This study is the first to show spontaneous gamma oscillations under NMDA antagonist in nonhuman primates. These oscillations appear in synchrony in the cortex and the basal ganglia. Phase analysis refutes the confounding effects of volume conduction and supports the funneling and amplifying architecture of the cortico-basal ganglia loops. These results suggest an abnormal network phenomenon with a unique spectral signature that could account for pathological mental and neurological states.
Keywords: gamma oscillations, ketamine, PCP, NMDA antagonist, basal ganglia
Abstract
N-methyl-d-aspartate (NMDA) antagonists are widely used in anesthesia, pain management, and schizophrenia animal model studies, and recently as potential antidepressants. However, the mechanisms underlying their anesthetic, psychotic, cognitive, and emotional effects are still elusive. The basal ganglia (BG) integrate input from different cortical domains through their dopamine-modulated connections to achieve optimal behavior control. NMDA antagonists have been shown to induce gamma oscillations in human EEG recordings and in rodent cortical and BG networks. However, network relations and implications to the primate brain are still unclear. We recorded local field potentials (LFPs) simultaneously from the primary motor cortex (M1) and the external globus pallidus (GPe) of four vervet monkeys (26 sessions, 97 and 76 cortical and pallidal LFPs, respectively) before and after administration of ketamine (NMDA antagonist, 10 mg/kg im). Ketamine induced robust, spontaneous gamma (30–50 Hz) oscillations in M1 and GPe. These oscillations were initially modulated by ultraslow oscillations (~0.3 Hz) and were highly synchronized within and between M1 and the GPe (mean coherence magnitude = 0.76, 0.88, and 0.41 for M1-M1, GPe-GPe, and M1-GPe pairs). Phase differences were distributed evenly around zero with broad and very narrow distribution for the M1-M1 and GPe-GPe pairs (−3.5 ± 31.8° and −0.4 ± 6.0°), respectively. The distribution of M1-GPe phase shift was skewed to the left with a mean of −18.4 ± 20.9°. The increased gamma coherence between M1 and GPe, two central stages in the cortico-BG loops, suggests a global abnormal network phenomenon with a unique spectral signature, which is enabled by the BG funneling architecture.
NEW & NOTEWORTHY This study is the first to show spontaneous gamma oscillations under NMDA antagonist in nonhuman primates. These oscillations appear in synchrony in the cortex and the basal ganglia. Phase analysis refutes the confounding effects of volume conduction and supports the funneling and amplifying architecture of the cortico-basal ganglia loops. These results suggest an abnormal network phenomenon with a unique spectral signature that could account for pathological mental and neurological states.
ketamine, an nmda antagonist, is best known as an anesthetic compound. Because of its stable hemodynamic profile on the one hand but dissociative effect on the other hand, it is mainly used for sedation and analgesia in prehospital and critical care settings, as well as in procedural sedation and pediatric sedation (Kurdi et al. 2014; Marland et al. 2013). Lately, its use as an analgesic agent has been extended to the fields of chronic pain and palliative care (Bredlau et al. 2013; Niesters et al. 2014). The dissociative effects exerted by ketamine have led to a recreational use of ketamine, especially as a club drug (Kalsi et al. 2011). In addition, ketamine and phencyclidine (PCP) induce psychotic symptoms, cognitive impairments, and behavioral changes that resemble the positive, negative, and cognitive symptoms of schizophrenia (Domino and Luby 2012; Driesen et al. 2013; Gilmour et al. 2012; Javitt 2007; Krystal et al. 1994); therefore, they are widely used in animal models for schizophrenia. Finally, in the last decade ketamine gained popularity as a potential antidepressant (Newport et al. 2015). A series of studies demonstrated reduction of depressive symptoms following single intravenous ketamine injection, with both a rapid (within hours) and a longer lasting (around a week) response (Murrough et al. 2013; Zarate et al. 2006). However, clinical data are still limited, and the efficacy and safety of repeated injections are still unknown (Caddy et al. 2015; Tsai 2007). In conclusion, NMDA antagonists, such as ketamine and PCP, are unique drugs with numerous clinical and scientific applications.
Ketamine’s mechanism of action in the neural network level is still unclear. In the depression literature (reviewed in Abdallah et al. 2015; Scheuing et al. 2015; Williams and Schatzberg 2016), it is believed that sustained stress and depression lead to overall synaptic depression and neuronal atrophy, particularly in the prefrontal cortex (Kang et al. 2012; Yuen et al. 2012). Ketamine is thought to act by blocking presynaptic NMDA receptor signaling, preferably on GABAergic interneurons, which results in increased glutamate release, pyramidal disinhibition, and activation of postsynaptic AMPA receptors (Homayoun and Moghaddam 2007; Miller et al. 2016). Further downstream, signaling pathways that include brain neurotrophic factor (BDNF) release and mammalian target of rapamycin (mTOR) complex activation facilitate synaptic formation and changes in synaptic strength that are thought to reverse the synaptic deficits of the depressive state (Abdallah et al. 2015; Li et al. 2010). The schizophrenia literature focuses on the effects of NMDA antagonists on the brain macro- and microcircuitry. In particular, ketamine-induced schizophrenia pathophysiology has been linked to alterations in gamma oscillations that are thought to be involved in the coordination of the activity of spatially distributed neural circuits (Gandal et al. 2012; Uhlhaas and Singer 2010).
In rodents, ketamine increases the spontaneous gamma oscillations in both cortical and subcortical structures (Ehrlichman et al. 2009; Hakami et al. 2009; Kocsis 2012; Kulikova et al. 2012; Lazarewicz et al. 2010; Ma and Leung 2007; Pinault 2008; Wood et al. 2012). Some studies report power increases in the 30- to 50-Hz frequency band (low-gamma band), whereas others report increase at the high-gamma band, as well. A few studies (Hunt et al. 2006; Kulikova et al. 2012; Nicolás et al. 2011) also show increases of high-frequency oscillations (HFO; 80–180 Hz). Studies of spontaneous gamma oscillations under ketamine in humans are sparse (Hong et al. 2010; Maksimow et al. 2006; Plourde et al. 1997; Purdon et al. 2015). Therefore, the exact subbands affected by ketamine remain elusive. Human electrophysiological studies are limited to EEG and MEG studies, which become more problematic when HFOs and subcortical structures are being studied. On the other hand, the considerably larger primate brain with longer conduction delays puts the relevance of rodents’ LFP oscillation studies into question (Buzsáki 2006; Uhlhaas and Singer 2012). This can be demonstrated by the different oscillatory frequencies seen in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) nonhuman primate model and the 6-hydroxydopamine (6-OHDA) rat model of Parkinson’s disease (Quiroga-Varela et al. 2013; Stein and Bar-Gad 2013). As such, studying the neural oscillations under ketamine in nonhuman primate could help to fill the translational gap between rodent and human studies.
The basal ganglia (BG) play a critical role in behavior modulation through their dopamine-modulated connectivity and excitability (Helie et al. 2013; Parush et al. 2011; Schultz et al. 1997). The current computational BG model holds that the cortex (including motor, associative, and emotional cortices) encodes the current state of the animal and uses glutamatergic synapses to project this information to the BG. In turn, the BG process and integrate this information and feed it to the brain motor centers (e.g., the frontal cortices, but also brain stem nuclei), which serve as the final common pathway for behavior expression. The striatum (the main BG input stage) is greatly enriched with dopamine and serotonin markers (Haber et al. 2012). Thus the current (dopaminergic and serotoninergic related) pharmacological treatment of schizophrenia, major depression, and other mental disorders supports the claim of the major role of the BG loop in the pathophysiology of these diseases. The primary motor cortex (M1) both projects to the BG and funnels the BG output to the muscular apparatus (Haber et al. 2012). By contrast, the GPe is the major central nucleus of the BG internal feedforward network (Gittis et al. 2014; Goldberg and Bergman 2011; Kita 2007). The GPe is the only BG structure that innervates the input stage (striatum and subthalamic nucleus, STN) as well as the output stage (globus pallidus internal segment and substantia nigra pars reticulata, GPi and SNr, respectively) of the basal ganglia circuit (Deffains et al. 2016). Therefore, the M1 and GPe represent the two extreme nodes of the cortical-BG network. Recently, a couple of studies have shown the induction of coherent oscillations at the low-gamma, high-gamma, and HFO bands between different nuclei of the BG and the motor cortex of ketamine-treated rats (Cordon et al. 2015; Nicolás et al. 2011). In the current study, we investigated the LFP activity in nonhuman primates treated with NMDA antagonists (ketamine, PCP) by performing multiple-electrode recordings from M1 and GPe. Our aim was to study the induced alterations in the gamma activity of the primate brain, with an emphasis on intra- and interstructure coherence and phase, and to shed light on the involvement of the BG network in NMDA antagonist-induced abnormal brain activity.
MATERIALS AND METHODS
Animals, Surgery, and MRI
The experiments were conducted on four African Green monkeys (Cercopithecus aethiops aethiops, females, weighing between 3.3 and 4.4 kg). All procedures were conducted in accordance with the Hebrew University guidelines for animal care and the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The Hebrew University is an AAALAC (Association for Assessment and Accreditation of Laboratory Animal Care)-approved institute.
The animals underwent surgical implantation of a Cylux MRI-compatible 27 × 27-mm recording chamber (Alpha Omega Engineering, Nazareth, Israel) and head holder (Crist Instrument, Hagerstown, MD). The location of the chamber was determined by a primate stereotaxic atlas (Contreras et al. 1981; Martin and Bowden 2000) to be above the right M1 and GPe. A few days after surgery, an MRI scan was performed to determine the chamber’s exact stereotaxic location (Fig. 1A).
Fig. 1.
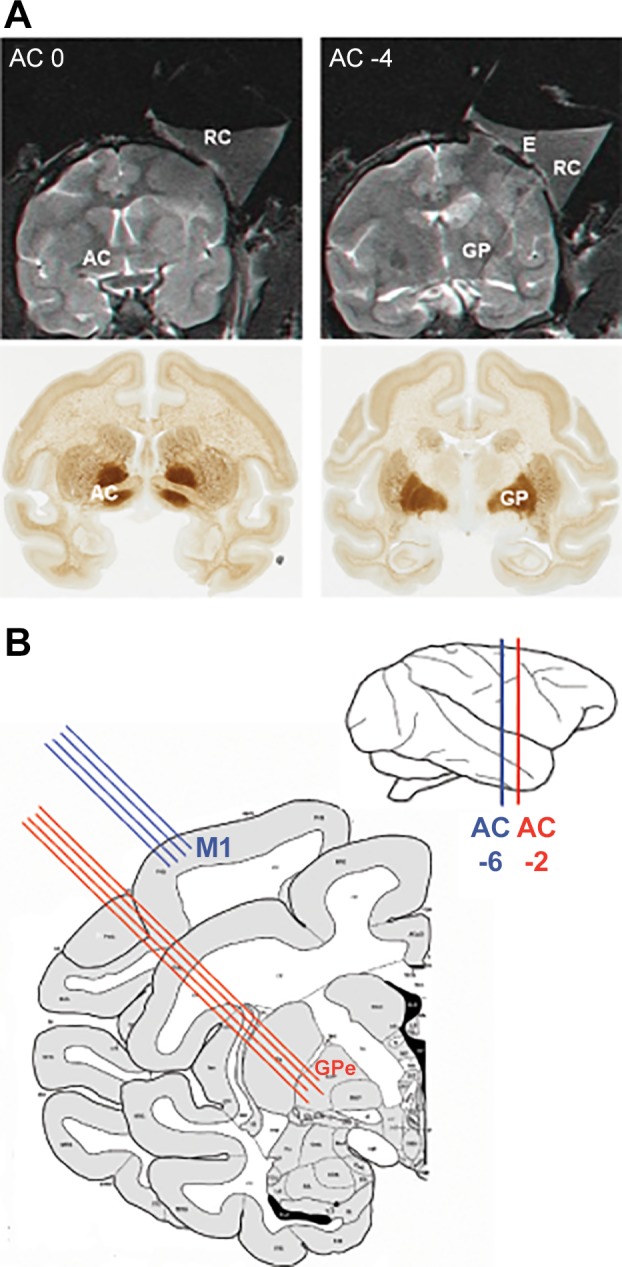
Anatomical localization and experimental setup. A: coronal MRI images (top) numbered with respect to distance (in mm) from the anterior commissure. The MRI scan was performed with 5 tungsten electrodes inserted at known coordinates of the recording chamber (2 electrodes can be seen at top right). The coronal MRI images are aligned with coronal sections (bottom) of the African green monkey atlas (www.brainmaps.org). B: experimental setup. Extracellular activity from M1 and the GPe was recorded simultaneously by 4 electrodes in each structure. The atlas scheme is adapted from BrainInfo (1991–present), National Primate Research Center, University of Washington (http://www.braininfo.org). AC, anterior commissure; E, electrode; GP, globus pallidus; GPe, external globus pallidus; M1, primary motor cortex; RC, recording chamber.
Recording and Data Acquisition
During recording sessions, the animals were seated in a primate chair with their head and hands restrained but were free to move their trunk and legs. In each recording session, two arrays of four glass-coated tungsten microelectrodes (impedance measured at 1 kHz, range 0.2–0.5 MΩ) were positioned in the upper limb area of M1 and in the GPe, with one array in each structure (Fig. 1B). The four microelectrodes were confined within a cylindrical (1.36-mm internal diameter) metal guide, with horizontal distance of ~0.5–0.7 mm between pairs of microelectrodes. The verification of recording location (M1 and GPe) was carried out separately for the two target structures (Rivlin-Etzion et al. 2008). M1 was identified by detecting movement of the contralateral arm, forearm, or hand in response to cortical microstimulation (see Rivlin-Etzion et al. 2008). The GPe was identified by the typical high-frequency (with pauses) discharge of GPe neurons. The vertical position of each electrode was adjusted by the double microdriving terminal and electrode positioning system (double MT and EPS; Alpha Omega Engineering), which allows independent movement of each electrode (with a step size of 2–5 µm). Hence, the vertical distance between each pair of electrodes in each guide was between 0 and 2 mm.
After recording in the baseline state, one of the experimenters entered the animal room and administered an intramuscular injection of ketamine (Ketaset, Fort Dodge Animal Health, Fort Dodge, IA; Ketalar, Pfizer Healthcare, Dublin, Ireland). The monkeys were treated with intramuscular injections of 10, 5, and 2 mg/kg ketamine (see Table 1 for the detailed neural database). Local field potentials (LFPs) from M1 and the GPe were recorded simultaneously using one to four electrodes for each structure (Fig. 1, A and B). Activity was recorded from 10 min before up to 120 min after the administration of ketamine.
Table 1.
Neural database
| Dose of Ketamine, mg/kg | Monkey | No. of Recording Sessions | No. of Cortical LFPs | No. of Pallidal LFPs | No. of Cortical LFP Pairs | No. of Pallidal LFP Pairs | No. of Cortical-Pallidal LFP Pairs |
|---|---|---|---|---|---|---|---|
| 10 | F | 7 | 28 | 25 | 42 | 34 | 100 |
| Su | 4 | 12 | 16 | 18 | 24 | 48 | |
| Sa | 10 | 38 | 25 | 54 | 21 | 95 | |
| M | 5 | 19 | 10 | 27 | 9 | 40 | |
| Total | 26 | 97 | 76 | 141 | 88 | 283 | |
| 5 | Sa | 9 | 31 | 24 | 42 | 27 | 92 |
| M | 5 | 20 | 13 | 30 | 16 | 52 | |
| Total | 14 | 51 | 37 | 72 | 43 | 144 | |
| 2 | Sa | 4 | 10 | 9 | 10 | 6 | 24 |
| Total | 4 | 10 | 9 | 10 | 6 | 24 |
LFPs, local field potentials.
The electrical activity was amplified with a gain of 5K, bandpass filtered with a 1- to 5,000-Hz four-pole hardware Butterworth filter, and continuously sampled at 25 kHz. The data were stored by a data acquisition system (AlphaMap; Alpha Omega Engineering) for offline analysis. The animals’ behavior was monitored by a computerized video surveillance system (monkeys F and Su: GV-650, GeoVision, Taipei City, Taiwan; monkeys Sa and M: GV-2400, GeoVision) connected to four infrared digital cameras.
Data Analysis
LFP signal.
The neural raw data were divided into segments of 60 s in length. Each segment was digitally low-pass filtered at 150 Hz using an eight-pole Butterworth filter. The filtered signal was down sampled to 500 Hz, and the mean of the segment was subtracted.
Power spectral density.
The power spectral density (PSD) was calculated using Welch’s method whereby each 60-s segment was divided into 8 sections of equal length (each ~13.3 s, 50% overlap) windowed with a Hamming window. The frequency resolution was 0.01 Hz. Values within 0.6 Hz of the power supply artifacts (50 Hz) and their harmonics were removed and interpolated from the surrounding values.
Ultraslow oscillations are filtered out by the recording system hardware filter (1–5,000 Hz). To reveal significant modulations of the gamma activity at low frequencies (below the 1-Hz high-pass filter), each segment was bandpass filtered (8-pole Butterworth filter) at 30–48 Hz (the frequency range most significantly enhanced by ketamine administration) and full-wave rectified by the “absolute” operator (Moran and Bar-Gad 2010; Zaidel et al. 2010). The mean of the rectified signal was subtracted. The PSD values were calculated (using Welch’s method) as described above.
Autospectrogram.
The “autospectrograms” were created by concatenating the PSD of consecutive 1-min segments from 10 min before ketamine administration until the end of every recording session. Time 0 is defined as the time immediately after the injection. In monkeys F and Su, occasional narrowband artifacts lasting throughout the recording session (including the baseline period) were detected by visual inspection. These bands were discarded from the relevant recording sessions and interpolated from the surrounding data. In addition, in monkeys F and Su, the experiment also included short epochs in which proprioceptive perturbations to the left forearm of the animal were applied. This consisted of motor-induced periodic left forearm movement (mimicking artificial tremor) at discrete and known frequencies (2 and 5 Hz). Each motor testing epoch lasted 90–120 s. There were 3–10 epochs per recording session. Thus, for most of the recording time in monkeys F and Su, and throughout the entire recording sessions in monkeys Sa and M, the animals’ hands were at rest. However, during the proprioceptive perturbations, an increase in spectral power was seen at discrete frequencies corresponding to the motor frequency and its first harmonics. We therefore removed the PSD values within 0.2 Hz of the motor frequency and its first two harmonics (in monkeys F and Su) and interpolated them on the basis of adjacent values.
To better demonstrate the changes in neural activity following ketamine administration, we calculated the “normalized autospectrogram;” i.e., the average baseline power before ketamine administration was computed for each frequency bin. Each PSD value was then divided by its respective average baseline power. This was done for both high and low (<1 Hz) frequency ranges. The color scale of the autospectrograms and the normalized autospectrograms in this manuscript represents 10 log10(PSD) and 10 log10(PSD/average baseline power), respectively.
Coherence.
The magnitude and phase of the coherence between two simultaneously recorded LFPs were calculated using Welch’s method with the same parameters and conventions as for the PSD analysis. The “coherogram” was created by concatenating the values of the magnitudes of the coherence of consecutive 1-min segments. By definition, the coherence magnitude attained values between 0 and 1. The “normalized coherence” was created by subtraction of the average coherence magnitude for each frequency bin before the administration of ketamine (baseline coherence) from all time bins. Note that the normalized coherence values can therefore be out of the 0-to-1 range.
The distribution of the magnitude of the coherence of different LFP pairs at the low-gamma range (30–48 Hz) was calculated as follows. For each LFP pair, the magnitude-squared coherence was calculated in 1-min segments as described above and averaged across the range of 30–48 Hz. These values were then averaged across the 5- to 15-min postinjection period (the 10-min surrounding the gamma peak). The distribution was calculated separately for M1-M1, GPe-GPe, and M1-GPe pairs.
The phase between pairs of LFPs was calculated using two different methods:
1) The phase was derived from the cross-PSD. The cross-PSD calculation was carried out using Welch’s method with the same parameters as described above. For each pair, the resultant phase was averaged across the range of 30–48 Hz and over the 5- to 15-min postinjection period.
2) Both LFP signals were bandpass filtered at 30–48 Hz. The minimum points of both filtered signals were compared, and deviations (in ms) were divided by the cycle length (in ms), multiplied by 360°, yielding the phase shift in degrees.
Both methods yielded similar results. Thus only the first method is presented in this article. The distribution of average phase shifts was calculated separately for all M1-M1, GPe-GPe, and M1-GPe pairs.
Time course.
To assess the time course of the spectral and coherence changes, the normalized total power/coherence at the range of 30–48 Hz or 0.2–0.4 Hz was averaged across all (cortical or pallidal) recording sites to yield the PSD/coherence time course. The power/coherence values were transformed into Z scores that reflect the deviation of the power/coherence from the baseline in units of standard deviations.
The above analyses were carried out using custom software written in MATLAB (R2007a; The MathWorks, Natick, MA).
PCP Administration
A few recording sessions were conducted after the administration of PCP in a fifth monkey, monkey V (C. aethiops aethiops, female, 3.7 kg). Protocols for the surgery, MRI procedures, and ethical guidelines are the same as described above. These PCP sessions included 1, 3, and 2 sessions of 0.3, 0.5, and 0.7mg/kg im PCP administration, respectively. In each session, a single array of eight glass-coated tungsten microelectrodes (impedance 0.15–1 MΩ at 1,000 Hz) confined within a cylindrical guide (1.65-mm inner diameter) was targeted to the GPe. Electrical activity was amplified with a gain of 20, filtered using hardware Butterworth filters (high pass at 0.075 Hz, 2 poles; low pass at 10,000 Hz, 3 poles), and sampled at ~44.6 kHz (AlphaLab SnR; Alpha Omega Engineering). These data were then downsampled by a factor of 40. The autospectrograms and the coherograms were calculated using the Chronux toolbox v. 2.11 for MATLAB (Bokil et al. 2010). The analysis used five tapers with a time bandwidth product of 5. The power and coherence were calculated using a 60-s-wide sliding window with no overlap and smoothed using a two-dimensional rectangular window. Data analyses were performed using MATLAB (2014b release; The MathWorks).
RESULTS
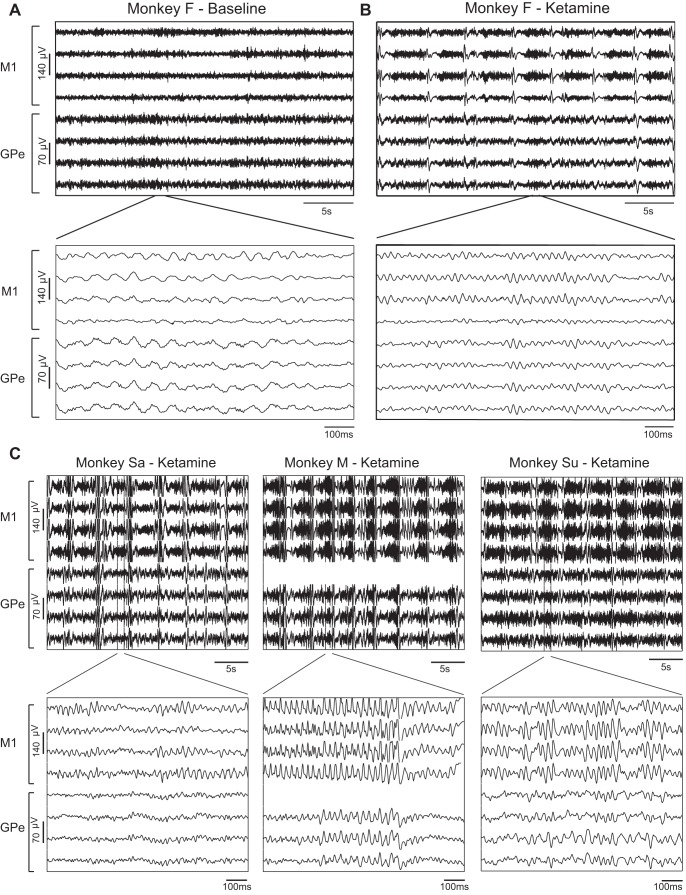
Figure 2 shows an example of the raw analog LFP recorded before (Fig. 2A, baseline state) and 12 min after (Fig. 2B) ketamine administration (10 mg/kg im) to monkey F. In the baseline state one can observe low-beta band (~15 Hz) oscillations and synchronization between the pallidal LFPs, but a lower degree of synchronization could be seen within the cortex and between cortical and pallidal LFPs. After ketamine administration, a periodic pattern with a frequency of ~0.25 Hz emerged in both the cortical and pallidal sites. This pattern was composed of alternating polyphasic high-amplitude peaks (resembling sleep K-complex and delta waves) and lower amplitude gamma band (~40 Hz) oscillations. These multifrequency (~0.25, 4, and 40 Hz) oscillatory phenomena were replicated in the three other animals treated with the same dose of ketamine (10 mg/kg; Fig. 2C).
Fig. 2.
Simultaneous recording of cortical and pallidal LFP following administration of ketamine. A and B, top: 30-s traces of the raw analog LFPs recorded from monkey F before (baseline) and 12 min after the administration of ketamine (10 mg/kg im). Bottom plots show an enlargement of 1-s strips from top plots. In each plot the top 4 traces and the bottom 4 traces were recorded from M1 and GPe, respectively. C: examples of the raw analog signal recorded from monkeys Sa, M, and Su 8, 10, and 6 min after ketamine administration, respectively. Same conventions as in B. GPe, external globus pallidus; LFP, local field potentials; M1, primary motor cortex.
Ketamine-Induced Changes in Spectral Content of Cortical and Pallidal LFPs
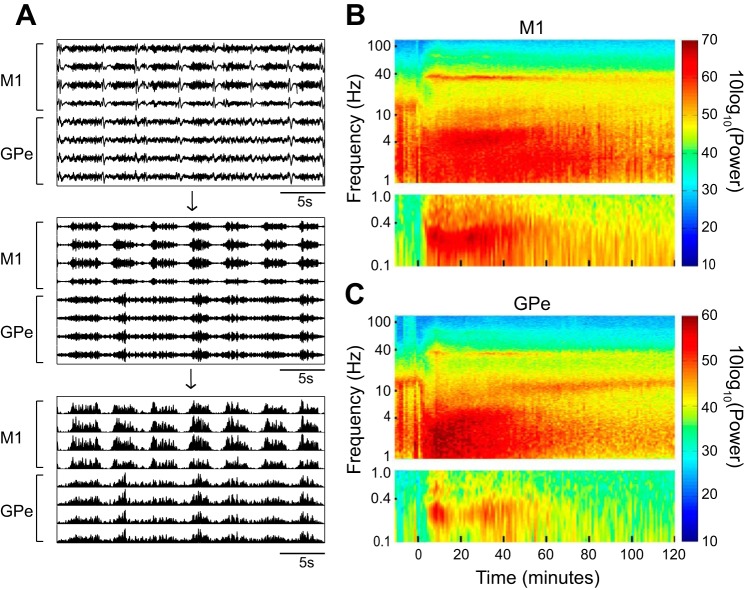
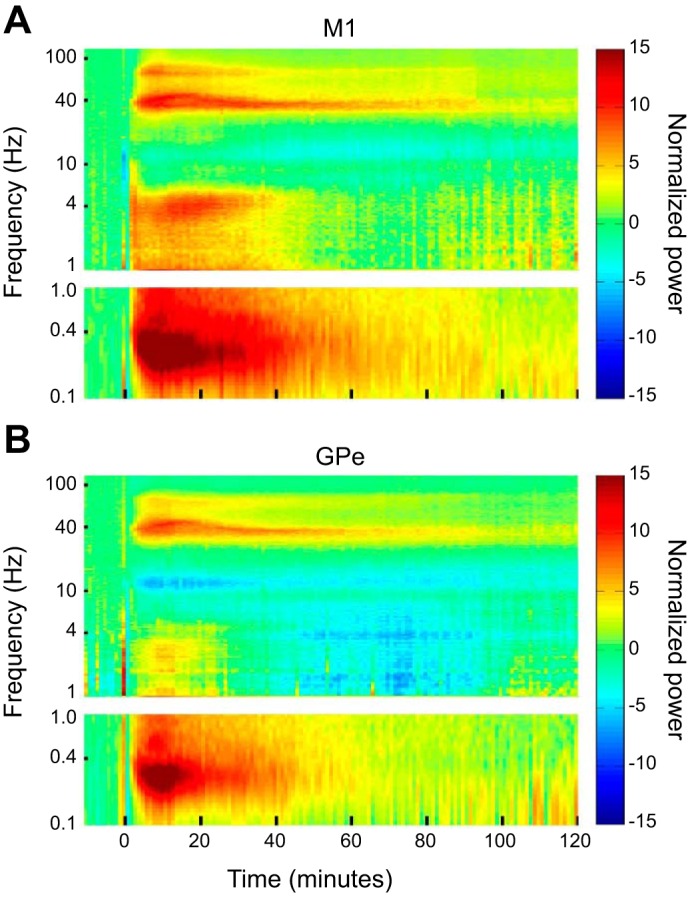
To quantitatively examine the changes in neural activity in the frequency domain over time, we calculated the autospectrograms of the cortical and pallidal LFPs. To detect oscillatory frequencies below 1 Hz that were filtered out by the 1-Hz high-pass hardware filter of the recording system, we first (Fig. 3A) bandpass filtered the signal at 30–48 Hz and full-wave rectified it (Moran and Bar-Gad 2010; Zaidel et al. 2010). Thus the power at <1 Hz frequencies in the autospectrograms of this study represents the envelope of the gamma band activity.
Fig. 3.
Cortical and pallidal LFPs exhibit changes in multifrequency bands following ketamine administration. A: the extraction of ultraslow frequency oscillations. Top, the raw analog LFP signal shown in Fig. 2A, top right. Middle, the same signal after bandpass filtering at 30–48 Hz. Bottom, the filtered signal after full wave rectification. B and C: examples of the autospectrogram of single cortical and pallidal LFPs recorded simultaneously from monkey M. The time axis initiated 10 min before the administration of ketamine (10 mg/kg im) at time 0. The frequency axis is presented in logarithmic scale. The oscillation power is color coded (right). For the ultraslow frequencies (<1 Hz) the power spectral density (PSD) analysis was performed on the signal following the extraction process described in A. Thus the PSD at 0.1–1 Hz represents the modulation of the gamma activity. M1, primary motor cortex; GPe, external globus pallidus; LFP, local field potentials.
Figure 3, B and C, shows an example of the autospectrogram of single cortical and pallidal LFPs recorded simultaneously, starting from 10 min before ketamine administration (10 mg/kg) and for the 120 min that followed. The autospectrograms are in line with the oscillatory phenomena illustrated in the snapshot of the analog data in Fig. 2. Narrowband gamma oscillations at 30–42 Hz (in this specific example) emerged in both the cortex and the GPe ~3 min after ketamine administration. Ultraslow modulation of the gamma activity with a frequency of 0.2–0.4 Hz appeared at the same time as the gamma oscillations. The gamma oscillations decayed as time elapsed but could still be detected 2 h subsequent to ketamine administration, exceeding the duration of the ultraslow modulation. In addition, an increase in cortical delta power at 3–5 Hz and in pallidal delta power at 1–4 Hz, as well as a decrease in alpha power (10–15 Hz), was observed following ketamine administration.
Figure 4 shows the normalized (by baseline activity) average spectral changes in cortical and pallidal LFPs. A prominent increase in the low-gamma band power (30–50 Hz) and in the ultraslow (0.2–0.4 Hz) gamma modulation power can be seen in both structures, starting from 2 min after ketamine (10 mg/kg) administration. As in the single examples (Fig. 3, B and C), the duration of the population gamma oscillations exceeded that of the ultraslow modulation. A small increase in the high-gamma band power (60–95 Hz) that followed the temporal structure of the low-gamma band oscillations can also be seen in both structures. In addition, there was a pronounced increase in cortical delta power (3–5 Hz) but only a modest increase in pallidal delta power and in lower (1–4 Hz) frequencies. Finally, a clear decrease of power in the alpha range (8–13 Hz) was found in both structures, albeit more prominently in the GPe.
Fig. 4.
Average spectral changes in cortical and pallidal LFPs following ketamine administration. A and B: the normalized average autospectrograms of the cortical LFPs and the pallidal LFPs, respectively. The average power values across all recording sessions at each time and frequency bin were divided by the average baseline power at each frequency bin. Color bar represents 10 log10 (normalized power). The number of recording sites in the cortex started at 97 recording sites before and at injection time, dropped to 89 recording sites after 60 min, and then gradually decreased to 53 after 120 min. The number of recording sites in the GPe started at 76 recording sites, dropped to 68 recording sites after 60 min, and then gradually deceased to 33 after 120 min. Other conventions are as in Fig. 3, B and C. M1, primary motor cortex; GPe, external globus pallidus; LFP, local field potentials.
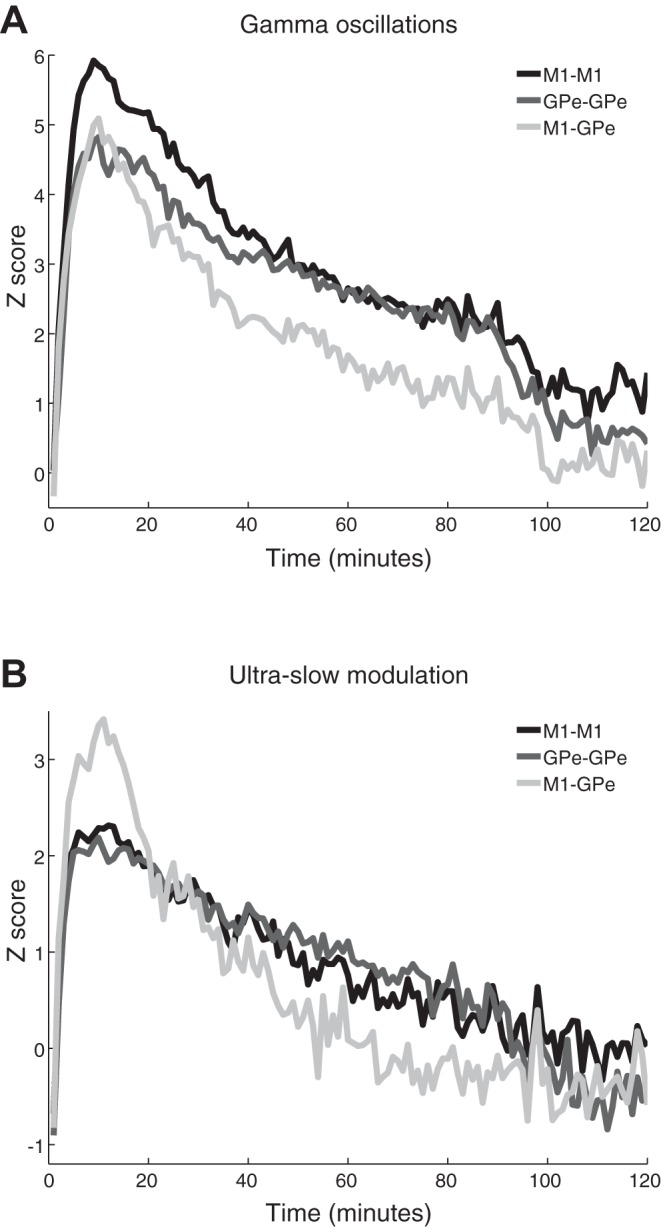
Figure 5 shows the time course of the power changes (in Z-score units) in the low-gamma band and its ultraslow modulation. The average total power at 30–48 Hz (Fig. 5A) and 0.2–0.4 Hz (Fig. 5B) relative to the baseline is depicted. Both the gamma oscillations and their ultraslow modulation reached their peak ~10 min after the 10 mg/kg im ketamine injection in both structures. However, the two oscillatory phenomena (the gamma oscillations and the ultraslow modulation) had different durations and decay rate. Fitting the decay of both phenomena (from the point of maximum amplitude) to a one-term exponential model Y = a·e−x/τ yielded (r2 ≥ 0.96), a mean lifetime (tau) of 47.2 and 42.7 min for cortical and pallidal gamma oscillations, respectively, and a tau of 11.7 and 8.6 min for cortical and pallidal ultraslow modulation (see Fig. 8B, 2 left pairs of columns).
Fig. 5.
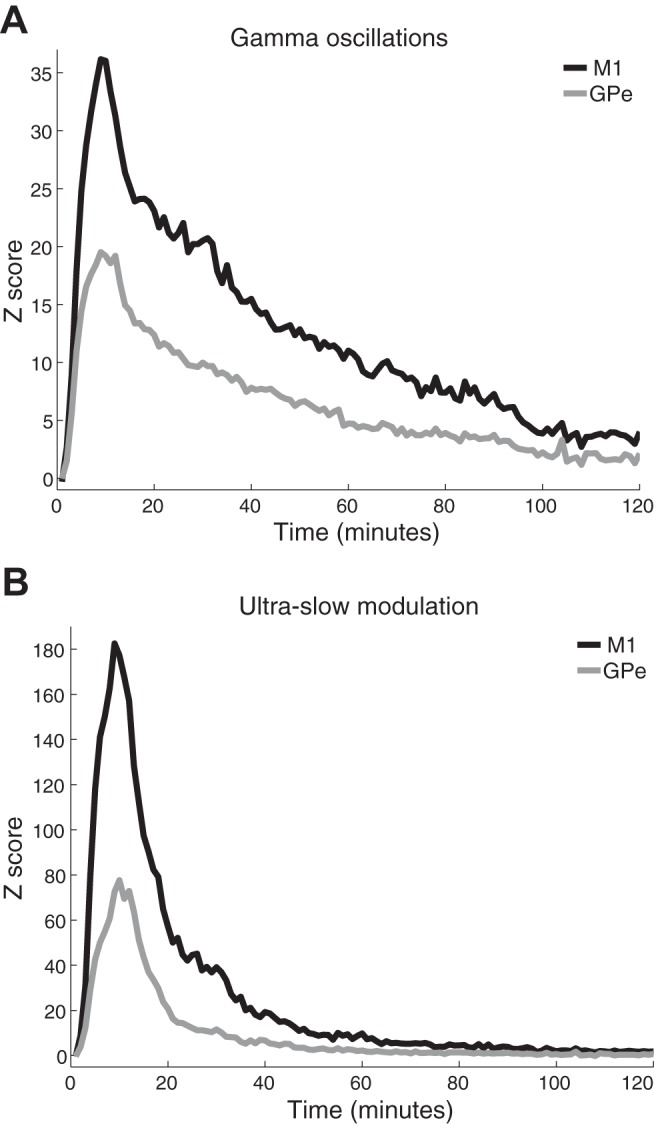
Time course of the gamma oscillations and their ultraslow modulation. A and B: the average increase in total power (M1, black; GPe, gray) at 30–48 Hz (A) and at the 0.2- to 0.4-Hz modulation of the gamma activity (B). The Z score represents the standard deviation of the average baseline normalized power. Time 0 is immediately after ketamine administration. Number of recording sites is the same as in Fig. 4. M1, primary motor cortex; GPe, external globus pallidus.
Fig. 8.
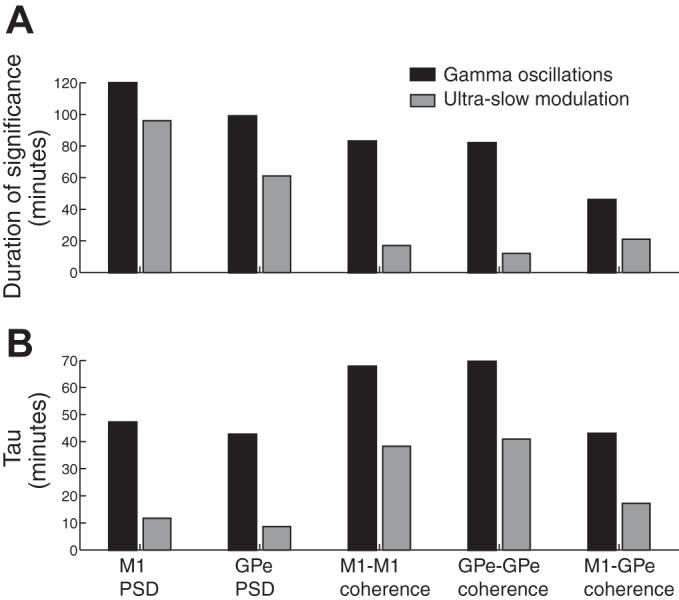
Duration of significant levels and mean lifetime (tau) of the gamma (black bars) and ultraslow (gray bars) synchronous oscillations. A: the duration of significant (>2 SD of baseline values) increases in power (first 2 pairs of columns) and coherence (last 3 pairs of columns), respectively. B: the mean lifetime (tau) of the exponential decay of the oscillatory power and coherence. M1, primary motor cortex; GPe, external globus pallidus.
Ketamine-Induced Changes in Synchronization of Cortical and Pallidal LFPs
We next examined the degree of synchronization within and between the M1 and GPe. Figure 6 shows the normalized average coherence between all pairs of simultaneously recorded cortical (Fig. 6A), pallidal (Fig. 6B), and cortical-pallidal LFPs (Fig. 6C). Figure 6A points out that the gamma, delta and ultraslow oscillations that were induced by ketamine in the cortical LFPs (Figs. 3B and 4A) were synchronized across different cortical sites. Figure 6B shows similar increases in the coherence between different pallidal LFPs in the gamma and ultraslow frequency bands. Finally, Fig. 6C illustrates the increased coherence between cortical and pallidal LFPs in the gamma and ultraslow bands following ketamine administration. The time course of the coherence changes for the different frequencies and loci pairs are depicted in Fig. 7.
Fig. 6.
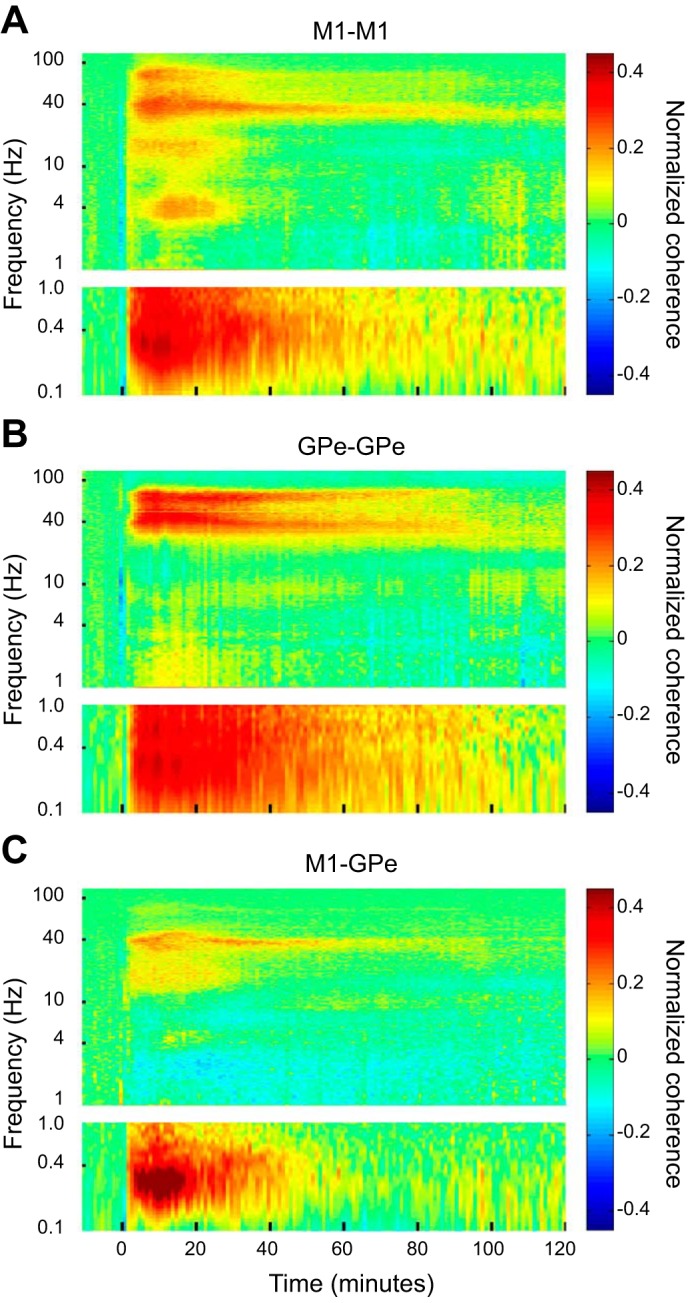
Ketamine-induced oscillations are synchronized within and between M1 and GPe. A–C: the normalized average coherence between all pairs of simultaneously recorded cortical LFPs (A), pallidal LFPs (B), and cortical-pallidal LFPs (C). The average baseline coherence was computed for each frequency and was subtracted from the average coherence at each time and frequency bin before and after ketamine administration. All other conventions are as in Fig. 3, B and C. The number of cortical pairs started at 141 at time 0, dropped to 129 at 60 min, and then gradually decreased to 75 after 120 min. The number of pallidal pairs started at 88 at time 0, dropped to 76 at 60 min, and then gradually decreased to 29 after 120 min. The number of cortical-pallidal pairs started at 283 at time 0, dropped to 251 at 60 min, and then gradually decreased to 127 after 120 min. M1, primary motor cortex; GPe, external globus pallidus; LFP, local field potentials.
Fig. 7.
Time course of the coherence of gamma oscillations and their ultraslow modulation. A and B: the average increase in total coherence (cortical pairs, black; pallidal pairs, dark gray; cortical-pallidal pairs, light gray) at 30–48 Hz (A) and at the 0.2- to 0.4-Hz modulation of the gamma activity (B). The Z score represents the standard deviation of the average baseline normalized coherence. Time 0 is immediately after ketamine administration. Number of pairs is the same as in Fig. 6. M1, primary motor cortex; GPe, external globus pallidus.
Figure 8A summarizes the difference in the duration of increased coherence within and between the two structures in the low-gamma band vs. the ultraslow modulation range (right 3 pairs of columns). The differences in the decay patterns between the two oscillatory phenomena are further illustrated by the mean lifetime (tau) in Fig. 8B (r2 ≥ 0.8 for the fit with 1-term exponential model). As for the autospectrograms, the changes in the gamma band in the coherence spectrograms were more long-lasting than their ultraslow modulation (0.2–0.4 Hz).
Figure 9 presents the distribution of the coherence magnitude and phase for the low-gamma frequency band oscillations. The magnitude-squared coherence at 30–48 Hz was averaged across the 5- to 15-min period following the ketamine injection. The mean coherence magnitude was 0.76 ± 0.13, 0.88 ± 0.06, and 0.41 ± 0.11 (means ± SD) for M1-M1 (Fig. 9A, left), GPe-GPe (Fig. 9B, left), and M1-GPe LFP pairs (Fig. 9C, left), respectively.
Fig. 9.
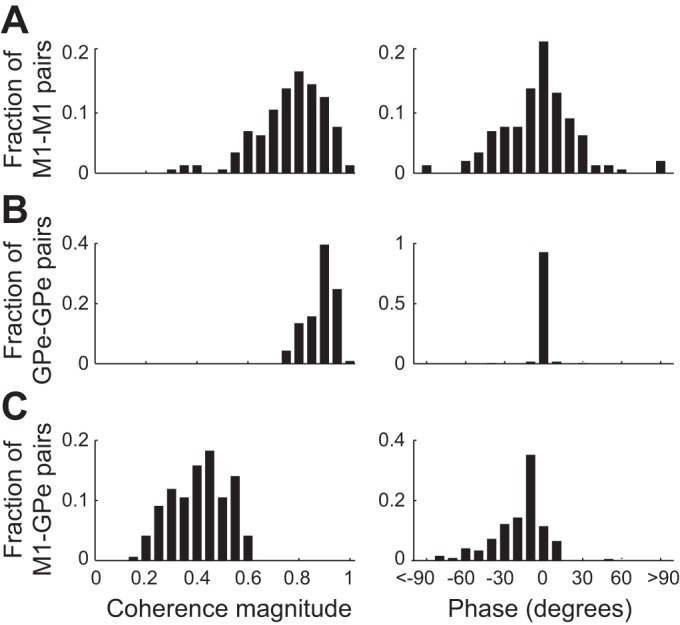
Distribution of the coherence magnitude and phase of the gamma synchronous oscillations. The magnitude-squared coherence (A–C, left) and the phase (A–C, right) between each pair of LFPs was averaged across time (5- to 15-min period following ketamine injection) and the low-gamma frequency range (30–48 Hz). The distribution of the average magnitude and phase was calculated separately for M1-M1 (A), GPe-GPe (B), and M1-GPe (C) LFP pairs. M1, primary motor cortex; GPe, external globus pallidus.
The distribution of the average phase (across the same time period and frequency range) was broadly and very narrow distributed around zero for the M1-M1 and the GPe-GPe pairs, respectively. The distribution of M1-GPe pairs was significantly different from zero and skewed toward negative phase shifts. Quantitatively, the phase shift distribution had a mean (±SD) of −3.5 ± 31.8°, −0.4 ± 6.0°, and −18.4 ± 20.9° (which correspond to 0.24, 0.03, and 1.28 ms) for M1-M1 (Fig. 9A, right), GPe-GPe (Fig. 9B, right), and M1-GPe LFP pairs (Fig. 9C, right), respectively.
Effects of Different Doses of Ketamine and PCP
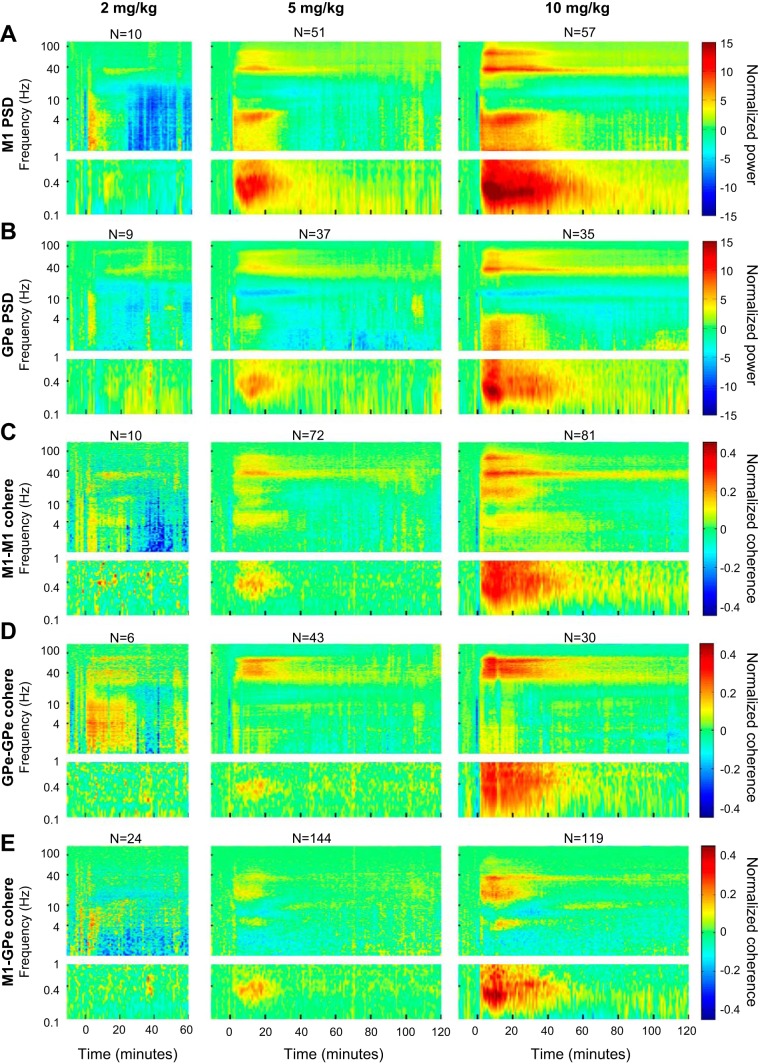
A limited number of recording sessions also explored the effects of lower doses of ketamine. Figure 10 demonstrates the existence of cortical and pallidal synchronous gamma oscillations in the average spectrograms of both 2 and 5 mg/kg ketamine doses. The 10 mg/kg spectrograms (Fig. 10, far right) depict the corresponding average of only the animals that were included in the lower dose examinations. It is clear that the difference between the 10 and 5 mg/kg doses is mainly quantitative rather than qualitative. In the 2 mg/kg dose, although much weaker than in the higher doses, gamma oscillatory activity can be noticed in both M1 and GPe. However, a clear ultraslow oscillation could not be observed. The doses used did not follow the classical scale of dose-response curves; rather, they were chosen to shed more light on the relationship between ketamine and gamma oscillations, and the possible appearance of unmodulated gamma oscillations at lower doses of ketamine.
Fig. 10.
Ketamine induces spectral and coherence changes in a dose-dependent manner. A and B: the normalized average autospectrogram of all cortical (A) and pallidal (B) LFPs according to the different doses of ketamine. C–E: the normalized average coherence between all pairs of cortical (C), pallidal (D), and cortical-pallidal (E) LFPs according to the different doses of ketamine. In A–E, left recordings follow administration of 2 mg/kg ketamine from monkey Sa only; middle recordings follow administration of 5 mg/kg ketamine from monkeys Sa and M; and right recordings follow administration of 10 mg/kg ketamine from monkey Sa and M. N represents the total (maximal) number of LFPs (PSD subplots) or LFP pairs (coherence subplots) recorded for each condition. In the 2 mg/kg dose, the number of LFPs remained constant in all time bins. In the 5 and 10 mg/kg doses, the number of LFPs or LFP pairs decreased throughout the recording; the average number (across all time bins) was 45, 34, 63, 40, and 130 in the 5 mg/kg dose (A–E, respectively) and 57, 35, 80, 30, and 118 in the 10 mg/kg dose (A–E, respectively). M1, primary motor cortex; GPe, external globus pallidus; cohere, coherence; PSD, power spectral density.
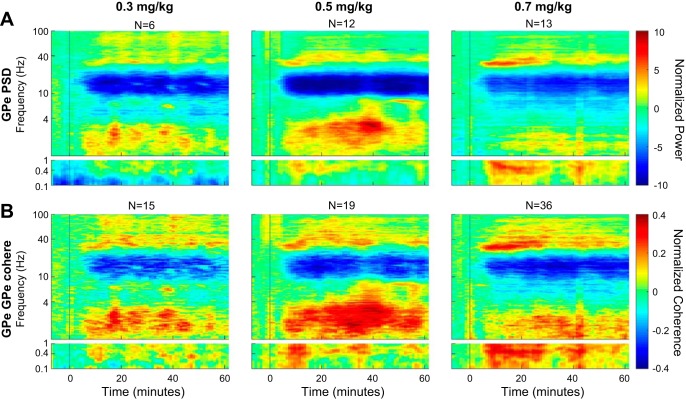
Finally, in one monkey (monkey V), we recorded the GPe activity with an array of 8 electrodes following intramuscular injection of 0.3, 0.5, and 0.7 mg of PCP. GPe normalized autospectrograms and coherograms are depicted in Fig. 11, A and B, respectively. As for the ketamine injections, PCP induced synchronous oscillations at the ultraslow (<1 Hz), delta (1–4 Hz), and gamma (mainly low gamma) domains, accompanied by a reduction of the power in the alpha-low beta domain (10–20 Hz).
Fig. 11.
Phencyclidine (PCP) induces spectral and coherence changes in a dose-dependent manner. A: normalized average autospectrograms of pallidal LFPs according to the different doses of PCP. B: normalized average coherence between all pairs of pallidal LFPs according to different doses of PCP. N represents the total number of LFPs (PSD subplots) or LFP pairs (coherence subplots) recorded for each condition. GPe, external globus pallidus; PSD, power spectral density; cohere, coherence.
DISCUSSION
In this article we have reported the emergence of sustained resting narrowband (30–50 Hz) synchronous gamma oscillations across cortical (M1) and basal ganglia (GPe) structures following the systemic administration of the NMDA antagonist ketamine to nonhuman primates. These oscillations were initially modulated by ultraslow oscillations (~0.3 Hz) and could still be detected 100–120 min later. The oscillations were synchronized within and between the M1 and GPe. Phase differences were distributed evenly around zero with broad distribution for the M1-M1 pairs but with narrow distribution for the GPe-GPe pairs. Finally, a non-zero and skewed distribution of phase shifts was found between the simultaneously recorded M1-GPe pairs. The administration of PCP (a different NMDA antagonist) also induced low-gamma band oscillations in the GPe, resembling the ketamine effects.
Low- vs. High-Gamma Band Oscillations Following the Administration of NMDA Antagonists
Recent EEG (Ehrlichman et al. 2009; Kittelberger et al. 2012; Kocsis 2012; Lazarewicz et al. 2010; Ma and Leung 2007), electrocorticography (Hakami et al. 2009; Pinault 2008), and LFP studies (Caixeta et al. 2013; Hakami et al. 2009; Kulikova et al. 2012; Nicolás et al. 2011; Wood et al. 2012) conducted on rodents have reported the emergence of spontaneous gamma oscillations under ketamine in various cortical and subcortical areas. Although there are fewer PCP oscillation studies, recent work has documented the appearance of low-gamma band oscillations in the cortical EEG of rats and monkeys after the administration of varied doses of PCP (Goonawardena et al. 2016; Hiyoshi et al. 2014). In the current study, LFP gamma band oscillations emerged under both ketamine (in M1 and GPe) and PCP (in GPe). Gamma oscillations under both substances began a few minutes after the injection and lasted for dozens of minutes thereafter. Most ketamine rodent studies indicate an increase in the power of both low- and high-gamma frequency bands. Similarly, we found an increase in power in both gamma frequency bands (Fig. 4). However, on the basis of the similar temporal pattern of spectral changes of the two gamma bands, we posit that the high-gamma band probably constituted the harmonic of the spectral power at the low-gamma band. The differences in the subbands involved between our study and other studies could be attributed to species differences, similar to the higher frequencies seen in the 6-OHDA model compared with the MPTP nonhuman model of Parkinson’s disease (Quiroga-Varela et al. 2013; Stein and Bar-Gad 2013). Alternatively, these differences may be attributed to different analysis methods, such as the use of summary measures (e.g., total power, percentage of power changes), which limits the differentiation between different subbands.
Studies of spontaneous gamma oscillations under ketamine in humans are sparse. These few studies tackle mainly the lower gamma range and show either no modulation (Plourde et al. 1997) or increases in subbands of 25–35 Hz (Purdon et al. 2015) or 30–50 Hz (Maksimow et al. 2006). Therefore, it is hard to form a unified picture of the ketamine-induced spectral changes in humans. Because electrophysiological data from humans are limited, our study provides a small step in closing the gap between the rodent and human studies.
Coherence and Phase Shifts of Cortical-Basal Ganglia Synchronous Gamma Oscillations
We showed that the gamma oscillations were synchronized within and between the M1 and GPe. Recent studies conducted on ketamine-treated rats showed an increase in power at the low-gamma, high-gamma, and the high-frequency (~150 Hz) range in the motor cortex and various nuclei of the BG (Cordon et al. 2015; Nicolás et al. 2011). These oscillations were synchronized between the different BG structures as well as between the motor cortex and the BG, similar to our findings. In line with previous cortical LFP studies, we found near-zero mean phase shift between M1-M1 and GPe-GPe LFPs recorded by two microelectrodes with a horizontal distance of ~0.5–0.7 mm (Gollo et al. 2011; Rajagovindan and Ding 2008; Viriyopase et al. 2012). Notably, we showed that the distribution of the phase shifts of M1-M1 pairs was much broader than that of the GPe-GPe pairs (Fig. 9, right). This probably reflects the anatomical convergence of cortical activity into the BG structures, creating a homogeneous subthreshold activity (common input) at different pallidal sites (Percheron et al. 1984). Moreover, the narrow distribution of phase shifts in the GPe emphasizes the notion of the BG as a funneling and amplifying system of the cortical activity (Bar-Gad et al. 2003). We hypothesize that the BG funneling system enables extraction of relevant information from the state-related activity in the input layers. To maximize the information content in the output layers of the basal ganglia, the system probably employs active decorrelation (e.g., through the lateral inhibitory system) to keep the pallidal units independent. However, under abnormal conditions the active decorrelation processes are not strong enough to overcome the common drives of the pallidal neurons, and abnormal synchronized activity emerges.
The findings of non-zero coupling and nonsymmetric distribution of phase shifts between the simultaneously recorded M1-GPe LFPs (Lachaux et al. 1999; Vinck et al. 2012) indicate that this synchronization is not merely due to volume conductance of the cortical gamma range LFP to the deep structures of the BG (Marmor et al. 2017; Wennberg and Lozano 2003). We found that the GPe gamma activity led the M1 by ~20°. However, because this is a cyclic phenomenon, it can also be concluded that the GPe lagged behind M1 activity by ~340°. This is consistent with a recent study of the 6-OHDA rodent model of Parkinson’s disease (Brazhnik et al. 2012), which found that neurons in the substantia nigra reticulata are phase-locked to cortical LFP oscillations and fire, on average, 17 ms after synchronized M1 spiking. Future theoretical and experimental studies of the complex closed-loop structure of the cortex-BG network will hopefully provide a better understanding of these phase shifts in the cortex-BG network.
Slow Modulation of Gamma Oscillations
The slow (~0.3 Hz) modulation of gamma activity in the current study resembles the oscillations that characterize the slow-wave stage of sleep in humans and states of anesthesia in animals (Amzica and Steriade 1995; Steriade et al. 1993) or the slow-2 regime in the natural logarithmic scale of brain oscillations (Penttonen and Buzsáki 2003). These slow oscillations are known to exist in both cortical and subcortical structures (Collins et al. 2001; Goldberg et al. 2003; Magill et al. 2000; Steriade et al. 1993). Qualitative observations of behavior of our animals indicated decreased limb movements following ketamine (10 mg/kg im) administration. In addition, while the animals’ eyes remained open, there was a prominent decrease in blinking rate and eye movements (data not shown). Thus the slow oscillations observed in this study could reflect a state of reduced arousal.
The dose administered (10 mg/kg) in the current study was similar to the doses given in the rodent studies in which ketamine administration was accompanied by a marked increase in locomotion, hyperactivity, and ataxic behavior (Kocsis 2012; Nicolás et al. 2011; Pinault 2008). However, in nonhuman primates, the injection of an equivalent dose of ketamine may result in an intermediate state between being fully awake (subanesthetic state) and complete loss of consciousness. Although the exact state of the animals in our study was not determined, we hypothesized that the ultraslow modulation (of the gamma activity) may have been due to the drowsiness caused by the drug, whereas the gamma oscillations themselves constituted the neural correlate of the cognitive and psychotic symptoms. This supposition is supported by the parallel emergence of sustained gamma oscillations as well as increased hyperactivity and stereotypic behavior in ketamine-treated rats (Caixeta et al. 2013; El Iskandrani et al. 2015; Jones et al. 2012; Kocsis 2012; Pinault 2008). Several studies have also found a direct temporal correlation between ongoing gamma oscillations and animals’ locomotor activity (Hakami et al. 2009; Nicolás et al. 2011). Moreover, the duration of the gamma oscillations in our study exceeded that of the ultraslow oscillations with 5 and 10 mg/kg doses, and the ultraslow modulation was almost imperceptible at the 2 mg/kg dose, suggesting that the gamma oscillation may appear alone (without the ultraslow modulation) at lower levels of ketamine.
Future studies should include full polysomnography recordings, including EEG, electrooculography (EOG), and electromyography (EMG) for a better assessment of the arousal state of the animals. Together with cognitive or behavioral tasks, the correlation between the arousal or cognitive state of the animal and the appearance of the ultraslow and gamma band oscillations could be investigated in more depth.
Functional and Computational Implications
In this study, we focused on M1 and GPe activity. The BG, once known for their participation in motor control alone, are now considered to play a role in integrating cognitive, emotional, motivational, and motor domains to achieve optimal behavioral (motor) policy (Helie et al. 2013; Parush et al. 2011). M1 is a unique point in the cortico-BG circuitry, representing both the input and output of the BG (Rivlin-Etzion et al. 2008). The GPe is emerging as the central nucleus of the BG (Deffains et al. 2016). The GPe receives inputs from the striatum and the subthalamic nucleus, and innervates back both the input level of the BG and the output level (GPi and SNr) (Abdi et al. 2015; Goldberg and Bergman 2011; Kita 2007; Mallet et al. 2012).
The BG are connected not only to the motor and premotor cortices but also to extensive associative and cognitive areas in the frontal cortex (Haber 2003). Previous studies in rodents have reported global cortical oscillatory activity (Hakami et al. 2009), as well as global cortico-BG oscillatory activity with complex intrastructure interactions under ketamine (Nicolás et al. 2011). In the current study, we only recorded from the motor cortex and the GPe. However (and based on the rodent studies cited above), it can be hypothesized that in primates as well, the entire cortico-BG network is engaged in abnormal oscillatory activity. Future recordings from other cortical areas (with an emphasis on the prefrontal cortex) and from the different BG structures and subregions (such as the various functional territories of the GPe, STN. etc.) are needed to reveal the full extent of cortico-BG network aberrant activity under NMDA antagonists.
It is well accepted that in Parkinson’s disease, excessive and coherent beta band activity appears across the whole cortico-BG circuit and is strongly linked to the disease pathophysiology. We hypothesize that synchronous gamma oscillations could be a neurophysiological feature of schizophrenia, similar to that reported in Parkinson’s disease (Boraud et al. 2005; Hammond et al. 2007; Weinberger et al. 2009) but with a different spectral signature. Hence, both dopamine depletion and NMDA antagonism could represent states of abnormal BG function characterized by exaggerated correlated activity that results in inefficient dimensionality reduction of information.
If network oscillation abnormalities are indeed the neural correlate of the schizophrenic symptomatology, deep brain electrical stimulation (DBS) that can interfere with the occurrence of pathological rhythms (Devos et al. 2004; Kühn et al. 2008; Miocinovic et al. 2013) could potentially lead to clinical improvement (Ewing and Grace 2013; George et al. 2007; Klein et al. 2013; Mikell et al. 2009; Perez et al. 2013). The funneling structure of the cortico-BG network, as suggested in this study by the phase distribution in Fig. 9, right, also explains the rationale of applying DBS directed to the GPi or STN structures (as opposed to cortical areas for example) in various neurological and mental disorders (Krack et al. 2010). Local stimulation could gain a stronger effect if applied at the “hub” of the network, where there is convergence of the many domains served by the cortex-BG neural networks. Thus future studies should reveal if one can use the BG oscillations as a trigger for closed-loop adaptive DBS therapy (Little and Brown 2012; Rosin et al. 2011), not only for Parkinson’s disease but also for other neurological and psychiatric BG-related disorders.
GRANTS
This work was supported by research grants of the European Research Council, Adelis Foundation, Rostrees Foundation, and Vorst Family Foundation, and the Simone and Bernard Guttman Chair of Brain Research. M. Slovik was supported by a Converging Technologies scholarship of the Israeli Council for Higher Education and by a Hebrew University scholarship. S. Moshel was supported by the Edmond and Lily Safra Center postdoctoral fellowship. R. Mitelman was supported by the Hoffman Leadership and Responsibility fellowship program at the Hebrew University. A. Raz was supported by a grant from the International Anesthesia Research Society Mentored Research Award.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.S., A.R., and H.B. conceived and designed research; M.S., B.R., S.M., R.M., E.S., and A.R. performed experiments; M.S. and E.S. analyzed data; M.S., A.R., and H.B. interpreted results of experiments; M.S. and E.S. prepared figures; M.S. drafted manuscript; M.S., R.E., A.R., and H.B. edited and revised manuscript; M.S., B.R., S.M., R.M., E.S., R.E., A.R., and H.B. approved final version of manuscript.
ACKNOWLEDGMENTS
Present address of R. Mitelman: Dept. of Neurobiology, Weizmann Institute of Science, Rehovot 76100, Israel.
Present address of E. Schechtman: Dept. of Psychology, Northwestern University, 2029 Sheridan Rd., Evanston, IL 60208-2710.
REFERENCES
- Abdallah CG, Averill LA, Krystal JH. Ketamine as a promising prototype for a new generation of rapid-acting antidepressants. Ann N Y Acad Sci 1344: 66–77, 2015. doi: 10.1111/nyas.12718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdi A, Mallet N, Mohamed FY, Sharott A, Dodson PD, Nakamura KC, Suri S, Avery SV, Larvin JT, Garas FN, Garas SN, Vinciati F, Morin S, Bezard E, Baufreton J, Magill PJ. Prototypic and arkypallidal neurons in the dopamine-intact external globus pallidus. J Neurosci 35: 6667–6688, 2015. doi: 10.1523/JNEUROSCI.4662-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amzica F, Steriade M. Short- and long-range neuronal synchronization of the slow (<1 Hz) cortical oscillation. J Neurophysiol 73: 20–38, 1995. [DOI] [PubMed] [Google Scholar]
- Bar-Gad I, Morris G, Bergman H. Information processing, dimensionality reduction and reinforcement learning in the basal ganglia. Prog Neurobiol 71: 439–473, 2003. doi: 10.1016/j.pneurobio.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Bokil H, Andrews P, Kulkarni JE, Mehta S, Mitra PP. Chronux: a platform for analyzing neural signals. J Neurosci Methods 192: 146–151, 2010. doi: 10.1016/j.jneumeth.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boraud T, Brown P, Goldberg JA, Gabriel AM, Magill PJ. Oscillations in the basal ganglia: the good, the bad, and the unexpected. In: The Basal Ganglia VIII, edited by Bolam JP, Ingham CA, Magill PJ, and SpringerLink (Online service). Boston: Springer Science+Business Media, 2005. [Google Scholar]
- Brazhnik E, Cruz AV, Avila I, Wahba MI, Novikov N, Ilieva NM, McCoy AJ, Gerber C, Walters JR. State-dependent spike and local field synchronization between motor cortex and substantia nigra in hemiparkinsonian rats. J Neurosci 32: 7869–7880, 2012. doi: 10.1523/JNEUROSCI.0943-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredlau AL, Thakur R, Korones DN, Dworkin RH. Ketamine for pain in adults and children with cancer: a systematic review and synthesis of the literature. Pain Med 14: 1505–1517, 2013. doi: 10.1111/pme.12182. [DOI] [PubMed] [Google Scholar]
- Buzsáki G. Rhythms of the Brain. New York: Oxford University Press, 2006. doi: 10.1093/acprof:oso/9780195301069.001.0001. [DOI] [Google Scholar]
- Caddy C, Amit BH, McCloud TL, Rendell JM, Furukawa TA, McShane R, Hawton K, Cipriani A. Ketamine and other glutamate receptor modulators for depression in adults. Cochrane Database Syst Rev 9: CD011612, 2015. doi: 10.1002/14651858.CD011612.pub2. [DOI] [PubMed] [Google Scholar]
- Caixeta FV, Cornélio AM, Scheffer-Teixeira R, Ribeiro S, Tort AB. Ketamine alters oscillatory coupling in the hippocampus. Sci Rep 3: 2348, 2013. doi: 10.1038/srep02348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins DR, Pelletier JG, Paré D. Slow and fast (gamma) neuronal oscillations in the perirhinal cortex and lateral amygdala. J Neurophysiol 85: 1661–1672, 2001. [DOI] [PubMed] [Google Scholar]
- Contreras CM, Mexicano G, Guzman-Flores C. A stereotaxic brain atlas of the green monkey (Cercopithecus aethiops aethiops). Bol Estud Med Biol 31: 383–428, 1981. [PubMed] [Google Scholar]
- Cordon I, Nicolas MJ, Arrieta S, Lopetegui E, Lopez-Azcarate J, Alegre M, Artieda J, Valencia M. Coupling in the cortico-basal ganglia circuit is aberrant in the ketamine model of schizophrenia. Eur Neuropsychopharmacol 25: 1375–1387, 2015. doi: 10.1016/j.euroneuro.2015.04.004. [DOI] [PubMed] [Google Scholar]
- Deffains M, Iskhakova L, Katabi S, Haber SN, Israel Z, Bergman H. Subthalamic, not striatal, activity correlates with basal ganglia downstream activity in normal and parkinsonian monkeys. eLife 5: 5, 2016. doi: 10.7554/eLife.16443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devos D, Labyt E, Derambure P, Bourriez JL, Cassim F, Reyns N, Blond S, Guieu JD, Destée A, Defebvre L. Subthalamic nucleus stimulation modulates motor cortex oscillatory activity in Parkinson’s disease. Brain 127: 408–419, 2004. doi: 10.1093/brain/awh053. [DOI] [PubMed] [Google Scholar]
- Domino EF, Luby ED. Phencyclidine/schizophrenia: one view toward the past, the other to the future. Schizophr Bull 38: 914–919, 2012. doi: 10.1093/schbul/sbs011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driesen NR, McCarthy G, Bhagwagar Z, Bloch M, Calhoun V, D’Souza DC, Gueorguieva R, He G, Ramachandran R, Suckow RF, Anticevic A, Morgan PT, Krystal JH. Relationship of resting brain hyperconnectivity and schizophrenia-like symptoms produced by the NMDA receptor antagonist ketamine in humans. Mol Psychiatry 18: 1199–1204, 2013. doi: 10.1038/mp.2012.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlichman RS, Gandal MJ, Maxwell CR, Lazarewicz MT, Finkel LH, Contreras D, Turetsky BI, Siegel SJ. N-methyl-d-aspartic acid receptor antagonist-induced frequency oscillations in mice recreate pattern of electrophysiological deficits in schizophrenia. Neuroscience 158: 705–712, 2009. doi: 10.1016/j.neuroscience.2008.10.031. [DOI] [PubMed] [Google Scholar]
- El Iskandrani KS, Oosterhof CA, El Mansari M, Blier P. Impact of subanesthetic doses of ketamine on AMPA-mediated responses in rats: An in vivo electrophysiological study on monoaminergic and glutamatergic neurons. J Psychopharmacol 29: 792–801, 2015. doi: 10.1177/0269881115573809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewing SG, Grace AA. Deep brain stimulation of the ventral hippocampus restores deficits in processing of auditory evoked potentials in a rodent developmental disruption model of schizophrenia. Schizophr Res 143: 377–383, 2013. doi: 10.1016/j.schres.2012.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandal MJ, Edgar JC, Klook K, Siegel SJ. Gamma synchrony: towards a translational biomarker for the treatment-resistant symptoms of schizophrenia. Neuropharmacology 62: 1504–1518, 2012. doi: 10.1016/j.neuropharm.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George MS, Nahas Z, Borckardt JJ, Anderson B, Foust MJ, Burns C, Kose S, Short EB. Brain stimulation for the treatment of psychiatric disorders. Curr Opin Psychiatry 20: 250–254, 2007. doi: 10.1097/YCO.0b013e3280ad4698. [DOI] [PubMed] [Google Scholar]
- Gilmour G, Dix S, Fellini L, Gastambide F, Plath N, Steckler T, Talpos J, Tricklebank M. NMDA receptors, cognition and schizophrenia–testing the validity of the NMDA receptor hypofunction hypothesis. Neuropharmacology 62: 1401–1412, 2012. doi: 10.1016/j.neuropharm.2011.03.015. [DOI] [PubMed] [Google Scholar]
- Gittis AH, Berke JD, Bevan MD, Chan CS, Mallet N, Morrow MM, Schmidt R. New roles for the external globus pallidus in basal ganglia circuits and behavior. J Neurosci 34: 15178–15183, 2014. doi: 10.1523/JNEUROSCI.3252-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JA, Bergman H. Computational physiology of the neural networks of the primate globus pallidus: function and dysfunction. Neuroscience 198: 171–192, 2011. doi: 10.1016/j.neuroscience.2011.08.068. [DOI] [PubMed] [Google Scholar]
- Goldberg JA, Kats SS, Jaeger D. Globus pallidus discharge is coincident with striatal activity during global slow wave activity in the rat. J Neurosci 23: 10058–10063, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollo LL, Mirasso CR, Atienza M, Crespo-Garcia M, Cantero JL. Theta band zero-lag long-range cortical synchronization via hippocampal dynamical relaying. PLoS One 6: e17756, 2011. doi: 10.1371/journal.pone.0017756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goonawardena AV, Heiss J, Glavis-Bloom C, Trube G, Borroni E, Alberati D, Wallace TL. Alterations in high-frequency neuronal oscillations in a cynomolgus macaque test of sustained attention following NMDA receptor antagonism. Neuropsychopharmacology 41: 1319–1328, 2016. doi: 10.1038/npp.2015.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN. The primate basal ganglia: parallel and integrative networks. J Chem Neuroanat 26: 317–330, 2003. doi: 10.1016/j.jchemneu.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Haber SN, Adler A, Bergman H. The basal ganglia. In: The Human Nervous System, edited by Mai JK, Paxinos G. Amsterdam: Elsevier Academic, 2012. [Google Scholar]
- Hakami T, Jones NC, Tolmacheva EA, Gaudias J, Chaumont J, Salzberg M, O’Brien TJ, Pinault D. NMDA receptor hypofunction leads to generalized and persistent aberrant gamma oscillations independent of hyperlocomotion and the state of consciousness. PLoS One 4: e6755, 2009. doi: 10.1371/journal.pone.0006755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond C, Bergman H, Brown P. Pathological synchronization in Parkinson’s disease: networks, models and treatments. Trends Neurosci 30: 357–364, 2007. doi: 10.1016/j.tins.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Helie S, Chakravarthy S, Moustafa AA. Exploring the cognitive and motor functions of the basal ganglia: an integrative review of computational cognitive neuroscience models. Front Comput Neurosci 7: 174, 2013. doi: 10.3389/fncom.2013.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiyoshi T, Kambe D, Karasawa J, Chaki S. Differential effects of NMDA receptor antagonists at lower and higher doses on basal gamma band oscillation power in rat cortical electroencephalograms. Neuropharmacology 85: 384–396, 2014. doi: 10.1016/j.neuropharm.2014.05.037. [DOI] [PubMed] [Google Scholar]
- Homayoun H, Moghaddam B. NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J Neurosci 27: 11496–11500, 2007. doi: 10.1523/JNEUROSCI.2213-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong LE, Summerfelt A, Buchanan RW, O’Donnell P, Thaker GK, Weiler MA, Lahti AC. Gamma and delta neural oscillations and association with clinical symptoms under subanesthetic ketamine. Neuropsychopharmacology 35: 632–640, 2010. doi: 10.1038/npp.2009.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt MJ, Raynaud B, Garcia R. Ketamine dose-dependently induces high-frequency oscillations in the nucleus accumbens in freely moving rats. Biol Psychiatry 60: 1206–1214, 2006. doi: 10.1016/j.biopsych.2006.01.020. [DOI] [PubMed] [Google Scholar]
- Javitt DC. Glutamate and schizophrenia: phencyclidine, N-methyl-d-aspartate receptors, and dopamine-glutamate interactions. Int Rev Neurobiol 78: 69–108, 2007. doi: 10.1016/S0074-7742(06)78003-5. [DOI] [PubMed] [Google Scholar]
- Jones NC, Reddy M, Anderson P, Salzberg MR, O’Brien TJ, Pinault D. Acute administration of typical and atypical antipsychotics reduces EEG γ power, but only the preclinical compound LY379268 reduces the ketamine-induced rise in γ power. Int J Neuropsychopharmacol 15: 657–668, 2012. doi: 10.1017/S1461145711000848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalsi SS, Wood DM, Dargan PI. The epidemiology and patterns of acute and chronic toxicity associated with recreational ketamine use. Emerg Health Threats J 4: 7107, 2011. doi: 10.3402/ehtj.v4i0.7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HJ, Voleti B, Hajszan T, Rajkowska G, Stockmeier CA, Licznerski P, Lepack A, Majik MS, Jeong LS, Banasr M, Son H, Duman RS. Decreased expression of synapse-related genes and loss of synapses in major depressive disorder. Nat Med 18: 1413–1417, 2012. doi: 10.1038/nm.2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita H. Globus pallidus external segment. Prog Brain Res 160: 111–133, 2007. doi: 10.1016/S0079-6123(06)60007-1. [DOI] [PubMed] [Google Scholar]
- Kittelberger K, Hur EE, Sazegar S, Keshavan V, Kocsis B. Comparison of the effects of acute and chronic administration of ketamine on hippocampal oscillations: relevance for the NMDA receptor hypofunction model of schizophrenia. Brain Struct Funct 217: 395–409, 2012. doi: 10.1007/s00429-011-0351-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein J, Hadar R, Götz T, Männer A, Eberhardt C, Baldassarri J, Schmidt TT, Kupsch A, Heinz A, Morgenstern R, Schneider M, Weiner I, Winter C. Mapping brain regions in which deep brain stimulation affects schizophrenia-like behavior in two rat models of schizophrenia. Brain Stimulat 6: 490–499, 2013. doi: 10.1016/j.brs.2012.09.004. [DOI] [PubMed] [Google Scholar]
- Kocsis B. Differential role of NR2A and NR2B subunits in N-methyl-d-aspartate receptor antagonist-induced aberrant cortical gamma oscillations. Biol Psychiatry 71: 987–995, 2012. doi: 10.1016/j.biopsych.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krack P, Hariz MI, Baunez C, Guridi J, Obeso JA. Deep brain stimulation: from neurology to psychiatry? Trends Neurosci 33: 474–484, 2010. doi: 10.1016/j.tins.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB Jr, Charney DS. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry 51: 199–214, 1994. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- Kühn AA, Kempf F, Brücke C, Gaynor Doyle L, Martinez-Torres I, Pogosyan A, Trottenberg T, Kupsch A, Schneider GH, Hariz MI, Vandenberghe W, Nuttin B, Brown P. High-frequency stimulation of the subthalamic nucleus suppresses oscillatory beta activity in patients with Parkinson’s disease in parallel with improvement in motor performance. J Neurosci 28: 6165–6173, 2008. doi: 10.1523/JNEUROSCI.0282-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulikova SP, Tolmacheva EA, Anderson P, Gaudias J, Adams BE, Zheng T, Pinault D. Opposite effects of ketamine and deep brain stimulation on rat thalamocortical information processing. Eur J Neurosci 36: 3407–3419, 2012. doi: 10.1111/j.1460-9568.2012.08263.x. [DOI] [PubMed] [Google Scholar]
- Kurdi MS, Theerth KA, Deva RS. Ketamine: current applications in anesthesia, pain, and critical care. Anesth Essays Res 8: 283–290, 2014. doi: 10.4103/0259-1162.143110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachaux JP, Rodriguez E, Martinerie J, Varela FJ. Measuring phase synchrony in brain signals. Hum Brain Mapp 8: 194–208, 1999. doi:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarewicz MT, Ehrlichman RS, Maxwell CR, Gandal MJ, Finkel LH, Siegel SJ. Ketamine modulates theta and gamma oscillations. J Cogn Neurosci 22: 1452–1464, 2010. doi: 10.1162/jocn.2009.21305. [DOI] [PubMed] [Google Scholar]
- Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, Duman RS. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 329: 959–964, 2010. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little S, Brown P. What brain signals are suitable for feedback control of deep brain stimulation in Parkinson’s disease? Ann N Y Acad Sci 1265: 9–24, 2012. doi: 10.1111/j.1749-6632.2012.06650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Leung LS. The supramammillo-septal-hippocampal pathway mediates sensorimotor gating impairment and hyperlocomotion induced by MK-801 and ketamine in rats. Psychopharmacology (Berl) 191: 961–974, 2007. doi: 10.1007/s00213-006-0667-x. [DOI] [PubMed] [Google Scholar]
- Magill PJ, Bolam JP, Bevan MD. Relationship of activity in the subthalamic nucleus-globus pallidus network to cortical electroencephalogram. J Neurosci 20: 820–833, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maksimow A, Sarkela M, Langsjo JW, Salmi E, Kaisti KK, Yli-Hankala A, Hinkka-Yli-Salomaki S, Scheinin H, Jaaskelainen SK. Increase in high frequency EEG activity explains the poor performance of EEG spectral entropy monitor during S-ketamine anesthesia. Clin Neurophysiol 117: 1660–1668, 2006. doi: 10.1016/j.clinph.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Mallet N, Micklem BR, Henny P, Brown MT, Williams C, Bolam JP, Nakamura KC, Magill PJ. Dichotomous organization of the external globus pallidus. Neuron 74: 1075–1086, 2012. doi: 10.1016/j.neuron.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marland S, Ellerton J, Andolfatto G, Strapazzon G, Thomassen O, Brandner B, Weatherall A, Paal P. Ketamine: use in anesthesia. CNS Neurosci Ther 19: 381–389, 2013. doi: 10.1111/cns.12072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmor O, Valsky D, Joshua M, Bick AS, Arkadir D, Tamir I, Bergman H, Israel Z, Eitan R. Local vs. volume conductance activity of field potentials in the human subthalamic nucleus. J Neurophysiol 117: 2140–2151, 2017. doi: 10.1152/jn.00756.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin RF, Bowden DM. Primate Brain Maps: Structure of the Macaque Brain. Amsterdam: Elsevier, 2000. [Google Scholar]
- Mikell CB, McKhann GM, Segal S, McGovern RA, Wallenstein MB, Moore H. The hippocampus and nucleus accumbens as potential therapeutic targets for neurosurgical intervention in schizophrenia. Stereotact Funct Neurosurg 87: 256–265, 2009. doi: 10.1159/000225979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller OH, Moran JT, Hall BJ. Two cellular hypotheses explaining the initiation of ketamine’s antidepressant actions: Direct inhibition and disinhibition. Neuropharmacology 100: 17–26, 2016. doi: 10.1016/j.neuropharm.2015.07.028. [DOI] [PubMed] [Google Scholar]
- Miocinovic S, Somayajula S, Chitnis S, Vitek JL. History, applications, and mechanisms of deep brain stimulation. JAMA Neurol 70: 163–171, 2013. doi: 10.1001/2013.jamaneurol.45. [DOI] [PubMed] [Google Scholar]
- Moran A, Bar-Gad I. Revealing neuronal functional organization through the relation between multi-scale oscillatory extracellular signals. J Neurosci Methods 186: 116–129, 2010. doi: 10.1016/j.jneumeth.2009.10.024. [DOI] [PubMed] [Google Scholar]
- Murrough JW, Iosifescu DV, Chang LC, Al Jurdi RK, Green CE, Perez AM, Iqbal S, Pillemer S, Foulkes A, Shah A, Charney DS, Mathew SJ. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry 170: 1134–1142, 2013. doi: 10.1176/appi.ajp.2013.13030392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newport DJ, Carpenter LL, McDonald WM, Potash JB, Tohen M, Nemeroff CB; APA Council of Research Task Force on Novel Biomarkers and Treatments . Ketamine and other NMDA antagonists: early clinical trials and possible mechanisms in depression. Am J Psychiatry 172: 950–966, 2015. doi: 10.1176/appi.ajp.2015.15040465. [DOI] [PubMed] [Google Scholar]
- Nicolás MJ, López-Azcárate J, Valencia M, Alegre M, Pérez-Alcázar M, Iriarte J, Artieda J. Ketamine-induced oscillations in the motor circuit of the rat basal ganglia. PLoS One 6: e21814, 2011. doi: 10.1371/journal.pone.0021814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niesters M, Martini C, Dahan A. Ketamine for chronic pain: risks and benefits. Br J Clin Pharmacol 77: 357–367, 2014. doi: 10.1111/bcp.12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parush N, Tishby N, Bergman H. Dopaminergic balance between reward maximization and policy complexity. Front Syst Neurosci 5: 22, 2011. doi: 10.3389/fnsys.2011.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penttonen M, Buzsáki G. Natural logarithmic relationship between brain oscillators. Thalamus Relat Syst 2: 145–152, 2003. doi: 10.1017/S1472928803000074. [DOI] [Google Scholar]
- Percheron G, Yelnik J, François C. A Golgi analysis of the primate globus pallidus. III. Spatial organization of the striato-pallidal complex. J Comp Neurol 227: 214–227, 1984. doi: 10.1002/cne.902270207. [DOI] [PubMed] [Google Scholar]
- Perez SM, Shah A, Asher A, Lodge DJ. Hippocampal deep brain stimulation reverses physiological and behavioural deficits in a rodent model of schizophrenia. Int J Neuropsychopharmacol 16: 1331–1339, 2013. doi: 10.1017/S1461145712001344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinault D. N-methyl d-aspartate receptor antagonists ketamine and MK-801 induce wake-related aberrant gamma oscillations in the rat neocortex. Biol Psychiatry 63: 730–735, 2008. doi: 10.1016/j.biopsych.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Plourde G, Baribeau J, Bonhomme V. Ketamine increases the amplitude of the 40-Hz auditory steady-state response in humans. Br J Anaesth 78: 524–529, 1997. doi: 10.1093/bja/78.5.524. [DOI] [PubMed] [Google Scholar]
- Purdon PL, Sampson A, Pavone KJ, Brown EN. Clinical electroencephalography for anesthesiologists. I. background and basic signatures. Anesthesiology 123: 937–960, 2015. doi: 10.1097/ALN.0000000000000841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiroga-Varela A, Walters JR, Brazhnik E, Marin C, Obeso JA. What basal ganglia changes underlie the parkinsonian state? The significance of neuronal oscillatory activity. Neurobiol Dis 58: 242–248, 2013. doi: 10.1016/j.nbd.2013.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagovindan R, Ding M. Decomposing neural synchrony: toward an explanation for near-zero phase-lag in cortical oscillatory networks. PLoS One 3: e3649, 2008. doi: 10.1371/journal.pone.0003649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivlin-Etzion M, Marmor O, Saban G, Rosin B, Haber SN, Vaadia E, Prut Y, Bergman H. Low-pass filter properties of basal ganglia cortical muscle loops in the normal and MPTP primate model of parkinsonism. J Neurosci 28: 633–649, 2008. doi: 10.1523/JNEUROSCI.3388-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosin B, Slovik M, Mitelman R, Rivlin-Etzion M, Haber SN, Israel Z, Vaadia E, Bergman H. Closed-loop deep brain stimulation is superior in ameliorating parkinsonism. Neuron 72: 370–384, 2011. doi: 10.1016/j.neuron.2011.08.023. [DOI] [PubMed] [Google Scholar]
- Scheuing L, Chiu CT, Liao HM, Chuang DM. Antidepressant mechanism of ketamine: perspective from preclinical studies. Front Neurosci 9: 249, 2015. doi: 10.3389/fnins.2015.00249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science 275: 1593–1599, 1997. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Stein E, Bar-Gad I. β oscillations in the cortico-basal ganglia loop during parkinsonism. Exp Neurol 245: 52–59, 2013. doi: 10.1016/j.expneurol.2012.07.023. [DOI] [PubMed] [Google Scholar]
- Steriade M, Nuñez A, Amzica F. A novel slow (< 1 Hz) oscillation of neocortical neurons in vivo: depolarizing and hyperpolarizing components. J Neurosci 13: 3252–3265, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai GE. Searching for rational anti N-methyl-d-aspartate treatment for depression. Arch Gen Psychiatr 64: 1099–1100; author reply 1100–1101, 2007. doi: 10.1001/archpsyc.64.9.1099. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci 11: 100–113, 2010. doi: 10.1038/nrn2774. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. Neuronal dynamics and neuropsychiatric disorders: toward a translational paradigm for dysfunctional large-scale networks. Neuron 75: 963–980, 2012. doi: 10.1016/j.neuron.2012.09.004. [DOI] [PubMed] [Google Scholar]
- Vinck M, Battaglia FP, Womelsdorf T, Pennartz C. Improved measures of phase-coupling between spikes and the local field potential. J Comput Neurosci 33: 53–75, 2012. doi: 10.1007/s10827-011-0374-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viriyopase A, Bojak I, Zeitler M, Gielen S. When long-range zero-lag synchronization is feasible in cortical networks. Front Comput Neurosci 6: 49, 2012. doi: 10.3389/fncom.2012.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger M, Hutchison WD, Dostrovsky JO. Pathological subthalamic nucleus oscillations in PD: can they be the cause of bradykinesia and akinesia? Exp Neurol 219: 58–61, 2009. doi: 10.1016/j.expneurol.2009.05.014. [DOI] [PubMed] [Google Scholar]
- Wennberg RA, Lozano AM. Intracranial volume conduction of cortical spikes and sleep potentials recorded with deep brain stimulating electrodes. Clin Neurophysiol 114: 1403–1418, 2003. doi: 10.1016/S1388-2457(03)00152-4. [DOI] [PubMed] [Google Scholar]
- Williams NR, Schatzberg AF. NMDA antagonist treatment of depression. Curr Opin Neurobiol 36: 112–117, 2016. doi: 10.1016/j.conb.2015.11.001. [DOI] [PubMed] [Google Scholar]
- Wood J, Kim Y, Moghaddam B. Disruption of prefrontal cortex large scale neuronal activity by different classes of psychotomimetic drugs. J Neurosci 32: 3022–3031, 2012. doi: 10.1523/JNEUROSCI.6377-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen EY, Wei J, Liu W, Zhong P, Li X, Yan Z. Repeated stress causes cognitive impairment by suppressing glutamate receptor expression and function in prefrontal cortex. Neuron 73: 962–977, 2012. doi: 10.1016/j.neuron.2011.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidel A, Spivak A, Grieb B, Bergman H, Israel Z. Subthalamic span of beta oscillations predicts deep brain stimulation efficacy for patients with Parkinson’s disease. Brain 133: 2007–2021, 2010. doi: 10.1093/brain/awq144. [DOI] [PubMed] [Google Scholar]
- Zarate CA Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK. A randomized trial of an N-methyl-d-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 63: 856–864, 2006. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]