muscle tendon proprioceptor in lobster. J Comp Neurol 363: 221–234, 1995. doi: 10.1002/cne.903630205. [DOI] [PubMed] [Google Scholar]
- Daghfous G, Green WW, Alford ST, Zielinski BS, Dubuc R. Sensory activation of command cells for locomotion and modulatory mechanisms: Lessons from lampreys. Front Neural Circuits 10: 18, 2016. doi: 10.3389/fncir.2016.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daur N, Diehl F, Mader W, Stein W. The stomatogastric nervous system as a model for studying sensorimotor interactions in real-time closed-loop conditions. Front Comput Neurosci 6: 13, 2012. doi: 10.3389/fncom.2012.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daur N, Nadim F, Bucher D. The complexity of small circuits: the stomatogastric nervous system. Curr Opin Neurobiol 41: 1–7, 2016. doi: 10.1016/j.conb.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLong ND, Beenhakker MP, Nusbaum MP. Presynaptic inhibition selectively weakens peptidergic cotransmission in a small motor system. J Neurophysiol 102: 3492–3504, 2009. doi: 10.1152/jn.00833.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Prisco GV, Pearlstein E, Robitaille R, Dubuc R. Role of sensory-evoked NMDA plateau potentials in the initiation of locomotion. Science 278: 1122–1125, 1997. doi: 10.1126/science.278.5340.1122. [DOI] [PubMed] [Google Scholar]
- Diehl F, White RS, Stein W, Nusbaum MP. Motor circuit-specific burst patterns drive different muscle and behavior patterns. J Neurosci 33: 12013–12029, 2013. doi: 10.1523/JNEUROSCI.1060-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi A, Ramirez JM. Neuromodulation and the orchestration of the respiratory rhythm. Respir Physiol Neurobiol 164: 96–104, 2008. doi: 10.1016/j.resp.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubuc R, Brocard F, Antri M, Fénelon K, Gariépy JF, Smetana R, Ménard A, Le Ray D, Viana Di Prisco G, Pearlstein E, Sirota MG, Derjean D, St-Pierre M, Zielinski B, Auclair F, Veilleux D. Initiation of locomotion in lampreys. Brain Res Brain Res Rev 57: 172–182, 2008. doi: 10.1016/j.brainresrev.2007.07.016. [DOI] [PubMed] [Google Scholar]
- Dubuc R, Grillner S. The role of spinal cord inputs in modulating the activity of reticulospinal neurons during fictive locomotion in the lamprey. Brain Res 483: 196–200, 1989. doi: 10.1016/0006-8993(89)90055-3. [DOI] [PubMed] [Google Scholar]
- Ezure K, Tanaka I. Convergence of central respiratory and locomotor rhythms onto single neurons of the lateral reticular nucleus. Exp Brain Res 113: 230–242, 1997. doi: 10.1007/BF02450321. [DOI] [PubMed] [Google Scholar]
- Fioravante D, Regehr WG. Short-term forms of presynaptic plasticity. Curr Opin Neurobiol 21: 269–274, 2011. doi: 10.1016/j.conb.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fort TJ, Brezina V, Miller MW. Modulation of an integrated central pattern generator-effector system: dopaminergic regulation of cardiac activity in the blue crab Callinectes sapidus. J Neurophysiol 92: 3455–3470, 2004. doi: 10.1152/jn.00550.2004. [DOI] [PubMed] [Google Scholar]
- Frost WN, Katz PS. Single neuron control over a complex motor program. Proc Natl Acad Sci USA 93: 422–426, 1996. doi: 10.1073/pnas.93.1.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia AJ III, Zanella S, Koch H, Doi A, Ramirez JM. Chapter 3–networks within networks: the neuronal control of breathing. Prog Brain Res 188: 31–50, 2011. doi: 10.1016/B978-0-444-53825-3.00008-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goaillard JM, Taylor AL, Pulver SR, Marder E. Slow and persistent postinhibitory rebound acts as an intrinsic short-term memory mechanism. J Neurosci 30: 4687–4692, 2010. doi: 10.1523/JNEUROSCI.2998-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golowasch J, Marder E. Ionic currents of the lateral pyloric neuron of the stomatogastric ganglion of the crab. J Neurophysiol 67: 318–331, 1992. [DOI] [PubMed] [Google Scholar]
- Graubard K, Raper JA, Hartline DK. Graded synaptic transmission between spiking neurons. Proc Natl Acad Sci USA 77: 3733–3735, 1980. doi: 10.1073/pnas.77.6.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez GJ, Grashow RG. Cancer borealis stomatogastric nervous system dissection. J Vis Exp 25: 1207, 2009. doi: 10.3791/1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrich UB, Smarandache CR, Stein W. Differential activation of projection neurons by two sensory pathways contributes to motor pattern selection. J Neurophysiol 102: 2866–2879, 2009. doi: 10.1152/jn.00618.2009. [DOI] [PubMed] [Google Scholar]
- Hurwitz I, Susswein AJ, Weiss KR. Transforming tonic firing into a rhythmic output in the Aplysia feeding system: presynaptic inhibition of a command-like neuron by a CpG element. J Neurophysiol 93: 829–842, 2005. doi: 10.1152/jn.00559.2004. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Martín-Cora FJ, Fornal CA. Activity of medullary serotonergic neurons in freely moving animals. Brain Res Brain Res Rev 40: 45–52, 2002. doi: 10.1016/S0165-0173(02)00187-X. [DOI] [PubMed] [Google Scholar]
- Jing J, Weiss KR. Generation of variants of a motor act in a modular and hierarchical motor network. Curr Biol 15: 1712–1721, 2005. doi: 10.1016/j.cub.2005.08.051. [DOI] [PubMed] [Google Scholar]
- Jordan LM, Liu J, Hedlund PB, Akay T, Pearson KG. Descending command systems for the initiation of locomotion in mammals. Brain Res Brain Res Rev 57: 183–191, 2008. doi: 10.1016/j.brainresrev.2007.07.019. [DOI] [PubMed] [Google Scholar]
- Katz PS, Eigg MH, Harris-Warrick RM. Serotonergic/cholinergic muscle receptor cells in the crab stomatogastric nervous system. I. Identification and characterization of the gastropyloric receptor cells. J Neurophysiol 62: 558–570, 1989. [DOI] [PubMed] [Google Scholar]
- Kintos N, Nadim F. A modeling exploration of how synaptic feedback to descending projection neurons shapes the activity of an oscillatory network. SIAM J Appl Dyn Syst 13: 1239–1269, 2014. doi: 10.1137/130943881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby MS, Nusbaum MP. Peptide hormone modulation of a neuronally modulated motor circuit. J Neurophysiol 98: 3206–3220, 2007. doi: 10.1152/jn.00795.2006. [DOI] [PubMed] [Google Scholar]
- Kozlov AK, Kardamakis AA, Hellgren Kotaleski J, Grillner S. Gating of steering signals through phasic modulation of reticulospinal neurons during locomotion. Proc Natl Acad Sci USA 111: 3591–3596, 2014. doi: 10.1073/pnas.1401459111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakatos A, Mahmood R, Ausborn J, Porres CP, Büschges A, El Manira A. Initiation of locomotion in adult zebrafish. J Neurosci 31: 8422–8431, 2011. doi: 10.1523/JNEUROSCI.1012-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyuyoung CL, Huguenard JR. Modulation of short-term plasticity in the corticothalamic circuit by group III metabotropic glutamate receptors. J Neurosci 34: 675–687, 2014. doi: 10.1523/JNEUROSCI.1477-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Jordan LM. Stimulation of the parapyramidal region of the neonatal rat brain stem produces locomotor-like activity involving spinal 5-HT7 and 5-HT2A receptors. J Neurophysiol 94: 1392–1404, 2005. doi: 10.1152/jn.00136.2005. [DOI] [PubMed] [Google Scholar]
- Marder E. Neurotransmitters and neuromodulators. In: The Crustacean Stomatogastric System (Selverston AI, Moulins M, eds.). Berlin, Germany: Springer-Verlag, 1987, p. 263–300. doi: 10.1007/978-3-642-71516-7_10. [DOI] [Google Scholar]
- Marder E. Neuromodulation of neuronal circuits: back to the future. Neuron 76: 1–11, 2012. doi: 10.1016/j.neuron.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder E, Bucher D. Understanding circuit dynamics using the stomatogastric nervous system of lobsters and crabs. Annu Rev Physiol 69: 291–316, 2007. doi: 10.1146/annurev.physiol.69.031905.161516. [DOI] [PubMed] [Google Scholar]
- Marder E, Eisen JS. Transmitter identification of pyloric neurons: electrically coupled neurons use different transmitters. J Neurophysiol 51: 1345–1361, 1984. [DOI] [PubMed] [Google Scholar]
- Marder E, Paupardin-Tritsch D. The pharmacological properties of some crustacean neuronal acetylcholine, gamma-aminobutyric acid, and L-glutamate responses. J Physiol 280: 213–236, 1978. doi: 10.1113/jphysiol.1978.sp012381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadim F, Bucher D. Neuromodulation of neurons and synapses. Curr Opin Neurobiol 29: 48–56, 2014. doi: 10.1016/j.conb.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Katakura N. Generation of masticatory rhythm in the brainstem. Neurosci Res 23: 1–19, 1995. doi: 10.1016/0168-0102(95)90003-9. [DOI] [PubMed] [Google Scholar]
- Norris BJ, Coleman MJ, Nusbaum MP. Recruitment of a projection neuron determines gastric mill motor pattern selection in the stomatogastric nervous system of the crab, Cancer borealis. J Neurophysiol 72: 1451–1463, 1994. [DOI] [PubMed] [Google Scholar]
- Nusbaum MP. Regulating peptidergic modulation of rhythmically active neural circuits. Brain Behav Evol 60: 378–387, 2002. doi: 10.1159/000067791. [DOI] [PubMed] [Google Scholar]
- Nusbaum MP. Modulatory projection neurons (Online). In: Encyclopedia of Neuroscience, edited by Binder MD, Hirokawa N, Windhorst U. Berlin, Germany: Springer, 2009, p. 2385–2388. http://www.springerlink.com/index/10.1007/978-3-540-29678-2_3538. [Google Scholar]
- Nusbaum MP, Blitz DM. Neuropeptide modulation of microcircuits. Curr Opin Neurobiol 22: 592–601, 2012. doi: 10.1016/j.conb.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien J. The ever-changing electrical synapse. Curr Opin Neurobiol 29: 64–72, 2014. doi: 10.1016/j.conb.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson KG. Generating the walking gait: role of sensory feedback. Prog Brain Res 143: 123–129, 2004. doi: 10.1016/S0079-6123(03)43012-4. [DOI] [PubMed] [Google Scholar]
- Peng YY, Horn JP. Continuous repetitive stimuli are more effective than bursts for evoking LHRH release in bullfrog sympathetic ganglia. J Neurosci 11: 85–95, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raper JA. Nonimpulse-mediated synaptic transmission during the generation of a cyclic motor program. Science 205: 304–306, 1979. doi: 10.1126/science.221982. [DOI] [PubMed] [Google Scholar]
- Rossignol S, Dubuc R, Gossard J-P. Dynamic sensorimotor interactions in locomotion. Physiol Rev 86: 89–154, 2006. doi: 10.1152/physrev.00028.2005. [DOI] [PubMed] [Google Scholar]
- Ryczko D, Dubuc R. The multifunctional mesencephalic locomotor region. Curr Pharm Des 19: 4448–4470, 2013. doi: 10.2174/1381612811319240011. [DOI] [PubMed] [Google Scholar]
- Severi KE, Portugues R, Marques JC, O’Malley DM, Orger MB, Engert F. Neural control and modulation of swimming speed in the larval zebrafish. Neuron 83: 692–707, 2014. doi: 10.1016/j.neuron.2014.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharples SA, Koblinger K, Humphreys JM, Whelan PJ. Dopamine: a parallel pathway for the modulation of spinal locomotor networks. Front Neural Circuits 8: 55, 2014. doi: 10.3389/fncir.2014.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu Y, Hasenstaub A, Duque A, Yu Y, McCormick DA. Modulation of intracortical synaptic potentials by presynaptic somatic membrane potential. Nature 441: 761–765, 2006. doi: 10.1038/nature04720. [DOI] [PubMed] [Google Scholar]
- Smarandache CR, Daur N, Hedrich UB, Stein W. Regulation of motor pattern frequency by reversals in proprioceptive feedback. Eur J Neurosci 28: 460–474, 2008. doi: 10.1111/j.1460-9568.2008.06357.x. [DOI] [PubMed] [Google Scholar]
- Spencer RM, Blitz DM. Network feedback regulates motor output across a range of modulatory neuron activity. J Neurophysiol 115: 3249–3263, 2016. doi: 10.1152/jn.01112.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Städele C, Stein W. The site of spontaneous ectopic spike initiation facilitates signal integration in a sensory neuron. J Neurosci 36: 6718–6731, 2016. doi: 10.1523/JNEUROSCI.2753-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein W. Modulation of stomatogastric rhythms. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 195: 989–1009, 2009. doi: 10.1007/s00359-009-0483-y. [DOI] [PubMed] [Google Scholar]
- Stein W. Sensory input to central pattern generators. In: Encyclopedia of Computational Neuroscience. New York: Springer, 2014. doi: 10.1007/978-1-4614-7320-6_465-3. [DOI] [Google Scholar]
- Swallie SE, Monti AM, Blitz DM. Anatomical organization of multiple modulatory inputs in a rhythmic motor system. PLoS One 10: e0142956, 2015. doi: 10.1371/journal.pone.0142956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swensen AM, Golowasch J, Christie AE, Coleman MJ, Nusbaum MP, Marder E. GABA and responses to GABA in the stomatogastric ganglion of the crab Cancer borealis. J Exp Biol 203: 2075–2092, 2000. [DOI] [PubMed] [Google Scholar]
- Velázquez-Ulloa N, Blackshaw SE, Szczupak L, Trueta C, García E, De-Miguel FF. Convergence of mechanosensory inputs onto neuromodulatory serotonergic neurons in the leech. J Neurobiol 54: 604–617, 2003. doi: 10.1002/neu.10184. [DOI] [PubMed] [Google Scholar]
- Vilim FS, Cropper EC, Price DA, Kupfermann I, Weiss KR. Release of peptide cotransmitters in Aplysia: regulation and functional implications. J Neurosci 16: 8105–8114, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weimann JM, Meyrand P, Marder E. Neurons that form multiple pattern generators: identification and multiple activity patterns of gastric/pyloric neurons in the crab stomatogastric system. J Neurophysiol 65: 111–122, 1991. [DOI] [PubMed] [Google Scholar]
- Whim MD, Lloyd PE. Frequency-dependent release of peptide cotransmitters from identified cholinergic motor neurons in Aplysia. Proc Natl Acad Sci USA 86: 9034–9038, 1989. doi: 10.1073/pnas.86.22.9034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White RS, Nusbaum MP. The same core rhythm generator underlies different rhythmic motor patterns. J Neurosci 31: 11484–11494, 2011. doi: 10.1523/JNEUROSCI.1885-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterer J, Stempel AV, Dugladze T, Földy C, Maziashvili N, Zivkovic AR, Priller J, Soltesz I, Gloveli T, Schmitz D. Cell-type-specific modulation of feedback inhibition by serotonin in the hippocampus. J Neurosci 31: 8464–8475, 2011. doi: 10.1523/JNEUROSCI.6382-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood DE, Manor Y, Nadim F, Nusbaum MP. Intercircuit control via rhythmic regulation of projection neuron activity. J Neurosci 24: 7455–7463, 2004. doi: 10.1523/JNEUROSCI.1840-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]