We show that insults with a NMDA receptor antagonist during neurodevelopment lead to suppressed evoked theta oscillations in ventral hippocampus in adult rats, while evoked gamma oscillations are enhanced and hypersensitive to an acute challenge with a NMDA receptor antagonist in prefrontal cortex. These observations reveal the significance of neurodevelopmental disturbances in the evolvement of schizophrenia-like symptoms and contribute to the understanding of the functional deficits underlying aberrant behavior in this disease.
Keywords: phencyclidine, NMDA receptor antagonist, development, gamma in prefrontal cortex, theta in hippocampus
Abstract
Symptoms of schizophrenia have been linked to insults during neurodevelopment such as NMDA receptor (NMDAR) antagonist exposure. In animal models, this leads to schizophrenia-like behavioral symptoms as well as molecular and functional changes within hippocampal and prefrontal regions. The aim of this study was to determine how administration of the NMDAR antagonist phencyclidine (PCP) during neurodevelopment affects functional network activity within the hippocampus and medial prefrontal cortex (mPFC). We recorded field potentials in vivo after electrical brain stem stimulation and observed a suppression of evoked theta power in ventral hippocampus, while evoked gamma power in mPFC was enhanced in rats administered with PCP neonatally. In addition, increased gamma synchrony elicited by acute administration of the NMDAR antagonist MK-801 was exaggerated in neonatal PCP animals. These data suggest that NMDAR antagonist exposure during brain development alters functional networks within hippocampus and mPFC possibly contributing to the reported behavioral symptoms of this animal model of schizophrenia.
NEW & NOTEWORTHY We show that insults with a NMDA receptor antagonist during neurodevelopment lead to suppressed evoked theta oscillations in ventral hippocampus in adult rats, while evoked gamma oscillations are enhanced and hypersensitive to an acute challenge with a NMDA receptor antagonist in prefrontal cortex. These observations reveal the significance of neurodevelopmental disturbances in the evolvement of schizophrenia-like symptoms and contribute to the understanding of the functional deficits underlying aberrant behavior in this disease.
evidence implicates a primary glutamatergic dysfunction in schizophrenia. Acute administration of noncompetitive N-methyl-d-aspartate receptor (NMDAR) antagonists, such as ketamine and phencyclidine (PCP), induces schizophrenia-like symptoms in healthy volunteers, including transient psychosis, disrupted affect, and cognitive deficits (Krystal et al. 1994; Malhotra et al. 1996), and exacerbates symptoms in patients with schizophrenia (Adler et al. 1999; Lahti et al. 2001). In addition, acute systemic administration of NMDAR antagonists leads to heightened gamma activity (Anver et al. 2011; Hakami et al. 2009) and behavioral responses that mimic positive symptoms such as hyperlocomotion and stereotypies (Adams and Moghaddam 1998). This correlates well with clinical findings demonstrating that cortical gamma activity is increased during hallucinations in individuals with schizophrenia (Mulert et al. 2011; Spencer et al. 2009) while in healthy volunteers acute administration of NMDAR antagonists induces hallucinations potentially stemming from the observed increase in gamma activity (Hong et al. 2010).
The medial prefrontal cortex (mPFC) is an associational cortical region that displays abnormal activity patterns during behavioral performance in patients with schizophrenia (Callicott et al. 2003; Weinberger et al. 1986). Under normal circumstances, gamma synchrony in mPFC can be modulated by hippocampal theta rhythm (Hyman et al. 2010; Sirota et al. 2008), most likely through a monosynaptic, excitatory hippocampal-prefrontal pathway (Thierry et al. 2000; Tierney et al. 2004). This hippocampal-prefrontal connectivity appears disrupted in schizophrenia: clinical studies report an altered correlation between hippocampal and prefrontal activity during working memory performance and psychosis in patients with schizophrenia (Fletcher 1998; Meyer-Lindenberg et al. 2005; Samudra et al. 2015), and several rodent models of schizophrenia display reduced coherence and phase locking between hippocampal and prefrontal neuronal populations corresponding to symptoms of compromised sensory gating and working memory performance (Dickerson et al. 2010; Sigurdsson et al. 2010).
The pathogenesis of schizophrenia is associated with altered neurodevelopment (Lewis and Levitt 2002), and exposure to PCP during fetal development enhances the risk of the child developing schizophrenia later in life (Deutsch et al. 1998; Green et al. 2004). In general, late second trimester insults are known to be a risk factor (Brown and Derkits 2010). Studies describing effects of acute NMDAR antagonist administration in adult animals do not model this neurodevelopmental aspect of schizophrenia. We and others have previously shown that NMDAR antagonist administration during the first two postnatal weeks, which in terms of rat development corresponds to the late second trimester (Bayer et al. 1993; Clancy et al. 2001), leads to schizophrenia-like behavioral symptoms such as impairments in attentional set shifting, sensory gating, and social and novelty discrimination in adult animals (Broberg et al. 2008; Jeevakumar et al. 2015; Kjaerby et al. 2013). In addition, this model displays histological and functional alterations in mPFC characterized by reductions in the number of parvalbumin-positive interneurons, deficits in the inhibitory functional input to pyramidal neurons, and an upregulated NMDAR-mediated input to parvalbumin-positive interneurons in mPFC (Jeevakumar and Kroener 2016; Kaalund et al. 2013; Kjaerby et al. 2014). Furthermore, neurodevelopmental ablation of NMDARs in mainly parvalbumin-positive interneurons within hippocampus and cortical regions led to cortical disinhibition and symptoms of schizophrenia (Belforte et al. 2010), whereas ablation in prefrontal excitatory neurons produced milder schizophrenia-like symptoms (Rompala et al. 2013). These findings among others have inspired the hypothesis that disruption of NMDAR neurotransmission in interneurons causes schizophrenia-like symptoms through disinhibition of excitatory circuits within the brain (Jackson et al. 2004; Suzuki et al. 2002). These changes in the excitation/inhibition balance of the brain have been linked to abnormal oscillatory rhythms in schizophrenia. Thus, NMDAR blockage during development could severely compromise neural networks during adulthood, but so far no one has looked at oscillatory changes in the neurodevelopmental PCP animal model.
The aim of this study was to explore the impact of developmental NMDAR antagonist administration on functional oscillatory activity in hippocampus and mPFC. We found that neonatal PCP administration in rats induced attenuation of evoked theta oscillations in the ventral hippocampus (vHPC), while increasing evoked gamma rhythms in the mPFC, and that the prefrontal gamma response to acute NMDAR antagonist exposure was exaggerated in neonatal PCP animals. These findings show that NMDAR-based insults during brain development induce functional deficits within hippocampal and mPFC brain networks that might be involved in the behavioral schizophrenia-like symptoms observed in this model.
MATERIALS AND METHODS
Animals.
All animal procedures employed in this study were carried out in accordance with the European Parliament and the Council of the European Union directive of 22 September 2010 (2010/63/EU) and were approved by the Danish State Research Inspectorate (J. No. 2009/561-1596).
Lister hooded rats were obtained from Charles River Laboratories and housed pairwise (pregnant dams were housed singly) in cages with standard sawdust bedding and environmental enrichment (plastic house and wooden chew blocks) under standard laboratory conditions with a 12:12-h light-dark cycle and ad libitum access to food and water. Neonatal PCP administration was performed as previously described (Broberg et al. 2008). Briefly, timed pregnant rats were obtained at gestational day 15, and the day of parturition was counted as postnatal day (PND) 0. On PND 5, male pups were cross-fostered and randomly assigned to a lactating dam in litters of 7–10. On PNDs 7, 9, and 11, pups were administered subcutaneously (sc) with either vehicle (0.9% isotonic saline) or PCP (20 mg/kg) (synthesized at H. Lundbeck A/S) in a 10 ml/kg dose volume. Rats were weaned at PND 24 and used for in vivo electrophysiological recordings after reaching adulthood on PNDs 56–80. A total of 31 rats (12 vehicle and 19 PCP treated) were used in this study.
In vivo field potential recordings.
In vivo field potentials were recorded simultaneously in mPFC and vHPC or dorsal hippocampus (dHPC). Rats were anesthetized with urethane (1.5 g/kg ip) and placed in a stereotaxic apparatus, and their temperature was maintained at 37°C via a heating pad. Stimulation and recording electrodes were implanted according to coordinates given by Paxinos and Watson (2007). A 400-µm tungsten concentric electrode (TM53CCINS, World Precision Instruments) was lowered into the pedunculopontine tegmental nucleus of the reticular formation (coordinates: AP, 7.6 mm posterior to bregma; ML, 1.6 mm lateral to the midline; DV, 6.0 mm below the surface of the brain). Unipolar stainless steel electrodes (125-µm diameter; E363/3, Plastics One) were inserted in mPFC (AP, 3.2 mm anterior to bregma; ML, 0.5 mm lateral to the midline; DV, 2.5 mm below the surface of the brain) and the ipsilateral vHPC (AP, 5.6 mm posterior to bregma; ML, 5.1 mm lateral to the midline, DV, 5.6 mm below the surface of the brain) or dHPC (AP, 3.5 mm posterior to bregma; ML, 2.2 mm lateral to the midline; DV, 2.0 mm below the surface of the brain). The signals were amplified (total gain: 1,000) with a two-channel amplifier (CyberAmp 320, Axon Instruments), band-pass filtered at 0.1 Hz to 0.1 kHz, digitized at a rate of 2,000 Hz (CED 1401, Cambridge Electronic Design), and stored for subsequent analysis with Spike2 software (Cambridge Electronic Design).
Every 100 s, a 6-s train of 0.3-ms pulses at 250 Hz was applied to the stimulating electrode via an A365 stimulus isolator (World Precision Instruments) controlled via Spike2 software. The depth of the recording electrode in the vHPC or dHPC was adjusted in order to obtain a maximal power of the evoked theta oscillations, which was typically obtained at the level of the hippocampal fissure as previously described (Kowalczyk and Konopacki 2002). After an optimal induction of hippocampal theta and prefrontal gamma was obtained, field potentials were recorded in mPFC and vHPC or dHPC in response to increasing intensities of stimulation (0–100 µA in 20-µA steps). Afterwards, the stimulation intensity was fixed at 60 µA to allow nonnormalized comparisons between the neonatal vehicle and PCP groups. Baseline recordings were then performed for a minimum of 30 min, and MK-801 was subsequently administered at 0.1 mg/kg sc followed 30 min later by an additional dose of 0.2 mg/kg sc (cumulative dose 0.3 mg/kg). A single vehicle (0.9% saline, 1.0 ml/kg sc) injection was performed before MK-801 as control.
Quantitative field potential analysis was performed with fast Fourier transformation by Spike2 (custom script provided by Cambridge Electronic Design). Power spectrum density of field potentials was calculated, and the total theta power was calculated by summation of the power within the 4.0–8.0 Hz frequency range. For the power of gamma band activity, field potentials recorded in mPFC were digitally band-pass filtered between 20 and 60 Hz, and the total power of the filtered signal was used for further analysis. The last 5 s of the 6-s stimulation period was used.
To evaluate the functional coupling between the hippocampus and mPFC, a coherence analysis was performed from local field potentials (LFPs) recorded in either dHPC or vHPC and mPFC. The cross-power spectra for each pair were used to evaluate the coherence, with a block size of 2,048 across the 0–100 Hz frequency range (user-defined script for Spike2, Cambridge Electronic Design). The coherence value varies between 0 and 1, a value of 1 indicating a perfect relationship between the two signals in the two areas and a value of 0 no correlation within the frequency domain of interest. Theta and gamma coherence were further analyzed using the area under the curve between 4 and 8 Hz and 20 and 60 Hz, respectively.
To quantify the effect of saline and MK-801, the power of theta and gamma waves was analyzed as described above and the average power between 20 and 30 min after drug administration was compared to the average power determined over a 15-min period immediately preceding the first injection. When recordings were finalized, a high current was applied to the three electrodes, rats were euthanized with a bolus injection of urethane, and brains were removed and frozen for histological verification of electrode placements.
Statistical analysis.
Two-way repeated-measures ANOVAs (rANOVAs) were used to compare either the power or frequency response to increasing stimulation intensity and the coherence between rats administered PCP (neoPCP) and vehicle (neoVEH) neonatally. A regular two-way ANOVA was used to compare spontaneous vs. induced hippocampal theta and prefrontal gamma power and coherence, while analysis of the normalized theta and gamma power in response to stimulation was performed by unpaired, two-tailed t-test. A correlation analysis was performed to establish whether normalized hippocampal theta and prefrontal gamma power correlated, and the Pearson correlation coefficient is reported. The effect of MK-801 on evoked oscillatory activity was computed by two-way ANOVA. Holm-Sidak multiple-comparison post hoc tests were performed after all ANOVA tests when a priori significance was reached at P < 0.05.
RESULTS
NeoPCP administration suppresses evoked hippocampal theta rhythm while enhancing evoked prefrontal gamma rhythm.
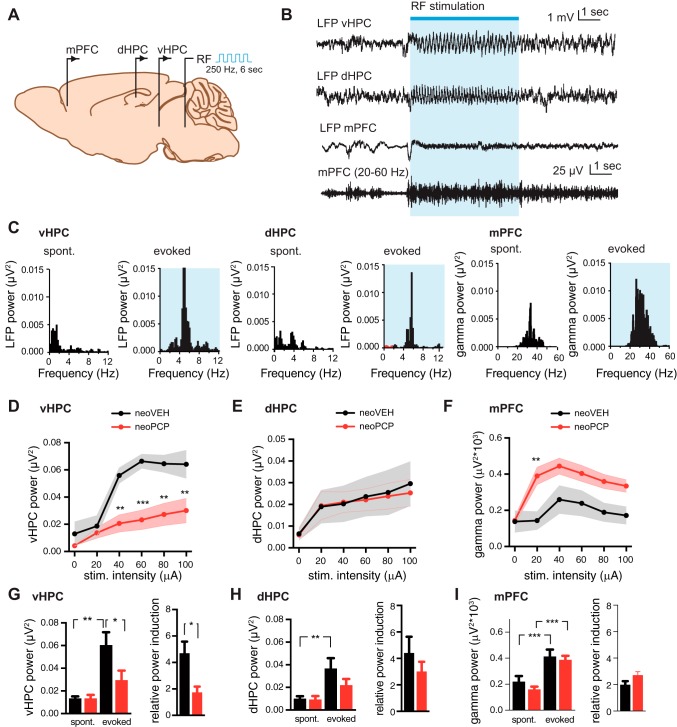
Field potential recordings were performed simultaneously in mPFC and either the dHPC or vHPC in anesthetized rats. A 6-s high-frequency stimulation train (250 Hz, 0.3-ms pulses) was applied in the pedunculopontine tegmental nucleus of the reticular formation every 100 s (Fig. 1A) and induced hippocampal theta and prefrontal gamma activity (Fig. 1, B and C). In control animals (neoVEH), hippocampal theta power was enhanced in a stimulation intensity-dependent manner in both vHPC [F(5,55) = 26.85, P < 0.001; Fig. 1D] and dHPC [F(5,70) = 11.45, P < 0.001; Fig. 1E] and reached saturation around 40- to 60-μA intensity. Similarly, an effect on prefrontal gamma power was evident as a consequence of increasing stimulation intensity in neoVEH rats [F(5,145) = 15.47, P < 0.001; Fig. 1F], reaching maximum power at 40 μA.
Fig. 1.
Developmental NMDA receptor antagonist administration suppresses evoked theta oscillations in vHPC and enhances gamma rhythm in mPFC. A: schematic illustration of experimental setup. A stimulating electrode is placed in the reticular formation (RF), and local field potentials (LFPs) are recorded from medial prefrontal cortex (mPFC) simultaneously with either the ventral (vHPC) or dorsal (dHPC) hippocampus in anesthetized rats. B: stimulation of the RF with pulse trains (250 Hz for 6 s every 100 s) increases LFP power within theta frequency (4–8 Hz) in both vHPC and dHPC and increases prefrontal gamma power (20–60 Hz). Example raw traces of the hippocampal and prefrontal signals as well as the digital filtered (20–60 Hz) prefrontal trace are shown. C: corresponding power spectra obtained with fast Fourier transform before and during stimulation for vHPC, dHPC, and mPFC. D: input-output curves validating the effect of increasing stimulus intensity on vHPC hippocampal theta power. The response to increasing stimulation intensities was significantly reduced in rats administered PCP neonatally (neoPCP) (Holm-Sidak multiple-comparison post hoc test, P = 0.0060). E: no difference was observed between neoPCP and neoVEH animals in dHPC. F: response to increased stimulation intensities was significantly increased with respect to prefrontal gamma power for neoPCP (Holm-Sidak multiple-comparison post hoc test, P = 0.0060) compared with neoVEH (Holm-Sidak multiple-comparison post hoc test, P = 0.0195) rats. G: stimulation intensity of 60 µA significantly increased vHPC theta power in both neoVEH and neoPCP rats (P = 0.0009), even though the effect was attenuated after neonatal PCP administration (unpaired t-test; P < 0.0001). Induced vHPC power normalized to spontaneous was significantly decreased in neoPCP compared with neoVEH (P = 0.0106). H: 60-µA stimulation induced dHPC theta power in neoVEH and neoPCP rats to similar extents (unpaired t-test; P = 0.0009). I: prefrontal gamma power was increased to the same extent in neoVEH and neoPCP rats. Data are represented as means ± SE. vHPC: neoVEH, n = 5; neoPCP, n = 6. dHPC: neoVEH, n = 6; neoPCP, n = 8. mPFC: neoVEH, n = 8; neoPCP, n = 8. *P < 0.05, **P < 0.01, ***P < 0.001.
In neoPCP rats the power of evoked vHPC theta was suppressed compared with neoVEH rats [F(1,11) = 11.54, P = 0.0060, Fig. 1D], while evoked prefrontal gamma power was increased in neoPCP compared with neoVEH rats [F(1,29) = 6.12, P = 0.0195; Fig. 1F].
A stimulating intensity of 60 µA resulted in significantly increased vHPC [F(1,10) = 21.57, P = 0.0009; Fig. 1G] and dHPC [F(1,12) = 19.39, P = 0.0009; Fig. 1H] theta power as well as prefrontal gamma power [F(1,27) = 63.25, P < 0.001; Fig. 1I] compared with their respective spontaneous power. No differences were observed between neoPCP and neoVEH rats for vHPC, dHPC, or mPFC; however, there was a significant interaction effect in vHPC [F(1,10) = 5.14, P < 0.05; Fig. 1G], illustrating the reduction in evoked theta power in neoPCP animals. When evoked theta and gamma power were normalized to the spontaneous power values, a decreased evoked theta power was observed for neoPCP animals in the vHPC (Student’s t value = 3.212, P = 0.0106; Fig. 1G) but not in the dHPC (Fig. 1H). A tendency toward an increased prefrontal gamma power was also observed in neoPCP compared with neoVEH rats (Student’s t value = 1.944, P = 0.0624; Fig. 1I).
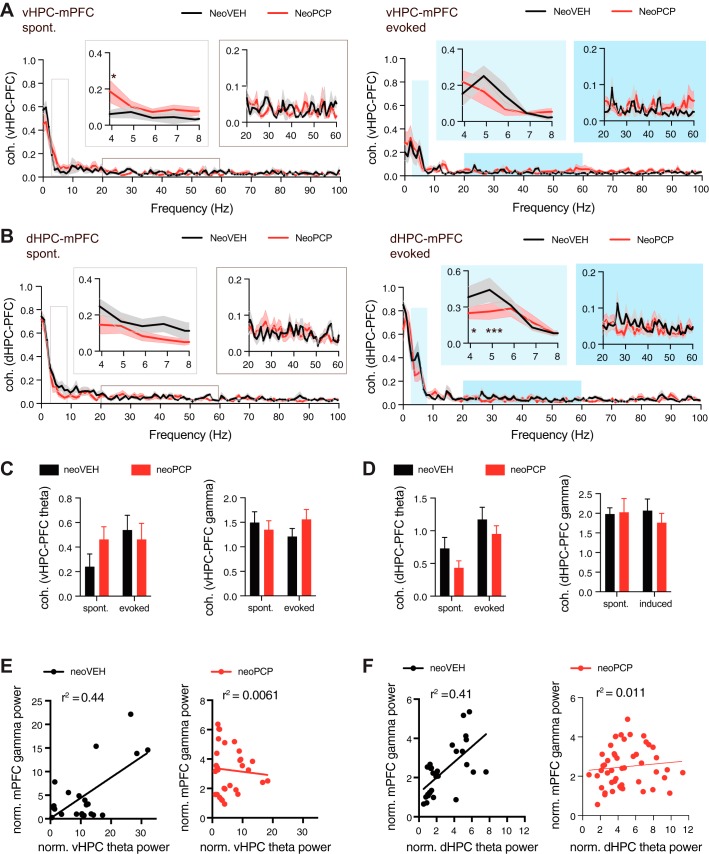
To determine whether synchronization between hippocampal and prefrontal regions was altered in neoPCP animals, the coherence was calculated for all frequencies between vHPC and mPFC and between dHPC and mPFC. There was an interaction effect between frequency and neoPCP administration for dHPC-mPFC coherence [F(102, 816) = 1.265, P < 0.05; Fig. 2B], illustrating that coherence was suppressed in neoPCP animals. This was also evident from a tendency toward reduced coherence in the theta frequency range between dHPC and mPFC observed after neoPCP administration [F(1,16) = 3.82, P = 0.068; Fig. 2D]. In addition, there was an interaction effect in vHPC-mPFC coherence in the gamma range [F(1,11) = 17.74, P < 0.01; Fig. 2C], indicating that spontaneous and evoked gamma coherence respond differently in neoVEH and neoPCP animals. In neoVEH rats, there was a positive correlation between evoked vHPC theta power and mPFC gamma power (r2 = 0.44, P = 0.0003; Fig. 2E) as well as between dHPC theta power and mPFC gamma power (r2 = 0.41, P = 0.0001; Fig. 2F). However, these correlations were lost in the neoPCP rats for both vHPC theta and mPFC gamma (r2 = 0.0061, P = 0.6824; Fig. 2E) and dHPC theta and mPFC gamma (r2 = 0.011, P = 0.4812; Fig. 2F).
Fig. 2.
Administration of PCP during development changes synchronization between hippocampus and mPFC. A: coherence between ventral hippocampus (vHPC) and prefrontal cortex (PFC) was calculated for all frequencies for both spontaneous (left) and evoked (right) field potentials. Data within the chosen theta and gamma frequency ranges are shown in white (spontaneous) and blue (evoked) boxes above the graph. Coherence was increased in neoPCP animals for certain frequencies (Holm-Sidak multiple-comparison post hoc test, P = 0.0118). B: coherence between dorsal hippocampus (dHPC) and PFC. Coherence was reduced in neoPCP animals for certain frequencies [Holm-Sidak multiple-comparison post hoc test, P = 0.0199 (freq. = 4) and P < 0.0001 (freq. = 5)]. C: summary graphs displaying area under the curve for vHPC-PFC coherence between 4 and 8 Hz (theta) and 20–60 Hz (gamma). D: area under the curve for dHPC-PFC coherence in the theta and gamma frequency ranges. E: the observed correlation between normalized vHPC theta power and normalized mPFC gamma power in neoVEH rats (linear regression; P = 0.003) was disrupted in neoPCP animals (P = 0.6824). F: a significant correlation was also found between dHPC theta power and prefrontal gamma power in neoVEH rats (linear regression; P = 0.001) but not in neoPCP rats (P = 0.4812). Data are represented as means ± SE. vHPC: neoVEH, n = 5; neoPCP, n = 6. dHPC: neoVEH, n = 6; neoPCP, n = 8. mPFC: neoVEH, n = 8; neoPCP, n = 8. *P < 0.05, ***P < 0.001.
neoPCP rats are hypersensitive to MK-801-induced increases in evoked prefrontal gamma oscillations.
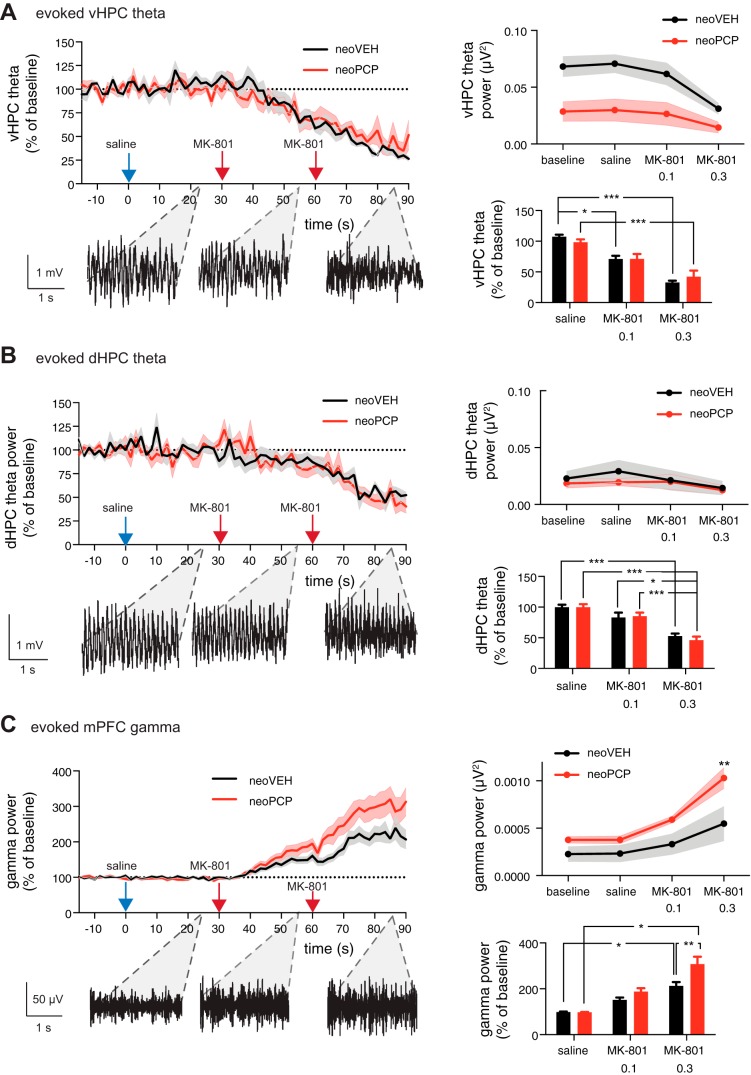
We also determined the effect of systemic administration of MK-801 on evoked hippocampal theta and prefrontal gamma power. In both neoPCP and neoVEH rats, MK-801 (0.1 mg/kg and 0.3 mg/kg sc, cumulative doses) dose-dependently decreased evoked theta in both the vHPC [F(63,618) = 16.09, P < 0.001; Fig. 3A, left] and dHPC [F(63,746) = 11.28, P < 0.001; Fig. 3B, left] when normalized to preinjection values. In the vHPC, neoPCP animals displayed reduced absolute theta power [F(1,40) = 29.19, P < 0.001; Fig. 3A, top right] but with similar relative reductions in theta power in response to MK-801 application for both neoVEH and neoPCP rats [F(3,40) = 4.275, P = 0.0104; Fig. 3A, bottom right]. In dHPC, no overall effect of neonatal PCP administration or MK-801 application on evoked absolute theta power values was observed (Fig. 3B, top right). However, when transformed to normalized values, a significant effect of MK-801 application was found [F(2,31) = 37.15, P < 0.001; Fig. 3B, bottom right], which was not significantly different between neoPCP and neoVEH rats.
Fig. 3.
Rats administered PCP neonatally display hypersensitive gamma oscillations in response to acute MK-801 application. A: animals were administered saline sc and then injected sc with 0.1 mg/kg and 0.2 mg/kg MK-801 at 30 and 60 min after the saline injection, respectively, while local field potentials in ventral hippocampus (vHPC), dorsal hippocampus (dHPC), and medial prefrontal cortex (mPFC) were recorded. MK-801 application reduced vHPC theta power (unpaired t-test, P < 0.001), with no difference between the relative reductions in neoVEH and neoPCP animals. The evoked absolute theta power baseline before MK-801 application was decreased in neoPCP compared with neoVEH rats (unpaired t-test, P = 0.0104; Holm-Sidak multiple-comparison post hoc test, P < 0.001). B: MK-801 administration reduced evoked normalized theta power in dHPC (unpaired t-test, P < 0.0001), with no difference between the baseline theta level and the relative size of the reduction between neoVEH and neoPCP rats. C: MK-801 increased gamma power in neoPCP animals (unpaired t-test, P < 0.0001; Holm-Sidak multiple-comparison post hoc test, P < 0.0001) to a greater extent than in control animals (Holm-Sidak multiple-comparison post hoc test, P = 0.0043). Data are represented as means ± SE. vHPC: neoVEH, n = 5; neoPCP, n = 7. dHPC: neoVEH, n = 9; neoPCP, n = 10. mPFC: neoVEH, n = 11; neoPCP, n = 14. *P < 0.05, **P < 0.01; ***P < 0.001.
Normalized prefrontal gamma power was significantly increased by MK-801 administration [F(63,1490) = 32.32, P < 0.001], and neoPCP rats displayed a hypersensitive response to the gamma power-enhancing effect of MK-801 compared with neoVEH rats [F(1,1490) = 81.39, P < 0.0001; Fig. 3C, left]. These differences were also represented in the absolute prefrontal gamma values, with respect to both MK-801 administration [F(3,92) = 13.92, P < 0.001; Fig. 3C, top right] and the exacerbated response of neoPCP rats [F(1,92) = 18.43, P < 0.0001; Fig. 3C, top right]. A dose-dependent increase in normalized gamma power was also found for both MK-801 application [F(2,65) = 39.64, P < 0.001] and the response to MK-801 for neoPCP compared with neoVEH rats [F(1,65) = 8.770, P = 0.0043; Fig. 3C, bottom right].
DISCUSSION
We investigated whether a NMDAR antagonist-based developmental rat model of schizophrenia displayed alterations in evoked oscillatory patterns within hippocampus and mPFC. We showed that oscillatory increases in hippocampal theta power and prefrontal gamma power evoked by stimulation of the reticular formation were diminished and enhanced, respectively, in adult rats administered neonatally or acutely with an NMDAR antagonist. In addition, the hyperoscillatory gamma responses elicited by acute administration of a NMDAR antagonist were exaggerated in rats administered neonatally with PCP.
Stimulation of reticular formation enhances hippocampal theta and prefrontal gamma power.
It is well known that high-frequency electrical stimulation of the reticular formation evokes theta oscillations in hippocampus (Kinney et al. 1999; Macadar et al. 1974; Oddie et al. 1994; Vertes 1981). The resulting theta synchronization has been proposed to involve an ascending polysynaptic pathway originating in the reticular formation and reaching the hippocampus through the supramammillary nucleus and medial septum (Bland 1986). We performed simultaneous recordings of LFPs in vHPC/dHPC and mPFC and showed that stimulation of the reticular formation in addition to enhancement of theta rhythms in hippocampus also elicits increases in gamma oscillations within mPFC. Interestingly, vHPC is strongly connected to mPFC (Ishikawa and Nakamura 2006; Verwer et al. 1997), and synchronization between these two regions increases during behaviors involving anxiety (Adhikari et al. 2010, 2011; Ciocchi et al. 2015; Padilla-Coreano et al. 2016), indicating that the evoked hippocampal theta rhythm could potentially be driving prefrontal gamma power. We found that theta coherence between the dorsal hippocampal region and mPFC was slightly reduced in neoPCP animals in comparison to neoVEH animals especially during evoked stimulation, indicating that the activation of the reticular formation induces changes in prefrontal oscillations through hippocampus.
Neonatal and acute NMDAR antagonist administrations reduce evoked hippocampal theta and enhance evoked prefrontal gamma rhythms.
We found that neoPCP administration reduced evoked theta power in vHPC and increased gamma power in mPFC. When we administered acute doses of a NMDAR antagonist in adult neoVEH rats the same pattern was evident, indicating that neonatal PCP administration induces changes in the neural network within vHPC and mPFC resembling the changes occurring after acute administration of NMDAR antagonists. In addition, neonatal PCP administration did not affect theta rhythm in dHPC, pointing toward a specific disruption of the vHPC-prefrontal pathway following NMDAR treatment. Recently, it was demonstrated that hippocampal theta bursts drive prefrontal oscillations already in the neonatal period (Brockmann et al. 2011), possibly directing maturation of cortical networks, which neonatal PCP administration could interfere with. In neoPCP animals, we observed suppressed coherence in the theta range between dHPC and mPFC as well as disrupted correlation between hippocampal theta and prefrontal gamma rhythms, indicating that neonatal PCP administration might indeed affect oscillatory power through compromised synchronization between hippocampal and prefrontal regions.
The reduced power of evoked theta oscillations in vHPC observed in neoPCP animals may relate to the observed reduction in hippocampal parvalbumin in this animal model (Nakatani-Pawlak et al. 2009; Okamoto et al. 2012), since genetic ablation of the NMDAR in parvalbumin-positive interneurons reduces hippocampal theta activity (Korotkova et al. 2010). Similarly, we and others have previously shown that parvalbumin expression and GABAergic function in mPFC are reduced in the neonatal PCP model (Jeevakumar and Kroener 2016; Kaalund et al. 2013; Kjaerby et al. 2014). Since prefrontal parvalbumin-positive interneurons play an important role in the generation of prefrontal gamma oscillations (Cardin et al. 2009; Sohal et al. 2009), the observed gamma hypersynchronies in the neonatal PCP model might reflect a loss of inhibitory tone.
Numerous studies have revealed aberrant gamma oscillations in patients with schizophrenia (Spencer et al. 2003, 2004; Uhlhaas and Singer 2010), likely leading to a failure to integrate the activity of local and distributed neural networks. In healthy subjects, gamma oscillations are believed to play an important role in cognitive tasks such as attention and working memory, and the aberrant gamma oscillatory activity in schizophrenia has been suggested to underlie both psychosis and cognitive impairments (Uhlhaas et al. 2008). In fact, cognitive deficits in patients with schizophrenia correlate with deficient gamma oscillatory activity (Cho et al. 2006; Lesh et al. 2011), and patients with schizophrenia display reduced gamma oscillatory activity during cognitive and sensory tasks (Basar-Eroglu et al. 2007). Resting-state cortical theta activity in patients with schizophrenia is reported to be increased (Moran and Hong 2011; Shreekantiah Umesh et al. 2016; Sponheim et al. 1994), while task-related theta oscillations are reduced (Başar and Güntekin 2013). We observe that in anesthetized neoPCP animals compared with vehicle control animals, evoked prefrontal gamma oscillations were increased while evoked vHPC theta oscillations were reduced. This indicates a complex effect of PCP on the interaction between hippocampal and prefrontal regions, where suppressed hippocampal input to mPFC might exacerbate prefrontal disinhibition leading to hyperoscillations.
neoPCP rats are hypersensitive to acute NMDAR antagonist administration.
Finally, we demonstrated that neoPCP rats are hypersensitive to NMDAR antagonist-induced increases in prefrontal gamma power. Increases in gamma oscillatory activity mediated by acute administration of NMDAR antagonists are observed in both rodents and humans (Hakami et al. 2009; Pinault 2008) and have been proposed to be responsible for psychosis-like states in healthy subjects (Krystal et al. 1994). Furthermore, positive symptoms in schizophrenia, particularly hallucinations, have been associated with cortical gamma hypersynchrony (Mulert et al. 2011; Spencer et al. 2009). Our results suggest that the deficits induced by PCP administration during neurodevelopment are further challenged with acute administration of NMDAR antagonists, leading to an exaggerated response of gamma rhythms. At the cellular level, acute systemic NMDAR blockade potentiates spike firing in pyramidal cells (Jackson et al. 2004; Suzuki et al. 2002), which has been linked to a reduction of interneuron function (Homayoun and Moghaddam 2007).
Conclusions.
In conclusion, the functional alterations in mPFC and vHPC oscillatory activity reported in the present study suggest that disruptions during neurodevelopment have a serious impact on later adult circuit function and further validate neonatal PCP administration as a promising model of schizophrenia, as it recapitulates several of the behavioral and neurophysiological alterations reported in schizophrenia.
GRANTS
The work performed in this study was financially supported by the Danish Agency for Science, Technology and Innovation.
DISCLOSURES
This work was performed at H. Lundbeck A/S, but no conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.K., N.H., N.O.D., and F.S. conceived and designed research; C.K. and N.H. performed experiments; C.K. and N.H. analyzed data; C.K., N.H., and F.S. interpreted results of experiments; C.K. and N.H. prepared figures; C.K. and N.H. drafted manuscript; C.K., N.H., N.O.D., and F.S. edited and revised manuscript; C.K. and N.H. approved final version of manuscript.
REFERENCES
- Adams B, Moghaddam B. Corticolimbic dopamine neurotransmission is temporally dissociated from the cognitive and locomotor effects of phencyclidine. J Neurosci 18: 5545–5554, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adhikari A, Topiwala MA, Gordon JA. Synchronized activity between the ventral hippocampus and the medial prefrontal cortex during anxiety. Neuron 65: 257–269, 2010. doi: 10.1016/j.neuron.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adhikari A, Topiwala MA, Gordon JA. Single units in the medial prefrontal cortex with anxiety-related firing patterns are preferentially influenced by ventral hippocampal activity. Neuron 71: 898–910, 2011. doi: 10.1016/j.neuron.2011.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler CM, Malhotra AK, Elman I, Goldberg T, Egan M, Pickar D, Breier A. Comparison of ketamine-induced thought disorder in healthy volunteers and thought disorder in schizophrenia. Am J Psychiatry 156: 1646–1649, 1999. doi: 10.1176/ajp.156.10.1646. [DOI] [PubMed] [Google Scholar]
- Anver H, Ward PD, Magony A, Vreugdenhil M. NMDA receptor hypofunction phase couples independent γ-oscillations in the rat visual cortex. Neuropsychopharmacology 36: 519–528, 2011. doi: 10.1038/npp.2010.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Başar E, Güntekin B. Review of delta, theta, alpha, beta, and gamma response oscillations in neuropsychiatric disorders. Suppl Clin Neurophysiol 62: 303–341, 2013. doi: 10.1016/B978-0-7020-5307-8.00019-3. [DOI] [PubMed] [Google Scholar]
- Basar-Eroglu C, Brand A, Hildebrandt H, Karolina Kedzior K, Mathes B, Schmiedt C. Working memory related gamma oscillations in schizophrenia patients. Int J Psychophysiol 64: 39–45, 2007. doi: 10.1016/j.ijpsycho.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Bayer SA, Altman J, Russo RJ, Zhang X. Timetables of neurogenesis in the human brain based on experimentally determined patterns in the rat. Neurotoxicology 14: 83–144, 1993. [PubMed] [Google Scholar]
- Belforte JE, Zsiros V, Sklar ER, Jiang Z, Yu G, Li Y, Quinlan EM, Nakazawa K. Postnatal NMDA receptor ablation in corticolimbic interneurons confers schizophrenia-like phenotypes. Nat Neurosci 13: 76–83, 2010. doi: 10.1038/nn.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland BH. The physiology and pharmacology of hippocampal formation theta rhythms. Prog Neurobiol 26: 1–54, 1986. doi: 10.1016/0301-0082(86)90019-5. [DOI] [PubMed] [Google Scholar]
- Broberg BV, Dias R, Glenthøj BY, Olsen CK. Evaluation of a neurodevelopmental model of schizophrenia—early postnatal PCP treatment in attentional set-shifting. Behav Brain Res 190: 160–163, 2008. doi: 10.1016/j.bbr.2008.02.020. [DOI] [PubMed] [Google Scholar]
- Brockmann MD, Pöschel B, Cichon N, Hanganu-Opatz IL. Coupled oscillations mediate directed interactions between prefrontal cortex and hippocampus of the neonatal rat. Neuron 71: 332–347, 2011. doi: 10.1016/j.neuron.2011.05.041. [DOI] [PubMed] [Google Scholar]
- Brown AS, Derkits EJ. Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatry 167: 261–280, 2010. doi: 10.1176/appi.ajp.2009.09030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callicott JH, Mattay VS, Verchinski BA, Marenco S, Egan MF, Weinberger DR. Complexity of prefrontal cortical dysfunction in schizophrenia: more than up or down. Am J Psychiatry 160: 2209–2215, 2003. doi: 10.1176/appi.ajp.160.12.2209. [DOI] [PubMed] [Google Scholar]
- Cardin JA, Carlén M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai LH, Moore CI. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature 459: 663–667, 2009. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho RY, Konecky RO, Carter CS. Impairments in frontal cortical gamma synchrony and cognitive control in schizophrenia. Proc Natl Acad Sci USA 103: 19878–19883, 2006. doi: 10.1073/pnas.0609440103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciocchi S, Passecker J, Malagon-Vina H, Mikus N, Klausberger T. Brain computation. Selective information routing by ventral hippocampal CA1 projection neurons. Science 348: 560–563, 2015. doi: 10.1126/science.aaa3245. [DOI] [PubMed] [Google Scholar]
- Clancy B, Darlington RB, Finlay BL. Translating developmental time across mammalian species. Neuroscience 105: 7–17, 2001. doi: 10.1016/S0306-4522(01)00171-3. [DOI] [PubMed] [Google Scholar]
- Deutsch SI, Mastropaolo J, Rosse RB. Neurodevelopmental consequences of early exposure to phencyclidine and related drugs. Clin Neuropharmacol 21: 320–332, 1998. [PubMed] [Google Scholar]
- Dickerson DD, Wolff AR, Bilkey DK. Abnormal long-range neural synchrony in a maternal immune activation animal model of schizophrenia. J Neurosci 30: 12424–12431, 2010. doi: 10.1523/JNEUROSCI.3046-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher P. The missing link: a failure of fronto-hippocampal integration in schizophrenia. Nat Neurosci 1: 266–267, 1998. doi: 10.1038/1078. [DOI] [PubMed] [Google Scholar]
- Green MF, Kern RS, Heaton RK. Longitudinal studies of cognition and functional outcome in schizophrenia: implications for MATRICS. Schizophr Res 72: 41–51, 2004. doi: 10.1016/j.schres.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Hakami T, Jones NC, Tolmacheva EA, Gaudias J, Chaumont J, Salzberg M, O’Brien TJ, Pinault D. NMDA receptor hypofunction leads to generalized and persistent aberrant gamma oscillations independent of hyperlocomotion and the state of consciousness. PLoS One 4: e6755, 2009. doi: 10.1371/journal.pone.0006755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homayoun H, Moghaddam B. NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J Neurosci 27: 11496–11500, 2007. doi: 10.1523/JNEUROSCI.2213-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong LE, Summerfelt A, Buchanan RW, O’Donnell P, Thaker GK, Weiler MA, Lahti AC. Gamma and delta neural oscillations and association with clinical symptoms under subanesthetic ketamine. Neuropsychopharmacology 35: 632–640, 2010. doi: 10.1038/npp.2009.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman JM, Zilli EA, Paley AM, Hasselmo ME. Working memory performance correlates with prefrontal-hippocampal theta interactions but not with prefrontal neuron firing rates. Front Integr Neurosci 4: 2, 2010. doi: 10.3389/neuro.07.002.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa A, Nakamura S. Ventral hippocampal neurons project axons simultaneously to the medial prefrontal cortex and amygdala in the rat. J Neurophysiol 96: 2134–2138, 2006. doi: 10.1152/jn.00069.2006. [DOI] [PubMed] [Google Scholar]
- Jackson ME, Homayoun H, Moghaddam B. NMDA receptor hypofunction produces concomitant firing rate potentiation and burst activity reduction in the prefrontal cortex. Proc Natl Acad Sci USA 101: 8467–8472, 2004. doi: 10.1073/pnas.0308455101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeevakumar V, Driskill C, Paine A, Sobhanian M, Vakil H, Morris B, Ramos J, Kroener S. Ketamine administration during the second postnatal week induces enduring schizophrenia-like behavioral symptoms and reduces parvalbumin expression in the medial prefrontal cortex of adult mice. Behav Brain Res 282: 165–175, 2015. doi: 10.1016/j.bbr.2015.01.010. [DOI] [PubMed] [Google Scholar]
- Jeevakumar V, Kroener S. Ketamine administration during the second postnatal week alters synaptic properties of fast-spiking interneurons in the medial prefrontal cortex of adult mice. Cereb Cortex 26: 1117–1129, 2016. doi: 10.1093/cercor/bhu293. [DOI] [PubMed] [Google Scholar]
- Kaalund SS, Riise J, Broberg BV, Fabricius K, Karlsen AS, Secher T, Plath N, Pakkenberg B. Differential expression of parvalbumin in neonatal phencyclidine-treated rats and socially isolated rats. J Neurochem 124: 548–557, 2013. doi: 10.1111/jnc.12061. [DOI] [PubMed] [Google Scholar]
- Kinney GG, Patino P, Mermet-Bouvier Y, Starrett JE Jr, Gribkoff VK. Cognition-enhancing drugs increase stimulated hippocampal theta rhythm amplitude in the urethane-anesthetized rat. J Pharmacol Exp Ther 291: 99–106, 1999. [PubMed] [Google Scholar]
- Kjaerby C, Broberg BV, Kristiansen U, Dalby NO. Impaired GABAergic inhibition in the prefrontal cortex of early postnatal phencyclidine (PCP)-treated rats. Cereb Cortex 24: 2522–2532, 2014. doi: 10.1093/cercor/bht109. [DOI] [PubMed] [Google Scholar]
- Kjaerby C, Bundgaard C, Fejgin K, Kristiansen U, Dalby NO. Repeated potentiation of the metabotropic glutamate receptor 5 and the alpha 7 nicotinic acetylcholine receptor modulates behavioural and GABAergic deficits induced by early postnatal phencyclidine (PCP) treatment. Neuropharmacology 72: 157–168, 2013. doi: 10.1016/j.neuropharm.2013.04.041. [DOI] [PubMed] [Google Scholar]
- Korotkova T, Fuchs EC, Ponomarenko A, von Engelhardt J, Monyer H. NMDA receptor ablation on parvalbumin-positive interneurons impairs hippocampal synchrony, spatial representations, and working memory. Neuron 68: 557–569, 2010. doi: 10.1016/j.neuron.2010.09.017. [DOI] [PubMed] [Google Scholar]
- Kowalczyk T, Konopacki J. Depth amplitude and phase profiles of carbachol-induced theta in hippocampal formation slices. Brain Res Bull 58: 569–574, 2002. doi: 10.1016/S0361-9230(02)00827-4. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB Jr, Charney DS. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry 51: 199–214, 1994. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- Lahti AC, Weiler MA, Tamara Michaelidis BA, Parwani A, Tamminga CA. Effects of ketamine in normal and schizophrenic volunteers. Neuropsychopharmacology 25: 455–467, 2001. doi: 10.1016/S0893-133X(01)00243-3. [DOI] [PubMed] [Google Scholar]
- Lesh TA, Niendam TA, Minzenberg MJ, Carter CS. Cognitive control deficits in schizophrenia: mechanisms and meaning. Neuropsychopharmacology 36: 316–338, 2011. doi: 10.1038/npp.2010.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Levitt P. Schizophrenia as a disorder of neurodevelopment. Annu Rev Neurosci 25: 409–432, 2002. doi: 10.1146/annurev.neuro.25.112701.142754. [DOI] [PubMed] [Google Scholar]
- Macadar AW, Chalupa LM, Lindsley DB. Differentiation of brain stem loci which affect hippocampal and neocortical electrical activity. Exp Neurol 43: 499–514, 1974. doi: 10.1016/0014-4886(74)90190-3. [DOI] [PubMed] [Google Scholar]
- Malhotra AK, Pinals DA, Weingartner H, Sirocco K, Missar CD, Pickar D, Breier A. NMDA receptor function and human cognition: the effects of ketamine in healthy volunteers. Neuropsychopharmacology 14: 301–307, 1996. doi: 10.1016/0893-133X(95)00137-3. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg AS, Olsen RK, Kohn PD, Brown T, Egan MF, Weinberger DR, Berman KF. Regionally specific disturbance of dorsolateral prefrontal-hippocampal functional connectivity in schizophrenia. Arch Gen Psychiatry 62: 379–386, 2005. doi: 10.1001/archpsyc.62.4.379. [DOI] [PubMed] [Google Scholar]
- Moran LV, Hong LE. High vs. low frequency neural oscillations in schizophrenia. Schizophr Bull 37: 659–663, 2011. doi: 10.1093/schbul/sbr056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulert C, Kirsch V, Pascual-Marqui R, McCarley RW, Spencer KM. Long-range synchrony of γ oscillations and auditory hallucination symptoms in schizophrenia. Int J Psychophysiol 79: 55–63, 2011. doi: 10.1016/j.ijpsycho.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani-Pawlak A, Yamaguchi K, Tatsumi Y, Mizoguchi H, Yoneda Y. Neonatal phencyclidine treatment in mice induces behavioral, histological and neurochemical abnormalities in adulthood. Biol Pharm Bull 32: 1576–1583, 2009. doi: 10.1248/bpb.32.1576. [DOI] [PubMed] [Google Scholar]
- Oddie SD, Bland BH, Colom LV, Vertes RP. The midline posterior hypothalamic region comprises a critical part of the ascending brainstem hippocampal synchronizing pathway. Hippocampus 4: 454–473, 1994. doi: 10.1002/hipo.450040408. [DOI] [PubMed] [Google Scholar]
- Okamoto M, Katayama T, Suzuki Y, Hoshino KY, Yamada H, Matsuoka N, Jodo E. Neonatal administration of phencyclidine decreases the number of putative inhibitory interneurons and increases neural excitability to auditory paired clicks in the hippocampal CA3 region of freely moving adult mice. Neuroscience 224: 268–281, 2012. doi: 10.1016/j.neuroscience.2012.08.013. [DOI] [PubMed] [Google Scholar]
- Padilla-Coreano N, Bolkan SS, Pierce GM, Blackman DR, Hardin WD, Garcia-Garcia AL, Spellman TJ, Gordon JA. Direct ventral hippocampal-prefrontal input is required for anxiety-related neural activity and behavior. Neuron 89: 857–866, 2016. doi: 10.1016/j.neuron.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates (6th ed.). London: Academic, 2007. [Google Scholar]
- Pinault D. N-methyl d-aspartate receptor antagonists ketamine and MK-801 induce wake-related aberrant gamma oscillations in the rat neocortex. Biol Psychiatry 63: 730–735, 2008. doi: 10.1016/j.biopsych.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Rompala GR, Zsiros V, Zhang S, Kolata SM, Nakazawa K. Contribution of NMDA receptor hypofunction in prefrontal and cortical excitatory neurons to schizophrenia-like phenotypes. PLoS One 8: e61278, 2013. doi: 10.1371/journal.pone.0061278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samudra N, Ivleva EI, Hubbard NA, Rypma B, Sweeney JA, Clementz BA, Keshavan MS, Pearlson GD, Tamminga CA. Alterations in hippocampal connectivity across the psychosis dimension. Psychiatry Res 233: 148–157, 2015. doi: 10.1016/j.pscychresns.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shreekantiah Umesh D, Tikka SK, Goyal N, Nizamie SH, Sinha VK. Resting state theta band source distribution and functional connectivity in remitted schizophrenia. Neurosci Lett 630: 199–202, 2016. doi: 10.1016/j.neulet.2016.07.055. [DOI] [PubMed] [Google Scholar]
- Sigurdsson T, Stark KL, Karayiorgou M, Gogos JA, Gordon JA. Impaired hippocampal-prefrontal synchrony in a genetic mouse model of schizophrenia. Nature 464: 763–767, 2010. doi: 10.1038/nature08855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirota A, Montgomery S, Fujisawa S, Isomura Y, Zugaro M, Buzsáki G. Entrainment of neocortical neurons and gamma oscillations by the hippocampal theta rhythm. Neuron 60: 683–697, 2008. doi: 10.1016/j.neuron.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature 459: 698–702, 2009. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer KM, Nestor PG, Niznikiewicz MA, Salisbury DF, Shenton ME, McCarley RW. Abnormal neural synchrony in schizophrenia. J Neurosci 23: 7407–7411, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer KM, Nestor PG, Perlmutter R, Niznikiewicz MA, Klump MC, Frumin M, Shenton ME, McCarley RW. Neural synchrony indexes disordered perception and cognition in schizophrenia. Proc Natl Acad Sci USA 101: 17288–17293, 2004. doi: 10.1073/pnas.0406074101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer KM, Niznikiewicz MA, Nestor PG, Shenton ME, McCarley RW. Left auditory cortex gamma synchronization and auditory hallucination symptoms in schizophrenia. BMC Neurosci 10: 85, 2009. doi: 10.1186/1471-2202-10-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sponheim SR, Clementz BA, Iacono WG, Beiser M. Resting EEG in first-episode and chronic schizophrenia. Psychophysiology 31: 37–43, 1994. doi: 10.1111/j.1469-8986.1994.tb01023.x. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Jodo E, Takeuchi S, Niwa S, Kayama Y. Acute administration of phencyclidine induces tonic activation of medial prefrontal cortex neurons in freely moving rats. Neuroscience 114: 769–779, 2002. doi: 10.1016/S0306-4522(02)00298-1. [DOI] [PubMed] [Google Scholar]
- Thierry AM, Gioanni Y, Dégénétais E, Glowinski J. Hippocampo-prefrontal cortex pathway: anatomical and electrophysiological characteristics. Hippocampus 10: 411–419, 2000. doi:. [DOI] [PubMed] [Google Scholar]
- Tierney PL, Dégenètais E, Thierry AM, Glowinski J, Gioanni Y. Influence of the hippocampus on interneurons of the rat prefrontal cortex. Eur J Neurosci 20: 514–524, 2004. doi: 10.1111/j.1460-9568.2004.03501.x. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Haenschel C, Nikolić D, Singer W. The role of oscillations and synchrony in cortical networks and their putative relevance for the pathophysiology of schizophrenia. Schizophr Bull 34: 927–943, 2008. doi: 10.1093/schbul/sbn062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci 11: 100–113, 2010. doi: 10.1038/nrn2774. [DOI] [PubMed] [Google Scholar]
- Vertes RP. An analysis of ascending brain stem systems involved in hippocampal synchronization and desynchronization. J Neurophysiol 46: 1140–1159, 1981. [DOI] [PubMed] [Google Scholar]
- Verwer RW, Meijer RJ, Van Uum HF, Witter MP. Collateral projections from the rat hippocampal formation to the lateral and medial prefrontal cortex. Hippocampus 7: 397–402, 1997. doi:. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Berman KF, Zec RF. Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia. I. Regional cerebral blood flow evidence. Arch Gen Psychiatry 43: 114–124, 1986. doi: 10.1001/archpsyc.1986.01800020020004. [DOI] [PubMed] [Google Scholar]